Abstract
Although there is a normal physiological rise in maternal lipids during pregnancy, excessive maternal hyperlipidemia during pregnancy increases cardiovascular disease risk for both the mother and offspring. There are limited safe lipid-lowering treatment options for use during pregnancy, therefore, we evaluated the influence of maternal phytosterol (PS) supplementation on lipid and lipoprotein metabolism in mothers and progeny. Female Syrian golden hamsters were randomly assigned to three diets throughout pre-pregnancy, gestation, and lactation (n=6/group): (i) Chow (Chow), (ii) chow with 0.5% cholesterol (CH), and (iii) chow with 0.5% cholesterol and 2% PS (CH/PS). Compared with newly-weaned pups from Chow dams, pups from dams fed the cholesterol-enriched diet demonstrated increases (p<0.05) in total-C, LDL-C, HDL-C, and total LDL and VLDL particle number. Pups from cholesterol-fed mothers also exhibited higher hepatic cholesterol concentration and differential mRNA expression pattern of cholesterol regulatory genes. Pups from PS-supplemented dams demonstrated reductions (p<0.05) in serum total-C, non-HDL-C, and LDL-C but also increased triglycerides compared with pups from CH-fed dams. Maternal PS supplementation reduced (p<0.05) hepatic cholesterol and increased the abundance of HMG-CoAr and LDLr protein in newly-weaned pups compared with the CH group. Results suggest that maternal PS supplementation is largely effective in normalizing cholesterol in pups born to mothers with hypercholesterolemia, however, the cause and long-term influence of increased TG is not known.
Keywords: Maternal hypercholesterolemia, phytosterols, dyslipidemia, offspring
Introduction
The tremendous impact of cardiovascular diseases (CVD) on the health and well-being of Americans cannot be over-emphasized as it presents major challenges to society due to increased disability, death, and a substantial financial burden on the health-care system. CVD is largely preventable as >75% of cases are attributed to preventable risk factors including dyslipidemia, smoking, physical inactivity, obesity, diabetes, and high blood pressure. As an established CVD risk factor, blood cholesterol levels are particularly troublesome as greater than 100 million US adults have total blood cholesterol levels that exceed the desirable target of ≤200 mg/dl [1].
Adding to the urgency of CVD as a global health issue is the rise in dyslipidemic risk factors amongst women of childbearing age, particularly hypertriglyceridemia and hypercholesterolemia. According to the Centers for Disease Control, 9.4% of females of childbearing age (20–44) have elevated total cholesterol (>240 mg/dl) [2] that is likely associated with numerous contributing factors including diet-induced obesity and underlying genetic influences including familial hypercholesterolemia (FH) [3]. Fetal exposure to excessive fat and cholesterol during pregnancy has been shown to increase fetal plasma cholesterol concentrations and aortic fatty streak formation and predispose adult offspring to diet-induced obesity, hyperlipidemia, and arterial plaque development [4, 5]. Women with FH who wish to become pregnant are advised to cease the use of lipid lowering medications (statins, ezetimbe, niacin) at least 4 weeks prior to discontinuing contraception [3]. The resulting increase in the absolute plasma cholesterol concentration, due to the cessation of medication as well as the typical increase in plasma total and LDL-cholesterol that is observed during a normal pregnancy (+25–70%), puts women with FH and their offspring at substantial risk of CVD [6, 7]. Therefore, limitations in acceptable therapeutic options result in a treatment dilemma and an acknowledged increase in CVD risk for dyslipidemic mothers.
Phytosterols (PS) are plant-based bioactive compounds that carry a health claim by the US Food and Drug Administration and are recommended by the National Cholesterol Education Program at a dose of 2 g/d for cholesterol lowering and CVD prevention. Over 50 years of cell culture, animal model, and human clinical investigations have repeatedly demonstrated the ability of PS to reduce cholesterol by up to 15% by limiting intestinal cholesterol absorption [8]. Furthermore, recent data suggest that PS may also be lower blood triglyceride (TG) concentrations through multiple mechanisms including interruption of intestinal fatty acid absorption [9] and modulation of hepatic lipogenesis and VLDL packaging and secretion [10].
Although a number of pre-clinical studies [11–13] and a human investigation [14] have suggested that PS supplementation during pregnancy is safe, the potential application of PS as a cholesterol-lowering therapy for use in diet-induced hypercholesterolemic pregnancies has not yet been investigated. Therefore, this study was undertaken with two aims: 1) to characterize the effect of maternal cholesterol feeding on blood lipid/lipoprotein responses and hepatic cholesterol metabolism in dams and their newly-weaned offspring and 2) to establish if maternal PS supplementation to a cholesterol-enriched diet could modulate these responses. We used the Syrian Golden hamster as it is an established model of human lipid and lipoprotein metabolism, is highly susceptible to diet-induced hypercholesterolemia, and demonstrates a well-characterized cholesterol-lowering responses to dietary PS supplementation.
Materials and Methods
Animals and Diets
3 month-old female (n=18) and male (n=18) Syrian golden hamsters were purchased from Harlan Laboratories (Indianapolis, Indiana). Animals were housed in the Animal Care Facility at the University at Buffalo in a temperature-controlled room (20°C, 12h light/dark cycle) in individual cages with shavings and enrichment housing with free access to water. For the following 2 week pre-pregnancy period, the animals (n=6/group) were randomly assigned to 1 of 3 standard chow-based diets (Teklad 2019, 19% protein, 51% carbohydrate, 8% fat): (i) chow only (Chow), (ii) chow with 0.5% cholesterol (CH), and (iii) chow with 0.5% cholesterol and 2% PS (CH/PS, PS sourced from Forbs Medi-Tech Corp, Kearny, NJ). All diets were prepared by Envigo (Madison, WI) (Suppl. Table 1). As a percent of total sterols, in-house analysis confirmed that the PS supplement was composed largely of β-sitosterol (>75%) with minor amounts of sitostanol (<15%) and campesterol (<7%). Following the 2-week pre-pregnancy period, females were mated with male breeders (1 male per female) and returned to their individual cages following confirmation of pregnancy based on daily weight gain. Following parturition, dams and pups were left undisturbed until postnatal (PN) day 5 as dams are prone to cannibalism of their young in the first few days after birth if disturbed. On PN day 5, litters were culled to 6 pups per dam by random selection to minimize variability in postnatal pup development influenced by litter size [15]. Throughout the suckling period the dams remained on their respective diets. To prevent the pups from consuming the diet as they matured, food pellets were provided on raised caged platforms that were only accessible by the mother. At weaning (d21), the dams and pups were anesthetized with isoflurane for blood collection. Fasting (15 h) blood (serum) was collected by cardiac puncture and stored at −80°C until further processing and analyses. The animals used in this experiment were cared for in accordance with the guidelines established by the Institutional Animal Care and Use Committee (IACUC). All procedures were reviewed and approved by the Animal Care Committee at the University at Buffalo (protocol # PTE16082N).
