Summary
The present study demonstrates that acute ozone exposure of healthy human subjects enhances lung immune responses to subsequent bacterial stimuli. This highlights common air pollutant exposures as modifiers of the intensity of pulmonary immune activation.
Keywords: innate immunity, LPS, TLR4, CD14
To the Editor
Our previous work in mouse models support that inhalation of ambient ozone can prime the biological response to inhaled bacterial LPS1. Mice exposed to inhaled ozone then subsequently challenged to low levels of LPS exhibited increased innate immune response as evidenced by higher levels of pro-inflammatory cytokines. In that study, alveolar macrophages from ozone-exposed animals demonstrated enhanced response to LPS and enhanced surface expression of the LPS receptor TLR4. Consistent with animal studies, evidence from sputum macrophages isolated from humans exposed to ozone identified enhanced surface expression of TLR4 and CD14 by flow cytometry2–4. However, the functional relevance of these initial observations in healthy human subjects remained unknown. Therefore, the current study was designed to test the hypothesis that in vivo inhalation of relevant levels of ambient ozone would enhance human alveolar macrophage responses ex vivo to bacterial stimulation with LPS.
Adult healthy human subjects (N=34) were exposed to either filtered air (FA) or ozone (200 ppb) in an exposure chamber for 135 minutes with intermittent exercise in a cross-over study design with at least a 21 day washout period between exposures as previously reported5. The full details of the study design, exposure and processing of samples are available in this article’s Online Repository at www.jacionline.org. The subjects enrolled were healthy and predominantly male (Table 1). Approximately 20 hours after each exposure, subjects underwent a flexible bronchoscopy with bronchoalveolar lavage. Alveolar macrophage cells were isolated with 95% purity and cultured. To determine innate immune response, cultured cells were stimulated with bacterial lipopolysaccharide (LPS), which is a prototypic toll-like receptor 4 (tlr4)-2 dependent ligand6. Cells were also challenged with phorbol-12-myristate-13-acetate (PMA), a specific activator of protein kinase C, which is directly involved in intracellular TLR-signaling7, but does not require intact surface TLR4. Ex vivo macrophages were challenged to saline (control), LPS (100 ng/mL), or phorbol-12-myristate-13-acetate (PMA, 1μM) for 2 hours. Cell lysates were analyzed by RT-PCR for mRNA expression level of TNFα. Inhalation of ozone resulted in increased mRNA expression of TNFα in alveolar macrophages, when compared to filtered air (Figure 1A/B). We also observed an enhanced response to bacterial LPS challenge in alveolar macrophages from subjects previously exposed to inhaled ozone (Figure 1A). Furthermore, we identified that inhalation of ozone enhanced macrophage response to PMA challenge (Figure 1B). These findings demonstrate that in healthy human subjects inhalation of ambient levels of ozone can prime the biological response of alveolar macrophages to both bacterial LPS and PMA.
Table 1.
Baseline characteristics of subjects
| Female | Male | |
|---|---|---|
| Subject Number | 6 | 28 |
| Age | 25.5 ± 2.81 | 24.71 ± 4.13 |
| Body Mass Index (kg/m2) | 22.27 ± 2.25 | 24.27 ± 2.67 |
| Ethnicity | ||
| Caucasian | 3 | 17 |
| African American | 3 | 1 |
| Hispanic | 0 | 2 |
| Asian | 0 | 8 |
Figure 1.
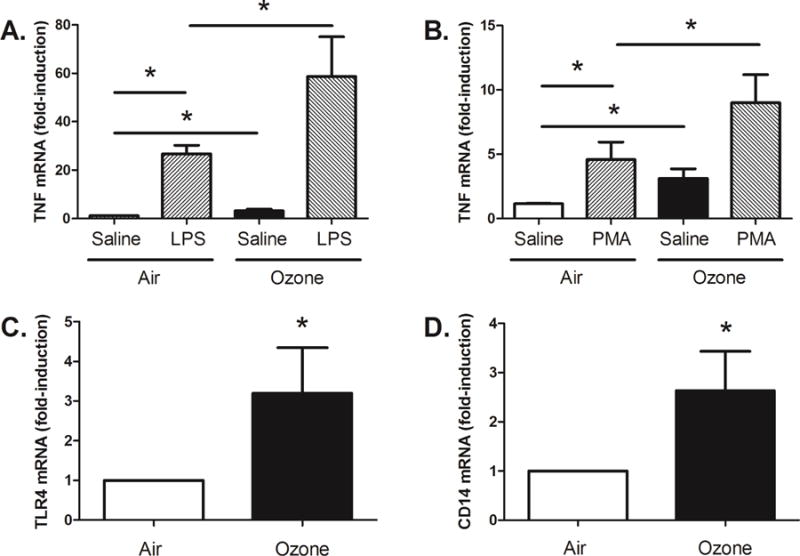
Ozone enhanced gene expression and functional response to LPS and PMA. Ozone exposure enhanced alveolar macrophage expression of TNFα and the functional response to both bacterial LPS (A) and PMA (B) (N=34, *p<0.05, two-tailed unpaired Student T test). Ozone exposure resulted in enhanced alveolar macrophage mRNA expression of both TLR4 (C) and CD14 (D), when compared to filtered air. (N=34, *p<0.05, one-tailed paired Student T-Test).
