Abstract
Background
Few studies of maternal prenatal fish intake have included biomarkers of exposure to mercury, long-chain n-3 fatty acids, and selenium, which are hypothesized to mediate associations with child neurodevelopment.
Objectives
Examine associations of maternal prenatal fish intake with child neurodevelopment accounting for biomarkers.
Methods
In 1999–2002 we enrolled pregnant women into the Project Viva cohort. At median 27.9 weeks gestation, we estimated maternal fish intake using food frequency questionnaires, and collected blood. We assayed erythrocytes for total mercury and selenium, and plasma for fatty acids including n-3 docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). In mid-childhood (median 7.7 years), we administered cognitive tests including the Kauffman Brief Intelligence Test (KBIT). We performed multivariable linear regression analyses adjusting for maternal and child characteristics including home environment and maternal intelligence.
Results
Among 1068 pairs (872 with blood), mean (SD) exposures were: maternal fish intake 1.7 (1.5) servings/wk, mercury 4.0 (3.6) ng/g, DHA+EPA 98.4 (41.8) mcg/ml, selenium 205.6 (34.6) ng/ml. Child KBIT verbal scores (mean 112.2, SD 15.0) were not related to any exposures: maternal fish intake (0.15; 95% CI: −0.50, 0.79), mercury (0.08; −0.18, 0.35), DHA+EPA (0.01; −0.22, 0.24), and selenium (0.20; −0.09, 0.50). Associations with KBIT nonverbal scores and tests of memory and visual motor abilities were similarly null. Mutual adjustment for each of the exposure measures did not substantially change estimates.
Conclusions
In this population with an average fish consumption of about 1½ weekly servings, , we did not see any evidence for an association of maternal prenatal fish intake, or of mercury, DHA+EPA, or selenium status, with verbal or non-verbal intelligence, visual motor function, or visual memory at median 7.7 years of age.
Keywords: Pregnancy, Fishes, Mercury, Child Development, n-3 fatty acids, Selenium
Introduction
Debate about the optimal amount and type of fish intake during pregnancy is ongoing and fervent. In 2001 the US Food and Drug Administration and Environmental Protection Agency released an advisory recommending that pregnant women and women of childbearing age limit their fish intake to less than 12 ounces per week to minimize fetal exposure to methylmercury, an environmental contaminant that is concentrated in fish and is a known fetal neurotoxicant.(Food and Drug Administration 2001; Goyer et al. 2000; Nesheim and Yaktine 2007) This initial guideline did not consider that fish is also a rich source of many beneficial nutrients including the elongated n-3 polyunsaturated fatty acids (PUFA) docosahexaenoic acid and eicosapentaenoic acid (DHA, EPA) that might benefit brain development,(Nesheim and Yaktine 2007) although updated guidance published in 2004 and 2014 discussed nutritional value of fish and recommended consumption of a minimum of 8 weekly ounces of fish lower in mercury.(US Department of Health and Human Services 2004; US Food and Drug Administration 2014) There was concern that the 2001 advisory resulted in lower overall intake of fish, perhaps resulting in net public health harm.(Oken et al. 2003; Shimshack and Ward 2010) Also, guidelines for methylmercury exposure were based on populations with high methylmercury exposure or very frequent seafood consumption. Whether the less frequent habitual fish intake typical of most US populations might also result in toxicity was not available in published literature.
Over the past 15 years, several investigations of populations with moderate fish consumption have found no evidence of neurodevelopmental harm, and some suggestion of benefit, from greater maternal fish intake, even above 12 weekly ounces.(Hibbeln et al. 2007; Lederman et al. 2008; Oken et al. 2008; Valent et al. 2013) Some of these studies also found statistical (but not clinical) evidence that prenatal mercury levels below the current benchmark dose were associated with poorer development.(Lederman et al. 2008) For example, in our previous analysis of 341 mother-child pairs enrolled in the Project Viva cohort in Massachusetts, we found that higher maternal fish intake >2 servings/week was associated with better child cognitive test performance in early childhood (3–5 years) compared with < 1 monthly fish meal, but higher mercury levels were associated with poorer test scores.(Oken et al. 2008) However, we performed only two cognitive tests in these young children, did not see an association of maternal red cell fatty acid concentration with child cognition, and did not have any information on selenium, a nutrient that may protect against mercury’s toxic effects.(Ralston and Raymond 2010)
In the present study, our primary aim was to examine associations of maternal prenatal fish consumption with multiple cognitive outcomes including overall intelligence in mid-childhood (6–10 years). Our secondary aim was to examine associations of the toxicant (mercury) and nutrients (DHA+EPA) richly sourced from fish, with the same outcomes. Additionally, we examined whether concentrations of elongated fatty acids or selenium modified the associations of mercury with cognitive outcomes.
