ABSTRACT
ADAR mediated A-to-I RNA editing is thought to be unique to animals and occurs mainly in the non-coding regions. Recently filamentous fungi such as Fusarium graminearum were found to lack orthologs of animal ADARs but have stage-specific A-to-I editing during sexual reproduction. Unlike animals, majority of editing sites are in the coding regions and often result in missense and stop loss changes in fungi. Furthermore, whereas As in RNA stems are targeted by animal ADARs, RNA editing in fungi preferentially targets As in hairpin loops, implying that fungal RNA editing involves mechanisms related to editing of the anticodon loop by ADATs. Identification and characterization of fungal adenosine deaminases and their stage-specific co-factors may be helpful to understand the evolution of human ADARs. Fungi also can be used to study biological functions of missense and stop loss RNA editing events in eukaryotic organisms.
Keywords: Adenosine deaminases, Fusarium, Inosine, Neurospora, RNA editing
A-to-I RNA editing occurs in F. graminearum and other filamentous fungi
A-to-I editing of mRNA catalyzed by Adenosine Deaminases Acting on RNA (ADAR) is considered to be unique to animals.1,2 In humans, over 1.4 million of A-to-I editing sites have been identified but only approximately 100 of them result in amino acid changes.3 In general, biological functions of mRNA editing in animals remains poorly understood because majority of editing events occur in the non-coding regions.4 To date, A-to-I editing of ion channels and neurotransmitter receptors are among the best-studied ADAR-mediated recoding events.5,6 Nevertheless, defects in A-to-I editing have been associated with human diseases, such as Dyschromatosis symmetrica hereditaria (DSH) caused by mutations in human ADAR1.7-9
Like plants, fungi have no predicted genes that encode proteins with both adenine deaminase domain and dsRNA binding domain, the hallmark of metazoan ADARs.1,10 However, the 2 tandem stop codons UA1831GUA1834G in the kinase domain of the PUK1 gene were found to be edited to UG1831 GUG1834G specifically during sexual reproduction in Fusarium graminearum, a causal agent of Fusarium head blight (FHB) of wheat and barley in the US and many other countries.11,12 F. graminearum is a haploid ascomycete that produces perithecia (sexual fruiting bodies) and ascospores (sexual spores) on infected plant debris. Ascospores are forcefully released from perithecia and function as the primary inoculum for FHB. Genome-wide analysis with strand-specific RNA-seq data generated with RNA isolated from perithecia identified 26,056 A-to-I editing sites.13 In RNA samples isolated from conidia (asexual spores) and vegetative hyphae, no A-to-I editing sites were identified.13 The average editing level is 14.8% in F. graminearum, which is similar to that of animals.14,15 However, genes that are functionally related to sexual reproduction or up-regulated during sexual reproduction tend to have editing sites with higher editing levels in fungi. The PUK1 kinase gene is essential for ascospore release and over 90% of its transcripts have both A1831 and A1834 edited to I in perithecia.
Stage-specific RNA editing also occurs during sexual reproduction in F. verticillioides.13 Analysis with published RNA-seq data16,17 showed that genome-wide A-to-I RNA editing also occurs in the model filamentous fungus Neurospora crassa 13 and its closely related species N. tetrasperma. However, because these published RNA-seq data of F. verticillioides and Neurospora species were un-stranded and generated from early sequencing platforms with short single end reads, they had high error rate and were not suitable for systematic identification of A-to-I editing sites. Recently, we generated strand-specific RNA-seq data of N. crassa. Our preliminary analysis showed that N. crassa has over 40,000 A-to-I editing events specifically occurred during sexual reproduction (our unpublished data). Therefore, sexual stage-specific A-to-I RNA editing likely is a common phenomenon in Sordariomycetes. Nevertheless, it will be important to determine the range of ascomycetes with stage-specific RNA editing when suitable RNA-seq data become available for other filamentous fungi. The budding yeast Saccharomyces cerevisiae and fission yeast Schizosaccharomyces pombe lack A-to-I editing.
