Abstract
While the cerebellum has traditionally been thought of as mainly involved in motor functions, evidence has been accumulating for cerebellar contributions also to non-motor, cognitive functions. The notion of a cerebellar internal model underlying prediction and processing of sensory events and coordination and fine-tuning of appropriate responses has put the cerebellum right at the interface of motor behavior and cognition. Along these lines, the cerebellum may critically contribute to performance monitoring, a set of cognitive and affective functions underlying adaptive behavior. This review presents and integrates evidence from recent neuroimaging and clinical studies for a cerebellar role in performance monitoring with focus on sensory prediction, error and conflict processing, response inhibition, and feedback learning. Together with evidence for involvement in articulatory monitoring during working memory, these findings suggest monitoring as the cerebellum’s overarching function.
Keywords: cerebellum, performance monitoring, cognition, internal model, executive functions
Introduction
The traditional view of the cerebellum as exclusively involved in motor functions has been challenged, amongst other findings, by early reports of cognitive and affective impairments following cerebellar damage [e.g. 1–4], ultimately leading to a fundamental paradigm shift. It is now widely recognized that the cerebellum contributes to motor behavior and cognition, the crucial question being how (rather than if) cerebellar involvement in the cognitive domain is implemented (for a review of current theories, see [5]). Anatomically and functionally, the cerebellum possesses vast connections to cerebral areas pivotally involved in non-motor functions, and these connections form uniform, closed loops [6, 7]. It has been proposed that these loops may underlie uniform processing habits for the different functional domains [7]. In other words, as the cerebellum is critical for coordination and timing in the motor domain, it may subserve similar functions also in the cognitive domain. Accordingly, motor dysmetria, that is, inability to perform accurate movements due to impaired coordination of the limbs, following cerebellar damage, may be paralleled by “dysmetria of thought” [8] or “cognitive dysmetria” [9], as reflected in affective disturbances, psychotic features and executive dysfunction, depending on the exact location of the cerebellar lesion [10]. In the present review, we will argue that by being right at the interface of motor behavior and cognition, the cerebellum is at an ideal position to crucially contribute to performance monitoring.
Cerebellar internal forward model
The notion of cerebellar internal models [11, 12] underlying prediction and processing of sensory events as well as coordination and fine-tuning of appropriate responses puts the cerebellum at the interface of motor behavior and cognition. Internal models enable diverse aspects of adaptive behavior, e.g. motor learning, maintenance of accurate performance despite feedback delays, and cancelling out of self-generated sensory effects. In the motor domain, forward models integrate current information about the motor effectors, e.g. proprioceptive information about the arm, with efference copies of motor commands e.g. for a reaching motion, yielding an estimate of the consequences of the movement before external feedback information is available [11]. Internal models have been proposed to apply to mental representations and cognition in an analogous manner [12, 13]. Given the importance of cerebellar internal models for adaptive behavior, it stands to reason that the cerebellum critically contributes to performance monitoring.
What is performance monitoring?
Performance monitoring refers to a diverse set of cognitive and affective functions underlying adaptive behavior. In order to adjust our behavior to meet ever-changing demands in a dynamic environment, we need to process (external and internal) performance-related feedback, detect erroneous responses, manage and inhibit conflicting response tendencies, allocate attentional resources accordingly, and regulate emotional responses to specific response outcomes such as rewards or punishment. Generally, performance monitoring is thought to recruit an extensive fronto-striatal network that makes use of dopamine-dependent coding of response outcomes (for a review, see [14]). However, neuroimaging studies have frequently also reported activations within the cerebellum during error processing, reward learning, and reversal learning [e.g. 15, 16], findings that strongly implicate the cerebellum in performance monitoring. Unfortunately, to date, these cerebellar activations have rarely been discussed in detail, so that the exact role of the cerebellum for performance monitoring has remained largely unclear. In the following paragraphs, we will review empirical data for cerebellar involvement in diverse aspects of performance monitoring.
Cerebellar contributions to sensory prediction
Early research has implicated the cerebellum in sensory acquisition. Activation in the dentate nucleus was observed for both passive and active sensory tasks, that is, for cutaneous stimulation and for tactile discrimination with and without finger movements, but not for finger movements without tactile discrimination [17, 18]. While these findings did not clarify the precise role of the cerebellum in sensory discrimination, they do support the notion that the cerebellum supports the control of sensory acquisition during a range of behaviors, not only motor but also perceptual and cognitive [18].
The cerebellum has since also been strongly implicated in sensorimotor prediction, and here specifically in the cancelling of self-induced sensory stimulation prediction (for a review, see [19]). Activation in the right cerebellar cortex was reduced during a self-generated movement that generated a tactile stimulus as compared to during an identical movement that did not trigger sensory stimulation [20]. These findings show that prediction of movement consequences modulated the cerebellar response. Moreover, cerebellar activation has been shown to contribute to activation decrease in the somatosensory cortex during self-produced tactile stimulation [21]. In the auditory domain, prediction of self-initiated sounds has been linked to suppression of the auditory N100 component in the event-related potential (ERP). In line with cerebellar involvement in sensory prediction, patients with cerebellar damage show largely attenuated or even a lack of N100 suppression [22, 23].
