Abstract
Background and aims
Substance use has been implicated in the onset and maintenance of risky sexual behaviors, which have particularly devastating consequences in young women. This study examined whether (i) adolescent onset of cannabis use is associated with repeated voluntary unprotected sex in women and (ii) whether this association persists after accounting for correlated familial influences.
Design
General population sample of female twins.
Setting
Midwestern United States.
Participants
2,784 sexually active twin women (15.5% African-American) aged 18-27 years (assessed 2002-2005), including 119 dizygotic (DZ) and 115 monozygotic (MZ) discordant pairs.
Measurements
Self-report interview data on cannabis use that first occurred prior to age 17 (27.1%) and repeated voluntary unprotected sex (27.2%). Key covariates included early onset of drinking, regular smoking, sexual debut and menstruation as well as conduct disorder symptoms and childhood sexual abuse.
Findings
Compared with never users and those who started using cannabis at a later age, adolescent cannabis users were more likely to report repeated voluntary unprotected sex (odds ratio [OR], 2.69; 95% Confidence Interval, CI 2.24-3.22). Genetic (rg= 0.57, 95% CI: 0.38-0.87) and non-shared environmental (re= 0.21, 95% CI: 0.02-0.38) factors contributed to the association. After accounting for correlated familial factors, there was a consistent elevation in the likelihood of repeated voluntary unprotected sex in the exposed twin relative to her genetically identical never/late-onset user co-twin (unadjusted OR 2.25, 95% CI 1.14-4.44), even after adjustment for covariates (adjusted OR 2.27, 95% CI 1.08-4.80).
Conclusions
Women who start using cannabis during adolescence appear to be more likely to report voluntary engagement in repeated unprotected sex than women who never use cannabis or who initiate cannabis use after adolescence. The results appear to be independent of shared genetic influences.
Keywords: Cannabis, Sexual, Twin, Unprotected, Cotwin-Control, Discordant
INTRODUCTION
Of current sexually active high school students, 13.7% report not using any methods to prevent pregnancy (1). Girls and young women are more likely to report unprotected sex than their male counterparts (15.7 vs. 11.5%) and the consequences of such risky sexual behavior are also greater in girls and women including profoundly elevated risks for unintended pregnancy and exposure to sexually transmitted infections. Substance use, during the lifetime as well as immediately preceding sexual intercourse, has been frequently implicated as an independent contributor to likelihood of such risky sexual behavior, including, early sexual debut, having multiple sexual partners and repeated voluntary unprotected sex (rVUS)(2-5). Use of cannabis, particularly during adolescence, has been cross-sectionally and prospectively linked to risky sexual behavior (6-11). However, no study to date has used a genetically informed design to disentangle the role of person-specific factors that may be of a causal nature from the contribution of common predisposing factors that might influence both adolescent cannabis use and rVUS.
As both cannabis use and sexual behaviors, both normative and risky (such as rVUS), are typically initiated during adolescence, it is likely that shared risk and protective influences at least partially contribute to their co-occurrence. This general liability to risk-taking and externalizing behaviors during youth is shaped by genetic and environmental factors (12-15). For instance, lack of self-regulation, which is associated with both reward-related (e.g. ventral striatum) and decision-making (e.g. dorsolateral prefrontal cortex) pathways in the brain (16), has been proposed as a common pathway to both substance use and risky sexual behaviors. Interactions between corticostriatal (i.e. ventral striatum) and corticolimbic (e.g. amygdala reactivity) circuitry have also been shown to modulate liability to substance use and risky sexual behaviors (17;18). Environmental factors (e.g. early trauma exposure; e.g. (19;20)) might also influence the likelihood of early onset of cannabis use and risky sexual behaviors, such as rVUS.
The action of these contributors, genetic or environmental, may be classified as being of a predisposing nature or person-specific. For instance, heightened corticostriatal responsivity may predate both early onset of cannabis use and risky sexual behavior (i.e. predisposing factor), or alternatively, early exposure to cannabis may result in altered ventral striatal reactivity which subsequently increases the likelihood of risky sexual behaviors (i.e. person-specific). Longitudinal data that span the developmental periods prior to onset of both cannabis use and rVUS and extend beyond their peak period of initiation are required to carefully address causal hypotheses. However, twin pairs discordant for early exposure to cannabis offer a unique opportunity for disarticulating person-specific influences (possibly, of a causal nature) from predisposing factors typically shared by members of twin pairs. In particular, for monozygotic (MZ) twins discordant for adolescent cannabis use, any excess likelihood of rVUS in the twin using cannabis at an early age, relative to her genetically identical co-twin who also shares a preponderance of her early familial environmental exposures, can be attributed to factors unique to adolescent cannabis use that may have causal characteristics. In contrast, no excess of rVUS in the twin who uses cannabis at an early age, relative to her non-using/later-onset user co-twin, would largely discount the possibility of there being any causal relationship between adolescent cannabis use and rVUS.
