Abstract
Background
S. aureus is an opportunistic pathogen that colonizes the skin of patients with atopic dermatitis (AD) and aggravates their disease. Neutrophils and the cytokines IL-17A and IL-17F, which drive the expression of the neutrophil-attracting chemokines are important for the clearance of S. aureus infection. The cytokine IL-22 is often co-produced by IL-17 secreting cells. The levels of IL-22 are elevated in AD skin lesions.
Objective
To determine the role of IL-22 in the clearance of S. aureus infection of mouse skin subjected to tape stripping, a surrogate for scratching, a cardinal feature of AD.
Methods
S. aureus was applied to the tape-stripped skin of WT and Il22-/- mice. Bacterial burden was evaluated by enumerating colony-forming units. qPCR and ELISA were performed to quantify Il22 mRNA and IL-22 protein in mouse and human skin. Flow cytometry was used to enumerate neutrophils in the skin.
Results
Scratching the skin of healthy adults and tape stripping of mouse skin induced local expression of Il22 mRNA and IL-22 protein. Induction of Il22 expression by tape stripping was dependent on IL-23 and γδ T cells. Clearance of S. aureus from tape-stripped skin was significantly impaired in Il22-/- mice. Neutrophil infiltration and upregulation of expression of genes encoding the antimicrobial peptides (AMPs) antigen-6/urokinase-type plasminogen activator receptor related protein-1 (SLURP1) and β-DEFENSIN 14 and the chemokine CXCL3 following tape stripping were significantly impaired in Il22-/- mice.
Conclusions
These findings show that IL-22 is important for limiting the growth of S. aureus on mechanically injured skin. They caution that IL-23 and IL-22 blockade in AD patients may enhance susceptibility to staphylococcal skin infection.
Keywords: IL-22, S. aureus, Neutrophils, AD
INTRODUCTION
Atopic Dermatitis (AD) is a chronic pruritic skin inflammatory disease that affects 15-20% of infants worldwide, and its incidence is still on the rise1. Microbial dysbiosis is a common feature of AD. In particular, colonization and infection of Staphylococcus aureus (S. aureus) is prevalent in the skin of AD patients1,2. Even in the absence of lesions, the density of S. aureus in AD skin can reach up to 106 colony-forming units (CFUs) per square centimeter of skin3. The increased susceptibility for S. aureus colonization and infection in AD skin is partly due to intense scratching, a characteristic feature of AD, which releases extracellular molecules such as collagens, fibronectins and elastin, that enhance the adhesiveness of S. aureus to the skin4.
S. aureus infection aggravates skin inflammation in AD. This is evidenced by the finding that cutaneous application of S. aureus products that include staphylococcal enterotoxin B (SEB), lipoteichoic acid and δ-toxin promotes local allergic inflammation and a systemic Th2 response in mice5, 6, 7, 8, 9, 10. Furthermore, co-application of SEB with ovalbumin (OVA) to tape stripped mouse skin potentiates the Th2 response to OVA9. In addition, application of S. aureus, its subcellular fractions, or its diacylated lipopeptides, to the skin upregulates the expression of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that plays a key role in the pathogenesis of AD11. Importantly, S. aureus skin infection in AD is associated with increased disease severity and an increased degree of allergic sensitization12. Moreover, double blind placebo-controlled studies have demonstrated that treatment with anti-staphylococcal antibiotics and bleach baths is beneficial in AD13. These observations stress the importance of understanding the mechanisms that lead to increased infection of S. aureus in the skin of AD patients.
The establishment of S. aureus infection involves its logarithmic growth during early stages of infection, which is stalled by the rapid mobilization of neutrophils14. Neutrophils block the growth of S. aureus via their phagocytic activity and release of reactive oxygen species14. The IL-17 cytokines IL-17A and IL-17F have been shown to be important for the recruitment of neutrophils and for defense against S. aureus15. These two cytokines are not released in isolation; often, another cytokine IL-22 is co-secreted by IL-17 producing cells16. IL-22 is a member of the IL-10 family of cytokines. It signals through the IL-22 receptor (IL-22R), which is highly expressed on epithelial cells, including keratinocytes, suggesting an important role for IL-22 signaling in promoting a healthy skin barrier17. Indeed, IL-22 induces the secretion of anti-microbial peptides by keratinocytes in vitro18, and is necessary for the expression of neutrophil attracting chemokines in a mouse model of psoriasis driven by topical application of the TLR7/8 agonist imiquimod19.
AD skin lesions exhibit increased IL22 expression and infiltration by IL-22 expressing T cells20. The role of IL-22 in controlling S. aureus infection in the skin is not well understood. We demonstrate that scratching human skin or tape stripping mouse skin, a surrogate for scratching, induces local IL-22 expression, and that IL-22 is important for limiting the growth of S. aureus on mouse skin mechanically injured by tape stripping. These findings have important implications for AD; they caution that IL-22 blockade in AD patients may enhance susceptibility to staphylococcal skin infection.
MATERIALS AND METHODS
Mice
C57BL/6J WT and Rorc-/- mice were purchased from the Jackson Laboratory (Bar Harbor, Me). Rag2-/- mice were purchased from Taconic. BALB/c WT mice were purchased from Charles River Laboratory. Il22-/- mice were previously described 21. Tcrd-/- mice were provided by Dr. Joonsoo Kang (University of Massachusetts Medical School, Worcester) and also purchased from Jackson labs. Il23- /- (p19-/-) mice were a gift of Dr. Georges Tsokos (Beth Israel Deaconess Medical Center). All mice were kept in a pathogen-free environment. All procedures performed on the mice were in accordance with the Animal Care and Use Committee of the Children’s Hospital Boston.
