Abstract
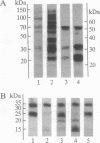
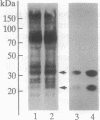
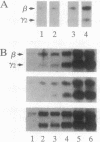
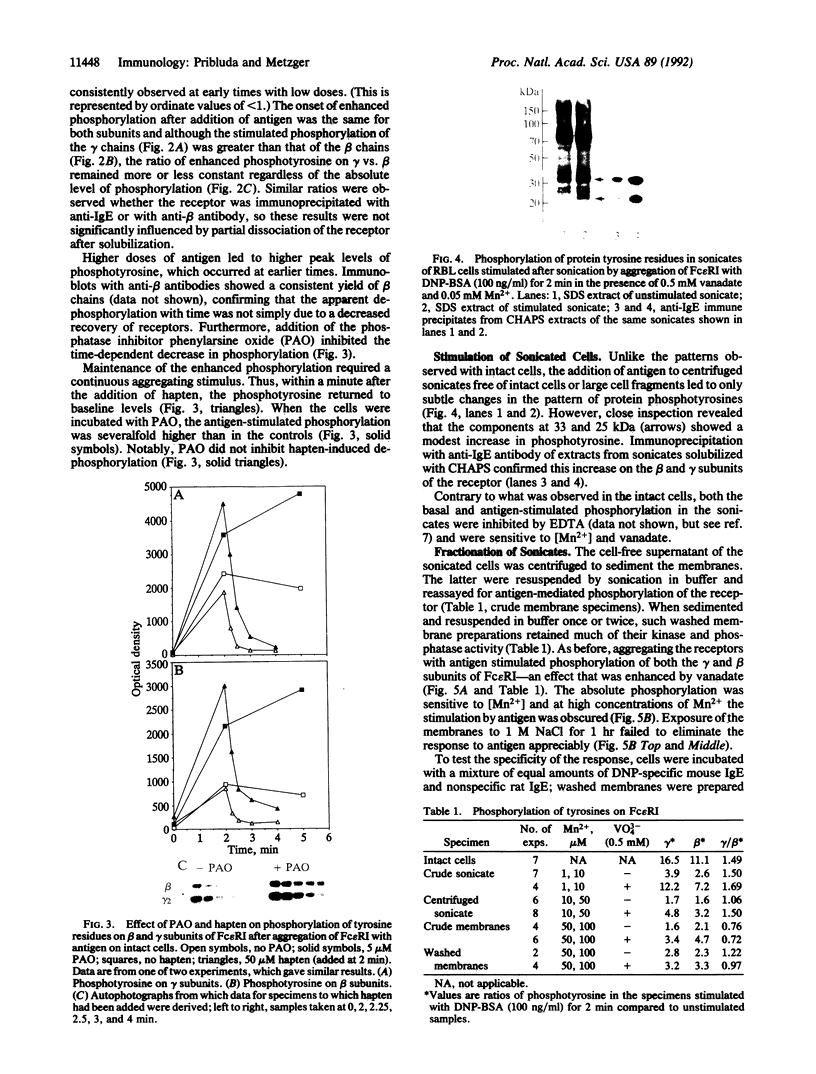
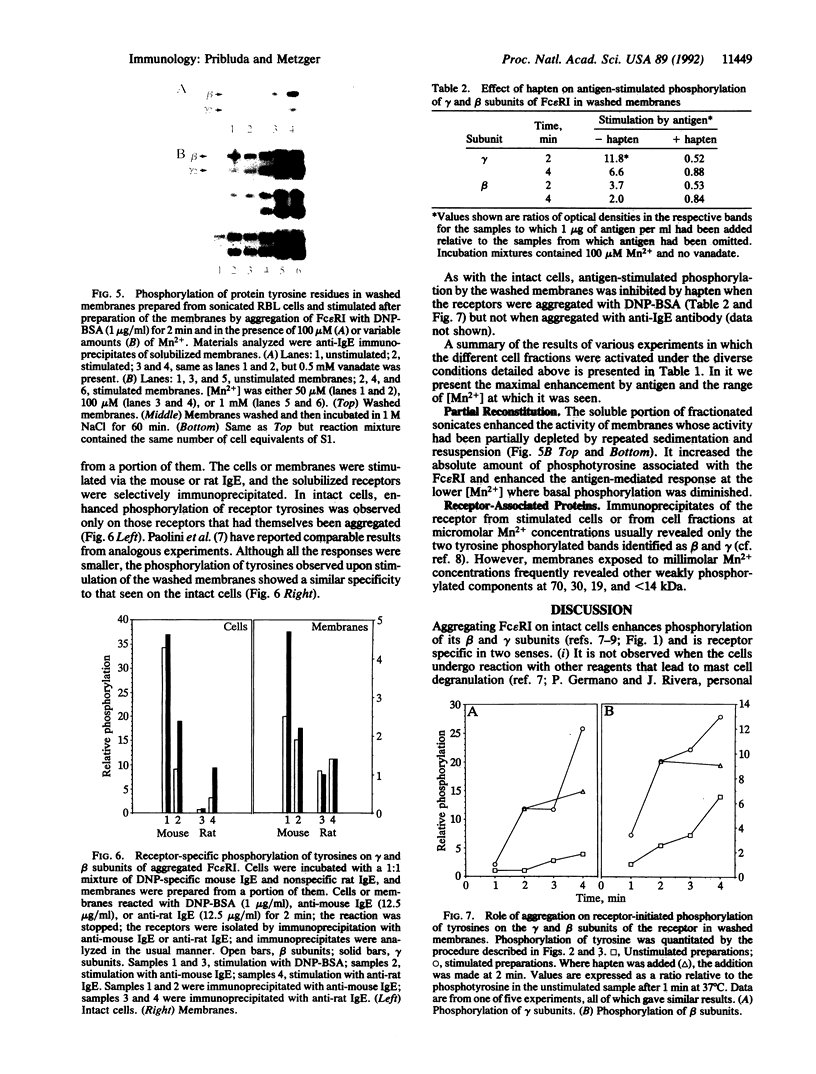
Aggregating the receptor with high affinity for IgE (Fc epsilon RI) stimulates a variety of phenomena in mast cells. Previous efforts to reproduce some of these events in broken-cell preparations such as isolated membranes have had limited success, possibly because the phenomena being monitored were too distal from the initial events. One of the earliest responses is now known to be the phosphorylation of tyrosine residues on several proteins, including the beta and gamma subunits of Fc epsilon RI. We show that in cell sonicates or on partially purified membranes derived from tumor mast cells, aggregating Fc epsilon RI stimulates phosphorylation of receptor tyrosine residues. As in the intact cells, receptor-mediated phosphorylation occurs only on receptors that are themselves aggregated. Because even in the unfractionated sonicates the phosphorylation of other cellular components was not detectably enhanced, and because the evidence is against the receptor itself being a kinase, our results suggest that phosphorylation of Fc epsilon RI is one of the earliest events stimulated by the receptor--an event that can now be investigated on simpler biological preparations than previously available.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber G., Kent U. M., Metzger H. Functional comparison of Fc epsilon RI, Fc gamma RII, and Fc gamma RIII in mast cells. J Immunol. 1992 Oct 1;149(7):2428–2436. [PubMed] [Google Scholar]
- Beaven M. A., Cunha-Melo J. R. Membrane phosphoinositide-activated signals in mast cells and basophils. Prog Allergy. 1988;42:123–184. [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou M., Siraganian R. P. Protein-tyrosine phosphorylation: an essential component of Fc epsilon RI signaling. Immunol Today. 1992 Jun;13(6):195–197. doi: 10.1016/0167-5699(92)90152-w. [DOI] [PubMed] [Google Scholar]
