Abstract
Solid organ transplantation provides life saving therapy for patients with end stage organ disease. In order that the transplanted organ survive, the recipient must take a lifelong cocktail of immunosuppressive medications that increase the risk for infections, malignancies and drug toxicities. Data from many animal studies have shown that recipients can be made tolerant of their transplanted organ by infusing stem cells, particularly hematopoietic stem cells, prior to the transplant. The animal data have been translated into humans and now several clinical trials have demonstrated that infusion of hematopoietic stem cells, along with specialized conditioning regimens, can permit solid organ allograft survival without immunosuppressive medications. This important therapeutic advance has been made possible by understanding the immunologic mechanisms by which stem cells modify the host immune system, although it must be cautioned that the conditioning regimens are often severe and associated with significant morbidity. This review discusses the role of hematopoietic stem cells in solid organ transplantation, provides an understanding of how these stem cells modify the host immune system and describes how newer information about adaptive and innate immunity might lead to improvements in the use of hematopoietic stem cells to induce tolerance to transplanted organs.
Introduction
Long-term outcomes in solid organ transplantation are limited by ongoing alloreactivity to the transplanted organ and non-specificity and toxicity of immunosuppressive medications. Current immunosuppressive medications are highly effective in suppressing host anti-donor responses, but they do so at the cost of increased risk for infectious and malignant complications. Therefore, it has been a long-time goal of physicians caring for patients with solid organ transplants to develop new therapies that essentially trick the recipient’s immune system into accepting the transplanted organ as it’s own (also called tolerance) and thereby avoid the need for immunosuppressive medications. Many investigators have shown that animals can be made tolerant to transplanted organs by the infusion of hematopoietic stem cells (HSCs) at the time of transplantation. It has in fact been known for many years that infusion of HSCs leads to engraftment of donor cells within the thymus and bone marrow of the recipient, which then modifies the recipient’s immune response to donor antigens. While HSC transplantation has been shown to induce transplantation tolerance in rodents, monkeys and swine models, recent studies now show that HSCs might also induce tolerance in humans that receive a solid organ transplant (1). The mechanisms by which HSC infusions induce tolerance have been the subject of multiple published studies. As newer information is acquired about the way in which HSCs modify the host immune system it is likely that novel therapies will emerge that improve the ability of HSCs to induce tolerance in human transplant recipients. This review focuses on the role HSCs in immunologic tolerance and describes new concepts applicable to HSC transplantation in human solid organ transplantation.
What are stem cells?
Stem cells are undifferentiated, pluripotent, precursors that are able to transform into mature cells with specialized functions. A common factor among all stem cells remains their ability to exhibit extensive self-renewal and differentiation. Four different types of stem cells have been described including: embryonic stem cells (ESCs); adult stem cells (ASCs); induced pluripotent stem cells (iPS); and cancer stem cells (CSCs). ESCs are derived from pre-implantation epiblasts and are distinguished by their ability to maintain pluripotency. ASCs are postnatal derivatives of ESCs located throughout the body, and classified by their tissue of origin (e.g., hematopoietic, mesenchymal, neural, etc.). It is these types of stem cells, particularly HSCs that are used to induce tolerance to transplanted organs and are the focus of this review. iPS cells are differentiated adult cells that have been reprogrammed to assume a stem cell-like state of pluripotency. Each of the different types of stem cells can be distinguished by their cell surface phenotype (Table 1) (2–5).
Table 1.
Phenotypic markers distinguishing stem cells
| Stem cell types* | Surface marker |
|---|---|
| ESC | SSEA-3, SSEA-4, CD9, CD56, Class-I HLA, Thy1 3,5 |
| HSC | CD34, CD59, Thy, CD38low, CD135, CD48, CD159 2,3 |
| MSC | STRO-1, VCAM-1, Sca-1, BMPR-IA/ALK3, BMPR-IB/ALK6, BMPR-II, CD73, c-kit, Class-I HLA, Thy-1, CD105/endoglin 3 |
| CSC | CD44, CD24, CD133, CD166, SSE-1, SSE-4 4 |
ESC-embryonic stem cell; HSC – hematopoietic stem cell; MSC – mesenchymal stem cell; CSC – cancer stem cell
Stem cells grow and differentiate in environments called “niches”; and duplicating these niches has been a challenge for clinical application. Niches are physiologically defined microenvironments with properties that regulate and support the balance of quiescence, self-renewal and differentiation. In the bone marrow, adhesion molecules and components of the extracellular matrix are important for anchoring adult HSCs to the stroma, allowing for regulation of survival, proliferation and differentiation (6–8). Both cellular and extracellular matrix elements of the stem cell niche are essential for normal stem cell function. For instance, the extracellular matrix molecules osteopontin and hyaluronic acid, as well as membrane-bound stem cell factor (mSCF), regulate HSC quiescence, homing, trans-marrow migration and lodgment in the niche (9–11). Inflammatory signaling molecules including interferons, tumor necrosis factor-alpha and toll-like receptors (TLRs) are also recognized to play an important role in regulating stem cell responses involving growth, proliferation and differentiation (12). Niche-associated elements mediate the many growth and development pathways of residing cells, and it is often the relationship between stem cells and their respective niches that direct phenotypic variations between stem cell types.
Why are stem cells of interest in solid organ transplantation?
Since the mid twentieth century, scientists have used HSC infusions (administered through the injection of donor bone marrow) to modify host immune responses in experimental models of autoimmunity and solid organ transplantation (13). Infusion of allogeneic donor bone marrow can lead to a state of mixed hematopoietic chimerism, where the genetically distinct donor HSCs engraft in the host and differentiate into lymphocytes of donor origin that coexist with those of the host.
