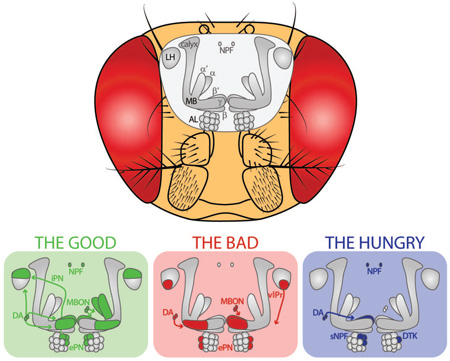
Abstract
All animals must eat in order to survive but first they must successfully locate and appraise food resources in a manner consonant with their needs. To accomplish this, external sensory information, in particular olfactory food cues, need to be detected and appropriately categorized. Recent advances in Drosophila point to the existence of parallel processing circuits within the central brain that encode odor valence, supporting approach and avoidance behaviors. Strikingly, many elements within these neural systems are subject to modification as a function of the fly’s satiety state. In this review we describe those advances and their potential impact on the decision to feed.
Graphical Abstract
Introduction
Accurate and timely appraisal of potential food resources is critical to survival. Prior to initiating feeding, neural systems must integrate food cue information from the external environment with information about internal satiety state to initiate motor programs that drive the search for food [1–3]. Odors are among the primary cues guiding such foraging behavior. Significant advances are being made in understanding how metabolic state alters the sensitivity of primary neurons along the olfactory axis, promoting foraging behavior [4,5*,6]. Yet comparatively little is known about how downstream elements of olfactory circuits shape this received sensory information and their effects on the locomotor behaviors that support eventual food acquisition. In recent years an increasing number of neural pathways were characterized that support the detection, categorization and evaluation of potential