Methods
Participants
We studied participants in Project Viva, a prospective longitudinal cohort study designed to examine prenatal diet and other health factors in relation to pregnancy and child health outcomes. From 1999 to 2002, Project Viva staff enrolled pregnant women attending prenatal care visits at 8 obstetrical offices of Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice in eastern Massachusetts. Exclusion criteria included multiple gestation, inability to answer questions in English, gestational age ≥ 22 weeks at initial prenatal care appointment, and plans to move away from the area prior to delivery. We completed in-person visits with mothers during pregnancy in the late first (median 9.9 weeks of gestation) and second (median 27.9 weeks) trimesters. We saw mothers and children in the hospital during the delivery admission and during infancy (median age 6.3 months), early childhood (median 3.2 years) and mid-childhood (median 7.7 years). Full details of recruitment and follow-up at through mid-childhood have been reported (Oken et al. 2015) and all study aims and questionnaires are available on our website (https://www.hms.harvard.edu/viva/). Institutional review boards of participating institutions approved the study protocols and mothers gave written informed consent at enrollment and child follow-up.
Of the 2128 live births, we obtained cognitive outcome measures at in-person visits with 1110 children (65% of the 1708 who had not disenrolled and thus remained eligible for that visit). Of these, we also had information on maternal mid-pregnancy exposures from 1068 pairs, who comprised the main cohort for our analysis of prenatal fish intake with cognitive outcomes. We also collected mid-pregnancy blood from 872 mothers, who form the analytic sample for our analyses of prenatal biomarkers and child cognition. The mothers included in the analytic sample were very similar in many respects to the overall cohort (Table S1) including in fish intake (13% with >=3 weekly fish servings), but were slightly older (mean 32.2 vs. 31.8 years), had higher annual household income at enrollment (64% vs. 58% >$70,000), and had higher IQ (KBIT 106.7 vs. 105.4 points).
Exposures
Maternal diet
At the mid-pregnancy and post-delivery visits, mothers completed semiquantitative food frequency questionnaires (FFQ), which we modified for pregnancy from a well-validated instrument used in several large cohort studies.(Rimm et al. 1992; Willett et al. 1985) The mid-pregnancy questionnaire quantified average frequency of consumption of over 140 foods and beverages during the previous three months. The limited post-delivery questionnaire had 9 questions focused on major dietary contributors to fatty acid intake in the month prior to delivery. Both instruments assessed consumption of fish and shellfish (hereafter “seafood”) with four questions: “canned tuna fish (3–4 oz.)”; “shrimp, lobster, scallops, clams (1 serving)”; “dark meat fish, e.g. mackerel, salmon, sardines, bluefish, swordfish (3–5 oz.)”; and “other fish, e.g. cod, haddock, halibut (3–5 oz.)”. Six frequency response options ranged from “never/less than 1 per month” to “1 or more servings per day.” We combined responses to the four questions to estimate average total fish intake.
We also used the FFQs to estimate intake of DHA and EPA. We used the Harvard nutrient database, which is based on US Department of Agriculture publications as well as other published sources and personal communications, and has been used in Project Viva and other cohort studies of n-3 fatty acid intake.(Hu et al. 2002; Oken et al. 2004; US Department of Agriculture Agricultural Research Service 1999) We have previously validated these questionnaires against erythrocyte and plasma levels of elongated n-3 fatty acids.(Fawzi et al. 2004; Oken et al. 2014)
Maternal blood
At the second trimester visit, we obtained blood specimens in vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). We centrifuged tubes at 2000 rpm for 10 minutes at 4°C to separate plasma from erythrocytes (red blood cells), which we then washed with chilled saline. We stored erythrocyte and plasma aliquots at −70°C, but did not store any whole blood. We collected umbilical cord blood by venipuncture after delivery of the infant, and stored plasma at −70°C. We did not retain cord erythrocytes.
We measured maternal and cord plasma fatty acids using liquid-gas chromatography, and report fatty acid concentrations in mcg/ml. Analytic methods have good within-run precision (coefficient of variance <5.4%) and been previously validated.(Lin et al. 2012) For this analysis, we used the sum of DHA and EPA, the two long-chain n-3 polyunsaturated fatty acids that are found in highest concentrations in fish and are of greatest interest for neurodevelopment.
We measured total mercury in erythrocytes using the Direct Mercury Analyzer 80 (Milestone Inc., Monroe, CT). A separate aliquot of erythrocytes was provided for mercury analysis, and we used the entire sample to minimize errors resulting from possible inhomogeneity resulting from the red cell membranes, which we have observed in the past when using sub-aliquots. Results were reported as mercury content in the original red cell sample. The detection limit was 0.5 ng/g of sample, and percent recovery for QC standards was 90–110%. For the selenium assay, we analyzed erythrocyte samples after digestion at room temperature for 24 hours with nitric acid, followed by hydrogen peroxide, using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) in Dynamic Reaction Cell (DRC) mode (DRCII, ICP-MS, Perkin Elmer, Norwalk CT). We used oxygen as the reaction gas and a solution of tellurium as the internal standard, and measured Se78. We validated the selenium analytic method in the erythrocyte samples using the DRC mode to remove interference on the selenium analysis by ICP-MS. The method was also validated using high resolution (HR) ICP-MS. We report mercury concentration as ng/g and selenium as ng/ml; we also calculated an erythrocyte selenium:mercury molar ratio(Al-Saleh et al. 2014; Ralston and Raymond 2010) as [Selenium (ng/ml)/79] / [mercury (ng/g)*5].