Skin cell preparation and flow cytometry
1cm2 skin pieces from unmanipulated or tape stripped mice, were obtained. Skin pieces were finely chopped using scissors after fat removal and digested for 90 minutes in the media containing Liberase (0.2mg/mlRoche) and DNAse II (Sigma), with continuous shaking at 37° C. Digested skin homogenates were filtered, washed and resuspended in PBS and used for flow cytometry. Cells were preincubated with FcγR-specific blocking mAb (2.4G2) and washed before staining with the following monoclonal antibodies (mAbs): APC-anti-CD45, BV605-anti-CD11b (Biolegend), FITC-anti-Gr1 (Biolegend), Percpcy5.5 γδ TCR (ebioscience), CD3 efluor-450 (ebioscience). Cells were analyzed on LSR Fortessa (BD Biosciences), and the data were analyzed with FlowJo software.
IL-22 detection
For IL-22 detection by flow-cytometry, isolated skin cells were incubated with PMA (10ng/ml) and Ionomycin (500ng/ml) and IL-23 (50ng/ml) with Golgi-stop and Golgi-plug for 5 hours in Iscove’s Modified DMEM media. Intracellular staining was performed using BD cytokine detection kit. APC-anti-IL-17A (ebioscience) and PE-anti-IL-22 (Biolegend) were added to permeabilized cells after surface staining with antibodies against CD3, CD11b, CD19, CD4 and γδ TCR.
For quantitative PCR, skin cells were isolated from tape-stripped skin using Liberase and DNAse as described earlier. Single-cell suspensions were stained with antibodies against CD45, CD3, CD49b, FcεRIα, CD90, CD117 and TCR δ. Antibodies against CD11b, CD11c, Class II, F4-80 and Gr1 were added in a single color to define the Lineage gate. Mast cells were identified as CD45+CD3-Lin-CD117+FcεRIα+ population; ILCs were identified as: CD45+CD3-Lin-CD49b-FcεRIα-CD90+ cells and γδ T cells were identified as: CD45+CD3+TCRδ+ cells. All populations were sorted on FACS Aria II with purity of >95%). Total RNA was extracted from sorted cells using RNeasy Micro Kit (Qiagen). cDNA preparation and quantitative PCR details are described below.
Tape stripping of mouse skin and quantitative RT-PCR
Ears or shaved back skin of anesthetized mice were tape stripped 6 times. At indicated times after tape stripping, total skin RNA was extracted with Total RNA Isolation Kit (Ambion). cDNA was prepared with iscript cDNA synthesis kit (Biorad). PCR reactions were run on ABI Prism 7300 (Applied Biosystems) sequence detection system platform. Taqman primers and probes were obtained from Life technologies. The housekeeping gene β2-microglobulin was used as an internal control. Relative mRNA expression was quantified using the 2-ΔΔCt method.
Skin explant culture and in vitro cytokine expression
Tape stripped skin explants (1 cm2) were chopped with scissors and cultured in complete RPMI. The supernatants were harvested after 18 hrs and IL-22 was measured by an ELISA kit according to the manufacturer’s instructions (sensitivity: 8 pg/ml, eBioscience).
Quantification of IL-22 levels in scratched human skin
After obtaining informed consent, the inner side of the forearm of two healthy non-allergic adult male subjects aged 35 and 69 was scratched 30 times with a #11 sterile blade, with care to not draw any blood. 6 hrs later a 4 mm punch biopsy was obtained from the scratched site and another one from an unscratched skin site on the contralateral forearm. The skin sample was chopped with scissors and cultured in complete RPMI medium for 18 hrs and IL-22 protein released in the supernatants was measured by ELISA (eBioscience).
S. aureus infection and quantification of skin infection
The community-acquired methicillin-resistant S. aureus (MRSA) USA300 SF8300 strain, a kind gift of Dr. Binh Diep (UCSF) was cultured in tryptic soy broth as previously described 15. Briefly, a S. aureus inoculum was streaked onto tryptic soy agar plate and grown overnight at 37°C. Single colonies were picked and inoculated into a 5 ml tube containing tryptic soy broth and cultured overnight in a shaking incubator. The following morning, 1:50 dilution of bacterial suspension was inoculated in 5 ml of tryptic soy broth and cultured for another 2 hrs. Bacterial concentrations were estimated by measuring absorbance at 600 nm. The bacteria were concentrated to 108 CFU/50 μl of PBS, and used for cutaneous infection. CFUs were verified by overnight culturing of inoculum on Chrom-agar plates. To enumerate the bacterial load from the skin, two 8 mm2 skin biopsies were obtained. After mechanical homogenization, serial dilutions of skin homogenates were cultured on Chrom-agar plates. The growth of USA300 strain was quantified by counting only pink colonies after overnight incubation.
Neutrophil depletion
Neutrophils were depleted 24 hrs before tape stripping by intra-peritoneal injection of anti-Ly6G antibody (1A8, BioXcell). The depletion was confirmed by FACS analysis for neutrophils in the blood and skin of tape-stripped mice.
Histological Analysis
H&E staining was performed using the formalin fixed skin biopsies by the Histology core of Children’s Hospital. ImageJ was used for the quantification of the epidermal thickness.
Statistical analysis
Depending on the number of sample groups and sample size statistical significance was determined by Mann-whitney’s test, Student’s t test or one-way anova analysis on graph-pad prism. A p value <0.05 was considered statistically significant.
RESULTS
Mechanical injury induces IL-22 expression in the skin
We examined Il22 mRNA expression in mouse skin mechanically injured by tape stripping. Il22 mRNA was not detectable in unmanipulated mouse skin, but became readily detectable in the skin 6 hrs after tape stripping, and was no longer detectable 24 hrs later (Fig.1A). More importantly, IL-22 was released into the supernatants by explants of tape stripped skin, but not unmanipulated control skin (Fig.1B).
Figure 1. Mechanical injury induces IL-22 expression in the skin.
