Abstract
Background
Gut microbiota may play a role in the natural history of cow’s milk allergy
Objective
To examine the association between early life gut microbiota and the resolution of cow’s milk allergy
Methods
We studied 226 children with milk allergy who were enrolled at infancy in the Consortium of Food Allergy (CoFAR) observational study of food allergy. Fecal samples were collected at age 3–16 months, and the children were followed longitudinally with clinical evaluation, milk-specific IgE levels, and milk skin prick test performed at enrollment, 6 months, 12 months, and yearly thereafter up until age 8 years. Gut microbiome was profiled by 16s rRNA sequencing and microbiome analyses performed using QIIME (Quantitative Insights into Microbial Ecology), PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States), and STAMP (Statistical Analysis of Metagenomic Profiles).
Results
Milk allergy resolved by age 8 years in 128 (56.6%) of the 226 children. Gut microbiome composition at age 3–6 months was associated with milk allergy resolution by age 8 years (PERMANOVA P = 0.047), with enrichment of Clostridia and Firmicutes in the infant gut microbiome of subjects whose milk allergy resolved. Metagenome functional prediction supported decreased fatty acid metabolism in the gut microbiome of subjects whose milk allergy resolved (η2 = 0.43, ANOVA P = 0.034).
Conclusions
Early infancy is a window during which gut microbiota may shape food allergy outcomes in childhood. Bacterial taxa within Clostridia and Firmicutes could be studied as probiotic candidates for milk allergy therapy.
Keywords: cow’s milk allergy, microbiome, microbiota, Clostridia, Firmicutes, Bacteroidetes, metagenome, fatty acid, food allergy, 16s rRNA sequencing
Introduction
Cow’s milk allergy is the most common food allergy in young children, affecting 2–3%.1,2 Individuals with milk allergy are at risk for allergic reactions and also face challenges in finding suitable replacements for the nutritional content that milk-based products provide.3 A child may have to live with the limitations imposed by milk allergy for several years, as recent studies have shown that milk allergy often continues into later childhood and adulthood.1,4 Parents of milk allergic children often ask allergists to gauge whether their child’s milk allergy will resolve.
We hypothesized that gut microbiota play a role in the natural history of milk allergy, as the etiology of food allergy is thought to involve deviation from the default state of mucosal immune tolerance that may be driven by diet, commensal microbiota, and interactions between them.5,6 Variations in infant gut flora have been associated with allergy skin prick test response,7 specific IgE levels,8 atopic dermatitis,8,9 and food allergy status.10 Much of this previous work has relied upon culture methods, which allow for examination of species specifically targeted and cultured, but exclude the large majority of bacterial organisms that cannot be cultured. These excluded organisms may play key roles in the natural history of milk allergy.
In this study, we used high-throughput sequencing to comprehensively characterize the gut microbiota of 226 children age 3–16 months with cow’s milk allergy. We followed these children up to age 8 years and examined for associations between early-life gut microbiota diversity, composition, and milk allergy resolution.
Methods
Study protocols were approved by the institutional review boards of the participating institutions.
Study design and subjects
The subjects of this study are a subset of a larger observational cohort study by the Consortium of Food Allergy Research (CoFAR) of 512 participants with milk allergy, egg allergy, and/or moderate-to-severe atopic dermatitis but without known peanut allergy.1,11 Participants were recruited at age 3–15 months from five study centers across the United States and were followed over time. The study sites included the Icahn School of Medicine at Mount Sinai, New York, New York; Duke University School of Medical Center, Durham, NC; Johns Hopkins University School of Medicine, Baltimore, MD; National Jewish Health, Denver, CO; and Arkansas Children’s Hospital, Little Rock, AR. The goal of the CoFAR observational study was to identify factors associated with the development of peanut allergy in a high risk cohort. Examination of the natural histories of milk and egg allergy were secondary objectives.
We collected stool from 234 of the 244 CoFAR observational study participants who had milk allergy at study entry. Stool samples were collected at or near the time of enrollment. Samples were collected during a study visit or by the parent at home using a stool collection kit provided by CoFAR. For this study, we excluded from the analysis 3 subjects who submitted stool samples after age 17 months, yielding 231 stool samples from 231 participants for DNA isolation.
DNA isolation and sequencing
DNA was isolated using the MoBio Power Soil DNA Isolation kit (Carlsbad, CA). The V4 region of the 16S rRNA gene was amplified with barcoded primers and 16S rRNA sequencing was performed on the Illumina MiSeq platform using 2×250bp paired-end read. Stool samples from 5 participants yielded less than 2,000 sequences/sample (2.2% of 231 samples sequenced) and these subjects were removed from the analysis, yielding a final sample size for analysis of 226 subjects. In total, 5,478,379 sequences were assigned to 16S rRNA gene sequences for the 226 samples, with a median of 23,682 sequences per sample.
Outcomes
Data were prospectively collected from study enrollment up through a visit at approximately age 8 years. Participants were considered to have milk allergy if they had either (1) positive physician-supervised oral food challenge or a convincing reaction and sensitization to milk by milk-specific IgE level ≥ 0.35 kUA/L and/or skin prick test (SPT) > 3mm, or (2) a flare of atopic dermatitis associated with milk ingestion along with a milk-specific IgE level > 5 kUA/L. At entry, dietary, medical, and social histories were obtained by questionnaires administered to the parents of participants. Milk-specific IgE levels and milk skin prick test were performed at entry and periodically thereafter as clinically indicated. Atopic dermatitis severity was graded based on criteria previously described.1 Participants were evaluated in person at enrollment, 6 months, 12 months, and yearly thereafter with additional telephone follow-up between each visit. Milk allergy was considered persistent if the subject had milk allergy according to the above definitions at the last documented encounter.