food sources. Here we focus on how central pathway components contribute to the hedonic classification of olfactory food cues and how an animal’s needs adaptively shape activity along the pathway length, thereby promoting exploration, approach or avoidance.
Initial representations of olfactory food cues
Food sources emit different types of odor cues that evoke attraction behavior while certain key odorants signal danger and induce strong aversion in flies [7–9*]. All these olfactory cues are detected first by olfactory receptor neurons (ORNs) housed in different sensilla on the fly’s olfactory organs, the antennae and the maxillary palps [10] and it is long-established that most ORNs are strongly activated by food odors and that many food odors activate multiple ORNs [11] (Figure 1). In contrast to the combinatorial code of complex food odor cues, odorants that indicate spoiled food are detected and mediated through highly selective and dedicated information pathways as shown for the odor geosmin, which is emitted by toxic microbes [8], and acids [12], often emanating from rotten food or unripe fruit (Figure 2). Additionally, potential food sources emit further discrete olfactory cues that drive behaviors other than feeding (Box. 1).
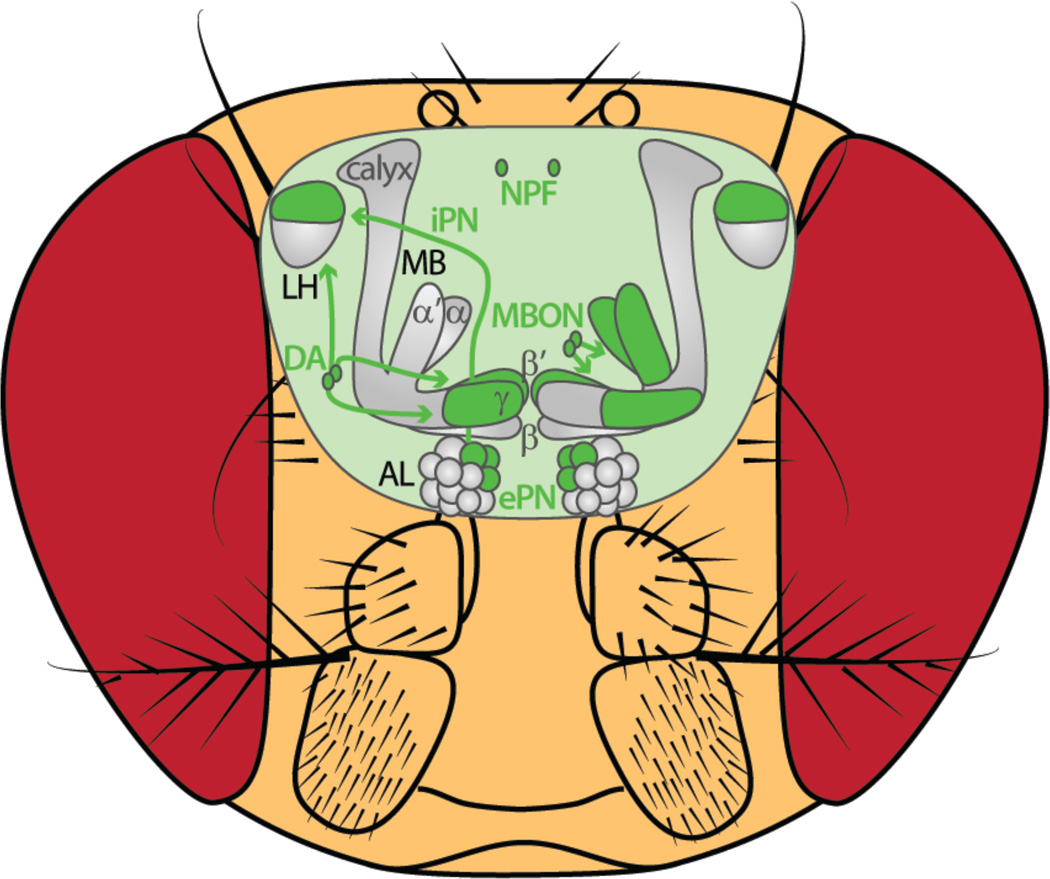
Figure 1. The good.
Elements along olfactory neural pathways that respond to food odorants or support approach behavior when activated. AL: antennal lobe, DA: dopamine, ePN: excitatory projection neuron, iPN: inhibitory PN, LH: lateral horn, MB: mushroom body, MBON: mushroom body output neuron, NPF: neuropeptide F.
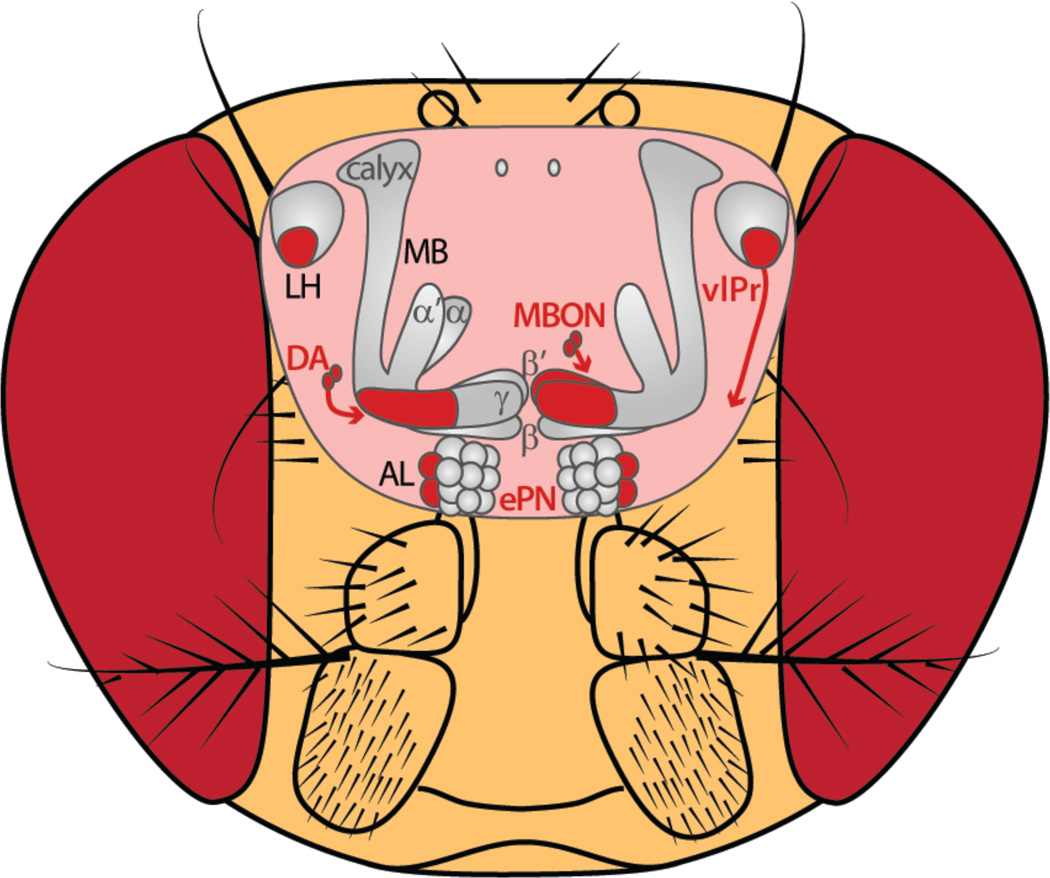
Figure 2. The bad.