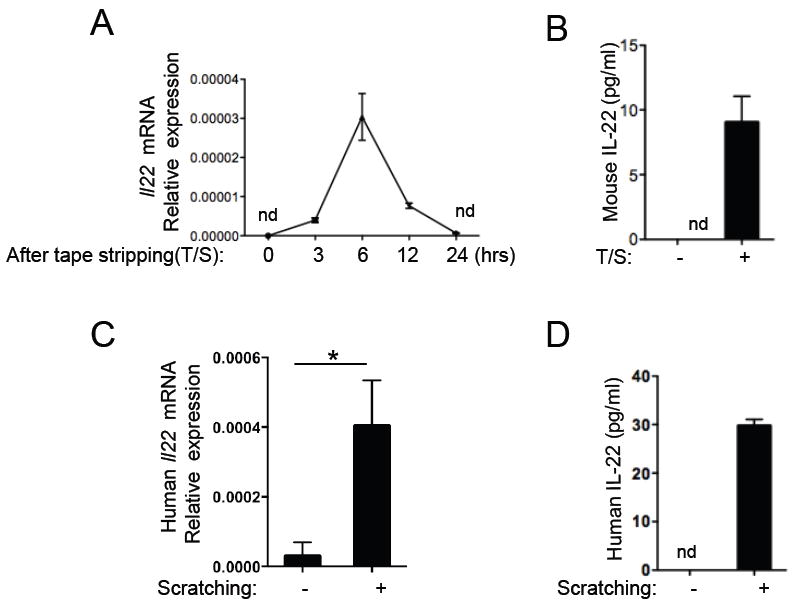
A. Il22 mRNA expression levels in the skin from WT (Balb/c) mice at 0, 3, 6, 12 and 24 hrs after tape-stripping, (n=3/group). The expression can be detected at 3 hrs, peaks at 6 hours and is undetectable (nd) at 24 hrs. B. IL-22 protein levels in the supernatants were quantitated after culturing the tape-stripped skin for 18hrs. C. Il22 mRNA expression in scratched versus unscratched human skin biopsies. One representative experiment out of two, with a total of 3 samples is shown. D. IL-22 protein levels quantitated by ELISA in supernatants from cultured scratched and control skin. Mean with SEM is shown *: p<0.05.
We also investigated whether mechanical injury inflicted by scratching causes IL-22 expression in human skin. Il22 mRNA expression was barely detectable in unmanipulated human skin, but was readily detected 6 hrs post-scratching (Fig.1C). IL-22 was released into the supernatants by explants of scratched human skin, but not control unmanipulated skin (Fig.1D). These results demonstrate that mechanical skin injury induces IL-22 expression in both mouse and human skin.
γδ T cells are the major IL-22 producing cells in response to mechanical skin injury
IL-22 is produced by adaptive αβ Th17/22 cells as well as by innate lymphocytes such as γδ T cells and ILC3s16. To determine the source of IL-22 released following tape stripping, we examined Rag2-/- mice, which lack all mature T and B cells. Il22 mRNA induction in the skin by tape stripping was virtually absent in Rag2-/- mice (Fig.2A), demonstrating an absolute requirement for mature T cells. Il22 mRNA induction by tape-stripping the skin was strikingly reduced in Tcrd-/- mice, indicating that γδ T cells contribute to the IL-22 induced by tape stripping (Fig.2B). We further determined if γδ T cells are the primary producers of IL-22 upon tape stripping in murine skin. Flow-cytometric analysis of skin cells after stimulation with PMA and Ionomycin and IL-23 for 5 hours demonstrated that lineage positive, but not lineage negative cells, stained intracellularly for IL-22 (Fig.S1A). Importantly, intracellular IL-22 expression was restricted to CD3+ γδ T cells, (Fig.S1A). Detection of intracellular IL-22 in the lineage positive cells from tape stripped skin required in vitro stimulation with IL-23 along PMA and Ionomycin. We therefore also sorted γδ T cells; ILCs and mast cells from tape stripped skin and examined their expression of Il22 mRNA without in vitro stimulation. Real time PCR analysis of RNA prepared from these cells confirmed the finding that γδ T cells were the major source of Il22 (Fig.S1B). ILCs, which represent a much smaller proportion of skin immune cells, compared to γδ T cells (50 fold lower number) demonstrated only weak expression of Il22 mRNA, whereas mast cells had no detectable Il22 mRNA.
Figure 2. γδ T cells are the major IL-22 producing cells in response to mechanical skin injury.
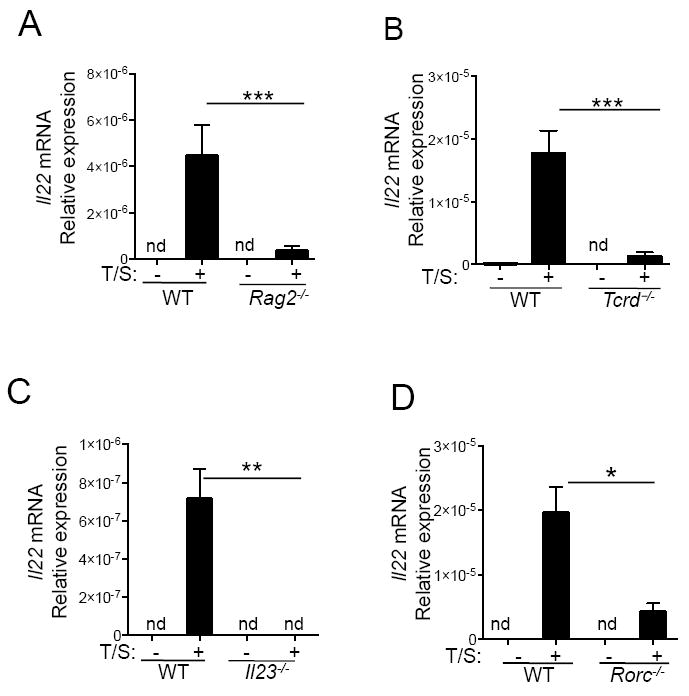
A. Il22 mRNA expression levels in the skin from unmanipulated WT (Balb/c), tape-stripped WT (Balb/c) mice, unmanipulated Rag2-/- and tape-stripped Rag2-/- mice (n=7/group). Il22 levels remain undetectable (nd) in unmanipulated skin. B. Il22 mRNA expression levels in the skin from unmanipulated WT (C57BL/6), tape-stripped WT mice, unmanipulated Tcrd-/- and tape-stripped Tcrd-/- mice (n=6, per group). C. Il22 mRNA expression levels quantitated in unmanipulated WT (C57BL/6), tape-stripped WT mice, unmanipulated Il23 (p19)-/- and tape-stripped Il23 (p19)-/- mice (n=3/ group). D. Il22 mRNA expression levels in unmanipulated WT (C57BL/6), tape-stripped WT mice, unmanipulated Rorc-/- and tape-stripped Rorc-/- mice (n=3/ group). Mean with SEM is shown *: p<0.05, **: p<0.01, ***: p<0.001
γδ T cells express Il22 rapidly following stimulation with IL-23; this is dependent on the transcription factor RORγt, which is activated following IL-23 stimulation22, 23. Induction of Il22 mRNA expression following tape stripping was markedly decreased in Il23-/- and Rorc-/- mice respectively (Fig.2C, 2D). These results suggest that IL-23 induces IL-22 expression in γδ T cells by activating the transcription factor RORγt.