- Benhamou M., Stephan V., Robbins K. C., Siraganian R. P. High-affinity IgE receptor-mediated stimulation of rat basophilic leukemia (RBL-2H3) cells induces early and late protein-tyrosine phosphorylations. J Biol Chem. 1992 Apr 15;267(11):7310–7314. [PubMed] [Google Scholar]
- Blank U., Ra C., Miller L., White K., Metzger H., Kinet J. P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989 Jan 12;337(6203):187–189. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- Canals F. Signal transmission by epidermal growth factor receptor: coincidence of activation and dimerization. Biochemistry. 1992 May 12;31(18):4493–4501. doi: 10.1021/bi00133a016. [DOI] [PubMed] [Google Scholar]
- Connelly P. A., Farrell C. A., Merenda J. M., Conklyn M. J., Showell H. J. Tyrosine phosphorylation is an early signaling event common to Fc receptor crosslinking in human neutrophils and rat basophilic leukemia cells (RBL-2H3). Biochem Biophys Res Commun. 1991 May 31;177(1):192–201. doi: 10.1016/0006-291x(91)91967-h. [DOI] [PubMed] [Google Scholar]
- Dreskin S. C., Metzger H. Fc epsilon RI-mediated hydrolysis of phosphoinositides in ghosts derived from rat basophilic leukemia cells. J Immunol. 1991 May 1;146(9):3102–3109. [PubMed] [Google Scholar]
- Dreskin S. C., Pribluda V. S., Metzger H. IgE receptor-mediated hydrolysis of phosphoinositides by cytoplasts from rat basophilic leukemia cells. J Immunol. 1989 Jun 15;142(12):4407–4415. [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992 Jan 2;355(6355):78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Holowka D., Metzger H. Further characterization of the beta-component of the receptor for immunoglobulin E. Mol Immunol. 1982 Feb;19(2):219–227. doi: 10.1016/0161-5890(82)90334-0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kawakami T., Inagaki N., Takei M., Fukamachi H., Coggeshall K. M., Ishizaka K., Ishizaka T. Tyrosine phosphorylation is required for mast cell activation by Fc epsilon RI cross-linking. J Immunol. 1992 Jun 1;148(11):3513–3519. [PubMed] [Google Scholar]
- Kinet J. P., Perez-Montfort R., Metzger H. Covalent cross-linking of subunits of the receptor for immunoglobulin E induced by immunoprecipitation. Biochemistry. 1983 Dec 6;22(25):5729–5732. doi: 10.1021/bi00294a008. [DOI] [PubMed] [Google Scholar]