Previous work supports that ozone can enhance the surface expression of TLR4 and CD14 on sputum macrophages isolated from human subjects3, 4. To extend prior observations from human subjects exposed to ozone, we measured the mRNA expression of TLR4 and CD14 in isolated alveolar macrophages from BAL samples. Inhalation of ozone, when compared to filtered air, resulted in significantly increased mRNA expression of both TLR4 and CD14 (Figure 1C/D). These findings demonstrate that human subjects exposed inhalation of ozone can induce the expression of genes that are required for the biological response to LPS.
We should highlight that there is a great degree of inter-individual variability in the biological response to ozone. There were clearly some individuals with extremely high response to ozone as measured by TNFα and by their enhanced priming of innate immunity. This variability is consistent with previous reports and further supports that host factors contribute to the biological response to ozone. Understanding of the specific host factors that regulate susceptibility to ozone will be an area of continued investigation.
Tight regulation of the intensity of pulmonary innate immunity is highly clinically relevant. It is recognized that intact innate immunity is required for effective antibacterial host defense. However, it is also recognized that over-exuberant innate immune responses can contribute to lung injury. Intensity of innate immunity can contribute to both allergic disease8 and emphysema9. Elevated short-term ozone exposures are associated with enhanced mortality and hospitalizations for respiratory disordersE1, E2. Mechanisms supporting this epidemiologic evidence are poorly understood; but it is plausible that altered innate pulmonary immune responses are important contributors. The present study supports that ozone exposure augments pulmonary innate immune responses in humans. Additionally, these responses are subject in inter-individual variability suggesting that host factors regulate ozone priming responses and potentially an individual’s susceptibility to ozone-derived health effects. Appreciation of the mechanisms that common environmental exposures can contribute to reprogramming of the intensity of innate immune response, in susceptible individuals, is therefore highly clinically significant. Our findings support that inhalation of environmentally relevant levels of ambient ozone in adult healthy subjects can result in increased mRNA expression of macrophage-derived TNFα, TLR4, and CD14. Furthermore, inhalation of ozone can enhance the functional response of alveolar macrophages to both bacterial LPS and PMA. Similar to our previous observations in rodent models, these new findings support that inhalation of ambient ozone can enhance pulmonary innate immunity in healthy human subjects.
Acknowledgments
This work was supported by National Institutes of Health grants ES016126, ES020350, AI081672 and HL105537
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Hollingsworth JW, Maruoka S, Li Z, Potts EN, Brass DM, Garantziotis S, et al. Ambient ozone primes pulmonary innate immunity in mice. J Immunol. 2007;179:4367–75. doi: 10.4049/jimmunol.179.7.4367. [DOI] [PubMed] [Google Scholar]
- 2.Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, et al. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. J Allergy Clin Immunol. 2007;120:719–22. doi: 10.1016/j.jaci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Alexis NE, Lay JC, Hazucha M, Harris B, Hernandez ML, Bromberg PA, et al. Low-level ozone exposure induces airways inflammation and modifies cell surface phenotypes in healthy humans. Inhal Toxicol. 2010;22:593–600. doi: 10.3109/08958371003596587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez ML, Lay JC, Harris B, Esther CR, Jr, Brickey WJ, Bromberg PA, et al. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 2010;126:537–44 e1. doi: 10.1016/j.jaci.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol (1985) 2011;111:679–87. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 7.Loegering DJ, Lennartz MR. Protein kinase C and toll-like receptor signaling. Enzyme Res. 2011;2011:537821. doi: 10.4061/2011/537821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–9. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]