A recent study with event-related functional magnetic resonance imaging (fMRI) has highlighted cerebellar coding of sensory prediction errors [24]. While in the scanner, subjects performed fast out and back reaching movements with a non-magnetic robotic arm that allowed for low friction two-dimensional movements in the horizontal plane. Movements were aimed at visual targets projected onto a back screen that was visible to the participant by means of a mirror. Movements were either aimed at targets which delivered a force pulse if intersected by the movement trajectory, or at a gap between two objects that both delivered force pulses if intersected by the movement trajectory. Missing the target could thus be signaled by either the presence or absence of a force pulse. Interestingly, errors were generally associated with greater activation in “hand areas” in cerebellar lobules V and VI, irrespective of which error signal had been received, indicating that the cerebellum similarly codes prediction errors based on unexpected presence as well as unexpected absence of sensory stimulation.
Prediction not only involves predicting if sensory stimulation will happen but also when it will occur. A number of studies have addressed cerebellar contributions to temporal prediction. Recently, Avanzino et al. [*25] had subjects predict the end of human body motion (a right hand writing a sentence) and inanimate object motion (a moving circle reaching a target) presented in video sequences that were interrupted by a dark interval while the motion was underway. Specifically, subjects indicated the end of the movement by button press during this dark interval. Inhibitory repetitive 1 Hz transcranial magnetic stimulation (TMS) over the lateral cerebellum immediately before completing the task was associated with increased timing errors for human body motion only, indicating that the cerebellum is specifically involved in predicting the consequences of observed motor acts, a cognitive domain tightly linked to the motor system.
Cerebellar internal models code temporal predictability also outside the motor domain. Early investigations by Ivry demonstrated that cerebellar damage also disrupts perceptual aspects of timing [26]. More recently, Kotz, Stockert and Schwartze [*27] investigated auditory deviance processing in cerebellar lesion patients with electroencephalography (EEG) and ERPs. The fronto-central N2b component which has been linked to attentive detection of a deviant tone in a sequence [28] did not differ between patients and healthy controls. In contrast, the P3b, a centro-parietal component associated with updating of mental models [29], was enhanced in controls for deviants in regular sequences. This effect was absent in patients, suggesting impaired processing of temporal predictability of auditory stimuli.
Cerebellar contributions to error processing
Generally, the cerebellum is thought to make rapid predictions about sensory consequences of self-generated movement at very low levels of movement execution, presumably without awareness [19]. Such fast, unconscious predictions crucially depend on efference copies of motor commands. A series of ERP studies with concurrent eye tracking recently showed that damage to the cerebellum is associated with altered processing of saccade-related efference copy signals. Patients with post-acute focal vascular cerebellar lesions showed altered neural responses but intact behavioral performance during saccadic updating, that is, remapping of spatial representations contingent upon saccades [*30,*31]. A post-saccadic positive deflection in the ERP presumably associated with saccadic updating was reduced in cerebellar lesion patients as compared to healthy controls or patients with focal vascular thalamic lesions. A similar pattern was also observed for evaluative saccade processing: Peterburs et al. [**32] reported altered processing of erroneous and correct saccades but preserved performance accuracy in an antisaccade task in cerebellar lesion patients. The error-correct ERP difference waveforms showed reduced amplitudes for patients in the time window of the error-related negativity (ERN) [33] or error negativity [34], an early post-response negative deflection in the ERP associated with error processing that is thought to depend on an efference copy of the motor command for the response [33, 35]. A recent EEG study with patients with progressive cerebellar degeneration complemented these findings [**36]. ERN amplitudes were reduced in patients, and error rates were increased. Voxel-based morphometry (VBM) analysis showed that performance monitoring abnormalities in patients were primarily associated with gray matter volume loss in posterolateral regions of the cerebellar hemispheres. This is consistent with engagement of lateral and inferior cerebellum during response conflict and error processing in a change-signal task [37], and also with a cerebellar functional topography that posits involvement of posterolateral cerebellar regions in complex motor and cognitive functions [38]. Interestingly, a functional magnetic resonance imaging (fMRI) study with Granger Causality Mapping established a causal relationship between cerebellar and ventrolateral prefrontal cortex (vlPFC) activations associated with errors and with post-error slowing in a stop signal task [39]. Results indicated that cerebellar activation directly mediated error and post-error processing in vlPFC via projections to thalamus and supplementary motor area (SMA).