Outcomes
At the mid-childhood visit, trained research assistants who were unaware of exposure status administered cognitive tests as previously reported.(Belfort et al. 2013; Harris et al. 2015; Oken et al. 2015) The Kaufman Brief Intelligence Test 2nd edition (KBIT-II). The KBIT-II measures two distinct cognitive functions through two subtests: Verbal and Nonverbal. The Verbal subtest contains two item types (Verbal Knowledge and Riddles) that measure crystallized ability; the Nonverbal subtest includes a Matrices subtest that measures fluid reasoning. The two subtests are used to generate an IQ Composite. The KBIT-2 provides valid and reliable results, has been normed among a culturally diverse population, and can be used between the ages of 4 and 90 years. The KBIT correlates with full length measures of intelligence such as the WISC-III (correlation coefficient = 0.63 for composite score).(Chin et al. 2001) We also administered the drawing scale of the Wide Range Assessment of Visual Motor Abilities (WRAVMA), which assesses visual motor abilities; we had previously seen associations of maternal fish consumption and mercury concentrations with the WRAVMA in early childhood.(Oken et al. 2008) The WRAVMA is an individually administered test for children aged 3.0 years to 17.9 years. The drawing scale is a series of line drawings that the child must copy. The drawings are arranged in developmental order and become increasingly more complex. The test is discontinued after three consecutive failed items. (Adams and Sheslow 1995) The WRAVMA and KBIT-II are each scaled to a mean score of 100 and SD of 15.
Additionally, we assessed visual memory with the Wide Range Assessment of Memory and Learning (WRAML). The WRAML is an individually administered test battery designed to assess memory ability. We included the Picture Memory and Design Memory subtests, which comprise the Visual Memory Index, as we have previously observed associations of fish consumption and mercury exposure with the same domain in infancy.(Oken et al. 2005) All subtests have scaled scores with an expected mean of 10 and standard deviation of 3. We summed the two subtests to yield a combined visual memory summary score.
Covariates
We collected data from mothers regarding parental and child demographic, social, economic, and health information through self-administered questionnaires and interviews in pregnancy and shortly after delivery, as described in detail previously.(Oken et al. 2015)
At the mid-childhood visit, prior to child cognitive testing, we asked the parent if the child’s primary language was English. We asked mothers to self-complete the Home Observation Measurement of the Environment short form (HOME-SF) questionnaire, which measures the cognitive stimulation and emotional support in the child’s environment.(Frankenburg and Coons 1986) Higher scores indicate more favorable environments; the range of possible scores is 0–22. We also administered the KBIT-II to mothers to use as a covariate.
Analysis
Following the analytic plans we outlined a priori, we first examined distributions of each of the exposures, and their correlations using Pearson correlation coefficients. We did not see any outliers or influential points, and thus included all available data. The primary exposure of interest was maternal mid-pregnancy fish intake, which we examined continuously and also categorized as 0, >0-<3, and >=3 weekly servings, in line with existing federal guidelines for fish consumption during pregnancy and as we have done previously.(Food and Drug Administration 2001; Oken et al. 2008) We performed separate linear regression analyses for each of the six cognitive outcomes (KBIT verbal, KBIT non-verbal, WRAVMA drawing, WRAML design memory, WRAML picture memory, and WRAML summary score). Model 1 was adjusted for child age and sex. Model 2 was additionally adjusted for maternal race/ethnicity, parity, smoking status and education; partner education and HOME score; and child gestation length, birth weight for gestational age z-score, breastfeeding duration, and primary language (English, other). Finally, Model 3 was additionally adjusted for maternal KBIT total score. We included these covariates a priori based on prior literature including our own previous work in this cohort at younger outcome ages. We additionally considered maternal use of alcohol and recreational drugs during pregnancy but both were rare and including them in our models did not influence estimates for exposures of interest, so we did not retain these covariates in final models. Outcomes were normally distributed, and models met assumptions for the use of linear regression.
Next, in the subset of participants with available maternal blood (n=872), we examined associations of mid-pregnancy maternal fish intake (continuous and in categories), DHA+EPA intake, mercury concentrations DHA+EPA concentrations, selenium concentrations, and the selenium/mercury molar ratio, with each of the outcomes. We report here only the fully adjusted (Model 3) results. We also repeated these analyses within the subset of children whom we included in our previously published analysis of early childhood outcomes (of 341 children with prior information on mercury and early childhood cognition, 278 also had mid-childhood outcomes).(Oken et al. 2008)
Finally, since brain growth and neuronal DHA uptake are most rapid in late gestation, we also examined associations of maternal intake of fish and DHA+EPA near delivery, and DHA+EPA in cord plasma, with the child outcomes (n=1068 with information on late pregnancy diet, n=538 with cord plasma fatty acid assay concentrations). We did not retain cord erythrocytes and so did not have an appropriate biomarker for assessment of late pregnancy mercury or selenium.