- Li W., Deanin G. G., Margolis B., Schlessinger J., Oliver J. M. Fc epsilon R1-mediated tyrosine phosphorylation of multiple proteins, including phospholipase C gamma 1 and the receptor beta gamma 2 complex, in RBL-2H3 rat basophilic leukemia cells. Mol Cell Biol. 1992 Jul;12(7):3176–3182. doi: 10.1128/mcb.12.7.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S. Y., Alber G., Rivera J., Kochan J., Metzger H. Interaction of aggregated native and mutant IgE receptors with the cellular skeleton. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):222–226. doi: 10.1073/pnas.89.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Paolini R., Jouvin M. H., Kinet J. P. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991 Oct 31;353(6347):855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- Perez-Montfort R., Fewtrell C., Metzger H. Changes in the receptor for immunoglobulin E coincident with receptor-mediated stimulation of basophilic leukemia cells. Biochemistry. 1983 Dec 6;22(25):5733–5737. doi: 10.1021/bi00294a009. [DOI] [PubMed] [Google Scholar]
- Quarto R., Metzger H. The receptor for immunoglobulin E: examination for kinase activity and as a substrate for kinases. Mol Immunol. 1986 Nov;23(11):1215–1223. doi: 10.1016/0161-5890(86)90154-9. [DOI] [PubMed] [Google Scholar]
- Rivnay B., Wank S. A., Poy G., Metzger H. Phospholipids stabilize the interaction between the alpha and beta subunits of the solubilized receptor for immunoglobulin E. Biochemistry. 1982 Dec 21;21(26):6922–6927. doi: 10.1021/bi00269a047. [DOI] [PubMed] [Google Scholar]
- WoldeMussie E., Maeyama K., Beaven M. A. Loss of secretory response of rat basophilic leukemia (2H3) cells at 40 degrees C is associated with reversible suppression of inositol phospholipid breakdown and calcium signals. J Immunol. 1986 Sep 1;137(5):1674–1680. [PubMed] [Google Scholar]
- Yu K. T., Lyall R., Jariwala N., Zilberstein A., Haimovich J. Antigen- and ionophore-induced signal transduction in rat basophilic leukemia cells involves protein tyrosine phosphorylation. J Biol Chem. 1991 Nov 25;266(33):22564–22568. [PubMed] [Google Scholar]