Cerebellar contributions to feedback processing
In the absence of internal error signals or in complex environments adaptive behavior critically depends on external feedback. In general, the neural systems underlying the processing of feedback and of performance errors largely overlap (for a review, see [14]). Analogous to the response-locked ERN, processing of feedback has been linked to a negative deflection in the ERP that occurs time-locked to feedback presentation, the feedback-related negativity (FRN) [e.g. 40, 41]. Rustemeier et al. [*42,9] investigated if feedback processing was altered in patients with focal cerebellar lesions using a probabilistic feedback learning task with monetary rewards. Although learning performance was preserved in patients, neural responses were altered, possibly indicating impaired outcome prediction as indexed by an altered FRN. The general result pattern, that is, preserved behavior and altered ERPs, resembles findings for error processing [**32], and may suggest post-acute functional reorganization and compensation in cerebellar lesion patients that presumably is hampered by disease progression in patients with progressive cerebellar degenerative disease [**36]. Alternatively, it has been proposed that cerebellar damage impairs error-based learning while leaving reinforcement mechanisms themselves intact [**43]. In this study, patients with cerebellar degeneration and controls learned a new reaching movement with error-based versus binary reinforcement feedback. Patients varied in reinforcement learning but showed intact retention rates. In contrast, there was a complete lack of error-based learning in patients. Mechanistic modelling of reinforcement learning revealed that learning success depended on a balance of exploration variability and motor noise, the latter being increased in patients, thus reducing the efficacy of reinforcement learning.
Outside the motor domain, evidence for cerebellar involvement in feedback learning has been provided in a study on probabilistic classification learning [**44]. Even though differential coding of positive and negative feedback was not found in the cerebellum in this study, activation in lateral regions (Crus I / lobule VII) increased with higher predictive values of stimulus combinations. It seems conceivable that cerebellar activation during learning of stimuli with high predictive value may reflect formation or updating of an internal model [**44]. In accordance with this notion, cerebellar activations are often reported in studies on probabilistic reversal learning [15, 16]. Reversal tasks present volatile environments which require constant adaptation of response strategies to changing response-reward contingencies. Von der Gablentz et al. [*45] reported lateral posterior cerebellar activations in concert with prefrontal activations for processing of error feedback versus switch feedback in a reversal task. Cerebellar contributions to acquisition and reversal of stimulus-reward representations are further supported by recent findings linking gray matter volume loss in cocaine-dependent subjects in partially overlapping posterolateral cerebellar clusters to both severity of cocaine use (lobule VIII) and to greater reversal costs (lobule VIIb/VIII), that is, larger differences in error rates between trial blocks with original and switched reward contingencies [*46]. While it is difficult to tease apart these two factors, partial rather than full overlap between cocaine use associated regions and reversal learning associated regions appears to suggest that reversal learning deficits are at least partially independent of neurotoxic effects of cocaine [*46].
Cerebellar activations have also been found during performance of different variants of the Wisconsin Card Sorting Test (WCST) [47], one of the most common tests to assess executive function. In this test, subjects have to match sample cards displaying colored shapes to different reference cards according to specific sorting criteria. Cerebellar activations were particularly pronounced in a task variant in which subjects were not informed about sorting dimensions but instead had to deduce sorting rules from post-response feedback [47]. In line with this, patients who had undergone surgery for removal of tumors affecting the cerebellar hemispheres have been shown to exhibit deficits in set-shifting on the WCST [48]. These findings further emphasize the role of the cerebellum for set-shifting which is critically needed for adaptive behavior.
The studies discussed above all have in common that feedback learning serves the purpose of selecting a motor output or a task response that will most likely be rewarded, thus requiring sensorimotor integration. Interestingly, lateral regions of the cerebellar hemispheres (Crus I and II) have recently also been implicated in feedback learning in a purely cognitive setting. Increased activation in these cerebellar regions was shown in the context of learning of higher order rules which specified the application of second-order rules and thus did not require integration of sensory information with motor effectors [**49].
Cerebellar contributions to response inhibition
Previous work has also explored the role of the cerebellum for response inhibition. An early study with positron emission tomography (PET) reported activation of the cerebellum in a well-controlled Stroop interference task [50]. Classic Stroop tasks require naming of the color of letters spelling a color word (e.g. “blue”) that, when the color word is semantically incongruent with the font color, involves an effortful, conscious response selection process that counteracts an automatic response tendency to read the word. Cerebellar involvement in Stroop performance was supported by findings of impaired color naming with and without interference in patients with recent cerebellar lesions [51]. Interestingly, impaired color naming with interference was also evident at a follow up one year after the lesion event [51].