Mixed hematopoietic chimerism was first associated with alloantigen tolerance by Owen in 1945 when freemartin cattle (fraternal twins sharing a placental circulation) were shown to be chimeric, and tolerant of one another (14, 15). Kashiwagi and Starzl introduced the concept of mixed chimerism in human transplantation in 1969 when they identified donor immunoglobulins circulating in the blood of recipients of liver allografts (16). Inducing tolerance through mixed chimerism became of great interest in experimental models of transplantation during the 1970s and 80s. However, it became clear that the induction of tolerance required host conditioning with harsh toxic agents, such as total lymphoid irradiation (TLI) and/or whole body irradiation (WBI) (17–25). Improving on the early experimental methods, Cobbold and Waldmann developed a preconditioning regimen using monoclonal antibodies to deplete the host of T cells; however, high doses of irradiation were still needed to achieve anything more than transient chimerism (26). Sharabi and Sachs took these early observations further and hypothesized that perhaps the monoclonal antibody regimen used by Cobbold failed to deplete mature T-cells residing in thymus. While a thymectomy could potentially overcome this problem, it would also leave the host without thymic stromal elements essential for educating naïve host T cells. They eventually reported successful long lasting chimerism by using a conditioning regimen of selective thymic irradiation (TI) and sub-lethal WBI along with T cell depletion (26, 27). Since these early days, a number of other preconditioning regimens have been tried along with infusion of donor HSCs to improve tolerance induction to transplanted organs. Other experimental models have been developed to eliminate irradiation preconditioning by either administering higher number of HCSs, selective T cell subset depletion and/or co-stimulatory blockade (28, 29).
Larger animal models, such as miniature swine, with greater similarities than rodents to human biology, were used in preparatory experiments for transitioning into clinical studies. In 1988, Pennington and Sachs pioneered the use of bone marrow transplantation in partially inbred, major histocompatibility complex (MHC)-defined miniature swine conditioned with total body irradiation (TBI). Initial attempts were associated with high rates of graft versus host disease (GVHD), but long lasting hematopoietic chimerism was achieved in the pig model when swine anti-CD3 antibody became available. In fact, the addition of host T cell depletion to the regimen achieved host tolerance to genetically disparate pig skin and kidney transplants (30–33). Non-human primates models have also been developed as a bridge to clinical practice. Cynomolgus recipient monkeys conditioned before transplant with donor bone marrow, sub-lethal TBI, TI and thymoglobulin, and then with cyclosporine A for four weeks after receiving an orthotopic histocompatibility antigen-mismatched kidney transplant developed clear evidence of hematopoietic chimerism. Eleven of 13 animals developed chimerism while 10 of 13 survived long-term without rejection (34).
The early animal studies led the way for HSC transplantation as a way to prolong human kidney allograft survival. In 1999 the Spitzer and Sachs team at the Massachusetts General Hospital treated a 55 year old female with end stage renal disease secondary to multiple myeloma with combined histocompatibility leukocyte antigen (HLA)-matched bone marrow and kidney transplant after conditioning with cyclophosphamide, antithymocyte globulin (ATG), and thymic irradiation. Cyclosporine, as the only post-transplant immunosuppressive therapy, was tapered and discontinued on day 73 after transplantation. No rejection episodes occurred and renal function remained normal 5 years after discontinuation of all immunosuppressive therapy (35, 36). Ciancio et al showed that infusion of donor bone marrow cells significantly improved long-term allograft survival and that the degree of hematopoietic chimerism correlated with the improvement in allograft function in both deceased and live kidney donor recipients, although in none of their subjects was immunosuppression discontinued (36, 40). Later, Millan and colleagues at Stanford reported four patients who were given combined kidney and HSC transplants following nonmyeloablative post-transplant conditioning with TLI and ATG. One patient had humoral rejection, one was able to wean off all immunosuppressive maintenance and the fourth patient did not reach the point of drug withdrawal (37). In 2006 the Massachusetts General group treated six patients with renal failure due to multiple myeloma with simultaneous kidney and bone marrow transplantation from HLA-identical sibling donors following nonmyeloablative conditioning with cyclophosphamide, peri-transplant ATG and thymic irradiation (38). Cyclosporine was given for approximately 2–3 months post-transplant in the majority of patients, followed by donor leukocyte infusions. Three patients lost detectable chimerism but accepted their kidney grafts off immunosuppression for 2 to >7 years. Two patients achieved full donor chimerism, but resumed immunosuppression to treat graft-versus-host disease. Only one patient experienced rejection, following cyclosporine withdrawal, but responded to acute immunosuppressant treatment, which was later successfully withdrawn. Following this experiment, Kawai and Sachs treated five end-stage renal disease patients with combined bone marrow and kidney transplants from HLA single-haplotype mismatched living related donors through use of a nonmyeloablative preparative regimen (39). Irreversible humoral rejection occurred in one patient. In the other four recipients, all immunosuppressive therapy was discontinued within 9 to 14 months after the transplant and renal function remained stable for 2 to 5.3 years. Ciancio, in another trial of haplotype mismatched donor stem cell infusions, used almetzumab as an induction agent and found the benefit of the donor lymphocyte infusions to be eliminated by the induction agent, possibly because chimerism was prevented by almetzumab (43). In a more recent trial, Leventhal & Ildstad reported fifteen HLA-mismatched living donor renal transplant recipients who underwent low intensity conditioning (fludarabine, cyclophosphamide, TBI), followed by a living donor kidney transplant (40). Maintenance of immunosuppression, tacrolimus and mycophenolate was weaned over one year. All but one patient demonstrated peripheral blood macrochimerism after transplantation. Engraftment failure occurred in one highly sensitized recipient, but complete immunosuppression withdrawal was successful by one year post-transplantation in all patients with durable chimerism. There was no evidence of graft versus host disease and renal transplantation loss occurred in only one patient who developed sepsis following an atypical viral infection. Table 2 provides a listing of these studies in which bone marrow infusions containing HSCs have been used to induce tolerance to solid organ transplants in humans (36–44). Other studies have certainly also been done support the importance of donor specific transfusions for transplant tolerance, including the Trivedi group in Hyderabad (45) and further studies on genomic markers is providing new information from groups that have been working in this area for some time (46, 47).