IL-22 limits cutaneous S. aureus infection
To test the potential role of IL-22 in host defense against S. aureus infection at sites of mechanical skin injury, we applied S. aureus (USA300 SF8300 strain) to the skin of WT and Il22-/- mice 24 hrs after tape stripping (Fig.3A). At the time of S. aureus application, Il22 mRNA expression was no longer detected in tape stripped skin; and S. aureus application to tape stripped skin did not upregulate Il22 mRNA expression (Fig.3B). We monitored skin lesions daily and quantitated bacterial growth 5 days after infection. On Day 5, no skin lesions were detected at the sites of S. aureus application in any of the WT mice (n=9). In contrast, erythematous skin lesions were detected at the sites of S. aureus application in 7 of 9 Il22-/- mice (Fig.3C). H&E staining of skin biopsies showed that S. aureus infection of tape stripped skin caused epidermal thickening in WT mice. Epidermal thickening in S. aureus infected tape stripped skin was modestly reduced in the Il22-/- mice (Figure S2A and S2B), 3 days after S. aureus infection. Reduced epidermal thickening may have contributed to the erythematous appearance of the skin lesions in Il22-/- mice. To ascertain whether the skin lesions observed in the absence of IL-22 were associated with overgrowth of S. aureus, we cultured skin homogenates on chrome agar, a selective medium that permits the growth of S. aureus strain USA300 colonies that can be identified by their pink coloration. Only sparse S. aureus colonies were recovered from WT mice. In contrast, significantly higher numbers of bacterial colonies were recovered from Il22-/- mice (Fig.3D, Left and Right). These results suggest that IL-22 induction is important for efficient clearance of S. aureus by mechanically injured skin.
Figure 3. IL-22 limits cutaneous S. aureus infection.
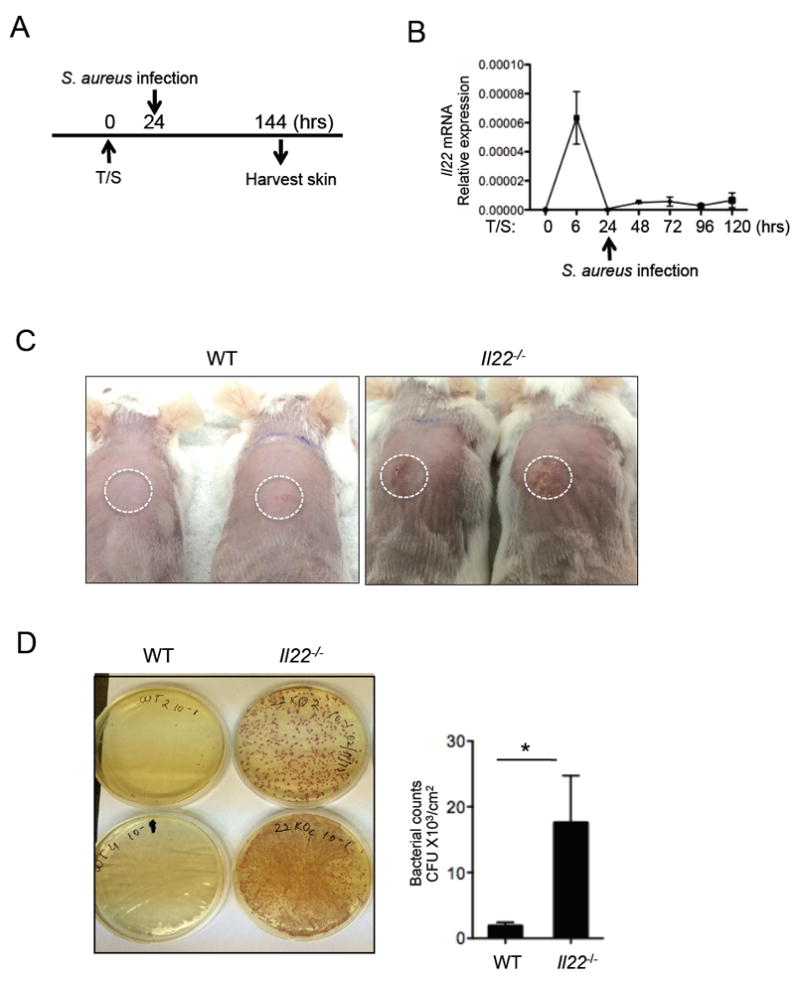
A. Schematic of S. aureus infection in tape-stripped mice is shown. B. Il22 mRNA expression determined at different times after tape stripping and after S. aureus infection (n=3/time-point). C. Representative pictures of WT and Il22-/- mice, 5 days after S. aureus infection. D. Left, Bacterial colonies obtained from culture of skin homogenates from WT or Il22-/- mice on Chrom-agar. Right, Bacterial CFU obtained from skin of WT or Il22-/- mice (n=9 /group). Mean with SEM is shown *: p<0.05.
IL-22 is often co-expressed with IL-17A in T cells, including γδ T cells16. The role of IL-17A in the clearance of S. aureus infections has been established in several models15. We tested if IL-17A also plays in the clearance of S. aureus in our model of superficial infection of tape stripped skin. As expected, we observed a significant increase in the bacterial skin burden in S. aureus infected Il17a-/- mice compared to WT controls 5 days after S. aureus infection (Fig.S2).