Serum Biochemistry
Serum cholesterol panel (total-C, HDL-C, and direct LDL-C) was measured by direct automated enzymatic assay while lipoprotein particle number and size was conducted by nuclear magnetic resonance spectroscopy (Liposcience, Raleigh, NC) [16]. Non-HDL cholesterol was calculated by subtracting HDL-C from the total cholesterol fraction. Serum activity of cholesterol-ester-transfer protein (CETP) in offspring was measured with a commercial kit fluorometric assay kit (Kamiya, KT-782).
PS (from diet and serum samples) and hepatic cholesterol were extracted and analyzed according to our previously published procedures [17, 18]. Approximately 0.5 mL of serum or 0.5 g of pulverized liver or diet was spiked with α-cholestane as internal standard and saponified in freshly prepared KOH–methanol at 100°C for 1 h. The non-saponifiable sterol fraction was extracted with petroleum diethyl ether and dried under N2 gas. Sterol fractions were analyzed on a Shimadzu GC-17A gas chromatograph fitted with a flame ionization detector using a SAC-5 capillary column (30m × 0·25mm × 0·25 mm, Supelco, Bellefonte, CA).
Hepatic lipids
Hepatic TG were analyzed with a commercial kit (ab65336, Abcam, Cambridge, MA, USA). Hepatic total fat was extracted by homogenization in aqueous Triton-X buffer (2%) and measured at OD570nm according to manufacturer instructions.
RNA preparation and real-time RT-PCR
Total RNA was isolated from whole liver tissue using TRIzol reagent (Invitrogen Inc., Grand Island, NY). RNA concentration and integrity was determined with spectrophotometry (260 nm) and agarose gel electrophoresis, respectively. RNA preparation and real-time RT-PCR was conducted using a one-step QuantiFast SYBR Green RT-PCR kit (Qiagen Inc., Valencia, CA) on a Biorad MyiQ real time PCR system according to previously established protocols [19]. Gene expression was analyzed using the 2(-delta delta Ct) method [20]. Sequences of sense and antisense primers for target and housekeeping genes were based on previously published reports for β-actin [21], ABCA1, ABCG1, ABCG5, ABCG8 [22], HMG-CoAr, LDLr, LXR, PCSK9, SREBP2, SREBP1C, ACC, FAS, CPT1 [23], PPARα [24], MTTP, DGAT [25], CD36 [26], and FABP2 [27], and CETP [28].
Hepatic total protein and nuclear/cytoplasmic fractions were prepared and extracts were probed for the abundance of target proteins with commercial antibodies for HMG-CoAr (sc-27578, Santa Cruz Biotechnology), LDLr (ab30532, abcam), and SREBP2 (ab30682, abcam) according to previously published procedures [29]. Target proteins were normalized to b-actin and quantified using Image lab (version 4.1, Biorad Laboratories, Hercules, CA).
Statistical analyses
Litters from each female were considered as a single observation. Data were analyzed with a general linear model ANOVA and multiple comparisons between treatment groups were analyzed with a Bonferonni post-hoc test [30]. Data were analyzed with SPSS 16 for Mac (SPSS Inc, Chicago, IL). Data are presented as mean ± SEM. Differences were considered significant at p≤ 0.05.
Results
Maternal response
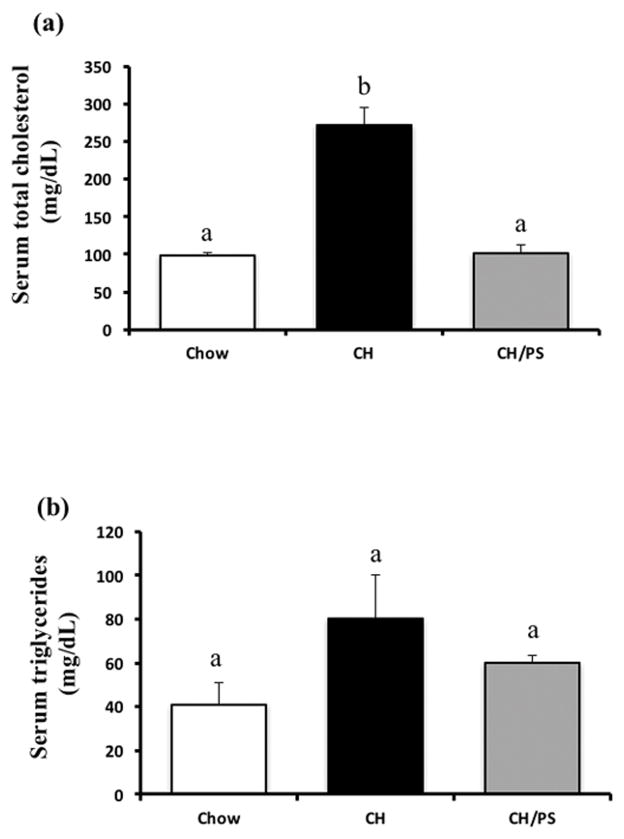
Maternal feed intake and body weight between the treatment groups did not differ (p>0.05) throughout the pre-pregnancy period, gestation, and lactation. Maternal cholesterol feeding (CH) during the 2-wk pre-pregnancy phase increased (p<0.05) serum total cholesterol (+178% compared with Chow), however dietary PS supplementation prevented this hypercholesterolemic response (−62% compared with the CH group, Fig 1a). No difference (p>0.05) was observed in pre-pregnancy serum TG concentrations between the treatment groups (Fig 1b).
Figure 1.
Serum total cholesterol (a) and triglycerides (b) at the end of the pre-pregnancy period in dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS). abGroups not sharing a superscript are significantly different (p<0.05).