We hypothesized that adolescent cannabis use would be associated with an increased likelihood of rVUS and that this association would be partly due to shared familial influences but would also persist after such shared familial influences were accounted for. In a large cross-sectional sample of 2,784 sexually active female twins aged 18 to 27 years, we examine the extent to which genetic and environmental factors contribute to the association between first cannabis use prior to age 17 and repeated (≥3 times) voluntary unprotected sex (rVUS). Analyses were further conducted within discordant pairs of twins, specifically in the subset of MZ twins discordant for adolescent cannabis use (Npairs= 115) to examine whether the association persisted after accounting for genetic similarity and familial environment. Finally, classical twin modeling was also used to quantify the extent to which genetic and environmental factors contributed to variance in and covariance between adolescent cannabis use and rVUS.
METHOD
Sample
The Missouri Adolescent Female Twin Study (MOAFTS) is a cohort of same-sex female twin pairs, identified from birth records, born between July 1st 1975 and June 30th 1985 to Missouri-born parents (see (21-23)). As a subset of the participants were 12-15 years of age at baseline, sensitive questions regarding cannabis use were not administered to them at that initial wave of data collection. During 2002-2005, all eligible twins who were then adults, regardless of whether they had participated in the baseline assessments or not (and as long as they had not declined to participate in future interviews), were invited to participate in the first full-length adult follow-up interview. This sample, which represented 80% of all live female twin births in the state, consists of 3,787 twins (including 964 monozygotic or MZ and 809 dizygotic or DZ pairs, as well as 241 un-paired twins who did not have a participating co-twin) aged 18-27 years, including 14.6% of African-American ancestry. Data on 2,950 (15.9% African-American) twin women who reported a lifetime history of any consensual sexual activity were included in the current report. However, as 166 of these women reported onset of rVUS prior to their first use of cannabis, they were excluded from analyses resulting in a final sample size of 2,784 twin women. Those reporting onset of rVUS prior to first cannabis use were more likely to be older and Black. They were also generally less likely engage in early-onset substance use behaviors but more frequently reported early sexual debut (Table S1).
Measures
A structured interview adapted from The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) was used to obtain interview data from respondents (24).
Adolescent Cannabis Use
Individuals who reported ever using cannabis were queried about the age at which they had first used it (median age 16 years). Consistent with prior studies of early-onset cannabis use (25;26), use for the first time prior to age 17 was defined as adolescent cannabis use, which was reported by 27.1% of the full sample (55% of users). Twin pairs were considered discordant for adolescent cannabis use if one twin reported onset of cannabis use prior to age 17 while the other reported either no lifetime history of cannabis use or use at age 17 and older. Sensitivity analyses that (a) excluded lifetime non-users and (b) examined other age cutoffs for adolescent onset as well as continuous age were also conducted.
Repeated Voluntary Unprotected Sex (rVUS)
Women were asked if they had ever engaged in heterosexual sexual intercourse when they or their partner had not used a condom. Those who reported even one such occurrence were further queried about whether they had ever had sexual intercourse when they and their partner had not used any form of birth control (e.g., contraceptive pill). Respondents were specifically asked to exclude occurrences when they were coerced into sexual activity or were voluntarily attempting conception. rVUS was coded as three or more instances of voluntary sexual intercourse without a condom or any form of birth control and was reported by 27.2% of the sample. Median age of onset of first instance of rVUS, in those reporting even one instance, was 17.0 years.
Features related to rVUS
The relationship between adolescent cannabis use and rVUS was also characterized based on other aspects of romantic relationships and reproductive history, including (a) number of romantic male partners across the lifetime (median split: ≥5); (b) age at first romantic date (prior to age 15); (c) ever pregnant, including non-viable pregnancies; (d) ≥3 pregnancies, including non-viable pregnancies; and (e) age at first pregnancy (prior to age 18).
Covariates
(a) Early regular alcohol use: drinking one or more drinks per month for six months in a row, prior to age 17; (b) Early regular cigarette smoking: smoking one day a week for at least 2 months in a row, prior to age 16; (c) Early sexual debut: first consensual sex, prior to age 16; (d) Early maturation: onset of menses, prior to age 12. (e) One or more symptoms of conduct disorder. (f) Childhood exposure to sexual abuse, prior to age 17. Analyses in the full sample were also adjusted for self-reported ethnicity (Black vs. White) and age (less than 22 years); as members of twin pairs are matched for ethnicity and age, this adjustment was not necessary in the within-pair comparisons.
Statistical Analyses
All regression analyses were conducted in SAS v9.2. Logistic regression was used to estimate the association between rVUS and adolescent cannabis use – standard errors were adjusted for clustered family data using the Taylor method in PROC SURVEYLOGISTIC. Conditional logistic regression was used to examine within-pair associations, including in pairs of MZ twins discordant for adolescent cannabis use (27). Regression analyses were also conducted with adjustment for covariates. For within-pair analyses, only those covariates that were significantly associated with rVUS in the full sample were included. The extent to which additive genetic (A), shared (C) and person-specific (E) environmental factors influenced the variance in and covariance between rVUS and adolescent cannabis use was estimated by using data on all available twins and fitting them to a bivariate twin model in the statistical software package Mx (28).