IL-22 is necessary for the early recruitment of neutrophils and the induction of Cxcl1, Cxcl2 and Cxcl3 expression
Neutrophils are critical for the clearance of intradermally inoculated S. aureus24. To ascertain the role of neutrophils in our model of epicutaneous S. aureus inoculation of tape stripped skin, WT mice were depleted of neutrophils by treatment with the anti-Ly6G mAb IA8 (Fig.4A). 24 hrs later, the skin was tape stripped and 24 hrs after tape stripping S. aureus was applied. Virtually complete neutrophil depletion was achieved in the skin and blood of mice treated with anti-Ly6G mAb IA8; there was no significant change in skin and blood neutrophil numbers in mice treated with IgG2a isotype control mAb (Fig.4B and Fig.S3). Neutrophil depleted mice showed a significant defect in clearing S. aureus from tape stripped skin compared to mice treated with isotype control mAb (Fig.4C). These results demonstrate that neutrophils are critical for the clearance of S. aureus infection from mechanically injured skin.
Figure 4. IL-22 is necessary for the early recruitment of neutrophils and the induction of Cxcl1, Cxcl2 and Cxcl3 expression.
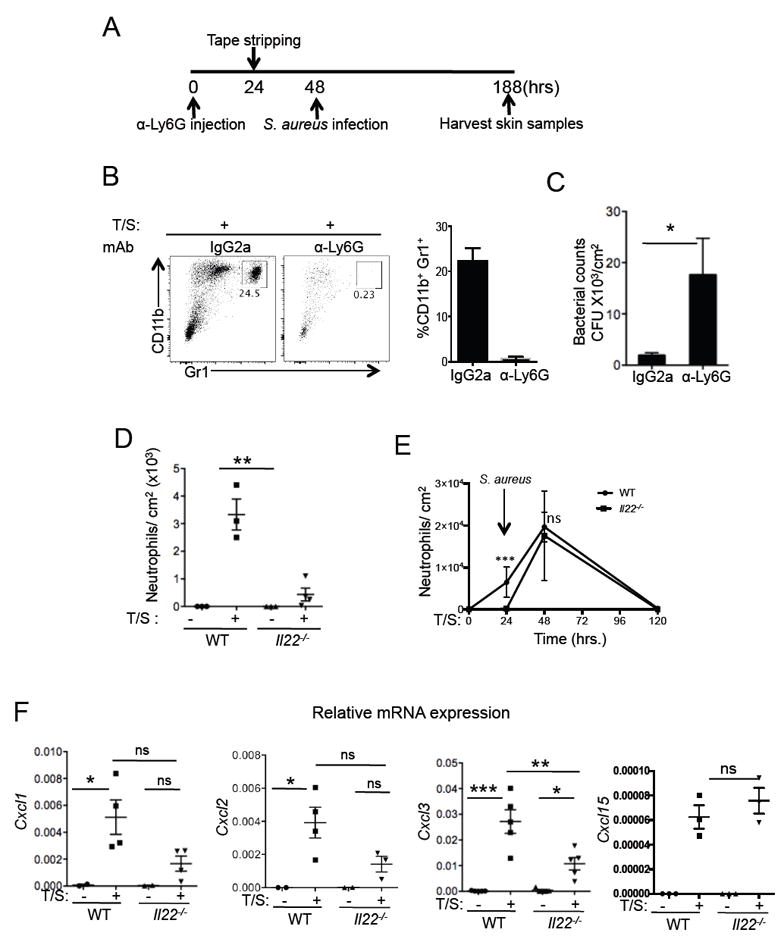
A. Schematic of S. aureus infection in neutrophil depleted mice. B. Left, Neutrophils, identified as CD11b+ Gr1hi cells are shown in the tape stripped skin of isotype treated or anti-Ly6G treated mice. Right, Percentage of neutrophils in the two groups of mice (n=4/group). C. Bacterial CFUs enumerated from the skin biopsies of WT and neutrophil depleted mice. D. Left, Neutrophils quantified in the skin of WT or Il22-/- mice in unmanipulated skin and 24 hrs after tape stripping, n=4/group. E. Neutrophil numbers in skin were quantitated in WT and Il22-/- mice at indicated time points after tape stripping and following S. aureus infection (n=3/time-point). F. mRNA expression levels of chemokines Cxcl1, Cxcl2, Cxcl3 and Cxcl15 determined in unmanipulated and tape-stripped skin of WT or Il22-/- mice, n=4/group. Mean with SEM is shown *: p<0.05, **: p<0.01, ***: p<0.001
We next determined whether IL-22 plays a role in neutrophil recruitment to mechanically injured skin. There was a robust infiltration of neutrophils in the skin of WT mice 24 hrs after tape stripping, the time when S. aureus was inoculated. In contrast, the number of skin infiltrating neutrophils 24 hrs after tape stripping was strikingly diminished in Il22-/- mice (Fig.4D). We also examined neutrophil infiltration in skin inoculated with S. aureus 24 hrs after tape stripping. There were significantly less neutrophils in Il22-/- mice at the time of S. aureus inoculation, compared to WT controls; however, neutrophil infiltration 24 hrs after inoculation was comparable in Il22-/- mice and WT controls (Fig.4E).
Expression of mRNA for the neutrophil attracting chemokines Cxcl1, Cxcl2 and Cxcl3 was not detectable in unmanipulated skin of WT or Il22-/- mice. Tape stripping induced a significant increase in the expression of Cxcl1 and Cxcl2 in the skin of WT mice, but not in the skin of Il22-/- mice (Fig.4F). Tape stripping induced a significant increase in Cxcl3 expression in both groups but the increase in Il22-/- mice was significantly less than in WT controls. Other chemokines such as Cxcl15 were similarly induced in both WT and Il22-/- mice upon tape stripping (Fig.4F). Overall, this data indicates that IL-22 is important for the expression of neutrophil chemoattractants and neutrophil recruitment in mechanically injured skin.