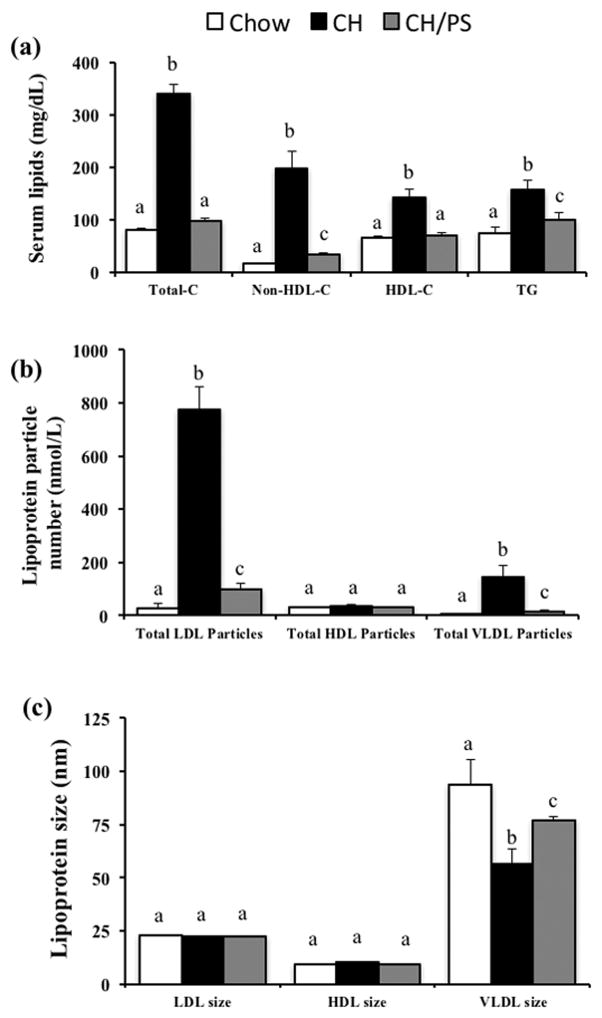
Maternal cholesterol feeding throughout pre-pregnancy, gestation, and lactation increased (p<0.05) in total-C (+316%), non-HDL cholesterol (+1135%), HDL-C (116%), and TG (+112%) compared with the chow fed mothers (Fig 2a). Furthermore, dams consuming the CH diet displayed an increased the total-C:HDL-C ratio (+103%) compared with the chow-fed dams. Supplementation of the maternal diet with PS (CH/PS) reduced total-C (-71%), non-HDL cholesterol (−83%), HDL-C (−50%), TG (−36%), and the total-C:HDL-C ratio (−40%) compared with the CH diet. Maternal cholesterol feeding also increased (p<0.05) total LDL (+2661%) and VLDL particles (+3678%) compared with the chow group, however, supplementation of PS largely protected (p<0.05) against this response by reducing total LDL (−87%) and VLDL particle number (−89%) compared with the CH group (Fig 2b). No difference (p>0.05) in HDL particle number (Fig 2b) was observed between the treatment groups. Although dietary treatment did no influence (p>0.05) LDL and HDL particle size, CH-fed dams demonstrated a reduction (p<0.05) in VLDL size compared with CH dams. Maternal PS-supplementation (p<0.05) increased VLDL size in relation to the CH-fed moms, however, not to chow levels (Fig 2c).
Figure 2.
Serum lipids (a), lipoprotein particle number (b), and lipoprotein size (c) in dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS) during the pre-pregnancy period and throughout gestation and lactation. Total-C, total cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; and TG, triglycerides. abGroups not sharing a superscript are significantly different (p<0.05).
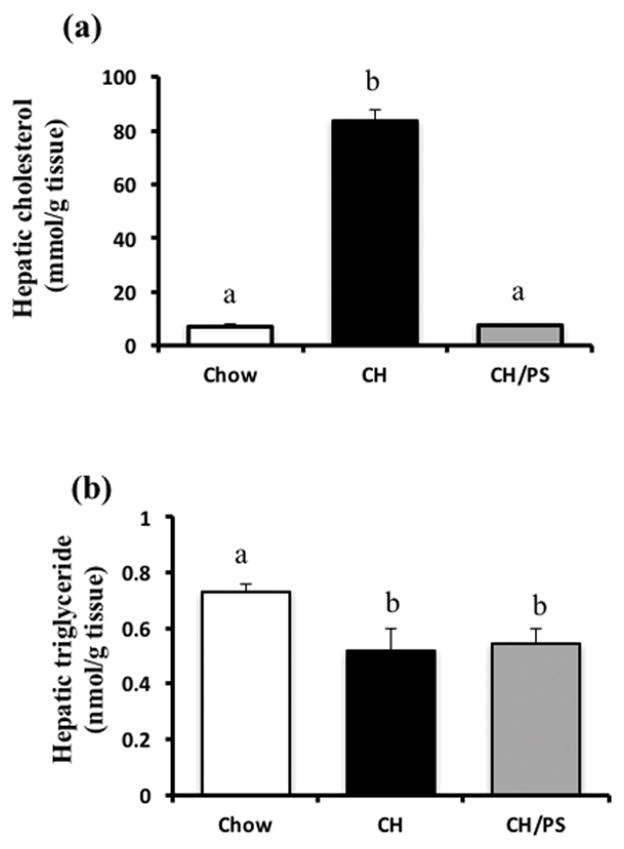
Compared with the chow-fed dams, cholesterol feeding increased (p<0.05) hepatic cholesterol concentrations, however, supplementation with PS normalized this response (Fig 3a). Hepatic TG concentrations were lower (p<0.05) in the CH and PS-supplemented dams compared with chow (Fig 3b).
Figure 3.
Hepatic concentration of cholesterol (a) and triglycerides (b) in dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS) during the pre-pregnancy period and throughout gestation and lactation. abGroups not sharing a superscript are significantly different (p<0.05).
Pup response
Litter size on PN day 0 and the fraction of males and females per litter (40–60%) were not affected (p>0.05) by maternal diet. Litter weights on PN day 5 did not differ with respect to maternal diet, however, litters from the CH and CH/PS dams weighed less (~13%, p<0.05) than litters from the chow-fed dams on PN day 10. Litter weights by PN day 20 were similar (p>0.05) among the treatment groups.