RESULTS
rVUS and adolescent cannabis use were not associated with ethnicity or zygosity, however younger individuals (age ≤ 21 years) were more likely to report adolescent cannabis use and less likely to report rVUS (Table 1). There was a marginally increased likelihood for MZ twins to report rVUS, relative to their DZ counterparts. With the exception of early age at pregnancy and early age at menstruation which were not associated with rVUS, both rVUS and adolescent cannabis use were associated with all other romantic and reproductive characteristics and covariates. Most prominently, rVUS was related to an almost 3.5 increased odds of ever being pregnant and, even within those reporting a pregnancy, an elevated likelihood of ≥3 pregnancies (OR=1.53, 95% C.I. 1.15-2.03). rVUS was also associated with early sexual debut. The strongest correlates of adolescent cannabis use were early regular use of alcohol and tobacco, as well as early sexual debut.
Table 1.
Univariate phenotypic associations (using logistic regression) between adolescent cannabis use, repeated voluntary unprotected sex (rVUS) and romantic/reproductive characteristics and individual covariates in 2,784 women aged 18-27 years. Estimates presented are Odds Ratios (OR) with their 95% Confidence Intervals [95% CI].
| Adolescent cannabis use | rVUS | |
|---|---|---|
| Demographics | ||
| Age ≤ 21 | 1.38 [1.17-1.63] | 0.60 [0.51-0.72] |
| Zygozity (MZ) | 0.94 [0.79-1.11]ns, p=0.45 | 1.19 [1.00-1.41]p=0.05 |
| Ethnicity (Black) | 0.86 [0.68-1.09]ns, p=0.22 | 0.96 [0.76-1.21]ns, p=0.71 |
| Other romantic and reproductive characteristics | ||
| Lifetime number of boyfriends ≥ 5 | 1.67 [1.43-1.96] | 1.55 [1.30-1.83] |
| Age of first romantic date ≤ 14 | 1.89 [1.61-2.22] | 1.58 [1.33-1.88] |
| Ever pregnant | 1.47 [1.25-1.72] | 3.48 [2.93-4.14] |
| ≥3 pregnancies in those pregnant (n=1079) |
0.93 [0.70-1.24]ns, p=0.61 | 1.53 [1.14-2.03] |
| Age at first pregnancy ≤ 17 in sthose pregnant (n=1079) |
1.62 [1.25-2.08] | 1.18 [0.92-1.52]ns, p=0.20 |
| Covariates | ||
| Early regular alcohol use | 5.35 [4.23-6.77] | 1.68 [1.32-2.12] |
| Early regular tobacco smoking | 9.21 [7.33-11.59] | 2.25 [1.82-2.79] |
| Early sexual debut | 4.06 [3.38-4.87] | 2.44 [2.03-2.93] |
| Early onset of menstruation | 1.40 [1.14-1.71] | 1.12 [0.91-1.37]ns, p=0.29 |
| Conduct Disorder symptoms | 3.12 [2.63-3.71] | 1.79 [1.51-2.12] |
| Childhood sexual abuse | 2.13 [1.70-2.65] | 1.74 [1.39-2.17] |
nsnot significant at p < 0.05.
In this sample of 2,784 sexually active female twins, adolescent cannabis use was strongly associated with rVUS (unadjusted OR 2.69, 95% CI 2.24-3.22; Table 2) and the association persisted even when never users were excluded (OR 2.55, 95% CI 2.01-3.24). Even after adjustment for covariates, women who used cannabis prior to age 17 were at over two times increased odds relative to their never user/late-onset user co-twin to report rVUS.
Table 2.
Pattern of associations (using conditional logistic regression) between adolescent cannabis use and repeated voluntary unprotected sex (rVUS) in female twins aged 18-27 years. Estimates presented are Odds Ratios (OR) with their 95% confidence intervals [95% CI] and represent the increased likelihood of rVUS in adolescent cannabis users (first use prior to age 17) relative to those who never used cannabis or used it at age 17 or later.
| All female twins | Discordant MZ & DZ pairs | Discordant MZ pairs only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Unadjusted | Adjusted* | Significant Covariates |
Npairs | Unadjusted | Adjusted# | Npairs | Unadjusted | Adjusted# | |
| Including never users (of cannabis) | 2784 | 2·69 [2.24-3.22] |
2.05 [1.65 -2.53] |
6, 7, 8a | 234 | 2.17 [1.34-3.51] |
1.98 [1.15-3.41] |
115 | 2.25 [1.14-4.44] |
2.27 [1.08-4.80] |
| In ever users only | 1368 | 2.55 [2.01-3.24] |
2.16 [1.64-2.83] |
6, 7, 8b | 105 | 2.80 [1.36-5.76] |
3.00 [1.29-7.00] |
52 | 3.00 [1.09-8.25] |
3.12 [1.01-9.61] |
Adjusted for (1) self-reported ethnicity (Black vs. White), (2) age at interview less than 22 years, (3) early regular smoking, (4) early regular alcohol use, (5) early onset of menses, (6) early sexual debut, (7) conduct disorder symptoms and (8) childhood sexual abuse.