Brunamonti et al. [*52] tested patients with focal cerebellar lesions with a countermanding task with go-trials requiring responses and stop-trials required withholding of responses. Patients showed increased reaction times on go-trials, especially following errors, and experienced deficits in responding to the stop signal, indicating difficulty in triggering the stop process. Impairment in patients was more severe if the deep cerebellar nuclei were affected. The authors speculated that these results may indicate cerebellar regulation of voluntary actions based on cerebellar influence on the cortico-striatal loop. Evidence to support this claim was recently provided by Picazio, Ponzo and Koch [53]. They showed modulation of functional connectivity between inferior frontal gyrus/left primary motor area M1 and right pre-SMA during response inhibition following repetitive TMS over the right cerebellar hemisphere. Hirose et al. [*54] reported learning-dependent changes in cerebro-cerebellar interactions during response inhibition as revealed by psycho-physical interaction (PPI) analysis. Performance improvement across two sessions of a go/nogo task was accompanied by decreased interaction from the right inferior frontal cortex to the cerebellar lobules VI or VII, while interaction from these cerebellar regions to the primary motor was increased. These findings appear to suggest that practice effects are supported by changes in the interplay between posterolateral cerebellum and frontal cortex.
Cerebellar monitoring functions in working memory
Monitoring functions have been ascribed to the cerebellum also outside the classic realm of learning and adaptive behavior. Working memory is one of the cognitive domains most readily associated with cerebellar contributions. Several studies have reported working memory impairment in patients with cerebellar damage [e.g. 55–59]. A growing number of neuroimaging studies have shown robust, load-dependent activations in the cerebellum for working memory [e.g. 60, 61]. Verbal working memory has been linked to discrete activations in superior/lateral (lobule VI, Crus I) and posterior/inferior regions (lobule VIIB/VIIIA) [61–63]. Bilateral superior cerebellar regions are thought to set up a memory trace and maintain it in an articulatory rehearsal loop, while the right inferior cerebellum serves as monitoring region that underlies error-correction by comparing the articulatory trace with representations in a short-term store [61–63, 65]. Along these lines, impaired immediate recall of non-words and impaired rhyme judgments in cerebellar lesion patients have been ascribed to deficient cerebellar-mediated articulatory monitoring [66]. Rhyme judgement and recall of non-words may rely on inner speech to provide input into an articulatory monitoring system that can detect errors in pronunciation. If this monitoring process is deficient, rhyme judgement for words with mismatching orthography and phonology (e.g. consider the rhyme judgement for “fear” vs. “bear”) should be particularly impaired. Consistent with a role for the cerebellum in articulatory monitoring, this result pattern was observed in cerebellar lesion patients [66].
Conclusions
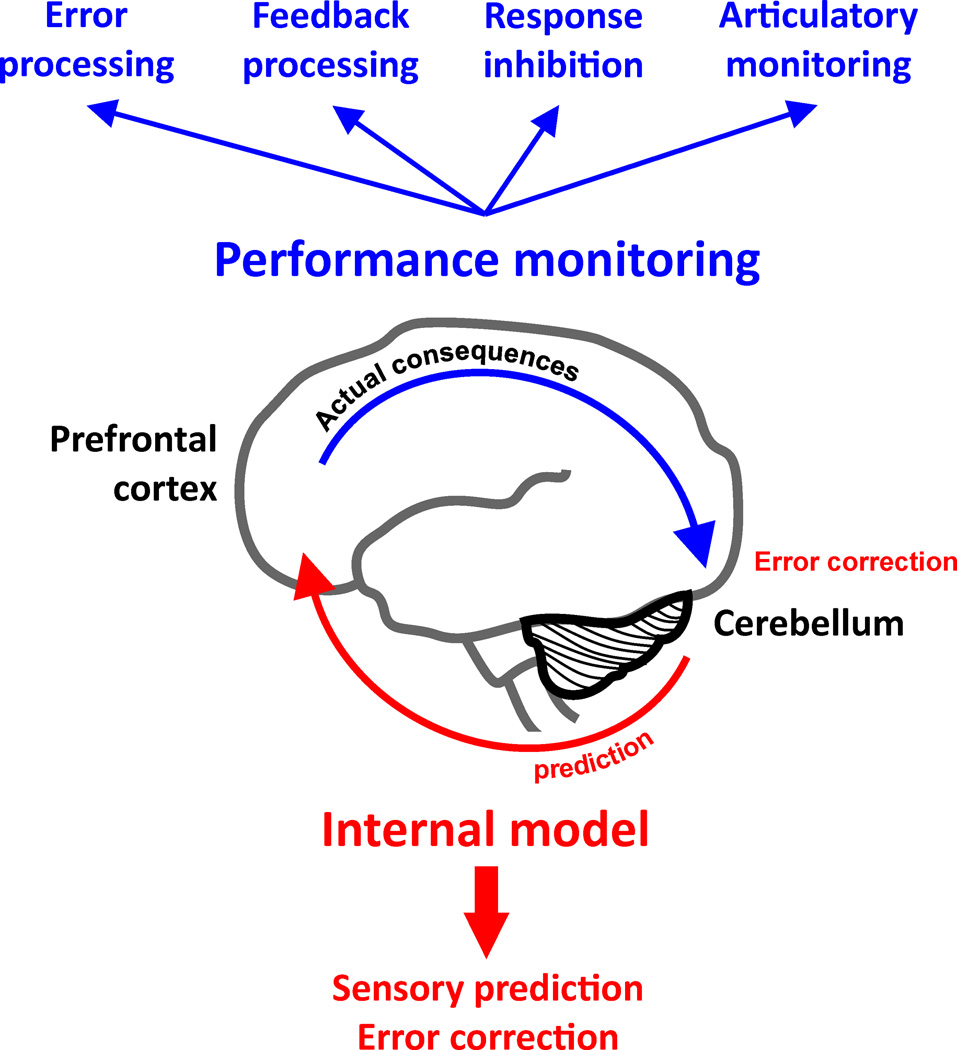
There is a wealth of evidence for cerebellar involvement in performance monitoring, specifically when a motor output is required, but also in cognitive tasks that do not require integration of sensory information with motor effectors. As illustrated in Figure 1, the cerebellum provides a feedforward sensory prediction to prefrontal areas which can then be compared to the actual consequences of an action. The discrepancy between expected and actual consequences creates an error signal that modifies subsequent predictions, thus allowing the cerebellum to achieve performance monitoring functions. We have reviewed evidence for cerebellar contributions to various aspects of performance monitoring such as sensory prediction, error and feedback processing, and response inhibition. Affective aspects of performance monitoring to date remain sparsely investigated, but there is some evidence for a role of the cerebellum in affective processing concerning the subjective perception of regret in a reward/punishment setting [67]. Moreover, lateral posterior cerebellar activations have also been observed in a study on theory of mind and empathy, although these activations were not further discussed [68]. It is conceivable that processes of mentalizing, that is, inferring one’s own and another person’s mental states, and here also self-other discrimination, are linked to affective aspects of performance monitoring. Another line of research posits a crucial role of the cerebellum in articulatory monitoring. Taken together, these findings highlight monitoring as the overarching function of the cerebellum. For systems neuroscience, understanding cerebro-cerebellar interactions is an important step in elucidating the complex networks engaged in complex motor and cognitive functions and in predicting and possibly mitigating effects of cerebellar damage on behavior.