Majority of fungal RNA editing sites are in the CDSs and result in amino acid changes
In humans and many other animals, A-to-I editing mainly occurs in the introns, 5′-UTRs, and 3′-UTRs.15,18-20 In contrast, 70.5% of A-to-I editing sites in F. graminearum occur in the coding regions (CDSs) and over 2-thirds of them result in amino acid changes.13 A total of 4,594 genes have at least one missense editing event during sexual reproduction. Because missense editing sites tend to have higher editing levels than synonymous editing sites and 76.2% of missense editing events cause changes to amino acids of different physicochemical properties,13 it is likely that A-to-I editing is important for adaptation and diversification protein functions during sexual reproduction in F. graminearum.
Although no nonsense editing was identified in F. graminearum, 503 genes had editing events occurred at the stop codon. Whereas 323 of them had the stop loss editing events that result in additional amino acid residues being added to the C-terminus, 69 genes had the UAG to UGG editing in CDSs that is necessary to encode full-length proteins in perithecia. Most of these genes were specifically expressed or upregulated during sexual reproduction. In N. crassa, like in F. graminearum, majority of the editing events are in the CDSs and cause missense or stop loss changes.
Sequence preference of fungal RNA editing differs from that of animal ADARs
When analyzed for the flanking sequences of the edited As, the −1 position has a strong preference for U in both F. graminearum 13 and F. verticillioides (Fig. 1A). In animals, such a strong preference for U at the −1 position was not observed.21 Fungal editing sites also have a preference for A or G at the +1 and +3 positions (Fig. 1A), which is also different from what has been observed in animals.21 The enrichment for U at −1 and A or G at +1 positions may be directly responsible for the high editing frequency of the UAG and UAA stop codons in F. graminearum.13 Therefore, fungal adenosine deaminases likely differ from animal ADARs in sequence preference for A-to-I RNA editing.
Figure 1.
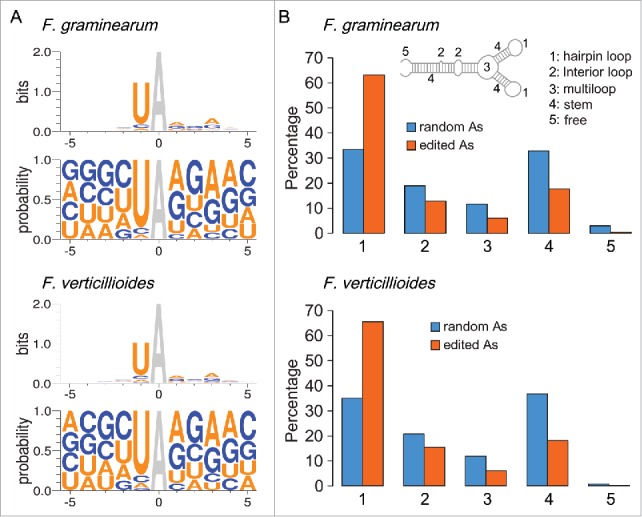
Sequence and structure preferences of A-to-I RNA editing in F. graminearum and F. verticillioides. (A) Frequency of different nucleotides at the −1 to −5 and +1 to +5 positions of the edited As (in gray) in F. graminearum (upper panel) and F. verticillioides (lower panel). (B) Preference of edited As in hairpin loops based on the distribution of 26,056 edited sites in F. graminearum (upper panel) and 7,659 editing sites in F. verticillioides (lower panel). Equal numbers of randomly chosen A sites from protein coding regions were used as the control (random As).
In animals, RNA structure is known to affect editing specificity and As in RNA stems (dsRNA) are preferentially targeted by ADARs for editing.22 In contrast, RNA editing preferentially targets As in hairpin loops (Fig. 1B) and the stability of hairpin loops affects editing efficiency in F. graminearum.13 Because all the sequenced fungal genomes lack orthologs of animal ADAR genes, preferential editing of As in hairpin loops suggest that A-to-I RNA editing in fungi likely involves novel mechanisms related to editing of tRNA in the anticodon loop by Adenosine Deaminases Acting on tRNA (ADAT) enzymes. Like other filamentous fungi, F. graminearum has 3 ADAT genes orthologous to yeast TAD1, TAD2, and TAD3.23,24 Target deletion of FgTAD1 had no obvious effects on A-to-I RNA editing in F. graminearum.13 However, deletion of FgTAD2 or FgTAD2 may be lethal, similar to what was reported in yeast.24 Because none of the ADATs and other putative adenosine deaminase genes were specifically expressed in perithecia, A-to-I editing in fungi is likely catalyzed by fungal adenosine deaminases together with their stage-specific co-factors during sexual reproduction. However, the intracellular compartmentalization of ADAR1 and ADAR2 is known to regulate the editing of their substrates in humans.25,26 In fungi, it is also possible that sexual stage-specific RNA editing is regulated by the intracellular compartmentalization of fungal adenosine deaminases in perithecia.