Table 2.
Tolerance induction studies utilizing donor bone marrow infusions.
| Study reference | Patient #s/gps | BM donor/kidney donor | Conditioning regimen/Induction | Post-transplant immunosuppression | Immunosuppression discontinued? | Follow-up time/Outcome |
|---|---|---|---|---|---|---|
| Spitzer et al, 1999(36) | 1 pt with multiple myeloma | BM - HLA identical sibling KD - HLA identical LKD sibling |
Cyclophosphamide (60mg/kg on d −5 and −4) Anti-thymocyte globulin (15mg/kg (on d −1, +1, +3 and +5) Thymic irradiation (700cGy on d −1) Cyclosporine (5mg/kg on d −1) |
Cyclosporine (6mg/kg Q12hr) | Yes | Follow-up – 170 days Creatinine - 0.7mg/dl |
| Ciancio et al, 2001(41) | Experimental group 63 pts – donor BM Control group 219 pts – no BM |
BM – HLA-DR-matched BM KD - HLA-DR-matched DKD |
OKT3 mAb | Tacrolimus Methylprednisolone Mycophenolate mofetil |
No | Follow-up – mean 4.7 years Creatinines - Control group –2.15mg/dl Experimental group –1.7mg/dl Acute rejection Control group - 15% Experimental group – 13% Chronic rejection Control group – 19% Experimental group –3% |
| Millan et al, 2002(37) | 4 pts | BM – HLA mismatched HSCs KD – HLA-mismatched LKD |
Anti-thymocyte globulin (1.5mg/kg on d0; ¾ pts given same dose of ATG on days +1,3,5,9,14) Total lymphoid irradiation (80 cGy on Day +1 and repeated x 10 to total dose of 800 cGy) |
Prednisone, Cyclosporine | Yes - 1 pt No – 3pts |
Follow-up- 116–374 days Creatinines – 1–1.7mg/dl Rejection - 1/4 humoral rejection |
| Ciancio et al, 2002(42) | Experimental group – 47 pts- donor BM Control group – 39 pts – no donor BM |
BM – matched to kidney donor KD – HLA mismatched LKD |
OKT3 mAb Daclizumab |
Tacrolimus Prednisone Mycophenolate mofetil |
No | Follow-up- 48 months Creatinines - no statistical differences Rejection – no statistical differences |
| Fudaba et al, 2006(38) | 6 pts with multiple myeloma | BM - HLA identical siblings KD - HLA identical LKD siblings |
Cyclophosphamide (60 mg/kd/d on d −5, −4) Thymic irradiation (700 cGy on day 1) Anti-thymocyte globulin (15–20mg/kg/d on day−1, +1,3,5) |
Cyclosporine Donor leukocyte infusions |
Yes – 4pts No – 2 pts |
Follow-up- 2–7.3 years Creatinines – 0.9 – 5.6mg/dl Rejection – 1 pt |
| Kawai et al, 2008(39) | 5 pts with multiple myeloma | BM - HLA haplotype mismatched KD – HLA haplotype mismatched |
Cyclophosphamide (60m/kg/d on d −5, −4) Anti CD2 Ab (0.6mg/kg on day −1, 0, +1) Thymic irradiation (700 cGy on d −1) Rituximab (375 mg/SA2 on d −7, −2) |
Cyclosporine Prednisone |
Yes – 4pts No – 1pt |
Follow-up – 2months – 9.2 years Creatinines - 1.2 – 1.8 mg/dl Rejection - 1/5 humoral rejection |
| Kawai et al, 2013(100) | 5 additional pts- extension of 2008 study | BM HLA haplotype mismatched KD HLA haplotype mismatched |
Cyclophosphamide(60mg/kg on d −5, −4) Anti CD2 Ab (0.6mg/kg on d −1, 0 and +1) Thymic irradiation (700 cGy on d –1) Rituximab (375mg/kg/BSA2 on d−7, −2) |
Cyclosporine Prednisone |
Yes – 3pts No – 2pts |
Follow-up – 2mo – 9.2yrs Creatinines – 0.8 mg/dl - ESRD Rejection - NR |
| Ciancio et al, 2013(44) | Experimental group – 4 pts donor HSCs Control group – 5 pts no – donor BM |
BM - 2–3 haplotype mismatch KD - 2–3 haplotype mismatch |
Almetzumab | Tacrolimus Mycophenolate mofetil (Converted to sirolimus in 1 patient) |
Yes- 4pts in HSC group | Follow-up – 31– 63 months Creatinines – Experimental group - 1.25 mg/dl Control group - 1.41 mg/dl Rejection – Experimental group--1/4 with rejection; Control group - 2/4 with rejection |
| Leventhal et al, 2013(40) | 15 pts | BM- HSCs enriched for facilitating cells KD - HLA mismatched LDK |
Fludarabine (30mg/kg on d −4, −3, −2) Cyclophosphamide(50mg/kg on d −3, +3) Total body irradiation(200 cGy) |
Tacrolimus Mycophenolate mofetil Sirolimus |
Yes – 6pts No – 4pts |
Follow-up 30 months Creatinine <2mg/dl in all Rejection NR |
How do HSCs induce tolerance?