Adjusted for significant covariates from analysis of all female twins. Results remain largely unchanged if all covariates are included.
Odds-ratio [95% C.I.] for (1) Black vs. White=0.89 [0.59-1.20], (2) age= 0.63 [0.49-0.81], (3) early regular smoking=1.19 [0.92-1.55], (4) early regular alcohol use=1.06 [0.78-1.41], (5) early onset of menses=0.91 [0.68-1.22], (6) early sexual debut=1.44 [1.11-1.88], (7) conduct disorder symptoms=1.47 [1.14-1.88] and (8) childhood sexual abuse=1.38 [1.02-1.86].
Odds-ratio [95% C.I.] for (1) Black vs. White=0.84 [0.60-1.21] (2) age= (3) early regular smoking=1.20 [0.89-1.61], (4) early regular alcohol use=1.02 [0.76-1.36], (5) early onset of menses=0.93 [0.70-1.24], (6) early sexual debut=1.48 [1.14-1.92], (7) conduct disorder symptoms [1.42 [1.11-1.82] and (8) childhood sexual abuse=1.42 [1.05-1.92].
Table 3 shows the prevalence of rVUS in MZ and DZ twin pairs concordant and discordant for adolescent cannabis use. Regardless of zygosity, twins from concordant adolescent-onset pairs were at the highest likelihood for reporting rVUS (MZ: 47.1%; DZ: 41.9%) while twin pairs where neither twin reported adolescent cannabis use exhibited the lowest rates of rVUS (MZ: 22.4%; DZ: 18.7%). The prevalence of rVUS was considerably higher in the adolescent-onset members of the discordant pairs (MZ: 39.1%; DZ: 34.5%) than in their never/late-onset user co-twins (MZ: 26.1%; DZ: 23.5%). Consistent with this observation, the adolescent onset cannabis-using twin member was at two times increased odds of reporting rVUS relative to their never or later-onset user co-twin (Table 2).
Table 3.
Prevalence of repeated voluntary unprotected sex (rVUS) in 2,784 sexually active female twins aged 18-27 years in monozygotic (MZ) and dizygotic (DZ) twin pairs concordant or discordant for adolescent cannabis use (first use prior to age 17).
| Monozygotic (MZ) | Dizygotic (DZ) | |||
|---|---|---|---|---|
| N | Prevalence % [95% C.I] | N | Prevalence % [95% C.I] | |
| Concordant never/late-onset user | 822 | 22.4 [19.5-25.2] | 530 | 18.7 [15.4-22.0] |
| Never/later-onset user of discordant pair | 115 | 26.1 [18.1-34.1] | 119 | 23.5 [15.9-31.2] |
| Adolescent onset user of discordant pair | 115 | 39.1 [30.2-48.1] | 119 | 34.5 [25.9-43.0] |
| Concordant adolescent onset user | 238 | 47.1 [40.7-53.4] | 132 | 41.7 [33.3-50.1] |
Note: Even though the prevalence of rVUS in DZ pairs consistently appears lower than that for MZ pairs, these differences are not statistically significant, as indicated by the 95% confidence intervals.
Importantly, even within MZ pairs discordant for adolescent cannabis use, there was a consistent elevation in the likelihood of rVUS in the adolescent-onset twin relative to their genetically identical never/later-onset user co-twin (OR 2.25, 95% CI [1.14-4.44]). Adjustment for covariates did not attenuate this association (Table 2), suggesting that factors other than those shared by members of MZ twin pairs, including shared genetic and environmental influences, significantly contributed to the relationship between adolescent cannabis use and rVUS.
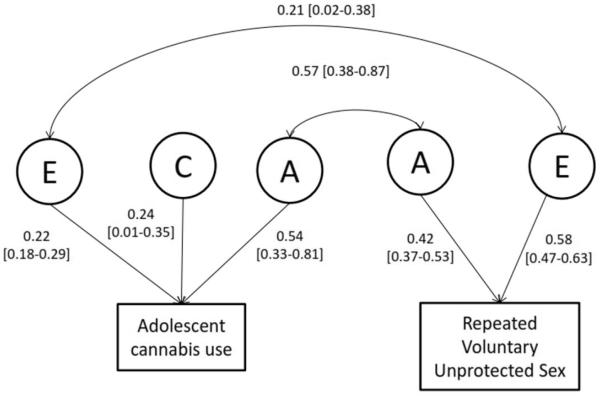
When analyzing data from all twins, MZ correlations were greater than the DZ correlations, and DZ correlations were greater than half the MZ correlations, suggesting the role of genetic and shared environmental influences respectively (Adolescent cannabis: rMZ=0.78, rDZ=0.54; rVUS: rMZ=0.41, rDZ=0.26). Results from the final bivariate genetic model are presented in Figure 1 (parameter estimates for the full model are in Table S5). Shared environmental (Δχ2(2df)=1.98), or additive genetic (Δχ2(2df)=2.32) influences, but not both (Δχ2(2df)=2.32) could be constrained to zero for rVUS – constraining shared environmental factors (estimated at 12%) to zero provided a modest improvement in fit. Correspondingly, variance in adolescent cannabis use was attributable to additive genetic (h2adol. cannabis: 54%), shared environmental (24%) and individual-specific environmental influences while variance in rVUS could be partitioned into additive genetic (h2rVUS: 42%) and individual-specific environmental sources. The covariance between adolescent cannabis use and rVUS was attributable to both genetic (rg=0.57) and, to a lesser extent, individual-specific (re=0.21) contributions.