Could A-to-I RNA editing occur in other developmental or infection stages?
In both F. graminearum and N. crassa, RNA editing was found in RNA-seq data of perithecia but not in RNA-seq data of conidia and hyphae. Preliminary analysis with public available RNA-seq data of F. graminearum showed that RNA editing was not detected in infected plant tissues. We also failed to identify A-to-I RNA editing during plant infection in publically available RNA-seq data of Colletotrichum graminicola (ERP011838), Sclerotinia sclerotiorum (SRP056176), Valsa mali (SRP034726), and Magnaporthe oryzae (DRP000568). However, there are only limited fungal reads in RNA-seq data of infected plant tissues of these 4 important plant pathogenic fungi. Therefore, it remains possible that missense editing of effector proteins in pathogenic fungi will provide sequence diversity to overcome host immunity, particularly in pathogens like Pneumocystis jirovecii, the causal agent of human Pneumocystis pneumonia that reproduces sexually in host tissues.27 Sexual reproduction also is co-regulated with pathogenesis in a number of plant pathogenic fungi, such as Ustilago maydis and Taphrina deformans.28,29 Furthermore, it is also possible that RNA editing occurs in other fungal differentiation or developmental stages, such as sclerotium formation and differentiation of specific fungal penetration or survival structures.
RNA editing and other sexual stage-specific genetic or epigenetic phenomena
Like RNA editing, RIP (repeat induced point mutation), MSUD (meiotic silencing by unpaired DNA), and spore killer are genetic and epigenetic phenomena that specifically occur during sexual reproduction in Neurospora, Fusarium, and other filamentous fungi.30-34 RIP is a genome defense mechanism that occurs premeiotically (Fig. 2) to introduce point mutations to repetitive sequences.35 RIPped sequences are often heavily methylated in sexual progeny and the putative cytosine methyltransferase gene rid is essential for RIP in N. crassa.30 In F. graminearum, the rid ortholog has the UA644G premature stop codon that requires stage-specific editing to encode a full-length functional protein during sexual reproduction (Fig. 3A). The corresponding stop codon (UA590G) was also found in the rid ortholog in F. verticillioides (Fig. 3B). The editing level for this editing site is over 60% in both F. graminearum and F. verticillioides. The rid orthologs in many Fusarium and other Nectriaceae species have the same stop codon in the CDS that requires A-to-I editing to encode the full-length protein (Fig. 3B). Therefore, RNA editing may be essential for RIP in F. graminearum, F. verticillioides, and other Nectriaceae species.
Figure 2.
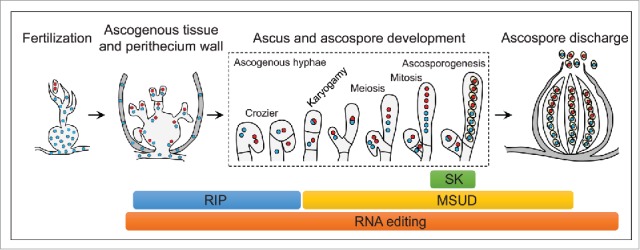
The occurrence of A-to-I RNA editing and other stage-specific genetic and epigenetic phenomena during sexual reproduction in Sordariomycetes and other filamentous ascomycetes. The approximate stages in which the genetic and epigenetic processes occur are indicated as colored bars. Whereas repeat-induced point mutation (RIP) occurs premeiotically, meiotic silencing of unpaired DNA (MSUD) occurs during meiosis and is important for ascospore formation. Spore killer (SK) is functional at late stages of ascospore development and maturation. RNA editing, however, like occurs before RIP and is also important for ascospore formation and release in F. graminearum.
Figure 3.