Elements along olfactory neural pathways that respond to aversive odorants or support avoidance behavior when activated. AL: antennal lobe, DA: dopamine, ePN: excitatory projection neuron, LH: lateral horn, MB: mushroom body, MBON: mushroom body output neuron, vlPr: ventrolateral protocerebrum.
Box 1. Feeding versus oviposition.
Food-derived odor cues do not solely indicate a food source. A mated female fly for example has to evaluate -- in addition to the nutritional value of the food -- whether this substrate represents a favorable environment where her offspring, i.e. the larvae, will be able to feed and develop. Both aspects are not necessarily combined within the same substrate. Most studies published so far have merely considered only one aspect. Flies prefer for example citrus fruits as an oviposition substrate [13] but seem not to feed on it. Flies detect terpenes which are characteristic of these fruits via a single class of ORNs, expressing the odorant receptor Or19a. These neurons are necessary and sufficient for selective oviposition. A separate olfactory pathway has recently been shown to specifically inhibit oviposition [9*]. Drosophila adult females as well as their larvae avoid sites smelling of the main parasitoid enemies, Leptopilina wasps. This avoidance is mediated via a highly specific ORN type that is tuned to detect three odors of the parasitoid, including the wasps’ sex pheromone iridomyrmecin. Also geosmin serves as an indicator for bad oviposition sites and strongly inhibits egg laying in female flies [8]. Interestingly, yeast-produced ethylphenols which are derived from dietary antioxidants represent odor cues that induce both, feeding as well as oviposition behavior [14]. Dietary antioxidants are abundant in fruits and thus constitute a significant nutritional reward. Flies are able to detect the presence of dietary antioxidants with ORNs expressing the odorant receptor Or71a. These ORNs are located on the maxillary palps and are tuned to detect ethylphenols. Activation of these neurons in adult flies induces attraction behavior, oviposition, and increases feeding [14].
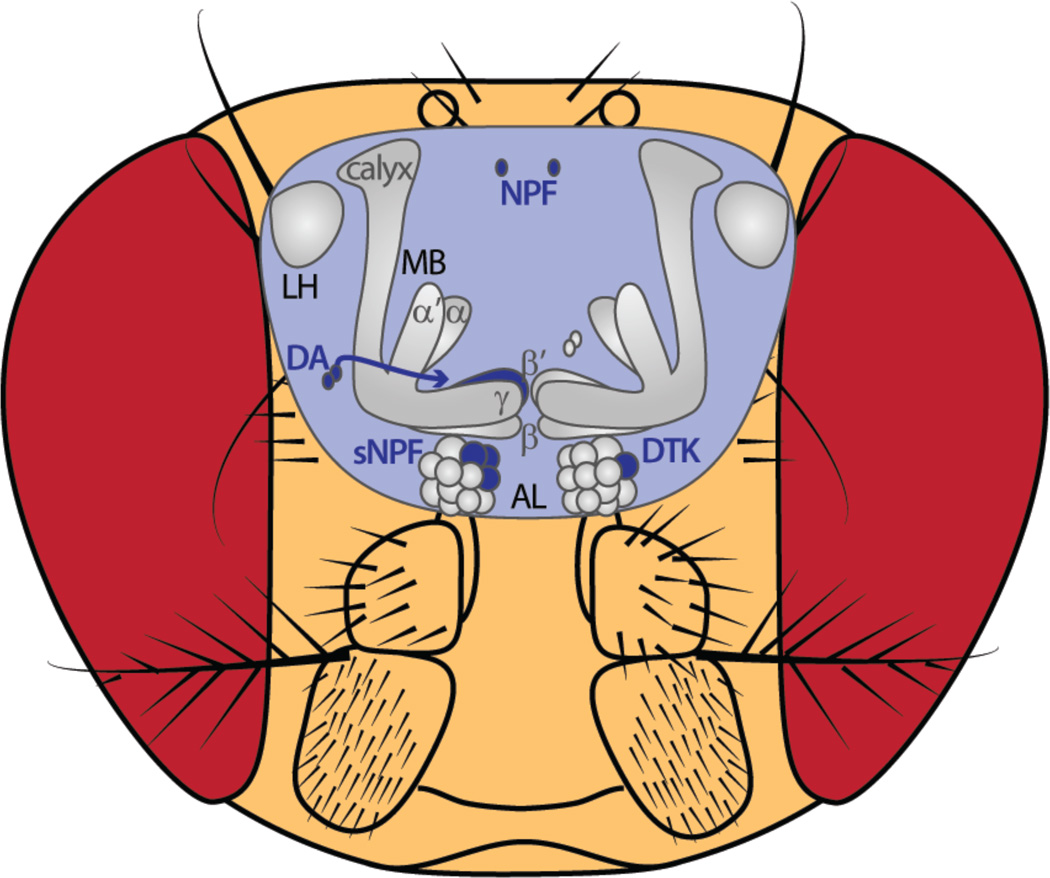
Even at this initial stage of processing, satiety state affects odor representation (Figure 3). Starvation increases on one hand the sensitivity of ORNs to food odors through neuromodulatory mechanisms [4,6]. Short neuropeptide F (sNPF), which is expressed in ORNs, facilitates synaptic transmission in specific ORNs, while the expression level of the sNPF receptor is increased by a reduction of insulin signaling [4]. Both mechanisms lead to robust food-search behavior. In addition to sNPF, the neuropeptide receptor CCHamide1 is involved in starvation-induced modulation at the ORN level [6].On the other hand, food deprivation reduces avoidance behavior to innately aversive odors [15] and increases the tolerance for noxious stimuli [16]. The reduction of aversive odor sensitivity during starvation has been shown to occur at the first olfactory synapse driven by the tachykinin receptor [5*]. Hence, diverse neuropeptide signaling systems act in opposing directions on olfactory attraction and aversion to adjust food approach to the satiety state of the fly [5*].