Figure 1.
Twin model of rVUS and adolescent cannabis use.
Twin model showing standardized path estimates for the role of genetic (A), shared environmental (C) and person-specific environmental (E) influences on adolescent cannabis use and repeated voluntary unprotected sex (rVUS) in 2,784 women.
Exclusion of lifetime abstainers
Our primary analyses coded both women who had never used cannabis and women who had used cannabis but at age 17 or older as unexposed members of twin pairs. Discordant twin analyses were repeated in women who reported a history of sexual activity as well as cannabis use (N=1368). Even in the modest number of 52 MZ pairs discordant for adolescent cannabis use (Table 2), the twin who used cannabis prior to age 17 was at 3 times increased odds of reporting rVUS relative to her genetically identical co-twin who reported cannabis use but at age 17 or later. Thus, even within cannabis users, earlier onset of cannabis use is associated with a unique increase in likelihood of rVUS.
Other definitions of adolescent onset
A continuous measure of age at onset of cannabis use was associated with rVUS, regardless of covariate adjustment (OR=0.58, 95% CI 0.49-0.68; i.e. lower age associated with higher rVUS; Table S2). When twin pairs with differing ages at first use were considered, rVUS was more commonly reported in the twin reporting the earlier age at first use (Table S3 for details). Other dichotomous definitions of adolescent cannabis use (e.g. <16 years, <18 years) showed similar results; however, the significance of the point estimate relied heavily on the discordant pair sample size (Table S4).
DISCUSSION
In the present study, the relationship between adolescent onset of cannabis use and repeated voluntary unprotected sex (rVUS) could be partially attributed to common genetic influences, but also to factors not shared by members of identical twin pairs. Problem behavior theory has previously organized early substance use and adolescent sexual activity into a common and heritable framework of impulsive and disinhibited behaviors (29;30). We identified the role of shared genetic influences on adolescent cannabis use and rVUS as well, substantiating the importance of considering shared predisposition in studies relating substance use to risky sexual behaviors.
The co-twin’s adolescent cannabis use status is indicative of genetic loading and the pattern of prevalence of rVUS in twins as a function of their concordance status for adolescent cannabis use was quite striking (Table 3). Twins concordant for adolescent cannabis exposure, presumably representing the highest degree of familial vulnerability, were most likely to report rVUS while twins concordantly unexposed (i.e. both twins either never used cannabis or used at a later age) were least likely to report rVUS. Within discordant pairs, whether for a dichotomous definition (Table 3) or continuously reported age (Table S3), the exposed (i.e. adolescent onset) twin was at consistently greater likelihood of reporting rVUS than her unexposed (never/late-onset) co-twin. Notably, the adolescent-onset twins from discordant pairs were marginally less likely to report rVUS than their counterparts from concordant exposed pairs indicating an attenuation of vulnerability to rVUS as a function of co-twin’s adolescent-onset cannabis use status. Similarly, even though they did not start using cannabis during adolescence, unexposed members of discordant pairs were somewhat more likely to report rVUS than concordantly never/late-onset users. These results indicated that the presence of an unexposed co-twin marginally reduced the likelihood of rVUS and vice versa. Familial effects were further corroborated by the moderate genetic correlation between adolescent cannabis use and rVUS. However, the upper confidence limit on this correlation estimate did not encompass 1.0 indicating the significant role of specific genetic influences on rVUS that were unrelated to adolescent cannabis use.
Our measure of rVUS is unique in that it involves repeated non-use of standardized methods of birth control, a behavior less subject to “spur of the moment” sexual activity which is often related to concomitant substance use. Thus, we did not expect indices of impulsivity to be the sole common feature underlying adolescent cannabis use and rVUS – correspondingly, adjustment for early sexual debut or conduct problems, both aspects of disinhibited behavior, did not alter the significance of the relationship. Other indices of impulsive sensation-seeking, such as low conscientiousness, have been reliably related to sexual risk-taking and promiscuity (31) as well as to substance use (32). Secondary analyses suggested that while rVUS was strongly associated with lower conscientiousness (from the NEO-PI-R(33)) in our data as well (mean score of 2.78 vs. 2.65; p<.0001), the addition of a measure of low conscientiousness (bottom quartile of scores) did not account for the association between adolescent cannabis use and rVUS (results available upon request).