γδ T cells are necessary for neutrophil recruitment upon tape stripping and for suppressing S. aureus infections in mice
Since γδ T cells were the primary source of Il22 in tape stripping, we examined if their absence reduced neutrophil recruitment and promoted S. aureus growth in mechanically injured skin. Similar to Il22-/- mice, we observed a 3 fold decrease in the numbers of neutrophils in the skin of Tcrd-/- mice following tape stripping (Fig.5A and 5B). We also observed increased growth of S. aureus in tape stripped skin of Tcrd-/- mice compared to WT controls after 5 days of infection (Fig.5C and 5D), with the difference approaching statistical significance (p=0.058, n=6/group). This result is consistent with the demonstrated role of γδ T cells in limiting the growth of intradermally delivered S. aureus 15.
Figure 5. γδ T cells are necessary for neutrophil recruitment and suppression of S. aureus infection in to tape-stripped skin of mice.
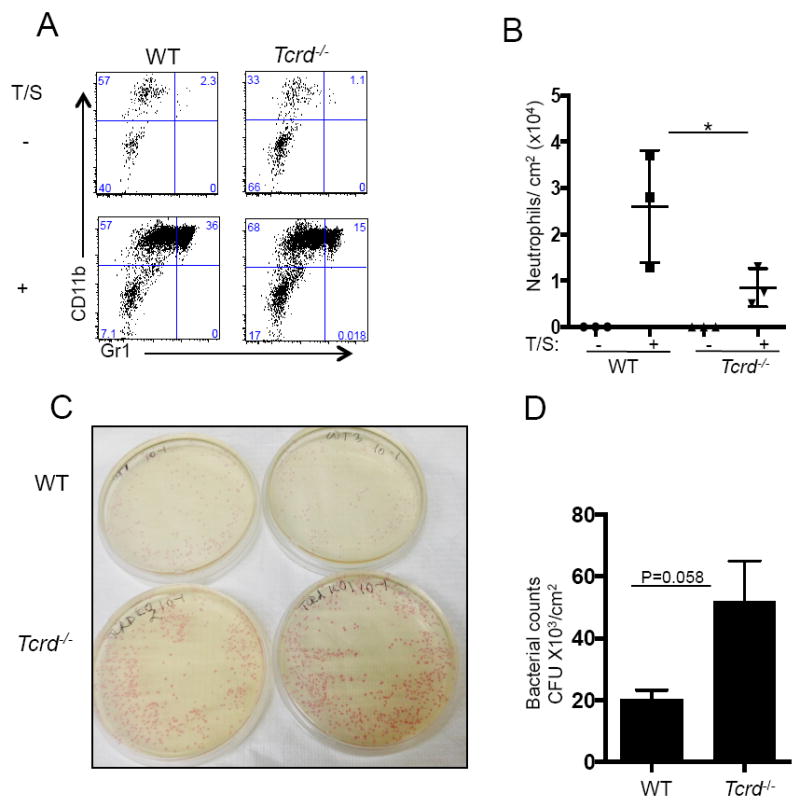
A and B Percentages and numbers of neutrophils in unmanipulated and tape-stripped skin of Tcrd-/- mice and WT controls, 24 hours after tape-stripping (n=3/group) *: p<0.05. C. Bacterial colonies obtained from homogenates of tape-stripped skin from Tcrd-/- mice and WT controls cultured on Chrom-agar, 5 days after infection. D. Bacterial CFUs obtained from tape-stripped skin of Tcrd-/- mice and WT controls (n=6/group). Mean with SEM are shown, p=0.058.
IL-22 regulates the expression of antimicrobial peptides (AMPs) Slurp1 and Defensin-beta14
AMPs play a role in the skin defense against S. aureus25. IL-22 is reported to induce the expression of AMPs by keratinocytes18. Tape stripping upregulated the expression of multiple AMPs in both WT and Il22-/- mice. We observed a significant reduction in the induction of Slurp1 and Defb14, but not Camp, Defb1, Lcn2 and Reg3g in the tape stripped skin of Il22-/- mice as compared to WT controls (Fig.6A, 6B. and data not shown). Mouse β-DEFENSIN 14 is the orthologue of human β-DEFENSIN 3. The expression of Defb3 mRNA is strikingly diminished in patients with recurrent S. aureus infections26 suggesting its importance in controlling the severity of S. aureus infections. The secreted lymphocyte antigen-6/urokinase-type plasminogen activator receptor related protein-1 (SLURP1) encoded by Slurp1, has been shown to induce proliferation of keratinocytes and suppression of growth of S. aureus27. Hence, our finding that the induction of Slurp1 and Defb14 in tape stripped skin is regulated by IL-22 demonstrates that IL-22 possesses a pleiotropic role in the control of S. aureus infection in mechanically injured skin of mice.
Figure 6. Reduced induction of Slurp1 and Defb14 anti-microbial peptides in WT and Il22-/- mice upon tape stripping.
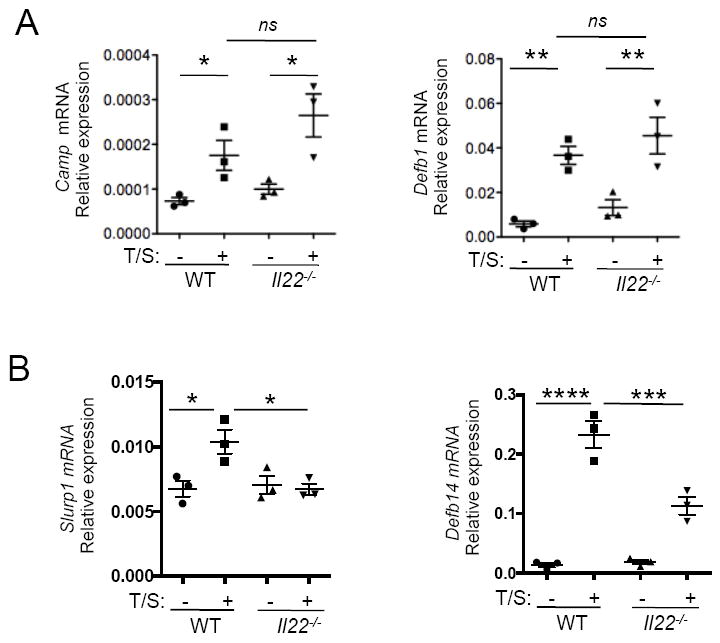
A. mRNA expression levels of genes encoding antimicrobial peptides Camp and Defb1 from unmanipulated versus tape stripped WT and Il22-/- mice. B. mRNA expression levels of genes encoding antimicrobial peptides Slurp1 and Defb14 from unmanipulated versus tape stripped WT and Il22-/- mice. Mean with SEM is shown *: p<0.05, **: p<0.01, ***p<0.001, ****p<0.0001.