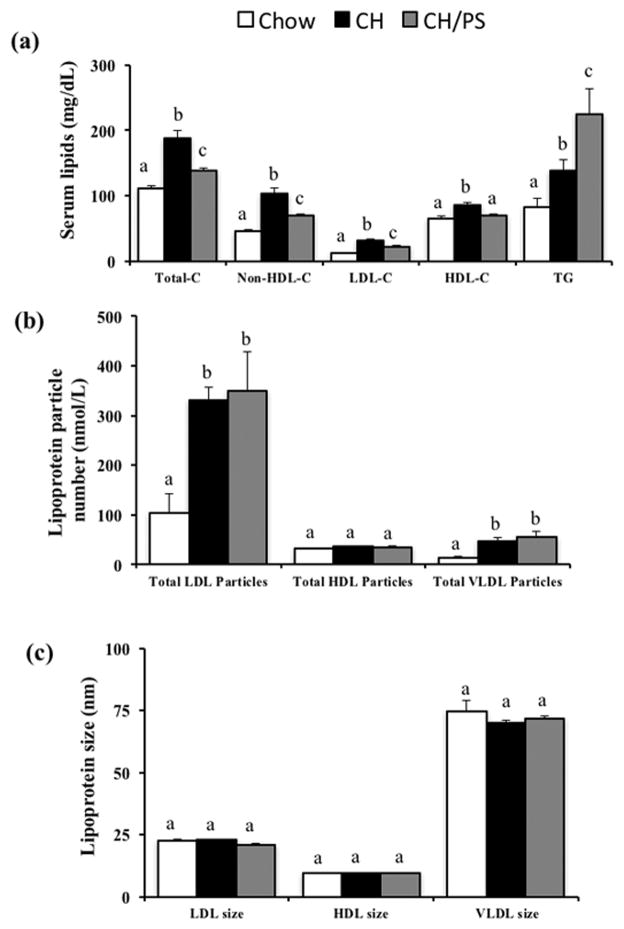
Compared with pups from chow-fed dams, pups from dams fed the cholesterol-enriched diet displayed increased (p<0.05) total-C (+68%), non-HDL-C (+123%), LDL-C (+154%), HDL-C (30%) but no change (p>0.05) in TG on PN day 21 (Fig 4a). The total-C:HDL-C ratio increased (+29%, p<0.05) in pups from cholesterol-fed dams compared with chow pups. Pups from PS-supplemented dams exhibited reductions (p<0.05) in total-C (−26%), non-HDL-C (−32%), LDL-C (−29%), and HDL-C (−19%), but increased TG (+62%, p=0.05) compared with pups from cholesterol-supplemented dams (Fig 4a). The total-C:HDL-C ratio decreased (−9%, p<0.05) in the CH/PS pups compared with the CH pups.
Figure 4.
Serum lipids (a), lipoprotein particle number (b), and (c) lipoprotein size in 21 day old offspring from dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS) during a 14 day pre-pregnancy period and throughout gestation and lactation. Total-C, total cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; and TG, triglycerides. abGroups not sharing a superscript are significantly different (p<0.05).
Although increases (p<0.05) were observed in total LDL particle number (+216%) and VLDL particle number (+254%) in pups from dams consuming the cholesterol-enriched diet compared with pups from chow-fed dams, maternal PS-supplementation was not effective (p>0.05) in ameliorating this response (Fig 4b). Total HDL particle number did not differ (p>0.05) among the treatment groups (Fig 4b). Maternal diet did not alter (p>0.05) LDL, HDL or VLDL particle size in pups (Fig 4c). Serum activity of CETP (pmol/μL/hr) was measured as a potential contributor to the observed lipoprotein responses, however, activity measurements did not different (p>0.05) between the Chow (33.2±9.0), CH (32.3±12.1), and CH/PS (37.3±14.5) groups.
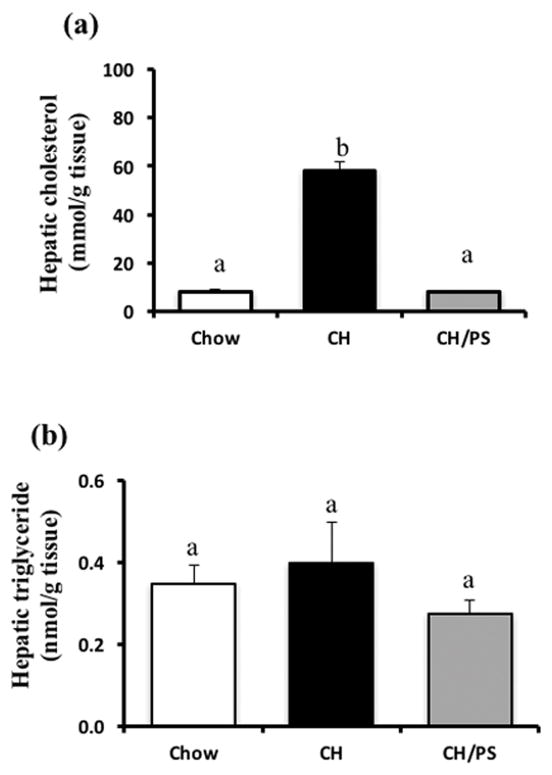
Hepatic cholesterol concentration was increased (p<0.05) in pups reared from CH-fed mothers compared with chow-fed dams but was normalized (p<0.05) to chow levels in pups from mothers supplemented with PS (Fig 5a). No difference (p<0.05) in hepatic TG concentrations was noted between pups in any treatment group (Fig 5b).
Figure 5.
Hepatic concentration of cholesterol (a) and triglycerides (b) in 21 day old offspring from dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS) during the pre-pregnancy period and throughout gestation and lactation. abGroups not sharing a superscript are significantly different (p<0.05).
The expression of a host of hepatic cholesterol and TG regulatory targets were examined in pups from each treatment group (Table 1). In comparison with the chow group, pups from cholesterol-supplemented dams displayed a differential mRNA expression pattern with increased (p<0.05) expression of ABCG1 (1.6 fold) and LDLr (1.6 fold) and decreased expression in a number of regulatory targets including LXR (0.60 fold), PCSK9 (0.56 fold), SREBP2 (0.43 fold), CPT1 (0.60 fold), DGAT (0.61 fold), and CETP (0.43 fold). Surprisingly, no differences were noted in the mRNA expression patterns between the CH and CH/PS groups.
Table 1.