We used logistic regression implemented in R 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) to test for the associations between early life exposures that shaped the infant gut microbiome (mode of delivery, breastfeeding, solid food intake, antibiotic use) and milk allergy resolution by age 8 years or milk allergen sensitization.
Microbiome analyses
Based on observations from previous studies12,13 of marked shifts in the gut microbiome composition in early life associated with developmental stages (e.g. predominant nutrition from breastmilk and formula until age 6 months, predominant nutrition from solid food and transition to whole milk or milk alternatives around 12 months), we a priori stratified the participants into the following 3 groups for microbiome analysis: participants whose stool samples were obtained at (1) age 3 to 6 months, (2) age 7 to 12 months, and (3) age 13 to 16 months. The members of the investigative team who conducted the microbiome and bioinformatic analyses had no role in acquiring or assessing the clinical results of the cohort study.
Unless noted otherwise, we performed all analyses using QIIME 1.8.0 (Quantitative Insights into Microbial Ecology), an open-source bioinformatics pipeline for performing analysis of microbiome sequence data. Operational taxonomic units (OTUs), defined as taxonomic units based on DNA sequences that share high identity14 were constructed using a >97% similarity threshold. Within the three age groups for analysis, alpha diversity (the richness of a sample in terms of the diversity of OTUs observed in it) was estimated using Faith’s phylogenetic diversity15. Beta diversity (distance between samples based on differences in OTUs present in each sample) was measured using unweighted UniFrac16. Principal coordinate analysis (PCoA) was used to visualize clustering patterns between samples based on beta diversity distances. Linear discriminant analysis effect size (LEfSe)17, a method for biomarker discovery, was used to determine taxa that best characterize each population, i.e. bacterial groups associated with milk allergy resolution or persistence. LEfSe scores measure the consistence of differences in relative abundance between taxa in the groups analyzed (i.e. resolution vs. persistence), with a higher score indicating higher consistency. Association between microbiome composition and covariates was tested using PERMANOVA, a non-parametric test similar to ANOVA but that does not require the data to be normally distributed. Significance of PERMANOVA tests was determined using 999 permutations with adjustment for multiple testing.
Prediction of metagenome functional content
We used PICRUSt 1.0.0 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States)18 to predict metagenome function from the 16S rRNA data. Bray-Curtis distances were used to determine similarity of samples based on metagenomic composition. We used STAMP (Statistical Analysis of Metagenomic Profiles)19 to analyze the predicted metagenome and identify pathways associated with resolution or persistence of milk allergy. The Benjamini-Hochberg procedure was used to control the false discovery rate due to multiple testing.
Baseline characteristic comparisons and logistic regression results were considered statistically significant with unadjusted P <0.05, significant results from linear discriminant analysis (LDA) required P < 0.05 and LDA score > 2.0, and PERMANOVA and metagenome analyses were considered statistically significant with adjusted P < 0.05.
Results
Study population
The baseline characteristics of the participants are shown in Table 1. The study population was mostly white (77%) with more male (65.9%) than female children. The majority of children had been born vaginally (61.9%) with about half breastfeeding and almost all (96.9%) taking solid foods at entry. The median age of participants at the time of stool collection was 10.6 months (IQR = 4.2) with a range of 3.6 to 16.9 months. The median age at last follow-up was 80.0 months (IQR = 18.0), with no significant difference in age at last follow-up between those with resolution or persistence of milk allergy. Participants whose milk allergy resolved by age 8 years had significantly smaller milk SPT, lower milk sIgE levels, and milder atopic dermatitis at entry.
Table 1.
Baseline characteristics of the participating subjects with milk allergy
| Characteristics | All subjects with milk allergy included in the analysis |
Milk allergy persistent at age 8 years |
Milk allergy resolved by age 8 years |
P value* |
|---|---|---|---|---|
| Subjects | 226 | 98 (43.4%) | 128 (56.6%) | |
| Age at stool collection | 0.83 | |||
| 3–6 months | 29 | 11 (37.9%) | 18 (62.1%) | |
| 7–12 months | 144 | 64 (44.4%) | 80 (55.6%) | |
| 13–16 months | 53 | 23 (43.4%) | 30 (56.6%) | |
| Age at last followup (months) – median (IQR) |
80.0 (18.0) | 79.9 (18.4) | 80 (15.8) | 0.68^ |
| Sex | 1.0 | |||
| Female | 77 | 33 (42.9%) | 44 (57.1%) | |
| Male | 149 | 65 (43.6%) | 84 (56.4%) | |
| Race | 0.13 | |||
| White | 174 | 72 (41.4%) | 102 (58.6%) | |
| Black/African-American | 30 | 12 (40.0%) | 18 (60.0%) | |
| Asian | 19 | 13 (68.4%) | 6 (31.6%) | |
| Other | 3 | 1 (33.3%) | 2 (66.7%) | |
| Baseline milk IgE level | 2.1 × 10–9 | |||
| <2 | 91 | 20 (22.0%) | 71 (78.0%) | |
| ≥ 2–10 | 68 | 30 (44.1%) | 38 (55.9%) | |
| >10 | 67 | 48 (71.6%) | 19 (28.4%) | |
| Baseline milk SPT response (wheal in mm) |
1.1 × 10–5 | |||
| <5 | 63 | 12 (19.0%) | 51 (81.0%) | |
| 5–10 | 82 | 41 (50.0%) | 41 (50.0%) | |
| >10 | 81 | 45 (55.6%) | 36 (44.4%) | |
| Baseline AD severity | 0.02 | |||
| None | 29 | 6 (20.7%) | 23 (79.3%) | |
| Mild | 32 | 11 (34.4%) | 21 (65.6%) | |
| Moderate | 126 | 62 (49.2%) | 64 (50.8%) | |
| Severe | 39 | 19 (48.7%) | 20 (51.3%) | |
| Breast-feeding at entry | 0.47 | |||
| Never | 36 | 14 (38.9%) | 22 (61.1%) | |
| Yes, currently | 115 | 47 (40.9%) | 68 (59.1%) | |
| Yes, but no longer | 75 | 37 (49.3%) | 38 (50.7%) | |
| Mode of delivery** | 0.78 | |||
| Vaginal | 140 | 62 (44.3%) | 78 (55.7%) | |
| C-section | 85 | 36 (42.4%) | 49 (57.6%) | |
| Solid food intake | 0.70 | |||
| Yes | 219 | 96 (43.8%) | 123 (56.2%) | |
| No | 7 | 2 (28.6%) | 5 (71.4%) | |
| Antibiotics – lifetime prior to sampling |
0.89 | |||
| Yes | 142 | 61 (43.0%) | 81 (57.0%) | |
| No | 84 | 37 (44.0%) | 47 (56.0%) |
Fisher Exact test
Wilcoxon rank sum test
Data N/A for 1 subject
The baseline characteristics of the participants stratified by age at stool collection (age 3–6 months (n=29), 7–12 months (n=144), 13–16 months (n=53)) are shown in Supplementary Table 1.