DISCUSSION
We demonstrate a critical role for IL-22 in limiting S. aureus infection of mechanically injured skin. We show that mechanical injury induces IL-22 release in the skin that is dependent on IL-23 and γδ T cells, and demonstrate that IL-22 is critical for the rapid mobilization of neutrophils to the skin and the containment of S. aureus infection.
Mechanical injury to mouse skin inflicted by tape stripping induced a rapid burst of Il22 mRNA expression and the release of IL-22 from skin explants. γδ T cells were the major source of IL-22 released in the skin following mechanical injury. The release of IL-22 in the skin was dependent on both IL-23 and on the transcription factor RORγt. Il23 mRNA expression is induced rapidly (within 3 hrs.) by the application of an antigen patch to shaved skin28. We have observed a similarly rapid induction of Il23 mRNA in tape stripped skin (data not shown). RORγt is activated by IL-23R signaling to drive Il22 gene expression. We demonstrated that RORγt is critical for the induction of il22 expression in tape stripped skin. RORγt is expressed in γδ T cells and ILC3s. We demonstrated that γδ T cell are the major source of IL-22 in tape stripped skin. γδ T cells are present in both the dermis and the epidermis23. IL-23R and RORγt are constitutively expressed by dermal γδ T cells whereas there is conflicting data regarding their expression by epidermal γδ T cells29. Thus, dermal γδ T cells are likely the major contributor to IL-22 production in mechanically injured skin23. Apart from γδ T cells, other innate cells such as type-3 innate lymphoid cells (ILC3s) and mast cells have been shown to be important sources of IL-22 in mice and humans29, 30. We observed no IL-22 induction upon tape stripping in Rag2-/- mice, which lack T cells but have normal numbers of ILCs and mast cells. Furthermore, we observed normal Il22 induction in Kit-Wsh/Wsh mice, which lack mast cells (data not shown). Mechanical injury to human skin caused by scratching also induced Il22 mRNA and protein expression. This suggests that the results we have obtained in the mouse model are applicable to humans. Potential sources of IL-22 in human skin include αβ T cells, γδ T cells and mast cells29, 31, 32. We have not been able to obtain the necessary samples to determine the source of Il22 mRNA in the scratched skin of human volunteers.
We show that IL-22 plays a protective role in suppressing the growth of S. aureus on tape stripped mouse skin. This was evidenced by a one-log increase in the bacterial burden in Il22-/- mice compared to WT controls. Notably, Il22 mRNA expression at the time of S. aureus application to tape stripped skin had returned back to undetectable levels and was not induced by S. aureus application. This suggests that the IL-22 induced by tape stripping is responsible for the protective effect against the growth of S. aureus in our model. This is consistent with the importance of bacterial replication early after inoculation in the establishment of S. aureus infection. Consistent with γδ T cells being the major source of IL-22 in tape stripped skin, we demonstrated a critical role for γδ T cells in limiting the growth of S. aureus on tape stripped mouse skin.
The receptor for IL-22 is highly expressed on keratinocytes 17. IL-22 signaling induces keratinocytes to release AMPs and neutrophil attracting chemokines 18. We observed a decrease in the expression of Slurp1 and Defb14. Both of these proteins have been shown to suppress the growth of S. aureus26, 27. These findings suggest that IL-22 acts pleiotropically to inhibit S. aureus infections. Neutrophils were essential for limiting S. aureus infection of tape stripped skin, consistent with their well-established role in protecting against the growth of intradermally injected S. aureus bacteria24. 24 hours after tape stripping, which corresponds to the time when S. aureus was inoculated there was a robust influx of neutrophils and significant induction of Cxcl1, Cxcl2 and Cxcl3 in the skin of WT mice, which were severely attenuated in Il22-/- mice. However, 24 hrs after S. aureus inoculation of tape stripped skin neutrophil infiltration was comparable in Il22-/- mice and WT controls. These findings suggest that IL-22 driven neutrophil infiltration in tape stripped skin at the time of bacterial application is important for the containment of cutaneous infection by S. aureus. They imply that IL-22 is important for closing a critical window that restricts early the replication S. aureus in mechanically injured skin.
In summary we show a protective role of IL-22 in limiting the growth of S. aureus in mechanically injured skin of mice. Induction by IL-22 of AMP gene expression, neutrophil attracting chemokines, neutrophil infiltration and epidermal thickening may all contribute to this protective role. Using Il22-/- mice, it was previously reported that IL-22 is dispensable for the protection against the growth of intradermally injected S. aureus33. However, recent studies show that neutralization of IL-22 increases the bacterial burden following intradermal inoculation of S. aureus34. Our model of epicutaneous application of S. aureus to skin mechanically injured by tape stripping mimics the natural route of S. aureus infection of skin mechanically injured by scratching in patients with AD. Our demonstration of the role of IL-22 in limiting S. aureus infection of mechanically injured skin suggests that IL-22 expression in AD skin lesions may counterbalance the effect of IL-4 and IL-13 in promoting cutaneous S. aureus infection12. Our findings have also important clinical implications because they caution that IL-23 and IL-22 blockade in AD patients may aggravate staphylococcal skin infection. Hence, careful monitoring for cutaneous S. aureus infection should accompany the use of these biological therapeutic approaches.