Figure 3. The hungry.
Elements along olfactory neural pathways that are modulated as a function of satiety state. AL: antennal lobe, DA: dopamine, DTK: tachykinin receptor, MB: mushroom body, NPF: neuropeptide F, sNPF: short neuropeptide F.
ORN processes converge in the fly brain on glomeruli at the level of the antennal lobe and there synapse with second-order projection neurons (PNs) [17]. Here the representation of odor valence begins to spatially segregate, with attractive and aversive odor cues activating predominantly medial- and lateral-projecting PNs, respectively [18,19].
Hedonic processing in the lateral horn
PNs form two classes, excitatory and inhibitory, and send spatially distinct processing pathways to the higher brain [20]. Excitatory PNs are uniglomerular and relay the odor information from the antennal lobe to the mushroom body, a center long-studied in learning and memory [21], and the lateral horn, a brain region assumed to be involved in innate olfactory behavior [22]. Inhibitory PNs, on the other hand, integrate odor-induced activity of several glomeruli, bypass the mushroom body, and innervate the lateral horn exclusively. Together, these PN populations process information on dual olfactory pathways [20,23]. Considering the axonal projections of PNs in the lateral horn, excitatory PNs spatially segregate in regions responding to pheromones or food odors [22], as well as attractive amines or aversive acids/CO2 [24]. Inhibitory PNs target the posterior-medial part of the lateral horn, which is tuned to attractive odors only, and have been shown to be necessary for odor attraction behavior [25**] (Figure 1). The anterior-lateral area of the lateral horn is innervated by third-order neurons that further innervate the ventrolateral protocerebrum and are responsive to aversive odors [25**] (Figure 2). A third region in the lateral horn, also targeted by inhibitory PNs, mediates odor intensity independent of valence [25**]. Hence, the lateral horn represents a feature-based, spatially segregated activity map decoding opposing hedonic valences and odor intensity. Notably, pheromone-responsive inhibitory PNs do not target a spatially separate area in the lateral horn as observed for excitatory PNs, but terminate together with PNs encoding attractive odors [25**]. This finding seems plausible, since pheromones also induce attraction behavior in both male and female flies [26]. However, inhibitory PNs reveal a differential inhibition onto third-order neurons which are selective for food odors and pheromones [20].
These data suggest that odor identity might be lost at the level of the lateral horn and odor information seems to be categorized according to their behavioral relevance. An alternative hypothesis has also been proposed: both odor features are still present at the lateral horn level, but are processed separately by the two PN pathways. Excitatory PNs encode odor identity and mainly determine innate odor discrimination, whereas inhibition from inhibitory PNs, which scales with olfactory stimulus strength, enhances the contrast between closely related odors by stretching the distance between overlapping odor representations of excitatory PNs [27].