Figure 1.
The cerebellum provides a feedforward sensory prediction to prefrontal areas. A comparison of this prediction with the actual consequences of an action, that is, a discrepancy between expected and actual consequences, creates an error signal that modifies subsequent predictions, thus allowing the cerebellum to achieve performance monitoring functions. In this manner, the cerebellum contributes to higher cognitive functions such as conflict processing, response inhibition, feedback processing, and articulatory monitoring during working memory.
Highlights.
-
-
Cerebellum involved in various aspects of performance monitoring
-
-
Contributions to sensory prediction, error and response conflict processing
-
-
Contributions to response inhibition, feedback learning, articulatory monitoring
-
-
Cerebellum at the interface of motor behavior and cognition
-
-
Monitoring as overarching cerebellar function
Acknowledgments
This work was supported by NIH RO1 MH104588 and NIAAA RO1 AA018694 to J.E.D. and DFG Pe2077/3-1 to J.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100(4):443–454. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- 2.Grafman J, Litvan I, Massaquoi S, Stewart M, Sirigu A, Hallett M. Cognitive planning deficit in patients with cerebellar atrophy. Neurology. 1992;42:1493–1496. doi: 10.1212/wnl.42.8.1493. [DOI] [PubMed] [Google Scholar]
- 3.Schmahmann JD, Sherman JC. Cerebellar cognitive affective syndrome. Int Rev Neurobiol. 1997;41:433–440. doi: 10.1016/s0074-7742(08)60363-3. [DOI] [PubMed] [Google Scholar]
- 4.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 5.Koziol LF, Budding D, Andreasen N, D'Arrigo S, Bulgheroni S, Imamizu H, Ito M, Manto M, Marvel C, Parker K, Pezzulo G, Ramnani N, Riva D, Schmahmann J, Vandervert L, Yamazaki T. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13(1):151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 7.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 8.Schmahmann JD. Dysmetria of thought: Correlations and conundrums in the relationship between the cerebellum, learning, and cognitive processing. Behav Brain Sci. 1996;19:472–473. 503–527. [Google Scholar]
- 9.Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 11.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cognit Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 12.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 13.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 14.Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- 15.Greening SG, Finger EC, Mitchell DGV. Parsing decision making processes in prefrontal cortex: response inhibition, overcoming learned avoidance, and reversal learning. Neuroimage. 2015;54:1432–1441. doi: 10.1016/j.neuroimage.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Remijnse PL, Nielen MMA, Uylings HBM, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26:609–618. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 18.Parsons LM, Bower JM, Gao JH, Xiong J, Li J, Fox RT. Lateral cerebellar hemispheres actively support sensory acquisition and discrimination rather than motor control. Learn Mem. 1997;4:49–62. doi: 10.1101/lm.4.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Blakemore SJ, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–245. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- 20.Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. 1998;1:635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- 21.Blakemore S-J, Frith CD, Wolpert DW. Spatiotemporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci. 1999;11:551–555. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- 22.Knolle F, Schröger E, Baess P, Kotz SA. The cerebellum generates motor-to-auditory predictions: ERP lesion evidence. J Cogn Neurosci. 2012;24(3):698–706. doi: 10.1162/jocn_a_00167. [DOI] [PubMed] [Google Scholar]
- 23.Knolle F, Schröger E, Kotz SA. Cerebellar contribution to the prediction of self-initiated sounds. Cortex. 2013;49(9):2449–2461. doi: 10.1016/j.cortex.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Schlerf J, Ivry RB, Diedrichsen J. Encoding of sensory prediction errors in the human cerebellum. J Neurosci. 2012;32(14):4913–4922. doi: 10.1523/JNEUROSCI.4504-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avanzino L, Bove M, Pelosin E, Ogliastro C, Lagravinese G, Martino D. The Cerebellum Predicts the Temporal Consequences of Observed Motor Acts. PLoS ONE. 2015;10(2):e0116607. doi: 10.1371/journal.pone.0116607. In this study on prediction of movement timing, subjects watched videos of biological (right hand writing) or inanimate object movements (ball reaching a target). The videos were interrupted by a dark screen while movements were underway, and subjects were asked to indicate by button press when the movement reached its end during this dark period. Subjects who had received repetitive inhibitory 1 Hz transcranial magnetic stimulation (TMS) over the lateral cerebellum before completing the task showed increased timing errors for biological motion but not for inanimate object motion as compared to subjects who had received sham TMS. The findings indicate that the cerebellum is directly involved in predicting the consequences of observed motor acts.