Hepatic mRNA expression of cholesterol and fatty acid/triglyceride regulatory targets in offspring from dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS) during a 14-day pre-pregnancy period and throughout gestation and lactation.
| Gene | Chow | CH | CH/PS |
|---|---|---|---|
| Cholesterol regulatory targets | |||
| ABCA1 | 1.0 ± 0.14a | 0.76 ± 0.18a | 0.95 ± 0.17a |
| ABCG1 | 1.0 ± 0.08a | 1.68 ± 0.28b | 1.09 ± 0.12ab |
| ABCG5 | 1.0 ± 0.25a | 0.82 ± 0.13a | 1.16 ± 0.20a |
| ABCG8 | 1.0 ± 0.16a | 1.05 ± 0.32a | 1.18 ± 0.26a |
| HMG-CoAr | 1.0 ± 0.13a | 1.54 ± 0.54a | 1.42 ± 0.16a |
| LDLr | 1.0 ± 0.16a | 1.60 ± 0.14b | 1.48 ± 0.13b |
| LXR | 1.0 ± 0.11a | 0.60 ± 0.04b | 0.79 ± 0.10ab |
| PCSK9 | 1.0 ± 0.12a | 0.56 ± 0.12b | 0.63 ± 0.56b |
| SREBP2 | 1.0 ± 0.15a | 0.43 ± 0.04b | 0.52 ± 0.09b |
| CETP | 1.0 ± 0.14a | 0.43 ± 0.03b | 0.42 ± 0.06b |
| Fatty acid/triglyceride regulatory targets | |||
| CD36 | 1.0 ± 0.26a | 1.06 ± 0.27a | 0.72 ± 0.08a |
| FABP2 | 1.0 ± 0.15a | 0.98 ± 0.21a | 1.10 ± 0.18a |
| SREBP1C | 1.0 ± 0.13a | 0.96 ± 0.12a | 0.78 ± 0.09a |
| PPARα | 1.0 ± 0.12a | 0.72 ± 0.08a | 0.65 ± 0.11a |
| ACC | 1.0 ± 0.23a | 0.64 ±0.03a | 0.91 ± 0.17a |
| FAS | 1.0 ± 0.14a | 0.75 ± 0.13a | 0.74 ± 0.19a |
| CPT1 | 1.0 ± 0.05a | 0.60 ± 0.08b | 0.67 ±0.08b |
| MTTP | 1.0 ± 0.15a | 0.65 ± 0.08a | 0.64 ± 0.14a |
| DGAT | 1.0 ± 0.09a | 0.61 ± 0.11b | 0.63 ± 0.09b |
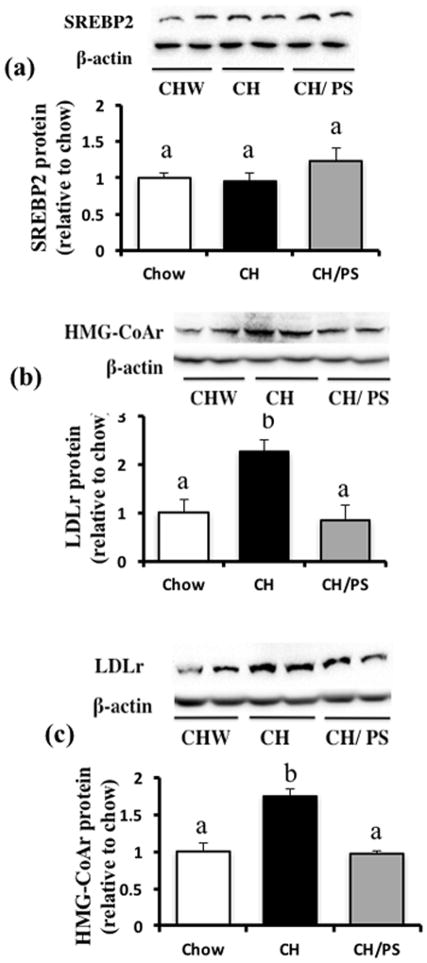
Hepatic nuclear SREBP2 protein expression did not differ between the treatment groups (Fig 6a). Compared with chow pups, pups from CH-fed dams demonstrated increased protein expression of both the LDLr (~2 fold, Fig 6b) and HMG-CoAr (~1.8 fold, Fig 6c) that was normalized to chow levels in pups from PS-supplemented dams.
Figure 6.
Hepatic protein expression of cholesterol regulatory targets in 21 day-old offspring from dams fed a chow diet (chow) or chow diet supplemented with cholesterol (CH) or cholesterol and phytosterols (CH/PS) during the pre-pregnancy period and throughout gestation and lactation. (a) SREBP2 in nuclear extract, (b) HMG-CoAr in total tissue fraction, (c) LDLr in total tissue fraction. abGroups not sharing a superscript are significantly different (p<0.05).
Discussion
We noted several novel findings that warrant further discussion. First, newly-weaned offspring from dams with diet-induced hypercholesterolemia throughout pregnancy and lactation were dyslipidemic compared with pups from chow-fed mothers. Second, maternal PS supplementation to a cholesterol-enriched diet during pre-pregnancy and throughout gestation/lactation protected pups against adverse blood and hepatic cholesterol profiles but also triggered an unexpected increase in serum TG concentrations compared with pups from cholesterol-fed mothers. Finally, pups from cholesterol-fed dams demonstrated differential mRNA and protein expression patterns of hepatic lipid regulators with a notable increase in the protein abundance of the LDLr and HMG-CoA reductase that was normalized by maternal PS-supplementation.
Serum lipid and lipoprotein profiles in children have been shown to be predictive of those in adulthood [31–33] and there is strong evidence to suggest that fetal and neonatal lipid status is influenced by that of the mother [34–36]. In a human study with aborted fetuses and premature newborns who died shortly after birth, Napoli et al. (1997) reported a strong association between fetal (<6 months) and maternal serum cholesterol and observed an increased number of oxidized LDL-containing lesions in fetal aortas from hypercholesterolemic versus normal cholesterolemic mothers [37]. Similarly, animal studies support an association between maternal cholesterol status and serum cholesterol levels in offspring [38, 39]. Burke et al. (2009) reported increased placental LDL-C fetal transfer in hypercholesterolemia dams compared with control dams [38]. Our results support the relationship between maternal and offspring cholesterol in that pups from cholesterol-supplemented dams displayed increase cholesterol in all serum lipoprotein fractions at weaning compared with pups from chow-fed dams. Moreover, offspring from cholesterol-fed dams exhibited an increased number of total LDL and VLDL particles compared with chow offspring, an important observation as very few studies have accessed how early exposure to cholesterol influences advanced lipoprotein measures even though these endpoints are considered valuable CVD predictive biomarkers [40].