Food cue processing along the lateral horn pathway
Arborizing within the lateral horn region [28*], neurons expressing Drosophila Neuropeptide F (NPF) are anatomically positioned to exploit the odor valence information encoded by the lateral horn. NPF is the functional homolog of mammalian orexigenic Neuropeptide Y (NPY), and both of these peptides are long-understood to play a critical role in regulating motivational aspects of food consumption [29–32]. NPF-positive neurons also respond specifically to food odors, and these responses are increased by hunger, indicating that these neurons integrate signals relating to both olfactory food cues and appetite control [33**] (Figures 1 and 3). Further, the level of activity observed in NPF neurons shows a strong correlation with foraging behavior: the greater the food odor-evoked NPF neural response, the greater the attraction to that odor. Strongly driving NPF neurons is sufficient to flip the valence of an odorant from aversive to attractive [33**]. Overall these results suggest that NPF neurons, rather than simply coding for hunger, play a key role in signaling the appetitive strength of food odors.
The activity of NPF neurons is not only necessary for food odor-induced foraging [33**] but also for food-odor stimulated feeding [28*]. Brief exposure to a banana-like odor leads to impulsive feeding in larvae. Both NPF neurons and neurons expressing its receptor NPFR1 are essential for the expression of this behavior [28*]. Interestingly, this effect occurs only in the presence of a palatable, readily available food source, indicating that it is not simply a reflexive feeding response, but an effect in the sensitivity to trigger an extant behavior. This may, at least in part, be explained by changes in gustatory sensitivity that accompany stimulation of NPF neurons. Activating NPF cells enhances sugar sensitivity, increasing the acceptance of lower concentrations of sweet tastants, in fed flies while leaving bitter sensitivity unchanged [16].
For both food odor-stimulated feeding and increased gustatory sensitivity, NPF cells act upstream of dopamine-positive neurons [16,28*]. Stimulating NPF cells is not sufficient to increase sugar sensitivity when dopamine receptors expressed in sugar-sensing gustatory receptor neurons [34] are absent [16] and food odor stimulated feeding is reduced after NPFR1 knockdown on dopamine-positive cells [28*]. Odor-driven feeding can also be elicited by activation of a small number of dopaminergic neurons, DL2-LH, so-named given their putative synaptic connectivity with the lateral horn. DL2-LH neurons also respond to the banana-like odor that drives impulsive feeding and knocking down NPF receptors on these cells attenuates this effect [28*] (Figure 1).
Hedonic and food cue processing along the mushroom body pathway
In addition to the lateral horn, olfactory information from the excitatory PNs is directly transmitted to the mushroom body which is thought to code for odor identity rather than valence [33**,35]. The approximately 2000 intrinsic neurons, so-called Kenyon cells, of the mushroom body converge onto a total of only 34 output neurons (MBONs) [36]. Unlike the highly stimulus-specific Kenyon cells and the stereotyped odor response properties of PNs [17,35], MBONs are broadly- and uniquely-tuned across animals with their response profiles likely dependent on experience [37]. Indeed, learning does support changes to the Kenyon cell-MBON odor drive [38–40].
Although Kenyon cells don’t appear to carry a representation of odor valence (but see [41]), photoactivation of individual MBON types induces robust attraction or aversion behavior (Figures 1 and 2). Furthermore, the type of behavioral response triggered by different MBONs is related to the neurotransmitter each uses: activating glutamatergic MBONs elicits aversion while activating either GABAergic or cholinergic MBONs elicits attraction [42*]. In line with this observation, a small subset of glutamatergic MBONs (projecting from γ5β’2a and β’2mp) are necessary to mediate aversion behavior to CO2 [43**]. CO2, generally repellent to the fly [44], is also a byproduct of fermenting fruit, a strong natural attractant and food source for the fly. A good deal of evidence describes how the olfactory periphery can accommodate the integration of these opposing signals [3] but higher order neural circuits also play a role [15,43**]. Overlapping with the same region of the mushroom body as the CO2-responsive glutamatergic MBONs [43**], are a subset of vinegar odor-responsive mushroom body-projecting dopamine-positive PAM neurons that when activated support attraction behavior. The responses of these PAM neurons are heightened with hunger and provide a mechanism by which to depress the activity of CO2-responsive MBONs [43**] (Figures 1 and 3). This local circuit provides a means to reconcile conflicting olfactory food cues and demonstrates that the mushroom body pathway is not limited to learning and memory processing.