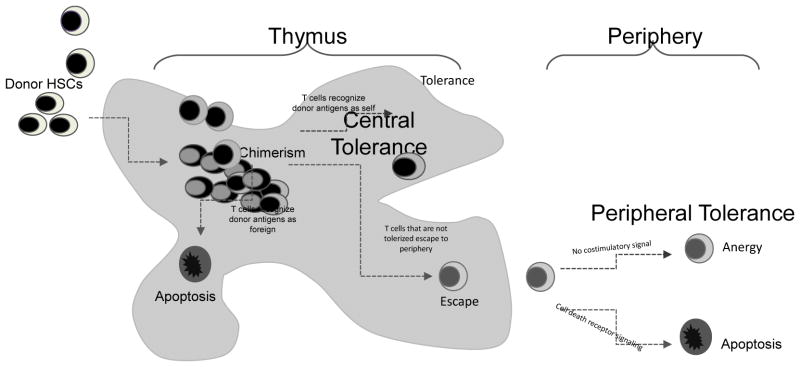
Several immunologic mechanisms have been observed in experimental models that help to explain how donor HSCs might induce host tolerance to alloantigens. When donor bone marrow is infused into the host, HSCs engraft within the recipient bone marrow and thymus and repopulate the host immune system with lymphocytes of donor origin. As shown in the schematic in Figure 1, the presence of donor progenitors within the thymus leads to the development of T cells that recognize donor antigens expressed by the transplanted organ as self, and thus the host becomes tolerant of the allograft. The coexistence of host and donor hematopoietic cells is called chimerism and it is this chimerism within the host that drives central tolerance mechanisms. Central tolerance is defined as tolerance that occurs while developing immune cells are still present in the thymus (48). Increasing evidence highlights central tolerance as the key mechanism of long-lasting HSC-induced allograft tolerance (49). This type of tolerance in many experimental models is dependent on engraftment of the allogeneic HSCs in the recipient thymus, and many experimental models have shown that newly developed donor-reactive T cells are deleted, resulting in systemic donor-specific tolerance (22, 29, 50, 51). Central tolerance lasts as long as the HSCs of donor origin persist in the host (52, 53).
Figure 1. Schematic of mechanisms of HSC-induced tolerance.
Donor HSCs home to the recipient thymus, where they integrate with recipient thymic cells and develop donor/recipient chimerism. The result of chimerism is that central tolerance mechanisms ensue allowing mature T cells to develop that can recognize donor antigens as self (tolerized). T cells that recognize donor antigens as foreign undergo apoptosis. T cells that escape to the periphery undergo peripheral tolerance mechanisms that result in either anergy (in the absence of costimulation) or apoptosis (in the presence of costimulation).
Due to the dynamic environment of the hematopoietic system following HSC transplantation, levels of chimerism exist and are categorized as either complete (full) or mixed chimerism. As implied, full chimerism exists when all hematopoietic elements are of donor origin, while mixed chimerism is the coexistence of both donor and recipient hematopoietic elements in varying proportions (28). The recipient preconditioning regimen determines the amount of chimerism that can be achieved. In general, complete chimerism requires a more extensive preconditioning regimen, which is associated with greater risk of GVHD and a lower retention of immunocompetence than mixed chimerism (52). Therefore, induction of mixed rather than complete chimerism is favored in clinical protocols that test HSC-induced immunologic tolerance to allografts.
Although central tolerance is a dominant mechanism for HSC-induced tolerance to alloantigens, it may not be complete, in part because not all donor antigens are expressed by donor HSCs in the host thymus, and because T lymphocytes with low affinity for self-antigens may escape the selection process and enter the peripheral lymphoid circulation. When self-reactive T cell populations evade the intrathymic selection processes, peripheral mechanisms are needed to maintain immunologic tolerance. In a transplant setting, with a mild preconditioning regimen aimed at inducing mixed chimerism, the survival of mature alloreactive T cells in the periphery may also be controlled through peripheral mechanisms, such as extra-thymic deletion of alloreactive lymphocytes and T cell anergy (54–56). Deletion of alloreactive lymphocytes occurs by activation of death-domain-containing receptors that ultimately cause apoptotic cell death (53). T cell anergy occurs when the T cell receives incomplete activation signals. T cell activation requires the presentation of antigen (provided by antigen presenting cells) through the T cell surface receptor, in addition to a costimulatory signal provided through CD28 costimulatory surface molecules. In the absence of CD28 signaling the T cell becomes hyporesponsive to the antigen presented through the TCR, also termed anergy. (53). Studies from several laboratories demonstrated that co-stimulation blockade is an essential component of allograft tolerance protocols (29, 57–59). In some experimental tolerance studies, co-stimulatory blockade was associated with peripheral deletion of donor-reactive mature host CD4+ T cells (57). However, co-stimulation blockade in combination with mixed chimerism has been shown to also anergize alloreactive host T cells that survived preconditioning (60). In yet other studies, the absence of a co-stimulation signal has been shown to lead to both T cell anergy and apoptosis (54, 55, 60).
Additional proposed mechanisms of immune modulation that allow HSC-induced allograft tolerance have been proposed and involve regulatory and suppressor cells. The ability of transplanted HSCs to create populations of regulatory and suppressor T cells has in fact now become of interest for cellular therapies in clinical transplantation. Of particular interest are “veto cells”, and non thymic-derived “adaptive” T regulatory cells (Tregs) induced in the periphery in response to antigen. While multiple different lineages may make up the larger category of “veto cells”, activated CD8+ T cells with ‘veto’ activity have been implicated in the induction of peripheral tolerance in HSC transplant models (61). By definition, veto cells engage and remove T lymphocytes that are reactive to veto cell antigens through MHC class I ligation as well as through the Fas/FasL signaling pathway (62). In mixed chimerism transplant models, veto cells have gained particular interest due to the ability of peripheral blood HSCs of donor origin to create CD8+ veto cells that can remove anti-donor CD8+ T cells of host origin in the bone marrow and peripheral lymphoid tissues (63). Furthermore, recipient CD8+ T cells with veto power can eliminate donor CD8+ T cells that recognize recipient MHC class I molecules in order to attenuate GVHD (56). While recent animal studies have suggested promise for the use of veto cells to induce tolerance to solid organ transplants and GVHD, currently we lack sufficient in vivo data to support translation to a clinical setting. Regulatory T cells on the other hand have recently been recognized to be integral for maintaining donor-specific tolerance in transplant models and are the focus of several clinical trials in transplantation (64). Recent studies have highlighted the possibility of creating peripherally derived CD4+CD25+ Tregs through in vivo manipulations, and have illustrated the ability of extrathymic-derived Treg populations to induce donor-specific tolerance (65–70). Several studies have provided evidence that Foxp3 expression is induced in CD4+CD25+ T cells in the periphery upon encounter with antigen by way of non-professional APCs, while in the presence of transforming growth factor- β (TGF-β) (71). These data suggest that CD4 Tregs are promising targets for tolerance strategies aimed at alloreactive T cells during the induction of tolerance to alloantigens. While regulatory T cells are important early after tolerance induction, it is thought that long-term tolerance requires additional mechanisms, such as deletion or anergy (72). Recently T cell exhaustion, which has been well-known to arise during an immune response to chronic infections and cancers, has been added to the list of possible mechanisms of transplant tolerance (73). Whether through peripheral or central mechanisms, growing evidence continues to highlight transplanted HSCs as crucial elements for the induction of initial and lasting allograft tolerance in multiple different experimental transplant models.