Additionally, pups from cholesterol-fed mothers also had higher hepatic cholesterol concentrations compared with chow pups. Although data on cholesterol-balance in newly-weaned offspring is limited, a number of studies have shown that maternal cholesterol feeding is associated with increased cholesterol deposition in hamster fetal tissues (placenta, yolk sac) [38, 41] and in rabbit liver upon birth [42]. Considering the importance of the liver in regulating whole-body cholesterol metabolism in neonates [43], it stands to reason that this dramatic increase in liver cholesterol deposition in pups from cholesterol-fed dams was also associated with a differential mRNA expression pattern of hepatic cholesterol-regulators that included a decrease in SREPB2 mRNA compared with chow-pups. Despite this change, SREBP2 target gene expression did not follow a predicable pattern as evidenced by a reduction in PCSK9 mRNA, an increase in LDLr mRNA, and no change in HMG-CoAr mRNA expression compared with chow pups. Furthermore, the protein abundance of LDLr and HMG-CoA reductase was increased in pups from cholesterol-fed dams compared with chow-pups. Although this apparent increase in hepatic cholesterol uptake and synthesis may partially underlie the dyslipidemic response we observed in the CH offspring at weaning, it contradicts previous work suggesting that fetal sterol synthesis rates are reduced in response to maternal cholesterol feeding in hamsters [41]. However, it has been reported that maternal cholesterol feeding can program an enhanced transcriptional capacity of cholesterol synthesis genes as well as the LDLr in adult apoE offspring [44].
Similar to the results of our previous study in apoE mice [45], maternal PS supplementation was effecting in protecting pups hypercholesterolemia induced by maternal cholesterol feeding. A number of mechanisms may have contributed to this protective effect. First, by reducing hypercholesterolemia in mothers, maternal PS supplementation may have indirectly impeded excessive maternal cholesterol transfer to the offspring in utero. Although the developing fetus has a capacity for cholesterol synthesis, it also obtains a proportion of its cholesterol from the maternal circulation [46]. The yolk sac and placenta express a variety of receptors, including the low-density-lipoprotein receptor (LDLr) and scavenger receptor class B type 1 (SR-B1), that mediate maternal lipid and liproprotein transfer across the apical and basolaterol membranes of trophoblasts and endothelial cells into the fetal circulation [47, 48]. Secondly, early exposure to PS during fetal development and/or through postnatal milk consumption could have directly impacted cholesterol homeostasis in offspring. Maternal PS supplementation during lactation has previously been shown to increase PS concentrations in maternal milk and result in increased circulating PS levels in infants [49]. We detected 2-fold increase in serum β-sitosterol concentrations in offspring from PS-fed dams compared with pups from the CH group (data not presented). Enhanced circulating levels of PS have been shown to directly influence the expression of cholesterol-regulatory genes, possibly by acting as ligands for liver X receptor (LXR) in the liver and peripheral tissues [50, 51]. Although we noted few differences in the transcriptional control of genes that regulate cholesterol and/or TG metabolism in PS versus CH pups, we did observe a normalization in the protein abundance of the LDLr and HMG-CoA reductase which may be associated with the protective effect of maternal PS supplementation.
Although maternal PS-supplementation protected against hypercholesterolemia in pups, we also observed an unexpected rise in serum TG compared with pups born to unsupplemented mothers. This is particularly surprising given that PS supplementation reduced serum TG (Fig 2a) and VLDL particle number (Fig 2b) in the mothers, a lesser-known effect of PS that is receiving significant research attention [52–54]. Although the cause of this TG increase is not known, we previously reported an increase in hepatic de novo lipogenesis in PS-fed C57BL6 mice that we interpreted to be a compensatory response to interference with intestinal fatty acid absorption by PS [17]. Therefore, it is possible that by reducing maternal TG status, PS supplementation interrupted the transfer of TG to the offspring during prenatal development or altered the TG level and/or fatty acid composition of the milk. If this is the case, the offspring may have compensated for this reduced maternal transfer with increased hepatic TG synthesis. The high demand for fatty acids to support early growth and postnatal development has been shown to derive from both maternal lipid stores and also de novo synthesis in the fetus and neonate [55]. However, we did not detect any change in the mRNA expression of hepatic lipid synthesis genes between the treatment groups. Regardless of the mechanism, the implications of this early increase in serum TG and whether this response causes more permanent adaptations in TG metabolism in adulthood must be investigated.
In summary, we report that newly-weaned offspring born to mothers with elevated cholesterol display a dyslipidemic plasma lipid and lipoprotein profile and increased hepatic cholesterol infiltration with a differential expression of cholesterol regulatory targets. Maternal PS-supplementation was effective in normalizing hypercholesterolemia in these newly-weaned offspring but also exacerbated serum TG concentrations.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Laura Woollett, University of Cincinnati, Pathology and Laboratory Medicine for her advice in establishing a maternal programming model with Syrian golden hamsters. The phytosterol supplement used in this study was generously donated by Forbs Medi-Tech Corp, Kearny, NJ.
Financial Support
This research was supported by a KO1 grant (1K01AT007826-01A1) from the National Center for Complementary and Integrative Health (NCCIH) and a KO1 supplement from NCCIH and the Office of Dietary Supplements (3K01AT007826-03S1) (to TCR).
List of Abbreviations
- PS
phytosterols
- CH
cholesterol group
- CH/PS
cholesterol/phytosterol group
- CVD
cardiovascular disease
- TG
triglyceride
- FH
familial hypercholesterolemia
Footnotes
Author Contributions
JL, AI, and AR assisted with the animal trial and lab analysis; RWB assisted with serum lipid and tissue analysis; MSP assisted with experimental design and data interpretation; TCR designed the experiment, interpreted the data, and wrote the initial manuscript draft. All authors read and approved the final manuscript.