As secondary analyses, we examined associations of the same exposures with each of the cognitive outcomes dichotomized as the lowest quartile vs. the top 3 quartiles within our cohort. Additionally, we examined erythrocyte mercury as a dichotomous exposure, as the top decile vs. <90th %ile within the cohort, as we have done previously.(Food and Drug Administration 2001; Oken et al. 2008)
As is common in large longitudinal studies, many participants were missing data on one or more characteristics. Using Proc MI in SAS version 9.3 (Cary, NC) and including all 2128 mother-child pairs, we imputed 50 values for each missing observation to create 50 “completed” datasets. After imputation, we combined multivariable modeling estimates using Proc MI ANALYZE. Following Project Viva protocols, we set our analytic sample sizes based on those who provided information on diet (main analysis, n=1068) or blood samples (biomarkers analysis, n=872); we did not impute outcomes. Characteristics were similar in the unimputed and imputed datasets (Table S1).
Results
Among the 1068 Project Viva mothers, mean (SD) age at study enrollment in early pregnancy was 32.2 (5.3) years, and pre-pregnancy BMI was 24.6 (5.1) kg/m2. Mean mid-pregnancy fish intake was 1.7 (1.5) weekly servings, and 13% of participants reported consuming at least 3 weekly servings of fish. Children attended mid-childhood visits at an average of 7.9 (0.8) years, and 99% spoke English as a primary language. As detailed in Table 1, participant characteristics were similar in the subset of women with available mid-pregnancy biomarkers.
Table 1.
Characteristics of Project Viva mothers and children included in this analysis
| Participants with data on mid-pregnancy diet (n=1068) % or mean (SD, range) |
Participants with data on mid-pregnancy blood (n=872) % or mean (SD, range) |
|
|---|---|---|
| Maternal and family characteristics | ||
| Age at enrollment, years | 32.2 (5.3, 15,3–44.9) | 32.4 (5.1, 15.3–44.9) |
| Pre-pregnancy BMI, kg/m2 | 24.6 (5.1, 16.2–48.5) | 24.5 (5.0, 16.2–48.5) |
| Maternal race/ethnicity, % | ||
| . Asian | 5% | 5% |
| . Black | 16% | 14% |
| . Hispanic | 6% | 5% |
| . White | 69% | 72% |
| . Other | 4% | 4% |
| College graduate, % | ||
| . No | 31% | 29% |
| . Yes | 69% | 71% |
| Married or cohabitating, % | ||
| . No | 8% | 7% |
| . Yes | 92% | 93% |
| Annual household income $>70,000, % | ||
| . No | 38% | 36% |
| . Yes | 62% | 64% |
| Nulliparous, % | ||
| . No | 52% | 52% |
| . Yes | 48% | 48% |
| Smoking status, % | ||
| . Never | 71% | 71% |
| . Former | 19% | 20% |
| . During pregnancy | 10% | 9% |
| KBIT II, points | 106.7 (15.3, 60.0–154.0) | 107.7 (15.2, 60.0–154.0) |
| Partner college graduate, % | ||
| . No | 36% | 35% |
| . Yes | 64% | 65% |
|
Any 2nd trimester data (n=1068) % or mean (SD, range) |
2nd trimester blood drawn (n=872) % or mean (SD, range) |
|
| Exposures, mid-pregnancy | ||
| Maternal fish intake, servings/week | 1.7 (1.5, 0.0–12.0) | 1.6 (1.4, 0.0–12.0) |
| Maternal fish intake category, % | ||
| . 0 servings/week | 12% | 12% |
| . >0–<3.0 servings/week | 75% | 76% |
| . >=3.0 servings/week | 13% | 12% |
| DHA+EPA intake, mg/day | 165 (158, 0–2060) | 161 (159, 0–2060) |
| Erythrocyte mercury, ng/ga | 4.0 (3.6, 0–38.2) | 4.0 (3.6, 0–38.2) |
| Plasma DHA+EPA, mcg/mla | 98.4 (41.8, 16.8–327.1) | 98.4 (41.8, 16.8–327.1) |
| Erythrocyte selenium, ng/mla | 205.6 (34.6, 44.3–380.3) | 205.6 (34.6, 44.3–380.3) |
| Child characteristics | ||
| Sex | ||
| . Male | 50% | 51% |
| . Female | 50% | 49% |
| Gestation length, weeks | 39.6 (1.7, 27.1–42.7) | 39.6 (1.6, 30.9–42.7) |
| Birth weight for gestational age z-score | 0.20 (0.97, −2.58–2.58) | 0.22 (0.97, −2.58–2.58) |
| Breastfeeding duration, months | 6.4 (4.6, 0–12.0) | 6.5 (4.6, 0–12.0) |
| Age mid-childhood, years | 7.9 (0.8, 6.6–10.9) | 7.9 (0.8, 6.6–10.7) |