There were no associations between early life exposures that shaped the infant gut microbiome (mode of delivery, breastfeeding, solid food intake, antibiotic use) and milk allergy resolution by age 8 years or milk allergen sensitization (Supplementary Table 2).
Distinct gut microbiome in 3–6 month old subjects with milk allergy resolution by age 8 years
Subjects sampled at age 3–6 months who achieved milk allergy resolution by age 8 years had a distinct gut microbiome composition compared to subjects sampled at the same age whose milk allergy persisted (PERMANOVA P = 0.047). This distinction was not observed in subjects sampled later in life at age 7–12 months (PERMANOVA P = 0.39) or at age 13–16 months (PERMANOVA P = 0.96). There were no significant effects of antibiotic use or atopic dermatitis severity on bacterial composition in the subjects sampled at age 3- 6 months (PERMANOVA P = 0.53 and 0.80, respectively), indicating that neither antibiotic use nor atopic dermatitis were confounders of the association between gut microbiome composition and milk allergy resolution. Results from linear discriminant analysis effect size (LEfSe) and principal coordinate analysis (PCoA) confirmed the distinct gut microbiome composition associated with milk allergy resolution.
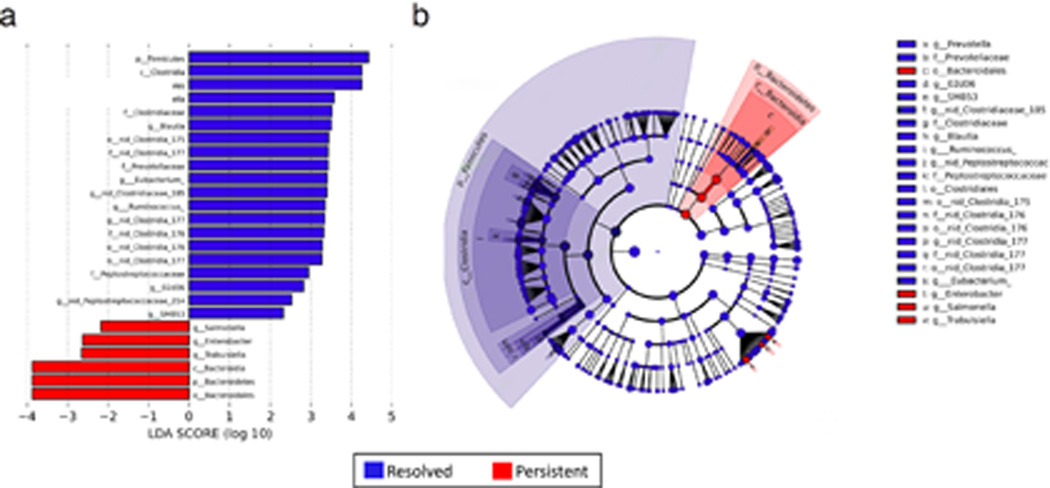
Linear discriminant analysis revealed distinct taxa in the microbiome of 3–6 month subjects with milk allergy resolution vs. milk allergy persistence (Figure 1). Among subjects sampled at age 3–6 months, the Firmicutes phylum and Clostridia class were enriched in the infant gut microbiome of subjects whose milk allergy resolved by age 8 years, while Bacteroidetes and Enterobacter, were characteristic of subjects whose milk allergy did not resolve by age 8 years (Figure 1b). These findings were consistent when covariates (antibiotics, solid food intake, mode of delivery, race, and breastfeeding) were taken into account in the LEfSe analysis (Supplementary Figure 1).
Figure 1. Linear discriminant analysis shows distinct gut microbiome composition associated with milk allergy resolution.
Taxa within Clostridia and Firmicutes were enriched in children sampled at age 3–6 months with milk allergy resolution vs. milk allergy persistence by age 8 years, while taxa within Bacteroidetes were underrepresented. (a) Linear Discriminant Analysis scores as calculated by LEfSe of taxa differentially abundant in milk allergy resolution and milk allergy persistence (b) LEfSe cladogram representing differentially abundant taxa. All taxa were significantly associated with either resolution or persistence of milk allergy (P < 0.05). Only taxa with LDA scores >2 are presented.