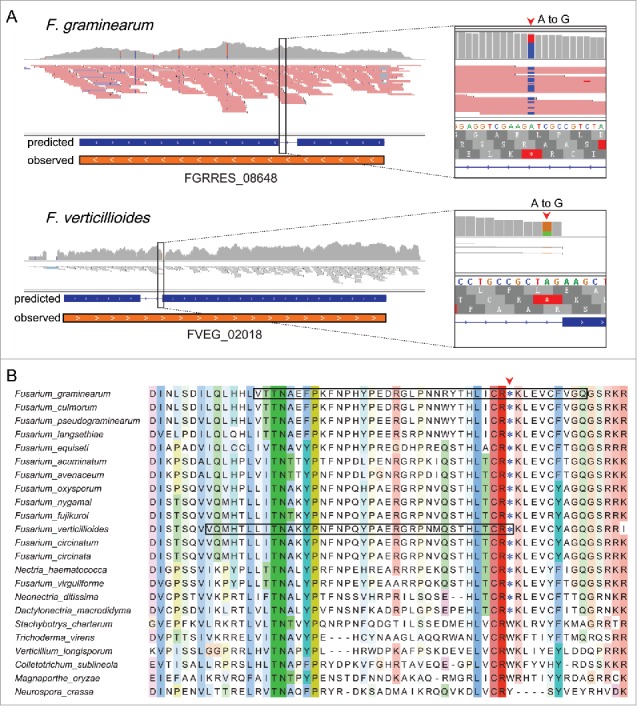
Editing of the premature stop codon in the rid orthologs from Fusarium and other Nectriaceae species. (A) IGV snapshots of RNA-seq reads from 8-day-old perithecia of F. graminearum 13 and 4-day-old perithecia of F. verticillioides 39 mapped to the genomic region of rid orthologs FGRRES_08648 (upper panel) and FVEG_02018 (lower panel). The gene structure based on automated annotation (predicted) differs from the actual cDNA sequence (observed) identified by RNA-seq analysis. The predicted intron is actually the coding region of rid orthologs that are represented by rectangle boxes with white arrowheads showing the direction. Red arrowheads in the zoom-in view boxes on the right mark the sites where the premature stop codons UA644G in FGRRES_08648 and UA590G in FVEG_02018 were edited to UGG. B. Sequence alignment of the edited premature stop codon and its flanking amino acid residues in rid orthologs from marked Fusarium and other Sordariomycetes species. Black boxes indicate the amino acid residues encoded by the incorrectly predicted intron in the rid orthologs of F. graminearum and F. verticillioides. Red arrowhead marks the premature stop codon (*) that requires A-to-I editing to encode a full-length protein by the rid orthologs from F. graminearum and other closely related Nectriaceae species.
MSUD is an epigenetic phenomenon that was first discovered in N. crassa but it also occurs in F. graminearum to silence unpaired genes during meiosis (Fig. 2).32,36 A number of genes required for MSUD have been identified in N. crassa,37 including sad-1 to sad-6, sms-2, sms-3, and qip. Interestingly, multiple A-to-I editing events occurred in the F. graminearum orthologs of sad-3, sad-6, sms-2, sms-3, and qip (Fig. 4). It is likely that MSUD, similar to RIP, is functionally related to RNA editing in F. graminearum. Therefore, characterization of their relationships during sexual reproduction is important to better understand these stage-specific genetic and epigenetic phenomena in N. crassa and other fungi. RNA editing may play a role in regulating stage-specific functions of RIP and MSUD.
Figure 4.
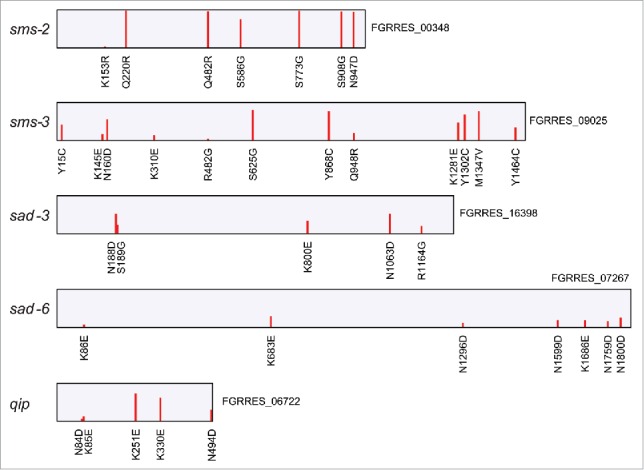
Missense RNA editing events in MSUD-related genes in F. graminearum. Missense editing events in the F. graminearum orthologs of N. crassa sms-2, sms-3, sad-3, sad-6, and qip are marked with red bars and labeled underneath for amino acid changes. The height of red bars is proportional to the editing level of individual sites, ranging from 3.6% to 98.1%.