Conflicts of Interest Statement
None
References
- 1.Capewell S, Ford ES, Croft JB, Critchley JA, Greenlund KJ, Labarthe DR. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in the United States of America. Bull World Health Organ. 2010;88:120–130. doi: 10.2471/BLT.08.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2013: With Special Feature on Prescription Drugs. Hyattsville, MD: 2014. [PubMed] [Google Scholar]
- 3.Kusters DM, Homsma SJ, Hutten BA, Twickler MT, Avis HJ, van der Post JA, Stroes ES. Dilemmas in treatment of women with familial hypercholesterolaemia during pregnancy. Neth J Med. 2010;68:299–303. [PubMed] [Google Scholar]
- 4.Palinski W, Napoli C. The fetal origins of atherosclerosis: maternal hypercholesterolemia, and cholesterol-lowering or antioxidant treatment during pregnancy influence in utero programming and postnatal susceptibility to atherogenesis. FASEB J. 2002;16:1348–1360. doi: 10.1096/fj.02-0226rev. [DOI] [PubMed] [Google Scholar]
- 5.Palinski W, D’Armiento FP, Witztum JL, de Nigris F, Casanada F, Condorelli M, Silvestre M, Napoli C. Maternal hypercholesterolemia and treatment during pregnancy influence the long-term progression of atherosclerosis in offspring of rabbits. Circ Res. 2001;89:991–996. doi: 10.1161/hh2301.099646. [DOI] [PubMed] [Google Scholar]
- 6.Ito MK, McGowan MP, Moriarty PM National Lipid Association Expert Panel on Familial H. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S38–45. doi: 10.1016/j.jacl.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Eapen DJ, Valiani K, Reddy S, Sperling L. Management of familial hypercholesterolemia during pregnancy: case series and discussion. J Clin Lipidol. 2012;6:88–91. doi: 10.1016/j.jacl.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Abumweis SS, Barake R, Jones PJ. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr Res. 2008:52. doi: 10.3402/fnr.v52i0.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rideout TC, Harding SV, Mackay D, Abumweis SS, Jones PJ. High basal fractional cholesterol synthesis is associated with nonresponse of plasma LDL cholesterol to plant sterol therapy. Am J Clin Nutr. 2010;92:41–46. doi: 10.3945/ajcn.2009.29073. [DOI] [PubMed] [Google Scholar]
- 10.Plat J, Mensink RP. Plant stanol esters lower serum triacylglycerol concentrations via a reduced hepatic VLDL-1 production. Lipids. 2009;44:1149–1153. doi: 10.1007/s11745-009-3361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryokkynen A, Kayhko UR, Mustonen AM, Kukkonen JV, Nieminen P. Multigenerational exposure to phytosterols in the mouse. Reprod Toxicol. 2005;19:535–540. doi: 10.1016/j.reprotox.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Waalkens-Berendsen DH, Wolterbeek AP, Wijnands MV, Richold M, Hepburn PA. Safety evaluation of phytosterol esters. Part 3. Two-generation reproduction study in rats with phytosterol esters--a novel functional food. Food Chem Toxicol. 1999;37:683–696. doi: 10.1016/s0278-6915(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker MH, Frankos VH, Wolterbeek AP, Waalkens-Berendsen DH. Two-generation reproductive toxicity study of plant stanol esters in rats. Regul Toxicol Pharmacol. 1999;29:196–204. doi: 10.1006/rtph.1999.1288. [DOI] [PubMed] [Google Scholar]
- 14.Laitinen K, Isolauri E, Kaipiainen L, Gylling H, Miettinen TA. Plant stanol ester spreads as components of a balanced diet for pregnant and breast-feeding women: evaluation of clinical safety. Br J Nutr. 2009;101:1797–1804. doi: 10.1017/S0007114508133608. [DOI] [PubMed] [Google Scholar]
- 15.Agnish ND, Keller KA. The rationale for culling of rodent litters. Fundam Appl Toxicol. 1997;38:2–6. doi: 10.1006/faat.1997.2318. [DOI] [PubMed] [Google Scholar]
- 16.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Rideout TC, Harding SV, Jones PJ. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol Nutr Food Res. 2010;54(Suppl 1):S7–13. doi: 10.1002/mnfr.201000027. [DOI] [PubMed] [Google Scholar]
- 18.Harding SV, Rideout TC, Jones PJ. Hepatic nuclear sterol regulatory binding element protein 2 abundance is decreased and that of ABCG5 increased in male hamsters fed plant sterols. J Nutr. 2010;140:1249–1254. doi: 10.3945/jn.109.120311. [DOI] [PubMed] [Google Scholar]
- 19.Rideout TC, Yuan Z, Bakovic M, Liu Q, Li RK, Mine Y, Fan MZ. Guar gum consumption increases hepatic nuclear SREBP2 and LDL receptor expression in pigs fed an atherogenic diet. J Nutr. 2007;137:568–572. doi: 10.1093/jn/137.3.568. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 22.Field FJ, Born E, Mathur SN. Stanol esters decrease plasma cholesterol independently of intestinal ABC sterol transporters and Niemann-Pick C1-like 1 protein gene expression. J Lipid Res. 2004;45:2252–2259. doi: 10.1194/jlr.M400208-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, Dong B, Cao A, Li H, Liu J. Delineation of molecular pathways that regulate hepatic PCSK9 and LDL receptor expression during fasting in normolipidemic hamsters. Atherosclerosis. 2012;224:401–410. doi: 10.1016/j.atherosclerosis.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Liu X, Zhu H, Huang L, Liu Y, Ma C, Qin C. Insulin resistance in insulin-resistant and diabetic hamsters (Mesocricetus auratus) is associated with abnormal hepatic expression of genes involved in lipid and glucose metabolism. Comp Med. 2009;59:449–458. [PMC free article] [PubMed] [Google Scholar]
- 25.Guo F, Yang X, Li X, Feng R, Guan C, Wang Y, Li Y, Sun C. Nuciferine prevents hepatic steatosis and injury induced by a high-fat diet in hamsters. PLoS One. 2013;8:e63770. doi: 10.1371/journal.pone.0063770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin B, Dawson H, Anderson RA. Elevation of tumor necrosis factor-alpha induces the overproduction of postprandial intestinal apolipoprotein B48-containing very low-density lipoprotein particles: evidence for related gene expression of inflammatory, insulin and lipoprotein signaling in enterocytes. Exp Biol Med (Maywood) 2010;235:199–205. doi: 10.1258/ebm.2009.009169. [DOI] [PubMed] [Google Scholar]