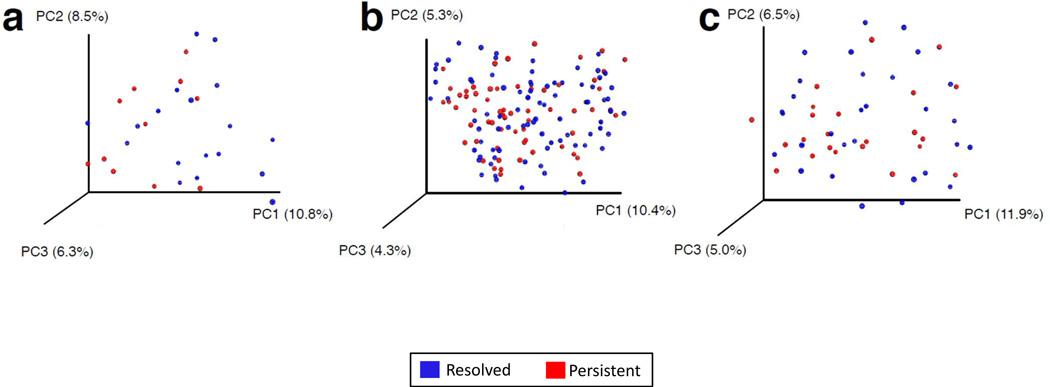
PCoA demonstrated separation in gut microbiome composition between subjects sampled at age 3–6 months who achieved milk allergy resolution vs. subjects with persistent milk allergy (Figure 2a). PCoA did not show separation reflecting distinct gut microbiome composition related to milk allergy resolution in subjects sampled at age 7–12 or 13–16 months (Figures 2b and 2c).
Figure 2. Principal coordinate analysis shows separation of bacterial composition at age 3–6 months associated with milk allergy resolution.
PCoA plots of bacterial composition and diversity of the gut microbiome of children sampled at age (a) 3–6 months, (b) 7–12 months, and (c) 13–16 months. Plots are based on unweighted UniFrac distances, with each point corresponding to a subject and colored by milk allergy resolution vs. milk allergy persistence by age 8 years. Only children sampled at age 3–6 months had a significantly distinct microbiome composition associated with milk allergy resolution by age 8 years (PERMANOVA P = 0.047).
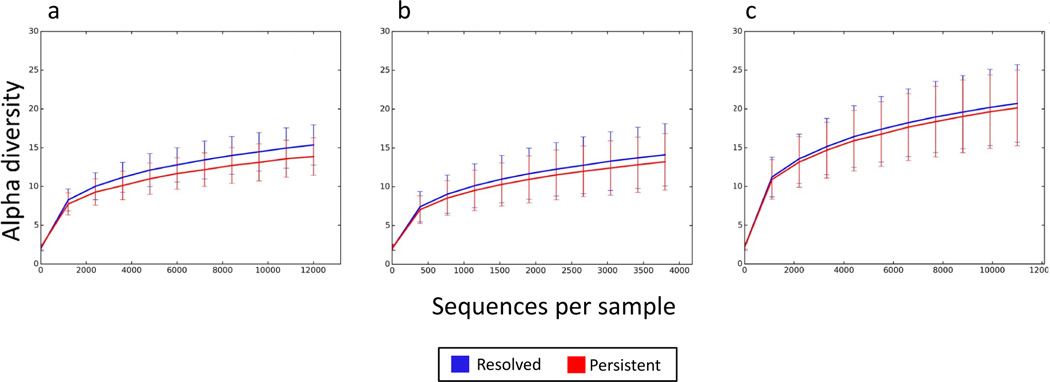
Trend toward increased bacterial diversity in subjects with milk allergy resolution by age 8 years
Compared to subjects whose milk allergy did not resolve by mid-childhood, the gut microbiome of participants with milk allergy resolution showed a trend of increased bacterial diversity. This trend was most pronounced in subjects sampled at age 3–6 months, with decreasing difference in those sampled at age 7–12 months and little difference at age 13–16 months (Figure 3).
Figure 3. Trend toward increased bacterial diversity in subjects with milk allergy resolution by age 8 years.
Rarefaction curves for bacterial diversity of the gut microbiome of children sampled at age (a) 3–6 months, (b) 7–12 months, and (c) 13–16 months. The vertical axis indicates alpha diversity measured using Faith’s phylogenetic diversity, while the horizontal axis indicates sequencing depth. Mean and standard deviation alpha diversity at each sequencing depth were estimated by subsampling all samples 10 times at the specified rarefaction.