The increased likelihood of rVUS in women who reported cannabis use during adolescence, relative to their genetically identical but unexposed co-twins (i.e. MZ odds ratio significantly greater than 1.0), and the significant person-specific environmental correlation indicated that the relationship between these behaviors might extend beyond familial predispositions, including (a) direct or indirect pathways from adolescent cannabis use to rVUS or (b) unmeasured common factors that precede both the adolescent onset of cannabis use and rVUS. Prior studies have proposed that providers of illicit substances, such as older peers, might be critical to the strong associations between adolescent cannabis and other illicit drug use (34;35). Such peer affiliations may be related to precocious adoption of adult roles (36), particularly in girls. In our sample as well, women reporting rVUS were more likely to report greater current affiliations with drug-using male peers, including romantic partners (OR 1.79 [95% CI 1.47-2.18]; also associated with adolescent cannabis use, OR 4.54 [95% CI 3.73-5.53]). In addition, both rVUS and adolescent cannabis use were associated with a greater number of romantic partners, an earlier age at first romantic date, and an earlier age at sexual debut. Early sexual maturation in women, which has been linked to substance use (37), risky sexual behaviors (38;39) and delinquencies (40-42), might also index increased likelihood of interactions with older male peers and romantic partners; however this measure did not account for our findings both when including a measure of early pubertal timing (Table 1) as well as a self-evaluation of early maturation (available upon request). While we cannot determine whether these sexual behaviors were associated with rVUS, our findings highlight the added vulnerability associated with rVUS over and above other indices of sexual maturity or risky sexual behavior.
Possibly, the specific characteristics of the male partners and the quality of sexual relationships may be the “causal” component in the observed association but we were unable to explore this hypothesis fully as women did not report on the characteristics of the partners with whom they engaged in rVUS. Based on self-reported reasons for condom non-use (Table S6), in addition to substance use, partner discomfort was frequently reported. It is, thus, possible that early discordance in cannabis exposure was a consequence of, or subsequently resulted in, exposure of one member of the twin pair to a more non-conforming cohort of male peers (with increased access to drugs and characterized by other risky behaviors), which increased her likelihood of rVUS.
Perhaps most critically, women who reported rVUS and adolescent cannabis use were more likely to also report ever being pregnant. While we did not have information on the outcomes of these individual pregnancies or whether they were unintended and related to rVUS, lack of contraceptive use is associated with 85% chance of pregnancy within a year (43). These findings raise the possibility that adolescent cannabis use may result in substantial risks associated with unintended pregnancy, including effects on maternal and offspring health (44).
It is also possible that unmeasured non-familial factors, particularly those that preceded the onset of cannabis use and rVUS and increased the likelihood of both behaviors, are responsible for the observed associations. For instance, early exposure to neglect and trauma (other than childhood sexual abuse; e.g.(45)) might increase susceptibility to adolescent-onset of cannabis use and later rVUS.
Finally, our finding of elevated odds of rVUS in adolescent cannabis users persisted even when they were only compared to their late-onset user co-twins (i.e. both twins had initiated use but at different ages). Even though the number of discordant MZ pairs was modest, these significant results suggest that women who use cannabis prior to age 17 are more likely to report rVUS relative, not only, to those who never use cannabis but also when compared to later-onset users. Thus, delaying the onset of cannabis use in women may have an appreciable impact on likelihood of rVUS.
Some limitations are noteworthy. First, our results may not extrapolate to other demographics. Our study did include a population-representative group of African-American women and they did not differ from their European-American counterparts in rates of rVUS or adolescent cannabis use; however they were more likely to report rVUS prior to cannabis onset (Table S1). Also, according to national surveys, Missouri is one of the states with higher rates of unprotected sex reported by youth (1) – findings in other states may differ. Second, legislation related to the Patient Protection and Affordable Care Act (healthcare.gov) may have modified women’s access to contraceptive alternatives, thus, optimistically reducing the prevalence of rVUS in current cohorts. Third, we did not collect data on partner characteristics or direct consequences of rVUS. Alcohol and drug use were considerably more frequently reported as reasons for condom non-use in those who also reported rVUS as well as in early-onset compared with never/late-onset cannabis users (Table S6); however, we are unaware of the extent to which they contributed to rVUS. Finally, reporting bias in age at initiation of cannabis use may have influenced assignment of twins to discordant pairs and our overall findings – this bias is likely to be modest as the average difference in age between early and late-onset twins was 3.6 years (2.2 years within discordant MZ pairs). We also show several additional analyses that demonstrate consistency of findings across age cutoffs (Table S2-S4). However, the cross-sectional nature of our data may have impacted these results in other, unobserved ways.