In summary, genome-wide A-to-I RNA editing occurs specifically during sexual reproduction in F. graminearum, F. verticillioides, N. crassa and probable other filamentous fungi. Fungi differ from animals in the sequence-preference and structure-selectivity of A-to-I editing sites. As a model organism, N. crassa will be a great system to study stage-specificity of RNA editing and biological consequences or functions of various RNA editing events in general. It has been suggested that ADARs in animals may evolve from the combination of ADAT-like enzymes with RNA-binding proteins.1,2,38 Identification and functional characterization of fungal adenosine deaminases will be useful to understand the evolution of human ADARs. It is also important to determine the functional relationship between RNA editing and other genetic or epigenetic phenomena during sexual reproduction in filamentous fungi and whether RNA editing occurs in other developmental or infection stages.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Cong Jiang for fruitful discussions.
Funding
This work was supported by grants from the National Basic Research Program of China (973 program) (2012CB114002 and 2013CB127703), US Wheat and Barley Scab Initiative, and Northwest A & F University Young Talent program for HQL.
References
- 1.Savva YA, Rieder LE, Reenan RA. The ADAR protein family. Genome Biol 2012; 13:252; PMID:23273215; http://dx.doi.org/ 10.1186/gb-2012-13-12-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem 2002; 71:817-46; PMID:12045112; http://dx.doi.org/ 10.1146/annurev.biochem.71.110601.135501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto Y, Cohen HY, Levanon EY. Mammalian conserved ADAR targets comprise only a small fragment of the human editosome. Genome Biol 2014; 15:R5; PMID:24393560; http://dx.doi.org/ 10.1186/gb-2014-15-1-r5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaswami G, Li JB. RADAR: a rigorously annotated database of A-to-I RNA editing. Nucleic Acids Res 2014; 42:D109-13; PMID:24163250; http://dx.doi.org/ 10.1093/nar/gkt996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palladino MJ, Keegan LP, O'Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. Rna-a Publication RNA Society 2000; 6:1004-18; PMID:10917596; http://dx.doi.org/9331360 10.1017/S1355838200000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patton DE, Silva T, Bezanilla F. RNA editing generates a diverse array of transcripts encoding squid Kv2 K+ channels with altered functional properties. Neuron 1997; 19:711-22; PMID:9331360; http://dx.doi.org/ 10.1016/S0896-6273(00)80383-9 [DOI] [PubMed] [Google Scholar]
- 7.Maas S, Kawahara Y, Tamburro KM, Nishikura K. A-to-I editing and human diseases. RNA Biol 2006; 3:1-9; PMID:17114938; http://dx.doi.org/ 10.4161/rna.3.1.2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slotkin W, Nishikura K. Adenosine-to-inosine RNA editing and human disease. Genome Med 2013; 5(11):105 29; PMID: 24289319; http://dx.doi.org/20192758 10.1186/gm508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo A, Locatelli F. ADARs: allies or enemies? The importance of A-to-I RNA editing in human disease: from cancer to HIV-1. Biol Rev 2011; 87:95-110; PMID:21682836; http://dx.doi.org/20192758 10.1111/j.1469-185X.2011.00186.x [DOI] [PubMed] [Google Scholar]
- 10.Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem 2010; 79:321-49; PMID:20192758; http://dx.doi.org/ 10.1146/annurev-biochem-060208-105251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai G, Shaner G. Management and resistance in wheat and barley to fusarium head blight. Annu Rev Phytopathol 2004; 42:135-61; PMID:15283663; http://dx.doi.org/ 10.1146/annurev.phyto.42.040803.140340 [DOI] [PubMed] [Google Scholar]