Distinct metagenome in subjects with milk allergy resolution
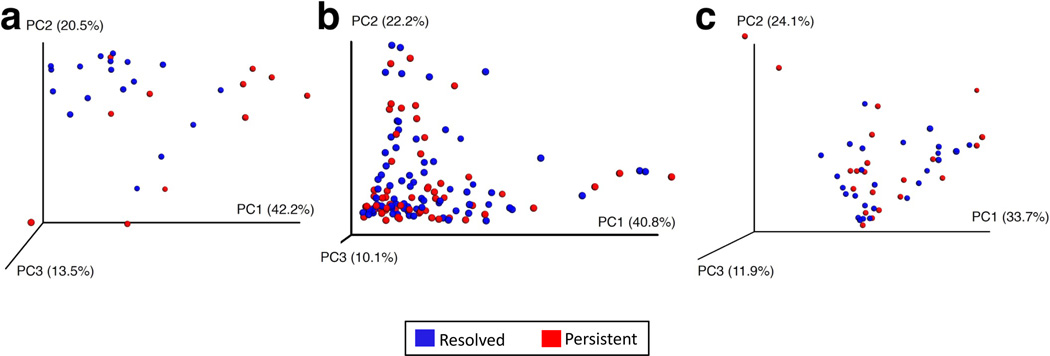
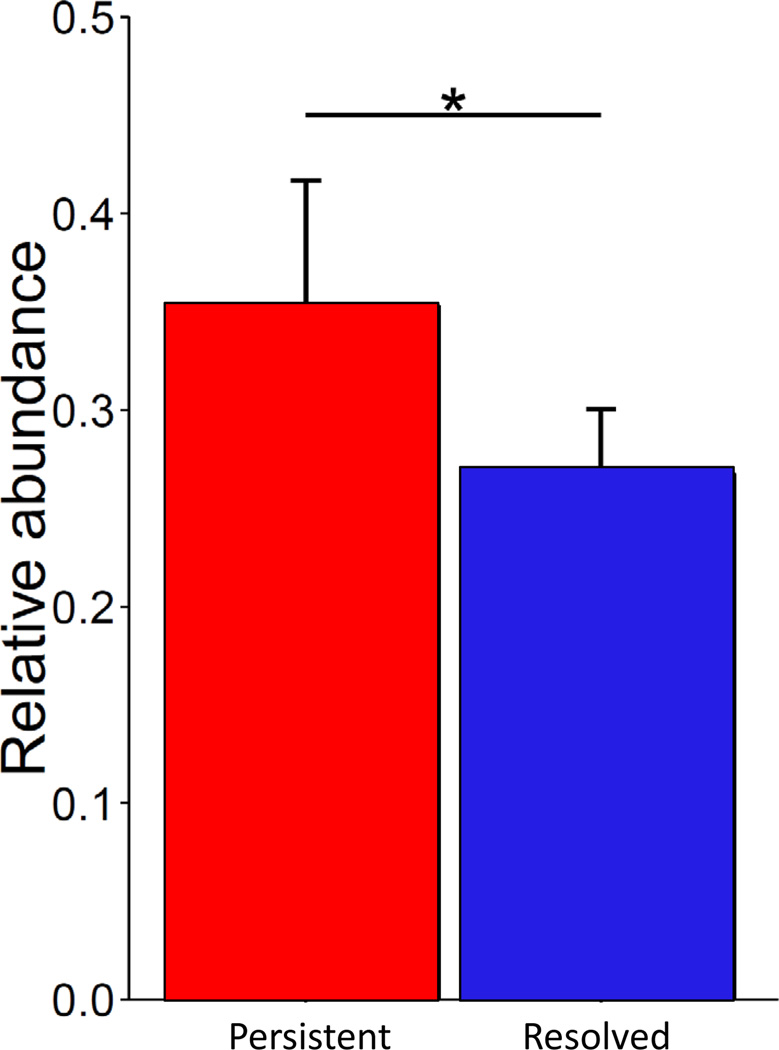
PCoA of predicted metagenome function showed a significant separation in bacterial functional pathways related to milk allergy resolution in subjects sampled at age 3–6 months (Figure 4a, PERMANOVA P = 0.004), but not in those sampled later in life (Figures 4b and 4c, PERMANOVA P = 0.79 at age 7–12 months and PERMANOVA P = 0.72 at age 13–16 months). The only metabolic pathway that was differentially enriched in the metagenome of subjects sampled at age 3–6 months was fatty acid metabolism, which was decreased (η2 = 0.43, P = 0.034) in subjects whose milk allergy resolved (Figure 5 and Supplementary Table 3). Subjects sampled at older ages did not show differential abundance of any metabolic pathways (Supplementary Table 3).
Figure 4. Principal coordinate analysis shows separation of metagenome functional content at age 3–6 months associated with milk allergy resolution.
PCoA plots of metagenome functional content of the gut microbiome of children sampled at age (a) 3–6 months, (b) 7–12 months, and (c) 13–16 months. Plots are based on Bray-Curtis distances, with each point corresponding to a subject and colored by milk allergy resolution vs. milk allergy persistence by age 8 years. Only children sampled at age 3–6 months had a significantly distinct metagenome composition associated with milk allergy resolution by age 8 years (PERMANOVA P = 0.004).
Figure 5. Metagenome functional enrichment of fatty acid metabolism in milk allergy persistence.
Prediction of metagenome functional content using PICRUSt showed differential enrichment of fatty acid metabolism pathways in children sampled at age 3–6 months (η2 = 0.43, ANOVA Pcorrected = 0.034). The vertical axis indicates the relative abundance of predicted KEGG orthologs annotated as being part of fatty acid metabolism. Error bars indicate standard deviation from the mean. Supplementary Table 3 includes information on all pathways.
Correlation between gut microbiome diversity, composition, and milk sIgE level or milk SPT
Milk sIgE levels or milk SPT size at baseline, change in levels, or slope of these measures over time were not significantly correlated with abundance of any microbial taxa. Alpha diversity was not significantly correlated with milk sIgE in any age group (PERMANOVA P = 0.40 in age 3–6 months; PERMANOVA P = 0.08 in age 7–12 months; PERMANOVA P = 0.18 in age 13–16 months), nor with milk SPT in any age group (PERMANOVA P = 0.32 in age 3–6 months; PERMANOVA P = 0.10 in age 7–12 months; PERMANOVA P = 0.27 in age 13–16 months).