| Primary language | ||
| . English | 99% | 99% |
| . Other | 1% | 1% |
| HOME Score, points | 18.4 (2.2, 9.0–22.0) | 18.5 (2.1, 9.0–22.0) |
| Child outcomes | ||
| KBIT verbal | 112.2 (15.0 , 44–147) | 112.9 (14.9 , 44–147) |
| KBIT nonverbal | 106.5 (17.0, 40–147) | 106.7 (16.9, 40–147) |
| WRAVMA drawing | 92.0 (16.7, 45–155) | 92.1 (16.8, 45–155) |
| WRAML design memory | 8.0 (2.8, 1–17) | 8.1 (2.8, 1–16) |
| WRAML picture memory | 8.9 (3.0, 1–17) | 8.9 (3.0, 1–17) |
| WRAML summary score | 16.9 (4.4, 2–31) | 17.0 (4.4, 2–31) |
Biomarker data are available only for the 872 mothers on whom second trimester blood was collected
KBIT, Kaufman Brief Intelligence Test; HOME - Home Observation Measurement of the Environment; WRAVMA - Wide Range Assessment of Visual Motor Abilities; WRAML - Wide Range Assessment of Memory and Learning; DHA - docosahexaenoic acid; EPA - eicosapentaenoic acid; BMI – body mass index
Mid pregnancy fish consumption, assessed by food frequency questionnaire, was highly correlated with DHA+EPA intake estimated from the FFQ (Pearson r=0.68), and moderately correlated with erythrocyte mercury (r=0.37) and plasma DHA+EPA (r=0.26), but only weakly correlated with erythrocyte selenium concentration (r=0.08) (Table 2). Erythrocyte mercury was moderately associated with both DHA+EPA intake (r=0.33) and plasma DHA+EPA (r=0.23) but less strongly with selenium concentration (r=0.15).
Table 2.
Correlations among exposure measures
| Second trimester |
Late pregnancy/Delivery |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fish intake (serv/wk) |
Erythrocyte mercury (ng/g) |
Erythrocyte selenium (ng/ml) |
Selenium: mercury molar ratioa |
DHA+EPA intake (mg/day) |
Plasma DHA+EPA (mcg/ml) |
Fish intake (serv/wk) |
DHA+EPA intake (mg/day) |
Cord plasma DHA+EPA (mcg/ml) |
|
| N | 1068 | 872 | 872 | 872 | 1068 | 872 | 1068 | 1068 | 538 |
| Mean (SD) | 1.7 (1.5) | 4.0 (3.6) | 206 (35) | 0.3 (1.0) | 161 (166) | 98.4 (42) | 1.8 (1.5) | 117 (145) | 35.9 (22.0) |
| Min, max | 0.0, 12.0 | 0, 38.2 | 44.3, 380.3 | 0, 74.3 | 0, 2718 | 16.8, 237.1 | 0, 14.4 | 0, 1719 | 3.2, 167.1 |
| Pregnancy | Pearson r | ||||||||
| Second trimester | |||||||||
| Fish intake | 1.00 | 0.37 | 0.08 | −0.14 | 0.68 | 0.26 | 0.40 | 0.29 | 0.10 |
| Erythrocyte mercury |
0.37 | 1.00 | 0.15 | −0.20 | 0.33 | 0.23 | 0.26 | 0.26 | 0.13 |
| Erythrocyte selenium |
0.08 | 0.15 | 1.00 | 0.03 | 0.11 | 0.07 | 0.07 | 0.08 | 0.02 |
| Selenium:mercury molar ratio |
−0.14 | −0.20 | 0.03 | 1.00 | −0.11 | −0.08 | −0.07 | −0.05 | −0.01 |
| DHA+EPA intake | 0.68 | 0.33 | 0.11 | −0.11 | 1.00 | 0.26 | 0.23 | 0.25 | 0.09 |
| Plasma DHA+EPA | 0.26 | 0.23 | 0.07 | −0.08 | 0.26 | 1.00 | 0.13 | 0.12 | 0.14 |
| Delivery | |||||||||
| Fish intake | 0.40 | 0.26 | 0.07 | −0.07 | 0.23 | 0.13 | 1.00 | 0.77 | 0.06 |
| DHA+EPA intake | 0.29 | 0.26 | 0.08 | −0.05 | 0.25 | 0.12 | 0.77 | 1.00 | 0.01 |
| Cord DHA+EPA | 0.10 | 0.13 | 0.02 | −0.01 | 0.09 | 0.14 | 0.06 | 0.01 | 1.00 |
Selenium:mercury molar ratio = [Selenium (ng/ml)/79] / [mercury (ng/g)*5]
DHA - docosahexaenoic acid; EPA - eicosapentaenoic acid; BMI – body mass index
In general, we did not observe associations of maternal fish consumption with any of the mid-childhood cognitive outcomes (Table 3), and effect estimates were only minimally affected by adjustment for maternal and child characteristics. Compared with those who reported no weekly fish servings, mothers who consumed 0-<3 weekly fish servings had children with slightly lower summary scores on the WRAML (−0.98 points, 95% CI: −1.84, −0.11 in the fully adjusted Model 3), but this association was in a direction contrary to our hypothesis, confidence limits for consumption of >=3 weekly servings included the null (−0.99, 95% CI: −2.11, 0.13), and there were no significant associations for the individual subscales of the WRAML (Table 3).