There is further evidence for context-dependent modulation of neural activity at the level of the mushroom body. Mushroom body-innervating dopamine-positive cells display spatially-segregated patterns of activity to aversive and appetitive stimuli [45*]. Electric shock heightens the activity of dopamine cells targeting γ2/γ3 and depresses activity in those targeting γ4/γ5. The reciprocal pattern is observed in response to sugar feeding. Intriguingly, even in the absence of overt external stimulation similar patterns are observed [45*]. When the fly is idle, dopamine responses mimic the sugar-feeding state. Conversely, during spontaneous expression of an escape-like behavior, patterns look like those elicited by electric shock [45*]. The compartmentalized nature of dopamine’s mushroom body modulation provides a means to differentially shape MBON activity in accordance with the fly’s behavioral state. When taken together [42*,43**,45*], there emerges a complementary segregation of the actions supported by mushroom body-projecting dopamine neurons and MBONs. On the whole, avoidance behavior is supported by dopamine-positive cells targeting the heel to the midline of the horizontal mushroom body lobes while avoidance-related MBONs cover the midline to the tip (Figure 2). The converse is true for approach behavior (Figure 1). It is also important to note that driving combinations of MBONs produces the strongest behaviors, be they approach or avoidance, supporting the idea that ultimate behavioral output results from the combination of multiple valence-specific signals [42*] even at this late level of processing. While MBONs and the dopamine neurons that modulate their activity clearly support the production of approach and avoidance behaviors [42*,45*], their response specificity for food odor cues and how elements of such cues might be integrated with satiety information to generate state-appropriate behavior remains unknown.
Conclusions
The conventional wisdom that olfactory information leaving the first brain relay bifurcates to form neural systems that separately code for odor identity and odor valence, along the mushroom body and lateral horn pathways respectively, needs to be revisited in light of recent discoveries. Within the central brain, valence-coding neurons that promote approach or avoidance behavior appear in both these parallel processing streams [19,25**,28*,33**,42*,43**,45*]. Indeed, food odor responsive neurons that promote attraction or feeding are present downstream of both the lateral horn and mushroom body [28*,33**,43**]. In light of decades of research solidifying its role in learning and memory [21], it is perhaps unsurprising that neurons downstream of the mushroom body appear more favorably tuned to alter their response properties as a function of acute learning [37–39,42*,45*]. Plasticity along the lateral horn axis remains to be determined.
Additionally, nearly every element within both of these neural pathways faces some form of modification as a function of satiety state [4,5*,6,15,16,28*,33**,43**,45*]. Emerging evidence for the existence of neurons that directly detect nutrient quality [46–48] and those responsible for coding hunger more generally [49] leaves open the question of how and where this information is integrated with external sensory cues, such as food odors, to flexibly promote the appropriate expression of food approach behavior. It is also interesting to note that while the satiety state of the animal can alter sensitivity to external cues, influence appears to be bidirectional. Exposure to food odors concomitantly elicits changes in metabolic pathways controlling food intake [50]. Given the critical nature of food resource determination in the life of any animal, the fly being no exception, it makes sense that there exist multiple controls governing the discrimination between good and bad food odor cues. The accessibility of the Drosophila nervous system continues to provide fertile ground in understanding how neural systems process such cues and dynamically alter their representation as a function of state and experience.
Highlights.
Starvation reduces aversive odor sensitivity and increases activity to food odors
Odors are categorized according to their behavioral relevance in the lateral horn
Dopamine and Neuropeptide F cells respond to food odors, directing approach/feeding
State-dependent dopamine modulates mushroom body output neurons coding odor valence
Acknowledgments
We would like to thank Hany Dweck, Markus Knaden, and Glenn Turner for helpful comments on the manuscript. This work is supported by the Max Plank Society and the National Institute of Deafness and Other Communication Disorders (RO1 DC013071).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Itskov PM, Ribeiro C. The dilemmas of the gourmet fly: the molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front Neurosci. 2013;7:12. doi: 10.3389/fnins.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pool AH, Scott K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 2014;29:57–63. doi: 10.1016/j.conb.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su CY, Wang JW. Modulation of neural circuits: how stimulus context shapes innate behavior in Drosophila. Curr Opin Neurobiol. 2014;29:9–16. doi: 10.1016/j.conb.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ko KI, Root CM, Lindsay SA, Zaninovich OA, Shepherd AK, Wasserman SA, Kim SM, Wang JW. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. Elife. 2015;4 doi: 10.7554/eLife.08298. This study describes two opposing neuromodulatory mechanisms following starvation to increase the hedonic value of food odors: short neuropeptide F increases the sensitivity to attractive odors, while tachykinin reduces activity to aversive odors.