Discussion
We found that gut microbiome composition of milk allergic children was associated with resolution of milk allergy by age 8 years, with enrichment of Clostridia and Firmicutes in the 3–6 month old gut microbiome of subjects whose milk allergy later resolved. Our examination of predicted metagenome function showed differential bacterial function associated with milk allergy resolution, with decreased fatty acid metabolism in resolvers.
Comparison with other studies
Our study is the first to examine the relationship between the microbiome and food allergy resolution. Previous studies have examined associations between the gut microbiome and food allergy status. Using bacterial cultures, Thompson-Chagoyan et al. found that compared to healthy infants, cow’s milk allergic infants had higher total bacteria and anaerobic counts; after 6 months during which milk allergic subjects took extensively hydrolyzed formula and healthy control subjects took cow’s milk formula, the milk allergic group had higher proportions of lactobacilli and lower proportions of enterobacteria and bifidobacteria.10 Major limitations of this study included reliance on bacterial culture (which cannot characterize the majority of bacterial taxa, which are unculturable) and differential diet as a confounder of the association between gut microbiome and food allergy status. Ling et al. reported enrichment of Clostridium sensu stricto, Butyricicoccus, Clostridicaceae 1, and Ruminococcaceae sequences in 34 Chinese infants with food allergy to a variety of foods compared to 45 healthy controls, but half of the “food allergic” had non-IgE-mediated food allergy.20 Employing a murine model of food allergy, Noval Rivas et al. reported that OVA-sensitized Il4raF709 mice had differential abundance of Lachnospiraceae, Lactobacillacaeae, Rikenallaceae, and Porphyromononadaceae compared to wild type mice, and reconstitution of wildtype mice with the microbiota from these allergen-sensitized mice promoted OVA-specific IgE responses.21 Although the above studies reported differences in bacterial composition related to food allergy status, none examined resolution of food allergy as we did in this study.
We found taxa from the Clostridia class and Firmicutes phylum to be enriched in human subjects with milk allergy resolution. Such taxa have been previously linked to immune tolerance in mouse models of allergy and protection from allergic inflammation in subjects with eosinophilic esophagitis. Atarashi et al. identified 17 strains of bacteria from human stool that enhanced Treg cell abundance and induced key anti-inflammatory markers such as IL-10 and inducible T-cell co-stimulator (ICOS) in Treg cells upon inoculation into germ-free mice, with genome sequencing revealing that all 17 strains were taxa within Clostridia.22 Oral administration of these 17 Clostridia strains into mice attenuated disease in models of colitis and allergic diarrhea.22 Stefka et al. found that gnotobiotic mice colonized with Clostridium clusters protected against sensitization to peanut/cholera toxin, with reduced levels of peanut-specific and total IgE compared to germ-free controls and no temperature drop upon allergen challenge.23 Additionally, Clostridia-colonized mice had significantly increased proportions of Foxp3+ Tregs in their colonic lamina propria and increased concentrations of fecal IgA compared to germ-free mice.23 Clostridia colonization induced IL-22 production by RORgt+ innate lymphoid cells and T cells, which reduced the concentration of serum allergen, supporting the concept that Clostridia play a critical role in regulating sensitization to food allergens.23 A strength of our study is that in contrast to these previous murine studies, it provides human data to support that Clostridia play a role in allergy and inflammation. With regards to Firmicutes, a study of 33 pediatric subjects with eosinophilic esophagitis and 35 normal controls showed that taxa from the Firmicutes phylum predominated the esophageal microbiota of control subjects.24
The gut microbiome of our 3–6 month subjects with persistent milk allergy was enriched with taxa from phylum Bacteroidetes, which is consistent with a prior study demonstrating a predominance of this phylum in infants with milk allergy and high risk of chronic allergic disease. Specifically, Kirjavainen et al. found high abundance of Bacteroides in the gut microbiome of infants with a high degree of milk allergy (as demonstrated by reaction to extensively hydrolyzed formula), early onset atopic eczema, and a strong family history of atopic disorders.25 Our results and those of Kirjavainen et al. are in contrast to findings from murine models, where colonization with Bacteroides were observed to correct Th1/Th2 imbalances26 and induce regulatory T cell development.27,28
The results of our predicted metagenome analyses showed that bacterial functional pathways related to fatty acid metabolism were significantly reduced in subjects whose milk allergy resolved with time. This finding suggests that decreased fatty acid metabolism by gut microbiota may be helpful toward reducing long-term allergy risk. Gas liquid chromatography studies of infant stool composition show lower levels of branched-chain short fatty acids in healthy infants relative to cow’s milk allergic infants.29 These levels were observed cross-sectionally, so it is not known if lower levels of fatty acids would be found in infants whose milk allergy resolved with time versus those with persistent milk allergy. Overall, lipids are known to facilitate the passage of food allergens through intestinal epithelial barriers and affect the allergenicity of food proteins.30 Lipids in cow’s milk itself can induce the expansion of iNKT cells and release of IL-4, IL-5, and IL-13, leading to a Th2-dominant milieu.31 Attenuation of such lipid-driven pro-inflammatory effects would be ostensibly beneficial in allergy resolution, and our results suggest that gut microbiota may contribute to this via reduced fatty acid metabolism.