Women who start using cannabis during adolescence are considerably more likely to repeatedly engage in risky sexual behaviors such as rVUS. We conclude that this relationship might not be entirely explained by a generalized predisposition to disinhibited behaviors. Reducing exposure to early life factors that increase person-specific liability to both substance use and risky sex might prevent onset of both behaviors. Furthermore, interventions that aim to create prosocial environments for female engagement, especially in women at high risk for adolescent cannabis use, may substantially attenuate the frequency of rVUS in women, a behavior with deleterious consequences for women’s health and well-being.
Supplementary Material
Acknowledgements
This research is supported by K02DA32573 and R01DA23368 from the National Institute on Drug Abuse as well as by AA17915, AA21492, AA017688, AA011998, from the National Institute on Alcohol Abuse and Alcoholism, grant HD049024 from the National Institute of Child Health and Human Development.
Footnotes
Declaration of competing interests: AA has previously (ended 12/2012) received peer-reviewed funds and an honorarium from ABMRF/Foundation for Alcohol Research which receives support from the brewing industry. These analyses are unrelated to that funding.
Reference List
- (1).Centers for Disease Control (CDC) Youth Risk Behavior Surveillance - United States, 2013. MMWR. 2014;63(4) [PubMed] [Google Scholar]
- (2).Bellis MA, Hughes K, Calafat A, Juan M, Ramon A, Rodriguez JA, et al. Sexual uses of alcohol and drugs and the associated health risks: a cross sectional study of young people in nine European cities. BMC Public Health. 2008;8:155. doi: 10.1186/1471-2458-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Poulin C, Graham L. The association between substance use, unplanned sexual intercourse and other sexual behaviours among adolescent students. Addiction. 2001 Apr;96(4):607–21. doi: 10.1046/j.1360-0443.2001.9646079.x. [DOI] [PubMed] [Google Scholar]
- (4).Guo J, Chung IJ, Hill KG, Hawkins JD, Catalano RF, Abbott RD. Developmental relationships between adolescent substance use and risky sexual behavior in young adulthood. J Adolesc Health. 2002 Oct;31(4):354–62. doi: 10.1016/s1054-139x(02)00402-0. [DOI] [PubMed] [Google Scholar]
- (5).Fortenberry JD. Adolescent substance use and sexually transmitted diseases risk: a review. J Adolesc Health. 1995 Apr;16(4):304–8. doi: 10.1016/1054-139X(94)00062-J. [DOI] [PubMed] [Google Scholar]
- (6).Duncan SC, Strycker LA, Duncan TE. Exploring associations in developmental trends of adolescent substance use and risky sexual behavior in a high-risk population. J Behav Med. 1999 Feb;22(1):21–34. doi: 10.1023/a:1018795417956. [DOI] [PubMed] [Google Scholar]
- (7).Valois RF, Oeltmann JE, Waller J, Hussey JR. Relationship between number of sexual intercourse partners and selected health risk behaviors among public high school adolescents. J Adolesc Health. 1999 Nov;25(5):328–35. doi: 10.1016/s1054-139x(99)00051-8. [DOI] [PubMed] [Google Scholar]
- (8).Shrier LA, Emans SJ, Woods ER, DuRant RH. The association of sexual risk behaviors and problem drug behaviors in high school students. J Adolesc Health. 1997 May;20(5):377–83. doi: 10.1016/S1054-139X(96)00180-2. [DOI] [PubMed] [Google Scholar]
- (9).Lowry R, Holtzman D, Truman BI, Kann L, Collins JL, Kolbe LJ. Substance use and HIV-related sexual behaviors among US high school students: are they related? Am J Public Health. 1994 Jul;84(7):1116–20. doi: 10.2105/ajph.84.7.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bryan AD, Schmiege SJ, Magnan RE. Marijuana use and risky sexual behavior among high-risk adolescents: trajectories, risk factors, and event-level relationships. Dev Psychol. 2012 Sep;48(5):1429–42. doi: 10.1037/a0027547. [DOI] [PubMed] [Google Scholar]
- (11).Andrade LF, Carroll KM, Petry NM. Marijuana use is associated with risky sexual behaviors in treatment-seeking polysubstance abusers. Am J Drug Alcohol Abuse. 2013 Jul;39(4):266–71. doi: 10.3109/00952990.2013.803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet. 2011 Jul;41(4):459–75. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Anokhin AP, Golosheykin S, Grant J, Heath AC. Heritability of risk-taking in adolescence: a longitudinal twin study. Twin Res Hum Genet. 2009 Aug;12(4):366–71. doi: 10.1375/twin.12.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005 Nov;8(11):1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- (15).Kendler KS, Prescott CA. Genes, Environment and Psychopathology. The Guilford Press; New York: 2006. Externalizing and Substance Use Disorders; pp. 81–113. [Google Scholar]