- 26.Ivry RB, Keele SW. Timing functions of the cerebellum. J Cog Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 27. Kotz SA, Stockert A, Schwartze M. Cerebellum, temporal predictability and the updating of a mental model. Philos Trans R Soc Lond B Biol Sci. 2014;369(1658):20130403. doi: 10.1098/rstb.2013.0403. In this study, auditory deviance processing was investigated in a sample of cerebellar lesion patients and healthy controls with electroencephalography (EEG). While attentive processing of deviant tones in sequences as indexed by the N2b component in the event-related potential (ERP) did not differ between groups, controls showed and enhanced P3b component to deviant tones in regular sequences. This component reflects updating of internal models. The results thus show impaired processing of temporal predictability likely due to deficient updating of internal models in cerebellar lesion patients.
- 28.Schwartze M, Rothermich K, Schmidt-Kassow M, Kotz SA. Temporal regularity effects on preattentive and attentive processing of deviance. Biol Psychol. 2011;87:146–151. doi: 10.1016/j.biopsycho.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterburs J, Koch B, Schwarz M, Hoffmann KP, Daum I, Bellebaum C. Cortical processing of saccade-related efference copy signals in patients with cerebellar lesion. Eur J Neurosci. 2013;37(5):804–815. doi: 10.1111/ejn.12081. This EEG and eyetracking study investigated saccade-related efference copy processing on the saccadic double-step task in cerebellar lesion patients and healthy controls. Despite intact behavioral performance, a positive ERP component between 150 and 450 ms after first saccade onset which has been interpreted in terms of the integration of efference copy signals with motor intentions for a subsequent saccade was markedly reduced in the patients when updating of visual space across eye movements was necessary. These findings suggest that the cerebellum contributes to on-line saccade monitoring, and that cerebellar lesions alter saccade-related efference copy processing.
- 31. Peterburs J, Koch B, Schwarz M, Hoffmann KP, Daum I, Bellebaum C. Updating of visual space across horizontal saccades in cerebellar and thalamic lesion patients. Cerebellum. 2013;12(1):1–15. doi: 10.1007/s12311-012-0386-2. This EEG and eye tracking study investigated saccade-related efference copy processing in cerebellar and thalamic lesions patients and controls in a perceptual context. The task was a perceptual localization task in which the location of a target had to be updated across a single horizontal saccade. Localisation precision did not differ between groups, but and updating-related positivity in the ERP occurring from about 300 to 500 ms after saccade onset in the updating condition reduced only in cerebellar lesion patients. This finding suggests that cerebellar damage altered the neural processes underlying saccadic updating in a perceptual context without causing overt behavioral deficits.
- 32. Peterburs J, Gajda K, Koch B, Schwarz M, Hoffmann KP, Daum I, Bellebaum C. Cerebellar lesions alter performance monitoring on the antisaccade task--an event-related potentials study. Neuropsychologia. 2012;50(3):379–389. doi: 10.1016/j.neuropsychologia.2011.12.009. This EEG and eye tracking study used an antisaccade task to investigate processing of correct and erroneous saccades in cerebellar lesion patients and controls. While error rates were comparable between groups, saccadic latencies were enhanced in patients. Patients also showed and altered pattern of the error and correct related negativity (ERN and CRN, respectively), two ERP components linked to efference copy processing during performance monitoring. Patients showed a smaller ERN and larger CRN. Moreover, the error positivity (Pe), an ERP component associated with error awareness, was increased in patients. These results suggest that the cerebellum is critically involved in fast classification of saccadic accuracy. Largely intact performance accuracy together with increased saccadic latencies and the altered Pe pattern may indicate a compensatory mechanism presumably related to slower, more conscious aspects of error processing in the patients.