Early infancy as a time window shaping the course of food allergy
The associations between microbiome composition, metagenome functional content, and milk allergy resolution were specific to subjects sampled at 3–6 months in our study, with no significant associations in subjects sampled at 7–12 months or 13–16 months. Two potential interpretations of this include: (1) early infancy (i.e. 3–6 months) is a particular developmental time window during which gut microbiota shapes the course of food allergy, and gut microbiota at later ages (e.g. 7–16 months) have little effect on food allergy course, and (2) gut microbiota is associated with food allergy course at different age stages, but confounders obscure this signal when subjects are sampled at > 6 months age. Early infancy could be a key developmental time window, as we and others have shown age-dependent associations between infant gut microbiome and allergy. Azad and colleagues found that gut microbial richness at age 3 months was associated with increased likelihood of food sensitization by 1 year, while there was no association between microbial richness at 12 months and food sensitization.32 Similarly, Abrahamsson et al. found that gut microbiota diversity at age 1 month but not at age 12 months was associated with atopic dermatitis during the first 2 years of life.33 Porcine models have shown that the postnatal environment from as early as the first day of life influences gut microbiota and subsequent metabolic phenotypes.34 Murine models also support that age-sensitive contact with microbiota is critical for establishing tolerance to later exposures.35 Our collective findings support the theory that factors influencing early immune system development may be particularly important for subsequent allergy development.36
However, the intestinal microbiome in early life is markedly dynamic with dramatic shifts after the introduction of solid foods leading to an “adult-like” profile.12,37 Thus, it is possible that associations between gut microbiota and allergy outcomes are obscured after the introduction of solid food in the diet, e.g. at approximately 6 months of age.
No association between gut microbiota and milk-specific IgE or milk SPT
We did not find an association between gut microbiota and sensitization (by sIgE or SPT) to milk at baseline, confirming previously reported results. Adlerberth et al. found no association between culturable bacteria and food sensitization by 18 months in 3 cohorts of European infants.8 Similarly, Kendler et al. reported no association between culturable gut microbiota and sensitization to common food allergens including milk, casein, egg, peanut and hazelnut.38 Experimental models suggest that food antigen sensitization results from inhibition of the immune system default of tolerance.39. The cross-sectional design of our study precludes us from determining whether changes over time in gut microbiota composition correlate with the decline in IgE levels associated with food allergy resolution. It is also possible that the mechanisms by which early life microbiome composition are associated with resolution are indirect through the production of bacterial metabolites. Indeed, short-chain fatty acids such as butyrate have been shown to have anti-inflammatory properties23 and to regulate macrophage function.40 Overall, our results suggest that gut microbiota may not play a direct role in tolerance inhibition leading to food allergen sensitization, but may instead help reestablish the default state of tolerance.
Limitations and future directions
Although we examined the gut microbiome of 226 subjects with milk allergy, significant associations between gut microbiome composition, predicted metagenome function, and milk allergy resolution were observed only in those sampled at age 3–6 months. This subset proved to be informative and supports the notion that the gut microbiome of young infants may be of particular interest and should be targeted with larger sample sizes in future studies that include longitudinal sampling. We note that previous studies of the early-life microbiome have had similar or smaller sample size for young infants41,42 A secondary analysis of microbiota associated with tolerance of baked milk would have been of interest, but we did not characterize baked milk consumption in a rigorous manner. Ability to consume products with baked milk is associated with accelerated resolution of milk allergy.43,44 Additionally, although our findings support the concept that specific gut microbial taxa are associated with milk allergy resolution, we caution that our results cannot determine causality and do not imply that probiotic supplementation with these taxa should be implemented as treatment for milk allergy without prospective study. Probiotic supplementation, particularly with Lactobacillus and Bifidobacterium species, has been explored as potential treatment for cow’s milk allergy in randomized controlled trials with mixed results.45,46 The taxa we identified differ from those already targeted and would require prospective study for efficacy as well as safety before their potential use as treatment for cow’s milk allergy.41
Conclusions
We found enrichment of Clostridia and Firmicutes in the infant gut microbiome of 3–6 month old subjects whose milk allergy resolved by age 8 years. The microbiota of these young infants whose milk allergy resolved were associated with decreased fatty acid metabolism. Our results are the first to address the relationship between gut microbiota and food allergy resolution, and our findings suggest directions for prospective examination of the highlighted taxa for potential therapeutic consideration. Early infancy is a window during which gut microbiota may shape food allergy outcomes in childhood.
Supplementary Material
Key messages.
Clostridia and Firmicutes were enriched in the gut microbiome of milk allergic children at age 3–6 months whose milk allergy resolved by age 8 years.
Metagenome functional prediction showed decreased fatty acid metabolism in the early infant gut microbiome of children whose milk allergy resolved.
Capsule summary.
Examination of early-life gut microbiota in milk allergic children from a longitudinal, multicenter observational study showed that Clostridia and Firmicutes are associated with resolution of milk allergy.
Acknowledgments
We thank Dr. Marshall Plaut, CoFAR scientific and medical officer and chief of the NIAID Food Allergy, Atopic Dermatitis, and Allergic Mechanisms Section. We thank the families who kindly participated. We thank the staff of the clinical research units at each institution and the Statistical and Clinical Coordinating Center including Alice Henning, whom we thank in particular.
Funding: This study was supported by the National Institutes of Health (U19AI066738, U01AI066560, K08AI093538, R01AI118833), SUCCESS, Crohn’s and Colitis Foundation of America (CCFA #362048), and the Department of Scientific Computing at the Icahn School of Medicine at Mount Sinai. The study was also supported by the National Center for Research Resources (NCRR)/National Institutes of Health (NIH), UL1 TR000154 from the NIH/National Center for Advancing Translational Sciences (National Jewish), UL1 TR000067 (Mount Sinai), UL1 TR000039 (Arkansas), UL 1 RR024128 (North Carolina), and UL1 RR 025005 (Johns Hopkins) from the NCRR.