How does the innate immune system influence hematopoietic stem cell transplantation?
While much of the interest in HSC-induced transplant tolerance has focused on the adaptive immune system, recent data suggest that innate immunological processes might also play an important role. The ability of transplanted HSCs to home and engraft in a myeloablated hematopoietic microenvironment appears to be modified by signals derived through the innate immune system. Following myeloblative preconditioning, irradiative damage to the bone marrow microenvironment leads to release of chemo-attractant cytokines and chemokines, which facilitate HSC homing (74). Recent data from Pitchford and Kim have highlighted the importance of complement activation during homing of HSCs to their niches following myeloablative preconditioning (75, 76). Using mice deficient in C3 and C5 complement fragment proteins, these investigators each showed impaired engraftment of HSCs from complement component deficient mice relative to their wild-type counterparts (77). The process of HSC homing to the niche microenvironment is highly dependent on membrane bound receptors, as well as chemo-attractant gradients produced by host cells in the niche microenvironment. The highly studied relationship between the bone marrow niche-associated α-chemokine CXCL12 (stromal-derived factor-1; SDF-1) and its respective HSC transmembrane receptor CXCR4 has consistently yielded evidence to suggest a vital role in homing, retention, and mobilization of HSCs to and from the niche (78–80). Kim and colleagues have documented how degradation of CXCL12 occurs due to induction of a highly proteolytic BM microenvironment following myeloablative preconditioning. Subsequently, activation of the innate immune response leads to upregulation of BM niche complement component cleavage fragments and cationic peptides (e.g. cathelicidin and β2-defensin), which act to preserve HSC responsiveness toward the lowered SDF-1 chemotactic gradient (75, 81). Additionally, prostaglandin E2 fibrinogen fragments, hyaluronic acid and bioactive lipids have all been recently placed on an emergening list of innate immune response-associated positive regulators of the CXCL12-CXCR4 axis during HSC homing to the BM niche (82–86). It is evident that the processes of homing/lodgment, and subsequently the engraftment of transplanted HSCs formulate a prerequisite of events needed to establish graft-derived hematopoiesis, mixed chimerism and, in turn, immunocompetence.
The influences of the innate immune response on the process of HSC transplantation extend past those seen during the process of homing and engraftment. Several pattern recognition receptors (PRRs) have been found to significantly alter HSC proliferation, differentiation and survival. PRRs are made up of four families of receptors known as toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type Lectin receptors (CLRs) and RIG-1 like receptors (RLRs). Recent data support that both TLRs and NLRs are expressed by HSCs and they play key roles as regulators of HSC activation in response to inflammation, danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) in vivo (87–89). TLRs are a family of innate immune receptors characterized as transmembrane proteins with the ability to recognize a variety of PAMPs and DAMPs (90). Experiments by Nagai et al found that murine HSCs expressed functional TLRs with the ability to activate HSC proliferation and differentiation upon receptor-specific stimulation (91). Furthermore, Sioud et al provided evidence for the presence of functional TLRs on human CD34+ bone marrow progenitors with capabilities of forcing differentiation and production of pro-inflammatory cytokines upon stimulation (92). In vivo murine models of chronic infection utilizing minimal-dose LPS treatments caused phenotypic changes to HSC populations through TLR dependent pathways and permanently reduced their capacities for repopulation and self-renewal following transplantation (93). Additionally, exposure of HSCs to Candida albicans leading to activation of TLR dependent signaling pathways directed HSCs toward proliferation, differentiation and diminished reconstitution ability (94). Similar to TLRs in their ability to recognize PAMPs and DMAPs, NLRs make up a body of intracytoplasmic innate receptors with the ability to mediate apoptosis and secretion of the pro-inflammatory cytokines. Developing evidence from recent clinical studies have found increased mortality and acute GVHD in recipients of allogeneic HSC transplants (HSCT) due to single nucleotide polymorphisms (SNPs) in the NOD2 receptor-coding gene (95–97). Strikingly, clinical findings showed that both donors and recipients with these SNP mutations in the NOD2 lead to increase recipient prevalence of transplant-related mortality and GVHD following HSCT (98, 99). The finding that donor and/or recipient NOD2 SNPs impact outcomes following HSC transplantation suggests that targeting NLRs might improve HSC engraftment, homing, lodgment and subsequent development of tolerance to transplanted allografts.
Conclusions
Given the potential for improving outcomes in solid organ transplantation, substantial efforts have been aimed at finding ways to improve the use of HSCs to tolerize recipients to transplanted allografts. Over the past two decades it has become clear from animal and human studies that transplant tolerance can be achieved through infusion of donor HSCs. The mechanisms by which tolerance is achieved have focused on alterations of central and peripheral adaptive immunity, and modifying these mechanisms has led to remarkable translation of the findings from animal studies into the clinic. Alteration of local stem cell niches, by decreasing the amount of irradiation and preserving thymic stromal cell content and structure, has been an important advance. Additionally, innovations in preconditioning protocols aimed at maximum elimination of pre-existing host T cells, by using targeted monoclonal antibodies, has been another advance that has allowed HSCs to become a viable therapeutic strategy for induction of tolerance. New data suggest that the innate immune system plays an important role in HSC survival in the host and therapies targeted at innate immunity are likely to emerge in the future. HSC transplantation is a promising therapy that allows specific downregulation of immune responses to transplanted organs. As our understanding of the mechanisms by which tolerance is achieved expands, HSC transplantation at the time of solid organ transplant will have broader applications and provide lifesaving therapy for patients with end-stage organ disease.