- 33.Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 34.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 35.Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 36. Peterburs J, Thürling M, Rustemeier M, Göricke S, Suchan B, Timmann D, Bellebaum C. A cerebellar role in performance monitoring - evidence from EEG and voxel-based morphometry in patients with cerebellar degenerative disease. Neuropsychologia. 2015;68:139–147. doi: 10.1016/j.neuropsychologia.2015.01.017. This study investigated processing of correct and erroneous saccades in patients with pure cerebellar degeneration and controls by means of an EEG-based antisaccade task and voxel-based morphometry (VBM). Error rates were increased, and the ERN was reduced while the Pe was preserved in patients. Results show that performance monitoring is altered in patients with cerebellar degeneration. In contrast to patients with focal lesions, post-acute functional reorganization and compensation presumably is hampered by disease progression, resulting in altered neural processing and impaired behavioural performance. VBM indicated correlations between gray matter volume reduction in bilateral posterolateral regions (left Crus II and right lobule VI) and increased error rates. Moreover, somewhat smaller correlations were found for volume loss in left lobule VIIb/VIIIa and right lobule V and ERN amplitude, and in right Crus I and Pe amplitude.
- 37.Becerril K, Barch DM. Conflict and error processing in an extended cingulo-opercular and cerebellar network in schizophrenia. Neuroimage Clin. 2013;3:470–480. doi: 10.1016/j.nicl.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 39.Ide JS, Li CR. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage. 2011;54:455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miltner WH, Braun CH, Coles MG. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwenhuis S, Holroyd CB, Mol N, Coles MG. Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neurosci Biobehav Rev. 2004;28:441–448. doi: 10.1016/j.neubiorev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 42. Rustemeier M, Koch B, Schwarz M, Bellebaum B. Processing of Positive and Negative Feedback in Patients with Cerebellar Lesions. Cerebellum. 2015 doi: 10.1007/s12311-015-0702-8. epub ahead of print. This EEG study investigated processing of performance feedback on a probabilistic learning task in cerebellar lesion patients. While patients showed intact learning performance, ERP difference wave amplitudes (rewards-losses) were increased in a time window between 250 and 450 ms after feedback presentation, possibly indicating impaired outcome prediction.
- 43. Therrien AS, Wolpert DM, Bastian AJ. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain. 2016;139:101–114. doi: 10.1093/brain/awv329. This study investigated learning of a new reaching movement with a robo-exoskeleton in cerebellar lesion patients and controls. A specific reinforcement schedule controlled for task difficulty produced learning in patients and controls, but patients varied in learning under reinforcement, with intact retention. In contrast, they showed a complete lack of error-based learning. Modelling of reinforcement learning indicated a balance between exploration variability and motor noise to be crucial for learning success. Along these lines, less efficient reinforcement learning in patients was attributed to greatly increased motor noise.
- 44. Lam JM, Wächter T, Globas C, Karnath HO, Luft AR. Predictive value and reward in implicit classification learning. Hum Brain Mapp. 2013;34(1):176–185. doi: 10.1002/hbm.21431. This fMRI study investigated probabilistic classification learning on the weather prediction task. Higher predictive value of card combinations during feedback presentation was associated with more activation in the lateral cerebellum, and may indicate the formation or updating of an internal model.
- 45. von der Gablentz J, Tempelmann C, Münte TF, Heldmann M. Neuroscience Performance monitoring and behavioral adaptation during task switching: an fMRI study. J Neurosci. 2015;285:227–235. doi: 10.1016/j.neuroscience.2014.11.024. This fMRI study investigated probabilistic reversal learning. Activation In cerebellar Crus II and prefrontal cortex was increased during processing of error feedback.
- 46. Moreno-López L, Perales JC, van Son D, Albein-Urios N, Soriano-Mas C, Martinez-Gonzalez JM, Wiers RW, Verdejo-García A. Cocaine use severity and cerebellar gray matter are associated with reversal learning deficits in cocaine-dependent individuals. Addict Biol. 2015;20(3):546–556. doi: 10.1111/adb.12143. This fMRI investigated probabilistic reversal learning in cocaine-dependent subjects and controls. VBM was applied VBM to determine links between structural changes and behavior in cocaine-dependent subjects. Severity of use correlated with gray matter volume reduction in left lobule VIII, and greater reversal costs were associated with volume loss in a partially overlapping cluster in VIIb and VIII.