Abbreviations
- CoFAR
Consortium of Food Allergy Research
- LDA
linear discriminant analysis
- LEfSe
Linear discriminant analysis effect size
- OTU
operational taxonomic units
- PCoA
principal coordinate analysis
- PICRUSt
Phylogenetic Investigation of Communities by Reconstruction of Unobserved; States
- QIIME
Quantitative Insights into Microbial Ecology
- sIgE
specific IgE
- SPT
skin prick test
- STAMP
Statistical Analysis of Metagenomic Profiles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wood RA, Sicherer SH, Vickery BP, et al. The natural history of milk allergy in an observational cohort. J Allergy Clin Immunol. 2013;131(3):805–812. doi: 10.1016/j.jaci.2012.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 3.Fleischer DM, Perry TT, Atkins D, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics. 2012;130(1):e25–e32. doi: 10.1542/peds.2011-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–1177. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol. 2013;23(9):R389–R400. doi: 10.1016/j.cub.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature reviews Immunology. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107(1):129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 8.Adlerberth I, Strachan DP, Matricardi PM, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol. 2007;120(2):343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Karlsson C, Olsson C, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008;121(1):129–134. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Thompson-Chagoyan OC, Vieites JM, Maldonado J, Edwards C, Gil A. Changes in faecal microbiota of infants with cow’s milk protein allergy--a Spanish prospective case-control 6-month follow-up study. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e394–e400. doi: 10.1111/j.1399-3038.2009.00961.x. [DOI] [PubMed] [Google Scholar]
- 11.Sicherer SH, Wood RA, Stablein D, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. J Allergy Clin Immunol. 2010;125(5):1077–1083. doi: 10.1016/j.jaci.2010.02.038. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaxter M, Mann J, Chapman T, et al. Defining operational taxonomic units using DNA barcode data. Philos Trans R Soc Lond B Biol Sci. 2005;360(1462):1935–1943. doi: 10.1098/rstb.2005.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faith DP, Baker AM. Phylogenetic diversity (PD) and biodiversity conservation: some bioinformatics challenges. Evolutionary bioinformatics online. 2006;2:121–128. [PMC free article] [PubMed] [Google Scholar]
- 16.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and environmental microbiology. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling Z, Li Z, Liu X, et al. Altered fecal microbiota composition associated with food allergy in infants. Applied and environmental microbiology. 2014;80(8):2546–2554. doi: 10.1128/AEM.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noval Rivas M, Burton OT, Wise P, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. Journal of Allergy and Clinical Immunology. 2013;131(1):201–212. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 23.Stefka AT, Feehley T, Tripathi P, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111(36):13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benitez AJ, Hoffmann C, Muir AB, et al. Inflammation-associated microbiota in pediatric eosinophilic esophagitis. Microbiome. 2015;3:23. doi: 10.1186/s40168-015-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51(1):51–5. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–440. doi: 10.1016/j.jaci.2011.10.025. 40 e1–2. [DOI] [PubMed] [Google Scholar]
- 29.Thompson-Chagoyan OC, Fallani M, Maldonado J, et al. Faecal microbiota and short-chain fatty acid levels in faeces from infants with cow’s milk protein allergy. Int Arch Allergy Immunol. 2011;156(3):325–332. doi: 10.1159/000323893. [DOI] [PubMed] [Google Scholar]
- 30.Bublin M, Eiwegger T, Breiteneder H. Do lipids influence the allergic sensitization process? J Allergy Clin Immunol. 2014;134(3):521–529. doi: 10.1016/j.jaci.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jyonouchi S, Abraham V, Orange JS, et al. Invariant natural killer T cells from children with versus without food allergy exhibit differential responsiveness to milk-derived sphingomyelin. J Allergy Clin Immunol. 2011;128(1):102–109. doi: 10.1016/j.jaci.2011.02.026. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azad MB, Konya T, Guttman DS, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 33.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Reply: To PMID 22153774. J Allergy Clin Immunol. 2013;131(1):248–249. doi: 10.1016/j.jaci.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 34.Merrifield CA, Lewis MC, Berger B, et al. Neonatal environment exerts a sustained influence on the development of the intestinal microbiota and metabolic phenotype. The ISME journal. 2015 doi: 10.1038/ismej.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Curr Opin Allergy Clin Immunol. 2003;3(2):125–132. doi: 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS biology. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendler M, Uter W, Rueffer A, Shimshoni R, Jecht E. Comparison of fecal microflora in children with atopic eczema/dermatitis syndrome according to IgE sensitization to food. Pediatr Allergy Immunol. 2006;17(2):141–147. doi: 10.1111/j.1399-3038.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 39.Berin MC. Mechanisms of allergic sensitization to foods: bypassing immune tolerance pathways. Immunology and allergy clinics of North America. 2012;32(1):1–10. doi: 10.1016/j.iac.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canani RB, Di Costanzo M. Gut microbiota as potential therapeutic target for the treatment of cow’s milk allergy. Nutrients. 2013;5(3):651–662. doi: 10.3390/nu5030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim ES, Zhou Y, Zhao G, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nature medicine. 2015;21(10):1228–1234. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122(2):342–347. doi: 10.1016/j.jaci.2008.05.043. 7 e1–2. [DOI] [PubMed] [Google Scholar]
- 44.Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow’s milk allergy in children. J Allergy Clin Immunol. 2011;128(1):125–131. doi: 10.1016/j.jaci.2011.04.036. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hol J, van Leer EH, Elink Schuurman BE, et al. The acquisition of tolerance toward cow’s milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol. 2008;121(6):1448–1454. doi: 10.1016/j.jaci.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Berni Canani R, Nocerino R, Terrin G, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580–582. doi: 10.1016/j.jaci.2011.10.004. 2 e1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.