Table 3.
Associations of maternal second trimester fish intake in categories with mid-childhood cognition among 1068 mother-child pairs in Project Viva
| Cognitive outcome | Model 1a β (95% CI) |
Model 2b β (95% CI) |
Model 3c β (95% CI) |
|---|---|---|---|
| KBIT verbal IQ | |||
| 0 serv/wk | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| >0-<3.0 serv/wk | 1.30 (−1.81, 4.40) | 1.37 (−1.21, 3.95) | 0.70 (−1.85, 3.25) |
| >=3.0 serv/wk | 0.68 (−3.35, 4.71) | 0.33 (−2.97, 3.63) | 0.48 (−2.76, 3.72) |
| KBIT nonverbal IQ | |||
| 0 serv/wk | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| >0-<3.0 serv/wk | 2.18 (−1.28, 5.64) | 2.29 (−1.00, 5.58) | 1.85 (−1.44, 5.13) |
| >=3.0 serv/wk | −1.11 (−5.54, 3.32) | −1.42 (−5.62, 2.78) | −1.32 (−5.49, 2.85) |
| WRAVMA drawing | |||
| 0 serv/wk | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| >0-<3.0 serv/wk | 1.85 (−1.51, 5.22) | 1.64 (−1.71, 5.00) | 1.40 (−1.97, 4.76) |
| >=3.0 serv/wk | 0.24 (−3.99, 4.47) | −0.31 (−4.53, 3.92) | −0.26 (−4.48, 3.96) |
| WRAML design memory | |||