Acknowledgments
The work presented in this manuscript was supported by grants awarded to DBM from the NIH R01 DK091136, R01 DK075718 and CIRM RB5-07379 and to RE from the NIH T32HL007261 and to ATS from CIRM TB1-01186.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harbor perspectives in medicine. 2014;4(1):a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(7):2804–8. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagano K, Yoshida Y, Isobe T. Cell surface biomarkers of embryonic stem cells. Proteomics. 2008;8(19):4025–35. doi: 10.1002/pmic.200800073. [DOI] [PubMed] [Google Scholar]
- 4.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem cells. 2009;27(5):1006–20. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao RR, Johnson AV, Stice SL. Cell surface markers in human embryonic stem cells. Methods in molecular biology. 2007;407:51–61. doi: 10.1007/978-1-59745-536-7_5. [DOI] [PubMed] [Google Scholar]
- 6.Zapata AG, Alfaro D, Garcia-Ceca J. Biology of stem cells: the role of microenvironments. Advances in experimental medicine and biology. 2012;741:135–51. doi: 10.1007/978-1-4614-2098-9_10. [DOI] [PubMed] [Google Scholar]
- 7.Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Experimental hematology. 2003;31(12):1284–91. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 9.Haylock DN, Nilsson SK. Stem cell regulation by the hematopoietic stem cell niche. Cell cycle. 2005;4(10):1353–5. doi: 10.4161/cc.4.10.2056. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106(4):1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 11.Heazlewood SY, Oteiza A, Cao H, Nilsson SK. Analyzing hematopoietic stem cell homing, lodgment, and engraftment to better understand the bone marrow niche. Annals of the New York Academy of Sciences. 2014 doi: 10.1111/nyas.12329. [DOI] [PubMed] [Google Scholar]
- 12.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends in immunology. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Copelan EA. Hematopoietic stem-cell transplantation. The New England journal of medicine. 2006;354(17):1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 14.Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science. 1945;102(2651):400–1. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 15.Sykes M. Hematopoietic cell transplantation for tolerance induction: animal models to clinical trials. Transplantation. 2009;87(3):309–16. doi: 10.1097/TP.0b013e31819535c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwagi N, Porter KA, Penn I, Brettschneider L, Starzl TE. Studies of homograft sex and of gamma globulin phenotypes after orthotopic homotransplantation of the human liver. Surgical forum. 1969;20:374–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307(5947):168–70. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 18.Morecki S, Leshem B, Weigensberg M, Bar S, Slavin S. Functional clonal deletion versus active suppression in transplantation tolerance induced by total-lymphoid irradiation. Transplantation. 1985;40(2):201–10. doi: 10.1097/00007890-198508000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Slavin S, Fuks Z, Kaplan HS, Strober S. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. The Journal of experimental medicine. 1978;147(4):963–72. doi: 10.1084/jem.147.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slavin S, Or R, Weshler Z, Fuks Z, Morecki S, Weigensberg M, et al. The use of total lymphoid irradiation for allogeneic bone marrow transplantation in animals and man. Survey of immunologic research. 1985;4(3):238–52. doi: 10.1007/BF02918677. [DOI] [PubMed] [Google Scholar]
- 21.Slavin S, Reitz B, Bieber CP, Kaplan HS, Strober S. Transplantation tolerance in adult rats using total lymphoid irradiation: permanent survival of skin, heart, and marrow allografts. The Journal of experimental medicine. 1978;147(3):700–7. doi: 10.1084/jem.147.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sykes M. Hematopoietic cell transplantation for the induction of allo- and xenotolerance. Clinical transplantation. 1996;10(4):357–63. [PubMed] [Google Scholar]
- 23.Sykes M. Mechanisms of transplantation tolerance in animals and humans. Transplantation. 2009;87(9 Suppl):S67–9. doi: 10.1097/TP.0b013e3181a2a6b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sykes M, Sachs DH. Mixed allogeneic chimerism as an approach to transplantation tolerance. Immunology today. 1988;9(1):23–7. doi: 10.1016/0167-5699(88)91352-7. [DOI] [PubMed] [Google Scholar]
- 25.Sykes M, Sheard MA, Sachs DH. Effects of T cell depletion in radiation bone marrow chimeras. II. Requirement for allogeneic T cells in the reconstituting bone marrow inoculum for subsequent resistance to breaking of tolerance. The Journal of experimental medicine. 1988;168(2):661–73. doi: 10.1084/jem.168.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986;323(6084):164–6. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- 27.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. The Journal of experimental medicine. 1989;169(2):493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sykes M, Sachs DH. Mixed chimerism. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2001;356(1409):707–26. doi: 10.1098/rstb.2001.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nature medicine. 2000;6(4):464–9. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 30.Pennington LR, Sakamoto K, Popitz-Bergez FA, Pescovitz MD, McDonough MA, MacVittie TJ, et al. Bone marrow transplantation in miniature swine. I. Development of the model. Transplantation. 1988;45(1):21–6. doi: 10.1097/00007890-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Popitz-Bergez FA, Sakamoto K, Pennington LR, Pescovitz MD, McDonough MA, MacVittie TJ, et al. Bone marrow transplantation in miniature swine. II. Effect of selective genetic differences on marrow engraftment and recipient survival. Transplantation. 1988;45(1):27–31. [PubMed] [Google Scholar]
- 32.Sakamoto K, Sachs DH, Shimada S, Popitz-Bergez FA, Pennington LR, Pescovitz MD, et al. Bone marrow transplantation in miniature swine. III. Graft-versus-host disease and the effect of T cell depletion of marrow. Transplantation. 1988;45(5):869–75. [PubMed] [Google Scholar]
- 33.Huang CA, Yamada K, Murphy MC, Shimizu A, Colvin RB, Neville DM, Jr, et al. In vivo T cell depletion in miniature swine using the swine CD3 immunotoxin, pCD3-CRM9. Transplantation. 1999;68(6):855–60. doi: 10.1097/00007890-199909270-00019. [DOI] [PubMed] [Google Scholar]