- 12.Goswami RS, Kistler HC. Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 2004; 5:515-25; PMID:20565626; http://dx.doi.org/ 10.1111/j.1364-3703.2004.00252.x [DOI] [PubMed] [Google Scholar]
- 13.Liu HQ, Wang Q, He Y, Chen LF, Hao C, Jiang C, Li Y, Dai Y, Kang Z, Xu JR. Genome-wide A-to-I RNA editing in fungi independent of ADAR enzymes. Genome Res 2016; 26(4):499-509; PMID: 26934920,24722121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JY, Peng Z, Zhang R, Yang XZ, Tan BC, Fang H, Liu CJ, Shi M, Ye ZQ, Zhang YE, et al.. RNA editome in rhesus macaque shaped by purifying selection. PLoS Genet 2014; 10:e1004274; PMID:24722121; http://dx.doi.org/ 10.1371/journal.pgen.1004274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahn JH, Lee JH, Li G, Greer C, Peng GD, Xiao XS. Accurate identification of A-to-I RNA editing in human by transcriptome sequencing. Genome Res 2012; 22:142-50; PMID:21960545; http://dx.doi.org/ 10.1101/gr.124107.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Lopez-Giraldez F, Lehr N, Farre M, Common R, Trail F, Townsend JP. Global gene expression and focused knockout analysis reveals genes associated with fungal fruiting body development in Neurospora crassa. Eukaryot Cell 2014; 13:154-69; PMID:24243796; http://dx.doi.org/ 10.1128/EC.00248-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehr NA, Wang Z, Li N, Hewitt DA, Lopez-Giraldez F, Trail F, Townsend JP. Gene expression differences among three Neurospora species reveal genes required for sexual reproduction in Neurospora crassa. PLoS One 2014; 9:e110398; PMID:25329823; http://dx.doi.org/ 10.1371/journal.pone.0110398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakurai M, Ueda H, Yano T, Okada S, Terajima H, Mitsuyama T, Toyoda A, Fujiyama A, Kawabata H, Suzuki T. A biochemical landscape of A-to-I RNA editing in the human brain transcriptome. Genome Res 2014; 24:522-34; PMID:24407955; http://dx.doi.org/ 10.1101/gr.162537.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Laurent G, Tackett MR, Nechkin S, Shtokalo D, Antonets D, Savva YA, Maloney R, Kapranov P, Lawrence CE, Reenan RA. Genome-wide analysis of A-to-I RNA editing by single-molecule sequencing in Drosophila. Nat Struct Mol Biol 2013; 20:1333-9; PMID:24077224; http://dx.doi.org/ 10.1038/nsmb.2675 [DOI] [PubMed] [Google Scholar]
- 20.Park E, Williams B, Wold BJ, Mortazavi A. RNA editing in the human ENCODE RNA-seq data. Genome Res 2012; 22:1626-33; PMID:22955975; http://dx.doi.org/ 10.1101/gr.134957.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggington JM, Greene T, Bass BL. Predicting sites of ADAR editing in double-stranded RNA. Nat Commun 2011; 2:319; PMID:21587236; http://dx.doi.org/ 10.1038/ncomms1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barraud P, Allain FH. ADAR proteins: double-stranded RNA and Z-DNA binding domains. Curr Top Microbiol Immunol 2012; 353:35-60; PMID:21728134; http://dx.doi.org/ 10.1007/82_2011_145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber A, Grosjean H, Melcher T, Keller W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. Embo J 1998; 17:4780-9; PMID:9707437; http://dx.doi.org/ 10.1093/emboj/17.16.4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber AP, Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 1999; 286:1146-9; PMID:10550050; http://dx.doi.org/ 10.1126/science.286.5442.1146 [DOI] [PubMed] [Google Scholar]
- 25.Desterro JM, Keegan LP, Lafarga M, Berciano MT, O'Connell M, Carmo-Fonseca M. Dynamic association of RNA-editing enzymes with the nucleolus. J Cell Sci 2003; 116:1805-18; PMID:12665561; http://dx.doi.org/ 10.1242/jcs.00371 [DOI] [PubMed] [Google Scholar]