| 0 serv/wk | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| >0-<3.0 serv/wk | −0.43 (−0.97, 0.12) | −0.45 (−0.99, 0.08) | −0.50 (−1.04, 0.04) |
| >=3.0 serv/wk | −0.66 (−1.36, 0.05) | −0.67 (−1.37, 0.02) | −0.67 (−1.36, 0.03) |
| WRAML picture memory | |||
| 0 serv/wk | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| >0-<3.0 serv/wk | −0.49 (−1.08, 0.11) | −0.45 (−1.05, 0.15) | −0.48 (−1.08, 0.12) |
| >=3.0 serv/wk | −0.38 (−1.15, 0.38) | −0.37 (−1.13, 0.40) | −0.36 (−1.13, 0.40) |
| WRAML summary score | |||
| 0 serv/wk | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| >0-<3.0 serv/wk | −0.91 (−1.78,-0.04) | −0.90 (−1.76,-0.04) | −0.98 (−1.84,-0.11) |
| >=3.0 serv/wk | −1.00 (−2.13, 0.13) | −1.00 (−2.13, 0.12) | −0.99 (−2.11, 0.13) |
Model 1. Adjusted for child age and sex
Model 2. Model 1 + for maternal race/ethnicity, parity, smoking status and education; partner education and HOME score; and child gestation length, birth weight for gestational age z-score, breastfeeding duration, and primary language (English, other).
Model 3. Model 2 + maternal KBIT score