- 6.Farhan A, Gulati J, Grobetae-Wilde E, Vogel H, Hansson BS, Knaden M. The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Sci Rep. 2013;3:2765. doi: 10.1038/srep02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 9. Ebrahim SA, Dweck HK, Stokl J, Hofferberth JE, Trona F, Weniger K, Rybak J, Seki Y, Stensmyr MC, Sachse S, et al. Drosophila Avoids Parasitoids by Sensing Their Semiochemicals via a Dedicated Olfactory Circuit. PLoS Biol. 2015;13:e1002318. doi: 10.1371/journal.pbio.1002318. This study uncovers the first neural circuitry involved in detecting life-threatening enemies for Drosophila melanogaster. Avoidance against sites smelling of parasitoid wasps is mediated via a highly selective olfactory pathway both in larvae and adults.
- 10.Joseph RM, Carlson JR. Drosophila Chemoreceptors: A Molecular Interface Between the Chemical World and the Brain. Trends Genet. 2015;31:683–695. doi: 10.1016/j.tig.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 12.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dweck HK, Ebrahim SA, Kromann S, Bown D, Hillbur Y, Sachse S, Hansson BS, Stensmyr MC. Olfactory preference for egg laying on citrus substrates in Drosophila. Curr Biol. 2013;23:2472–2480. doi: 10.1016/j.cub.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 14.Dweck HK, Ebrahim SA, Farhan A, Hansson BS, Stensmyr MC. Olfactory proxy detection of dietary antioxidants in Drosophila. Curr Biol. 2015;25:455–466. doi: 10.1016/j.cub.2014.11.062. [DOI] [PubMed] [Google Scholar]
- 15.Bracker LB, Siju KP, Varela N, Aso Y, Zhang M, Hein I, Vasconcelos ML, Grunwald Kadow IC. Essential role of the mushroom body in context-dependent CO(2) avoidance in Drosophila. Curr Biol. 2013;23:1228–1234. doi: 10.1016/j.cub.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Inagaki HK, Panse KM, Anderson DJ. Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron. 2014;84:806–820. doi: 10.1016/j.neuron.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–241. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaden M, Hansson BS. Mapping odor valence in the brain of flies and mice. Curr Opin Neurobiol. 2014;24:34–38. doi: 10.1016/j.conb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Knaden M, Strutz A, Ahsan J, Sachse S, Hansson BS. Spatial representation of odorant valence in an insect brain. Cell Rep. 2012;1:392–399. doi: 10.1016/j.celrep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Liang L, Li Y, Potter CJ, Yizhar O, Deisseroth K, Tsien RW, Luo L. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79:917–931. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21:519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Gong J, Wang Q, Li H, Cheng Q, Liu Y, Zeng S, Wang Z. Parallel pathways convey olfactory information with opposite polarities in Drosophila. Proc Natl Acad Sci U S A. 2014;111:3164–3169. doi: 10.1073/pnas.1317911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min S, Ai M, Shin SA, Suh GS. Dedicated olfactory neurons mediating attraction behavior to ammonia and amines in Drosophila. Proc Natl Acad Sci U S A. 2013;110:E1321–E1329. doi: 10.1073/pnas.1215680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strutz A, Soelter J, Baschwitz A, Farhan A, Grabe V, Rybak J, Knaden M, Schmuker M, Hansson BS, Sachse S. Decoding odor quality and intensity in the Drosophila brain. Elife. 2014;3:e04147. doi: 10.7554/eLife.04147. This study provides functional evidence for a feature-based spatial arrangement of the lateral horn decoding opposing hedonic valences and odor intensity. Furthermore, the authors demonstrate that inhibitory PNs mediate odor attraction behavior.