- 34.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59(2):256–62. [PubMed] [Google Scholar]
- 35.Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation. 2004;77(6):943–6. doi: 10.1097/01.tp.0000117779.23431.3f. [DOI] [PubMed] [Google Scholar]
- 36.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68(4):480–4. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 37.Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73(9):1386–91. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(9):2121–33. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine. 2008;358(4):353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013;95(1):169–76. doi: 10.1097/TP.0b013e3182782fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciancio G, Miller J, Garcia-Morales RO, Carreno M, Burke GW, 3rd, Roth D, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation. 2001;71(7):827–35. doi: 10.1097/00007890-200104150-00002. [DOI] [PubMed] [Google Scholar]
- 42.Ciancio G, Burke GW, Garcia-Morales R, Suzart K, Rosen A, Ricordi C, et al. Effect of living-related donor bone marrow infusion on chimerism and in vitro immunoregulatory activity in kidney transplant recipients. Transplantation. 2002;74(4):488–96. doi: 10.1097/00007890-200208270-00010. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Sachs DH, Sykes M, Cosimi AB Immune Tolerance N. HLA-mismatched renal transplantation without maintenance immunosuppression. The New England journal of medicine. 2013;368(19):1850–2. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciancio G, Sageshima J, Akpinar E, Gaynor JJ, Chen L, Zarak A, et al. A Randomized Pilot Study of Donor Stem Cell Infusion in Living-Related Kidney Transplant Recipients Receiving Alemtuzumab. Transplantation. 2013 doi: 10.1097/TP.0b013e3182a0f68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dave SD, Vanikar A, Trivedi HL, Gumber MR, Patel HV, Shah PR, et al. Stem cells versus donor specific transfusions for tolerance induction in living donor renal transplantation: a single-center experience. Transplantation. 2013;95(1):155–60. doi: 10.1097/TP.0b013e3182752bcc. [DOI] [PubMed] [Google Scholar]
- 46.Leventhal JR, Mathew JM, Salomon DR, Kurian SM, Suthanthiran M, Tambur A, et al. Genomic biomarkers correlate with HLA-identical renal transplant tolerance. Journal of the American Society of Nephrology: JASN. 2013;24(9):1376–85. doi: 10.1681/ASN.2013010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leventhal JR, Mathew JM, Salomon DR, Kurian SM, Friedewald JJ, Gallon L, et al. Nonchimeric HLA-Identical Renal Transplant Tolerance: Regulatory Immunophenotypic/Genomic Biomarkers. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(1):221–34. doi: 10.1111/ajt.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lederberg J. Genes and antibodies. Science. 1959;129(3364):1649–53. doi: 10.1126/science.129.3364.1649. [DOI] [PubMed] [Google Scholar]
- 49.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62(3):380–7. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 50.Manilay JO, Pearson DA, Sergio JJ, Swenson KG, Sykes M. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation. 1998;66(1):96–102. doi: 10.1097/00007890-199807150-00015. [DOI] [PubMed] [Google Scholar]
- 51.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. Journal of immunology. 1994;153(3):1087–98. [PubMed] [Google Scholar]
- 52.Janes S, Dhaliwal P, Wood K. Tolerance in renal transplantation: is mixed chimerism the missing link? Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(6):1726–9. doi: 10.1093/ndt/gfp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harbor perspectives in biology. 2012;4(6) doi: 10.1101/cshperspect.a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller DL. Mechanisms maintaining peripheral tolerance. Nature immunology. 2010;11(1):21–7. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 55.Womer KL. Transplantation tolerance. Saudi J Kidney Dis Transpl. 2005;16(4):498–505. [PubMed] [Google Scholar]
- 56.Zimring JC. Location, location, location: advancing veto cell therapies. Blood. 2013;121(7):1069–70. doi: 10.1182/blood-2012-12-472654. [DOI] [PubMed] [Google Scholar]
- 57.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. The Journal of experimental medicine. 1998;187(12):2037–44. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. Journal of immunology. 2001;167(2):1103–11. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- 59.Guo Z, Wang J, Dong Y, Adams AB, Shirasugi N, Kim O, et al. Long-term survival of intestinal allografts induced by costimulation blockade, busulfan and donor bone marrow infusion. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(9):1091–8. doi: 10.1034/j.1600-6143.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 60.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. Journal of immunology. 2009;182(12):7331–41. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 61.Bachar-Lustig E, Rachamim N, Li HW, Lan F, Reisner Y. Megadose of T cell-depleted bone marrow overcomes MHC barriers in sublethally irradiated mice. Nature medicine. 1995;1(12):1268–73. doi: 10.1038/nm1295-1268. [DOI] [PubMed] [Google Scholar]
- 62.Reich-Zeliger S, Zhao Y, Krauthgamer R, Bachar-Lustig E, Reisner Y. Anti-third party CD8+ CTLs as potent veto cells: coexpression of CD8 and FasL is a prerequisite. Immunity. 2000;13(4):507–15. doi: 10.1016/s1074-7613(00)00050-9. [DOI] [PubMed] [Google Scholar]
- 63.Miller RG, Derry H. A cell population in nu/nu spleen can prevent generation of cytotoxic lymphocytes by normal spleen cells against self antigens of the nu/nu spleen. Journal of immunology. 1979;122(4):1502–9. [PubMed] [Google Scholar]
- 64.Seyfert-Margolis V, Feng S. Tolerance: is it achievable in pediatric solid organ transplantation? Pediatr Clin North Am. 2010;57(2):523–38. doi: 10.1016/j.pcl.2010.01.015. table of contents. [DOI] [PubMed] [Google Scholar]
- 65.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109(2):827–35. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 66.Golshayan D, Wyss JC, Abulker CW, Schaefer SC, Lechler RI, Lehr HA, et al. Transplantation tolerance induced by regulatory T cells: in vivo mechanisms and sites of action. International immunopharmacology. 2009;9(6):683–8. doi: 10.1016/j.intimp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. The Journal of experimental medicine. 2002;195(12):1641–6. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heslan JM, Beriou G, Le Luduec JB, Guillonneau C, Anegon I, Soulillou JP, et al. Accumulation of T cells with potent regulatory properties and restricted Vbeta7-TCR rearrangements in tolerated allografts. Transplantation. 2005;80(10):1476–84. doi: 10.1097/01.tp.0000185198.07663.ba. [DOI] [PubMed] [Google Scholar]