- 26.Sansam CL, Wells KS, Emeson RB. Modulation of RNA editing by functional nucleolar sequestration of ADAR2. Proc Natl Acad Sci U S A 2003; 100:14018-23; PMID:14612560; http://dx.doi.org/ 10.1073/pnas.2336131100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freitag M, Williams RL, Kothe GO, Selker EU. A cytosine methyltransferase homologue is essential for repeat-induced point mutation in Neurospora crassa. Proc Natl Acad Sci USA 2002; 99:8802-7; PMID:12072568; http://dx.doi.org/ 10.1073/pnas.132212899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiu PK, Zickler D, Raju NB, Ruprich-Robert G, Metzenberg RL. SAD-2 is required for meiotic silencing by unpaired DNA and perinuclear localization ot SAD-1 RNA-directed RNA polymerase. Proc Natl Acad Sci USA 2006; 103:2243-8; PMID:16461906; http://dx.doi.org/ 10.1073/pnas.0508896103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son H, Min K, Lee J, Raju NB, Lee YW. Meiotic silencing in the homothallic fungus Gibberella zeae. Fungal Biol 2011; 115:1290-302; PMID:22115448; http://dx.doi.org/ 10.1016/j.funbio.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 30.Cuomo CA, Gueldener U, Xu JR, Trail F, Turgeon BG, Di Pietro A, Walton JD, Ma LJ, Baker SE, Rep M, et al.. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007; 317:1400-2; PMID:17823352; http://dx.doi.org/ 10.1126/science.1143708 [DOI] [PubMed] [Google Scholar]
- 31.Xu JR, Leslie JF. A genetic map of Gibberella fujikuroi mating population A (Fusarium moniliforme). Genetics 1996; 143:175-89; PMID:8722773; http://dx.doi.org/ 10.1017/S0016672300034066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selker EU, Cambareri EB, Jensen BC, Haack KR. Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell 1987; 51:741-52; PMID:2960455; http://dx.doi.org/ 10.1016/0092-8674(87)90097-3 [DOI] [PubMed] [Google Scholar]
- 33.Shiu PK, Raju NB, Zickler D, Metzenberg RL. Meiotic silencing by unpaired DNA. Cell 2001; 107:905-16; PMID:11779466; http://dx.doi.org/ 10.1016/S0092-8674(01)00609-2 [DOI] [PubMed] [Google Scholar]
- 34.Hammond TM, Xiao H, Boone EC, Decker LM, Lee SA, Perdue TD, Pukkila PJ, Shiu PK. Novel proteins required for meiotic silencing by unpaired DNA and siRNA generation in Neurospora crassa. Genetics 2013; 194:91-100; PMID:23502675; http://dx.doi.org/ 10.1534/genetics.112.148999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 2012; 25:297-317; PMID:22491773; http://dx.doi.org/ 10.1128/CMR.00013-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nadal M, Garcia-Pedrajas MD, Gold SE. Dimorphism in fungal plant pathogens. FEMS Microbiol Lett 2008; 284:127-34; PMID:18479435; http://dx.doi.org/ 10.1111/j.1574-6968.2008.01173.x [DOI] [PubMed] [Google Scholar]
- 37.Perez-Martin J, Castillo-Lluva S, Sgarlata C, Flor-Parra I, Mielnichuk N, Torreblanca J, Carbó N. Pathocycles: Ustilago maydis as a model to study the relationships between cell cycle and virulence in pathogenic fungi. Mol Genet Genomics 2006; 276:211-29; PMID:16896795; http://dx.doi.org/ 10.1007/s00438-006-0152-6 [DOI] [PubMed] [Google Scholar]
- 38.Gary MW. Evolutionary origin of RNA editing. Biochemistry 2012; 51:5235-42; PMID:22708551; http://dx.doi.org/ 10.1021/bi300419r [DOI] [PubMed] [Google Scholar]
- 39.Sikhakolli UR, Lopez-Giraldez F, Li N, Common R, Townsend JP, Trail F. Transcriptome analyses during fruiting body formation in Fusarium graminearum and Fusarium verticillioides reflect species life history and ecology. Fungal Genet Biol 2012; 49:663-73; PMID:22705880; http://dx.doi.org/ 10.1016/j.fgb.2012.05.009 [DOI] [PubMed] [Google Scholar]