- 47.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. NeuroImage. 2006;30:1038–1049. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 48.Karatekin C, Lazareff JA, Asarnow RF. Relevance of the cerebellar hemispheres for executive functions. Pediatr Neurol. 2000;22:106–112. doi: 10.1016/s0887-8994(99)00128-9. [DOI] [PubMed] [Google Scholar]
- 49. Balsters JH, Whelan CD, Robertson IH, Ramnani N. Cerebellum and cognition: evidence for the encoding of higher order rules. Cereb Cortex. 2013;23:1433–1443. doi: 10.1093/cercor/bhs127. This fMRI study reported that the prefrontal-projecting cerebellar lobules Crus I and II were commonly activated by processing of both rules that specify action and rules that merely specify application of other rules, thereby being devoid of integration of sensory information with motor effectors. These results show that the cerebellum can contribute to cognitive control independent of motor control.
- 50.Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- 51.Neau JP, Arroyo-Anllo E, Bonnaud V, Ingrand P, Gil R. Neuropsychological disturbances in cerebellar infarcts. Acta Neurol Scand. 2000;102:363–370. doi: 10.1034/j.1600-0404.2000.102006363.x. [DOI] [PubMed] [Google Scholar]
- 52. Brunamonti E, Chiricozzi FR, Clausi S, Olivito G, Giusti MA, Molinari M, Ferraina S, Leggio M. Cerebellar Damage Impairs Executive Control and Monitoring of Movement Generation. PLoS ONE. 2014;9(1):e85997. doi: 10.1371/journal.pone.0085997. This study investigated executive control in cerebellar lesion patients and controls with a countermanding task comprising go-trials requiring responses and stop-trials required withholding of responses. Patients showed increased reaction times on go-trials, especially following errors, and experienced deficits in responding to the stop signal, indicating difficulty in triggering the stop process. Impairment in patients was more severe if the deep cerebellar nuclei were affected.
- 53.Picazio S, Ponzo V, Koch G. Cerebellar Control on Prefrontal-Motor Connectivity During Movement Inhibition. Cerebellum. 2015 doi: 10.1007/s12311-015-0731-3. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54. Hirose S, Jimura K, Kunimatsu A, Abe O, Ohtomo K, Miyashita Y, Konishi S. Changes in cerebro-cerebellar interaction during response inhibition after performance improvement. Neuroimage. 2014;99:142–148. doi: 10.1016/j.neuroimage.2014.05.007. This fMRI study reported learning-dependent changes in cerebro-cerebellar interactions during response inhibition as revealed by psycho-physical interaction (PPI) analysis. Subjects completed a go/nogo task in two sessions. Performance improvement across these sessions was accompanied by decreased interaction from the right inferior frontal cortex to the cerebellar lobules VI or VII, while interaction from these cerebellar regions to the primary motor was increased. These findings may indicate that practice effects on a go/nogo task are supported by changes in the interplay between posterolateral cerebellum and frontal cortex.
- 55.Silveri MC, Di Betta AM, Filippini V, Leggio MG, Molinari M. Verbal short-termstore-rehearsal system and the cerebellum. Evidence from a patient with a right cerebellar lesion. Brain. 1998;121:2175–2187. doi: 10.1093/brain/121.11.2175. [DOI] [PubMed] [Google Scholar]
- 56.Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 57.Kirschen MP, Davis-Ratner MS, Milner MW, Chen SH, Schraedley-Desmond P, Fisher PG, Desmond JE. Verbal memory impairments in children after cerebellar tumor resection. Behav Neurol. 2008;20:39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterburs J, Bellebaum C, Koch B, Schwarz M, Daum I. Working memory and verbal fluency deficits following cerebellar lesions: relation to interindividual differences in patient variables. Cerebellum. 2010;9(3):375–383. doi: 10.1007/s12311-010-0171-z. [DOI] [PubMed] [Google Scholar]
- 59.Cooper FE, Grube M, Von Kriegstein K, Kumar S, English P, Kelly TP, Chinnery PF, Griffiths TD. Distinct critical cerebellar subregions for components of verbal working memory. Neuropsychologia. 2012;50(1):189–197. doi: 10.1016/j.neuropsychologia.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- 61.Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage. 2005;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 63.Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 64.Peterburs J, Cheng DT, Desmond JE. The Association Between Eye Movements and Cerebellar Activation in a Verbal Working Memory Task. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv187. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirschen MP, Chen SH, Schraedley-Desmond P, Desmond JE. Load- and practice-dependent increases in cerebrocerebellar activation in verbal working memory: an fMRI study. Neuroimage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Yehudah G, Fiez JA. Impact of cerebellar lesions on reading and phonological processing. Ann N Y Acad Sci. 2008;1145:260–274. doi: 10.1196/annals.1416.015. [DOI] [PubMed] [Google Scholar]
- 67.Clausi S, Coricelli G, Pisotta I, Pavone EF, Lauriola M, Molinari M, Leggio M. Cerebellar damage impairs the self-rating of regret feeling in a gambling task. Front Behav Neurosci. 2015;9:113. doi: 10.3389/fnbeh.2015.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Völlm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]