- 69.Akl A, Jones ND, Rogers N, Bakr MA, Mostafa A, El Shehawy el M, et al. An investigation to assess the potential of CD25highCD4+ T cells to regulate responses to donor alloantigens in clinically stable renal transplant recipients. Transplant international: official journal of the European Society for Organ Transplantation. 2008;21(1):65–73. doi: 10.1111/j.1432-2277.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 70.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Science translational medicine. 2011;3(83):83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all-trans retinoic acid in TGF-beta-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. The Journal of experimental medicine. 2009;206(2):343–57. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(6):1236–47. doi: 10.1111/j.1600-6143.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thorp EB, Stehlik C, Ansari MJ. T-cell exhaustion in allograft rejection and tolerance. Current opinion in organ transplantation. 2015;20(1):37–42. doi: 10.1097/MOT.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ratajczak MZ, Kim C, Ratajczak J, Janowska-Wieczorek A. Innate immunity as orchestrator of bone marrow homing for hematopoietic stem/progenitor cells. Advances in experimental medicine and biology. 2013;735:219–32. doi: 10.1007/978-1-4614-4118-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26(1):106–16. doi: 10.1038/leu.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4(1):62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 77.Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103(6):2071–8. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 78.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 79.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 80.Vagima Y, Lapid K, Kollet O, Goichberg P, Alon R, Lapidot T. Pathways implicated in stem cell migration: the SDF-1/CXCR4 axis. Methods in molecular biology. 2011;750:277–89. doi: 10.1007/978-1-61779-145-1_19. [DOI] [PubMed] [Google Scholar]
- 81.Wu W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, et al. The bone marrow-expressed antimicrobial cationic peptide LL-37 enhances the responsiveness of hematopoietic stem progenitor cells to an SDF-1 gradient and accelerates their engraftment after transplantation. Leukemia. 2012;26(4):736–45. doi: 10.1038/leu.2011.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–9. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 83.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–55. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kew RR, Penzo M, Habiel DM, Marcu KB. The IKKalpha-dependent NF-kappaB p52/RelB noncanonical pathway is essential to sustain a CXCL12 autocrine loop in cells migrating in response to HMGB1. Journal of immunology. 2012;188(5):2380–6. doi: 10.4049/jimmunol.1102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shirvaikar N, Marquez-Curtis LA, Ratajczak MZ, Janowska-Wieczorek A. Hyaluronic acid and thrombin upregulate MT1-MMP through PI3K and Rac-1 signaling and prime the homing-related responses of cord blood hematopoietic stem/progenitor cells. Stem cells and development. 2011;20(1):19–30. doi: 10.1089/scd.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105(1):40–8. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 87.Boiko JR, Borghesi L. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2012;57(1):1–8. doi: 10.1016/j.cyto.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115(10):1865–72. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 89.Schuettpelz LG, Link DC. Regulation of hematopoietic stem cell activity by inflammation. Frontiers in immunology. 2013;4:204. doi: 10.3389/fimmu.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochemical and biophysical research communications. 2009;388(4):621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 91.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24(6):801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sioud M, Floisand Y, Forfang L, Lund-Johansen F. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. Journal of molecular biology. 2006;364(5):945–54. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 93.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. Journal of immunology. 2011;186(9):5367–75. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yanez A, Flores A, Murciano C, O’Connor JE, Gozalbo D, Gil ML. Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol. 2010;12(1):114–28. doi: 10.1111/j.1462-5822.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 95.Holler E, Rogler G, Brenmoehl J, Hahn J, Greinix H, Dickinson AM, et al. The role of genetic variants of NOD2/CARD15, a receptor of the innate immune system, in GvHD and complications following related and unrelated donor haematopoietic stem cell transplantation. Int J Immunogenet. 2008;35(4–5):381–4. doi: 10.1111/j.1744-313X.2008.00795.x. [DOI] [PubMed] [Google Scholar]
- 96.Holler E, Rogler G, Brenmoehl J, Hahn J, Herfarth H, Greinix H, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood. 2006;107(10):4189–93. doi: 10.1182/blood-2005-09-3741. [DOI] [PubMed] [Google Scholar]
- 97.Penack O, Smith OM, Cunningham-Bussel A, Liu X, Rao U, Yim N, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. The Journal of experimental medicine. 2009;206(10):2101–10. doi: 10.1084/jem.20090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104(3):889–94. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]
- 99.van der Velden WJ, Blijlevens NM, Maas FM, Schaap NP, Jansen JH, van der Reijden BA, et al. NOD2 polymorphisms predict severe acute graft-versus-host and treatment-related mortality in T-cell-depleted haematopoietic stem cell transplantation. Bone marrow transplantation. 2009;44(4):243–8. doi: 10.1038/bmt.2009.21. [DOI] [PubMed] [Google Scholar]
- 100.Kawai T, Sachs DH. Tolerance induction: hematopoietic chimerism. Current opinion in organ transplantation. 2013;18(4):402–7. doi: 10.1097/MOT.0b013e328363621d. [DOI] [PubMed] [Google Scholar]