- 27.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izem L, Morton RE. Molecular cloning of hamster lipid transfer inhibitor protein (apolipoprotein F) and regulation of its expression by hyperlipidemia. J Lipid Res. 2009;50:676–684. doi: 10.1194/jlr.M800429-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrier B, Wen S, Zigouras S, Browne RW, Li Z, Patel MS, Williamson DL, Rideout TC. Alpha-Lipoic Acid Reduces LDL-Particle Number and PCSK9 Concentrations in High-Fat Fed Obese Zucker Rats. PLoS One. 2014;9:e90863. doi: 10.1371/journal.pone.0090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuehl RO. Design of Experiments: Statistical Principles of Research Design Analysis. 2. Brooks/Cole Publishing Company; 2000. [Google Scholar]
- 31.Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133:884–899. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- 32.Porkka KV, Viikari JS, Taimela S, Dahl M, Akerblom HK. Tracking and predictiveness of serum lipid and lipoprotein measurements in childhood: a 12-year follow-up. The Cardiovascular Risk in Young Finns study. Am J Epidemiol. 1994;140:1096–1110. doi: 10.1093/oxfordjournals.aje.a117210. [DOI] [PubMed] [Google Scholar]
- 33.Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–590. doi: 10.1016/j.jpeds.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Innis SM. Effect of diet during pregnancy and lactation on the activity of HMG-CoA reductase in the developing rat. J Nutr. 1988;118:1177–1183. doi: 10.1093/jn/118.10.1177. [DOI] [PubMed] [Google Scholar]
- 35.MacPherson RE, Castelli LM, Miotto PM, Frendo-Cumbo S, Milburn A, Roy BD, LeBlanc PJ, Ward WE, Peters SJ. A Maternal High Fat Diet Has Long-Lasting Effects on Skeletal Muscle Lipid and PLIN Protein Content in Rat Offspring at Young Adulthood. Lipids. 2015;50:205–217. doi: 10.1007/s11745-014-3985-5. [DOI] [PubMed] [Google Scholar]
- 36.Elahi MM, Cagampang FR, Mukhtar D, Anthony FW, Ohri SK, Hanson MA. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br J Nutr. 2009;102:514–519. doi: 10.1017/S000711450820749X. [DOI] [PubMed] [Google Scholar]
- 37.Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, Palinski W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke KT, Colvin PL, Myatt L, Graf GA, Schroeder F, Woollett LA. Transport of maternal cholesterol to the fetus is affected by maternal plasma cholesterol concentrations in the golden Syrian hamster. J Lipid Res. 2009;50:1146–1155. doi: 10.1194/jlr.M800538-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montoudis A, Simoneau L, Brissette L, Forest JC, Savard R, Lafond J. Impact of a cholesterol enriched diet on maternal and fetal plasma lipids and fetal deposition in pregnant rabbits. Life Sci. 1999;64:2439–2450. doi: 10.1016/s0024-3205(99)00201-5. [DOI] [PubMed] [Google Scholar]
- 40.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 41.McConihay JA, Horn PS, Woollett LA. Effect of maternal hypercholesterolemia on fetal sterol metabolism in the Golden Syrian hamster. J Lipid Res. 2001;42:1111–1119. [PubMed] [Google Scholar]
- 42.Marseille-Tremblay C, Gravel A, Lafond J, Mounier C. Effect of an enriched cholesterol diet during gestation on fatty acid synthase, HMG-CoA reductase and SREBP-1/2 expressions in rabbits. Life Sci. 2007;81:772–778. doi: 10.1016/j.lfs.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Yao L, Horn PS, Heubi JE, Woollett LA. The liver plays a key role in whole body sterol accretion of the neonatal Golden Syrian hamster. Biochim Biophys Acta. 2007;1771:550–557. doi: 10.1016/j.bbalip.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goharkhay N, Tamayo EH, Yin H, Hankins GD, Saade GR, Longo M. Maternal hypercholesterolemia leads to activation of endogenous cholesterol synthesis in the offspring. Am J Obstet Gynecol. 2008;199:273, e271–276. doi: 10.1016/j.ajog.2008.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rideout TC, Movsesian C, Tsai YT, Iqbal A, Raslawsky A, Patel MS. Maternal Phytosterol Supplementation during Pregnancy and Lactation Modulates Lipid and Lipoprotein Response in Offspring of apoE-Deficient Mice. J Nutr. 2015;145:1728–1734. doi: 10.3945/jn.115.215061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baardman ME, Erwich JJ, Berger RM, Hofstra RM, Kerstjens-Frederikse WS, Lutjohann D, Plosch T. The origin of fetal sterols in second-trimester amniotic fluid: endogenous synthesis or maternal-fetal transport? Am J Obstet Gynecol. 2012;207:202, e219–225. doi: 10.1016/j.ajog.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Woollett LA. Review: Transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32(Suppl 2):S218–221. doi: 10.1016/j.placenta.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baardman ME, Kerstjens-Frederikse WS, Berger RM, Bakker MK, Hofstra RM, Plosch T. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod. 2013;88:24. doi: 10.1095/biolreprod.112.102442. [DOI] [PubMed] [Google Scholar]
- 49.Mellies MJ, Ishikawa TT, Gartside PS, Burton K, MacGee J, Allen K, Steiner PM, Brady D, Glueck CJ. Effects of varying maternal dietary fatty acids in lactating women and their infants. Am J Clin Nutr. 1979;32:299–303. doi: 10.1093/ajcn/32.2.299. [DOI] [PubMed] [Google Scholar]
- 50.Calpe-Berdiel L, Escola-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Plat J, Nichols JA, Mensink RP. Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J Lipid Res. 2005;46:2468–2476. doi: 10.1194/jlr.M500272-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Rideout TC, Ramprasath V, Griffin JD, Browne RW, Harding SV, Jones PJ. Phytosterols protect against diet-induced hypertriglyceridemia in Syrian golden hamsters. Lipids Health Dis. 2014;13:5. doi: 10.1186/1476-511X-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schonewille M, Brufau G, Shiri-Sverdlov R, Groen AK, Plat J. Serum TG-lowering properties of plant sterols and stanols are associated with decreased hepatic VLDL secretion. J Lipid Res. 2014;55:2554–2561. doi: 10.1194/jlr.M052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rideout TC, Marinangeli CP, Harding SV. Triglyceride-Lowering Response to Plant Sterol and Stanol Consumption. J AOAC Int. 2015;98:707–715. doi: 10.5740/jaoacint.SGERideout. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth--a review. Placenta. 2002;23(Suppl A):S28–38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.