- 26.Steck K, Veit D, Grandy R, Badia SB, Mathews Z, Verschure P, Hansson BS, Knaden M. A high-throughput behavioral paradigm for Drosophila olfaction - The Flywalk. Sci Rep. 2012;2:361. doi: 10.1038/srep00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parnas M, Lin AC, Huetteroth W, Miesenbock G. Odor discrimination in Drosophila: from neural population codes to behavior. Neuron. 2013;79:932–944. doi: 10.1016/j.neuron.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Pu Y, Shen P. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 2013;3:820–830. doi: 10.1016/j.celrep.2013.02.003. The authors show that NPF and its receptor are necessary for food odor stimulated feeding. Here NPF neurons act to gate the activity of food odor responsive dopamine-positive cells.
- 29.Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, McCaleb ML. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 31.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;289:R29–R36. doi: 10.1152/ajpregu.00853.2004. [DOI] [PubMed] [Google Scholar]
- 32.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J Neurosci. 2013;33:15693–15704. doi: 10.1523/JNEUROSCI.2605-13.2013. This study demonstrates that rather than just a simple hunger signal, NPF-positive cells respond selectively to food odors. NPF food odor-evoked responses increase with hunger and predict the degree of food odor approach.
- 34.Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell RA, Honegger KS, Qin H, Li W, Demir E, Turner GC. Imaging a population code for odor identity in the Drosophila mushroom body. J Neurosci. 2013;33:10568–10581. doi: 10.1523/JNEUROSCI.0682-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, Dionne H, Abbott LF, Axel R, Tanimoto H, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3:e04577. doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hige T, Aso Y, Rubin GM, Turner GC. Plasticity-driven individualization of olfactory coding in mushroom body output neurons. Nature. 2015;526:258–262. doi: 10.1038/nature15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owald D, Waddell S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr Opin Neurobiol. 2015;35:178–184. doi: 10.1016/j.conb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hige T, Aso Y, Modi MN, Rubin GM, Turner GC. Heterosynaptic Plasticity Underlies Aversive Olfactory Learning in Drosophila. Neuron. 2015;88:985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 2015;86:417–427. doi: 10.1016/j.neuron.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, Waddell S. Different kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron. 2013;79:945–956. doi: 10.1016/j.neuron.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asos Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guerin G, Placais PY, Robie AA, Yamagata N, Schnaitmann C, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife. 2014;3:e04580. doi: 10.7554/eLife.04580. The authors show that optogenetic activation of glutamatergic MBONs elicits aversion and activation of either GABAergic or cholinergic MBONs elicits attraction. The projection zones of approach/avoidance-promoting MBONs are spatially segregated along the mushroom body lobes and the MBON odor responses are thought to code valence in a combinatorial manner.
- 43. Lewis LP, Siju KP, Aso Y, Friedrich AB, Bulteel AJ, Rubin GM, Grunwald Kadow IC. A Higher Brain Circuit for Immediate Integration of Conflicting Sensory Information in Drosophila. Curr Biol. 2015;25:2203–2214. doi: 10.1016/j.cub.2015.07.015. The authors identify vinegar responsive mushroom body-projecting dopamine neurons whose activity is increased with hunger. Activation of these neurons lessens innate avoidance of a fermenting fruit byproduct, CO2, by suppressing the activity of CO2-responsive MBONs.
- 44.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 45. Cohn R, Morantte I, Ruta V. Coordinated and Compartmentalized Neuromodulation Shapes Sensory Processing in Drosophila. Cell. 2015;163:1742–1755. doi: 10.1016/j.cell.2015.11.019. This study reveals that mushroom body-projecting dopamine neurons terminate in discrete compartments along the mushroom body lobes. Because responses of these dopamine-positive cells are sensitive to external and internal context, this organization allows the same Kenyon cell odor representation to evoke different patterns of downstream MBON activity. These in turn can affect approach/avoidance behavior (see also [42]).
- 46.Dus M, Lai JS, Gunapala KM, Min S, Tayler TD, Hergarden AC, Geraud E, Joseph CM, Suh GS. Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dus M, Ai M, Suh GS. Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nat Neurosci. 2013;16:526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albin SD, Kaun KR, Knapp JM, Chung P, Heberlein U, Simpson JH. A Subset of Serotonergic Neurons Evokes Hunger in Adult Drosophila. Curr Biol. 2015;25:2435–2440. doi: 10.1016/j.cub.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Lushchak OV, Carlsson MA, Nassel DR. Food odors trigger an endocrine response that affects food ingestion and metabolism. Cell Mol Life Sci. 2015;72:3143–3155. doi: 10.1007/s00018-015-1884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]