- (16).Bjork JM, Pardini DA. Who are those "risk-taking adolescents"? Individual differences in developmental neuroimaging research. Dev Cogn Neurosci. 2015 Feb;11:56–64. doi: 10.1016/j.dcn.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nikolova YS, Knodt AR, Radtke SR, Hariri AR. Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry. 2015 Jun 30; doi: 10.1038/mp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Victor EC, Sansosti AA, Bowman HC, Hariri AR. Differential patterns of amygdala and ventral striatum activation predict gender-specific changes in sexual risk behavior. J Neurosci. 2015 Jun 10;35(23):8896–900. doi: 10.1523/JNEUROSCI.0737-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Littleton H, Breitkopf CR, Berenson A. Sexual and physical abuse history and adult sexual risk behaviors: relationships among women and potential mediators. Child Abuse Negl. 2007 Jul;31(7):757–68. doi: 10.1016/j.chiabu.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000 Oct;57(10):953–9. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- (21).Heath AC, Howells W, Bucholz KK, Glowinski AL, Nelson EC, Madden PA. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin Res. 2002 Apr;5(2):107–12. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- (22).Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, et al. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005 May;35(5):625–35. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- (23).Waldron M, Doran KA, Bucholz KK, Duncan AE, Lynskey MT, Madden PA, et al. Parental separation, parental alcoholism, and timing of first sexual intercourse. J Adolesc Health. 2015 May;56(5):550–6. doi: 10.1016/j.jadohealth.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr., et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994 Mar;55(2):149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- (25).Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. JAMA. 2003 Jan 22;289(4):427–33. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- (26).Lynskey MT, Glowinski AL, Todorov AA, Bucholz KK, Madden PA, Nelson EC, et al. Major depressive disorder, suicidal ideation, and suicide attempt in twins discordant for cannabis dependence and early-onset cannabis use. Arch Gen Psychiatry. 2004 Oct;61(10):1026–32. doi: 10.1001/archpsyc.61.10.1026. [DOI] [PubMed] [Google Scholar]
- (27).Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993 Jan;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- (28).Neale MC. Statistical Modeling with Mx. Dept of Psychiatry, Box # 980710; Richmond VA 23298: 2004. [Google Scholar]
- (29).Donovan JE, Jessor R, Costa FM. Adolescent health behavior and conventionality-unconventionality: an extension of problem-behavior theory. Health Psychol. 1991;10(1):52–61. [PubMed] [Google Scholar]
- (30).Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002 Aug;111(3):411–24. [PubMed] [Google Scholar]
- (31).Schmitt DP. The Big Five Related to Risky Sexual Behavior Across 10 World Regions: Differential Personality Associations of Sexual Promiscuity and Relationship Infidelity. Eur J Personality. 2004;18:301–319. [Google Scholar]
- (32).Kotov R, Gamez W, Schmidt F, Watson D. Linking "big" personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull. 2010 Sep;136(5):768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- (33).Costa PT, McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychol.Asses. 1992;4:5–13. [Google Scholar]
- (34).Hall W, Lynskey M. Is cannabis a gateway drug: Testing hypotheses about the relationship between cannabis use and use of other illicit drugs. Drug and Alcohol Review. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- (35).Andrews JA, Tildesley E, Hops H, Li F. The influence of peers on young adult substance use. Health Psychol. 2002 Jul;21(4):349–57. doi: 10.1037//0278-6133.21.4.349. [DOI] [PubMed] [Google Scholar]
- (36).Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000 Nov;95(11):1621–30. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- (37).Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: associations between and within families across late adolescence. Dev Psychol. 2000 Mar;36(2):180–9. [PubMed] [Google Scholar]
- (38).Mendle J, Turkheimer E, Emery RE. Detrimental Psychological Outcomes Associated with Early Pubertal Timing in Adolescent Girls. Dev Rev. 2007 Jun;27(2):151–71. doi: 10.1016/j.dr.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is pubertal timing associated with psychopathology in young adulthood. J Am Acad Child Adolesc Psychiatry. 2004 Jun;43(6):718–26. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- (40).Caspi A, Moffitt TE. Individual differences are accentuated during periods of social change: the sample case of girls at puberty. J Pers Soc Psychol. 1991 Jul;61(1):157–68. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- (41).Caspi A, Lynam D, Moffitt T, Silva PA. Unraveling girls' delinquency: biological, dispositional, and contextual contributions to adolescent misbehavior. Dev Psychol. 1993;29:19–30. [Google Scholar]
- (42).Burt SA, McGue M, DeMarte JA, Krueger RF, Iacono WG. Timing of menarche and the origins of conduct disorder. Arch Gen Psychiatry. 2006 Aug;63(8):890–6. doi: 10.1001/archpsyc.63.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Trussell J. Contraceptive failure in the United States. Contraception. 2011 May;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Brown SS, Eisenberg L. The Best Intentions: Unintended pregnancy and the well-being of children and families. National Academy Press; Washington, D.C.: 1995. [PubMed] [Google Scholar]
- (45).Werner KB, McCutcheon VV, Agrawal A, Sartor CE, Nelson EC, Heath AC, et al. The association of specific traumatic experiences with cannabis initiation and transition to problem use: Differences between African-American and European-American women. Drug Alcohol Depend. 2016 May 1;162:162–9. doi: 10.1016/j.drugalcdep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.