Supplementary Material
Figure S1. γδ T cells are the major source of IL-22 in murine tape-stripped skin. A. Flow-cytometric analysis of intracellular IL-22 in CD3+ and Lineage- (CD3-CD11b-CD19-) CD45+ cells in the skin. B. Intracellular IL-22 and IL-17A in γδ T cells obtained from tape stripped of WT mice. Il22-/- mice were used as a negative control for IL-22 antibody staining. C. qPCR analysis of Il22 mRNA levels in γδ T cells ILCs and sorted Mast cells, sorted from tape-stripped skin of WT mice. One of the two representative experiments is shown.
Figure S2. A. H&E staining of Tape stripped or tape stripped and S. aureus infected skin of WT and Il22-/- mice, shown at 20X magnification B. Measurement of epidermal thickness performed using ImageJ software is shown mice (n=3 mice/group). C. Bacterial colonies obtained from skin homogenates from WT or Il17a-/- mice cultured on Chrom-agar. D. Bacterial CFU obtained from skin of WT or Il17a-/- mice (n=3 mice/group). Assays were performed 5 days after S. aureus infection. Mean with SEM is shown, *: p <0.05.
Figure S3. Depletion of circulating neutrophils in the blood of anti-Ly6G treated mice. Proportions of neutrophils identified as CD11bhiGr1hi in the blood of tape-stripped mice that were treated with isotype antibody or anti-Ly6G antibody are shown 24 hrs after depletion.
Key messages.
Mechanical injury induces IL-22 expression in the skin. This is dependent on IL-23 and γδ T cells. IL-22 is important for expression of AMPs and neutrophil attracting chemokines, neutrophil recruitment, and containment of S. aureus infection in tape stripped mouse skin.
Acknowledgments
We thank Dr. Binh Diep, UCSF, for his kind gift of S. aureus USA300, SF8300 strain. This work was supported by NIH grant HHSN272201000020C. Juan Manuel-Levya Castillo was supported by a postdoctoral fellowship from Consejo Nacional de Ciencia y Technologia (CONACYT, Mexico.)
Abbreviations
- AD
atopic dermatitis
- AMP
Anti-microbial peptide
- CFUs
Colony-forming units
- CXCL
Chemokine (C-X-C motif) ligand
- EC
epicutaneous
- IL
interleukin
- OVA
Ovalbumin
- S. aureus
Staphylococcus aureus
- SEB
Staphylococcal enterotoxin B
- TSLP
Thymic stromal lymphopoietin
- WT
wild type
- T/S
Tape stripping
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–27. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–9. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauber M, Balica S, Hsu C-Y, Jean-Decoster C, Lauze C, Redoules D, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Novak N, Bieber T, Leung DYM. Immune mechanisms leading to atopic dermatitis. J Allergy Clin Immunol. 2003;112:S128–39. doi: 10.1016/j.jaci.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–66. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huvenne W, Callebaut I, Plantinga M, Vanoirbeek JAJ, Krysko O, Bullens DMA, et al. Staphylococcus aureus enterotoxin B facilitates allergic sensitization in experimental asthma. Clin Exp Allergy. 2010;40:1079–90. doi: 10.1111/j.1365-2222.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsui K, Nishikawa A. Lipoteichoic acid from Staphylococcus aureus induces Th2-prone dermatitis in mice sensitized percutaneously with an allergen. Clin Exp Allergy. 2002;32:783–8. doi: 10.1046/j.1365-2222.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Muñoz-Planillo R, Hasegawa M, et al. Staphylococcus d-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503:397–401. doi: 10.1038/nature12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savinko T, Lauerma A, Lehtimäki S, Gombert M, Majuri M-L, Fyhrquist-Vanni N, et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J Immunol. 2005;175:8320–6. doi: 10.4049/jimmunol.175.12.8320. [DOI] [PubMed] [Google Scholar]
- 10.Laouini D, Kawamoto S, Yalcindag A, Bryce P, Mizoguchi E, Oettgen H, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immunol. 2003;112:981–7. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130:845–52. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boguniewicz M, Leung DYM. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125 doi: 10.1016/j.jaci.2009.11.027. 4–13–quiz14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808–14. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 14.Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nature Publishing Group. 2011;11:505–18. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–73. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–32. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 17.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Wolk K, Witte E, Wallace E, Döcke W-D, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 19.Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, et al. IL-22 Is Required for Imiquimod-Induced Psoriasiform Skin Inflammation in Mice. J Immunol. 2011;188:462–9. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 20.Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQF, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 22.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 InduceInnate IL-17 Production from gd T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–5. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mölne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68:6162–7. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L-J, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, et al. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanger P, Holzer J, Schleucher R, Scherbaum H, Schittek B, Gabrysch S. Severity of Staphylococcus aureus Infection of the Skin Is Associated with Inducibility of Human b-Defensin 3 but Not Human b-Defensin 2. Infect Immun. 2010;78:3112–7. doi: 10.1128/IAI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriwaki Y, Takada K, Nagasaki T, Kubo N, Ishii T, Kose K, et al. IL-22/STAT3-Induced Increases in SLURP1 Expression within Psoriatic Lesions Exerts Antimicrobial Effects against Staphylococcus aureus. PLoS ONE. 2015;10:e0140750. doi: 10.1371/journal.pone.0140750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masaki K, Suzuki Y, Kagawa S, Kodama M, Kabata H, Miyata J, et al. Dual role of interleukin-23 in epicutaneously-sensitized asthma in mice. Allergol Int. 2014;63(Suppl 1):13–22. doi: 10.2332/allergolint.13-OA-0632. [DOI] [PubMed] [Google Scholar]
- 29.Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2015;136:351–1. doi: 10.1016/j.jaci.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014;134:984–91. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123:1244–52. e2. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal Role of Dermal IL-17-Producing γδ T Cells in Skin Inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, et al. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1b and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat Immunol. 2013:1–10. doi: 10.1038/ni.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, et al. Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci. 2014;111:E5555–63. doi: 10.1073/pnas.1415610111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