KBIT, Kaufman Brief Intelligence Test; HOME - Home Observation Measurement of the Environment; WRAVMA - Wide Range Assessment of Visual Motor Abilities; WRAML - Wide Range Assessment of Memory and Learning
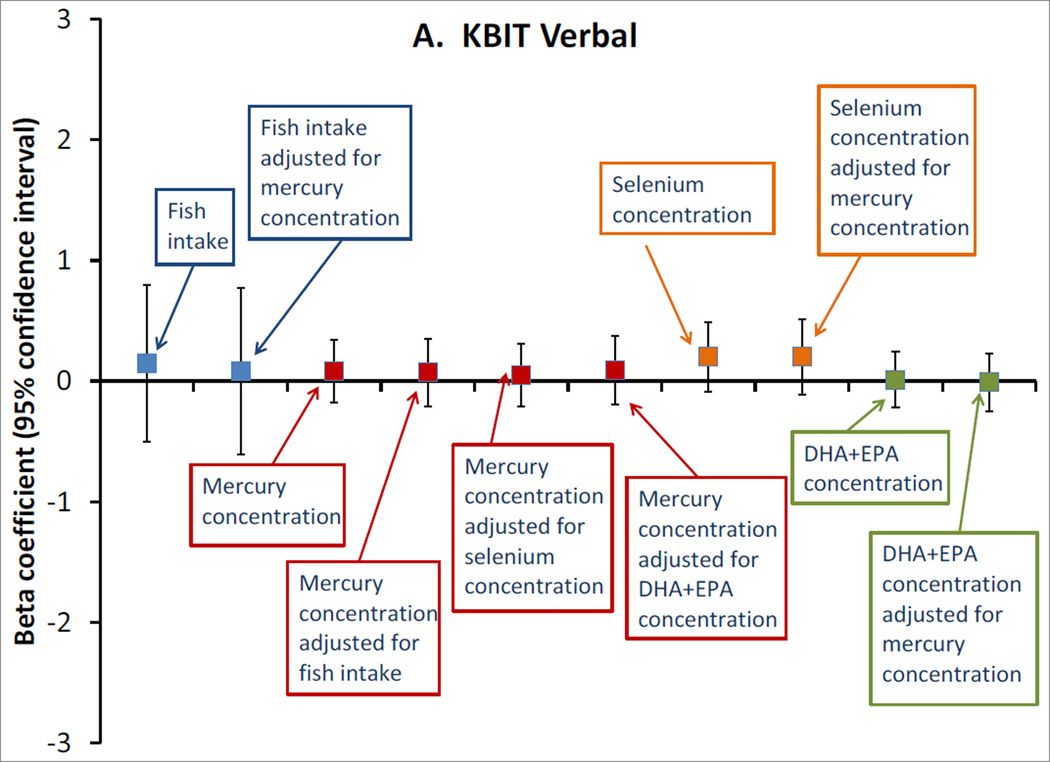
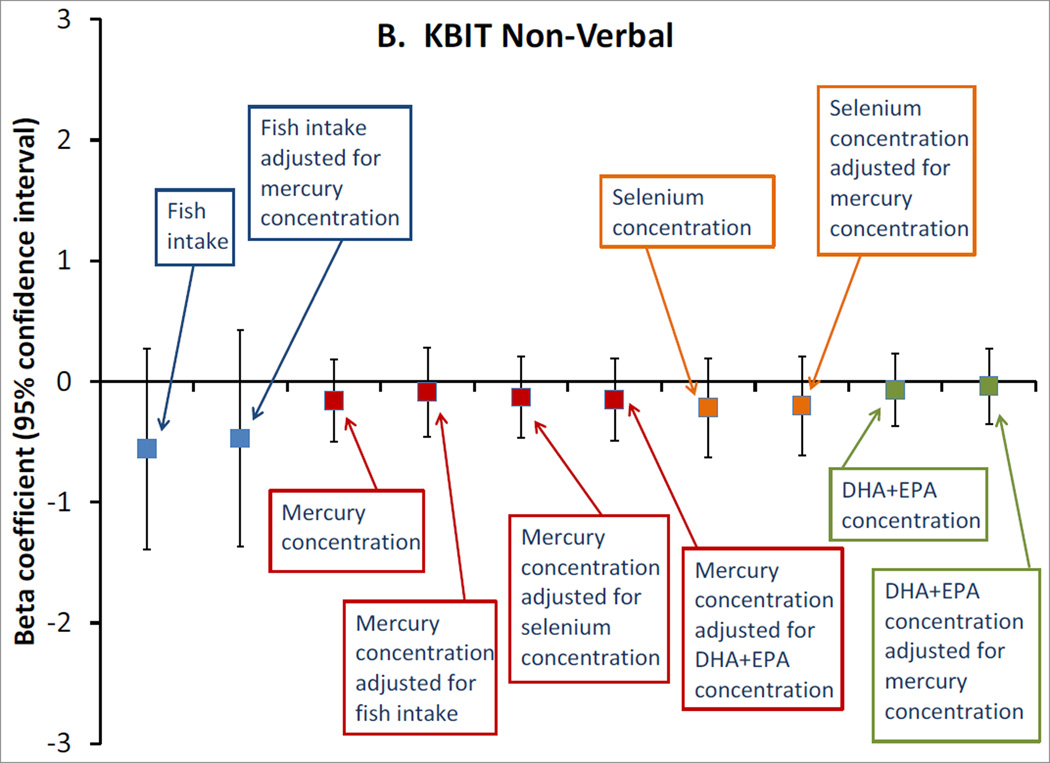
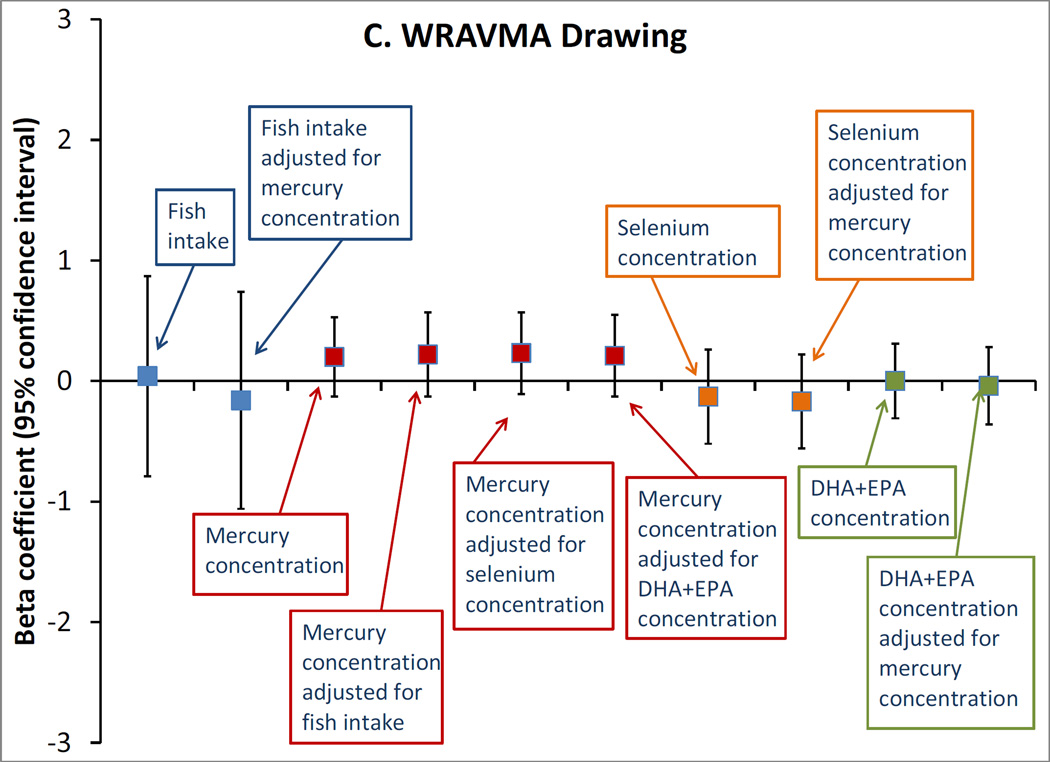
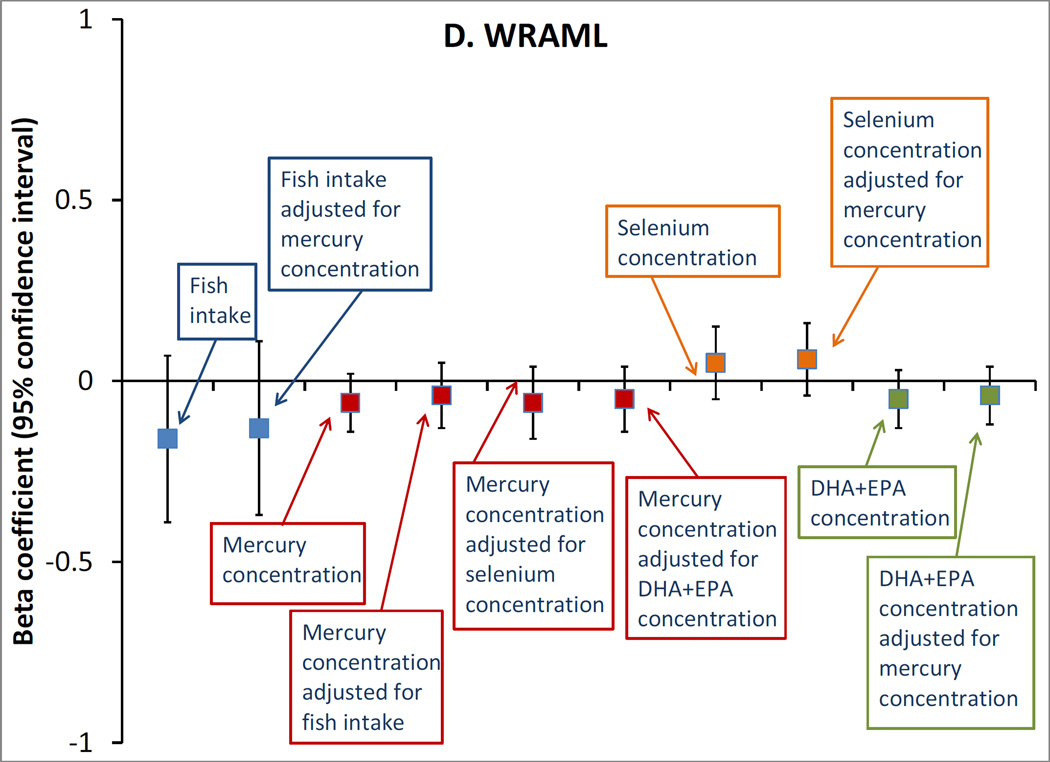
Among the 872 pairs with biomarker exposure data, fish consumption was similarly unassociated with the cognitive outcomes, whether modeled in categories (not shown) or as continuous servings/week (Figure). Also, we did not observe any associations of mid-pregnancy erythrocyte mercury or selenium concentrations, or plasma DHA+EPA concentrations, with any of the outcomes, either in unadjusted (not shown) or adjusted models (Figure). Mutual adjustment of each of the exposures for the others did not meaningfully change any estimates (Figure).
Figure.
Adjusted associations of mid-pregnancy exposures with A. Kaufman Brief Intelligence Test 2nd edition (KBIT) verbal score, n=865; B. KBIT non-verbal score, n=872; C. Wide Range Assessment of Visual Motor Abilities (WRAVMA) drawing score, n=867; D. Wide Range Assessment of Memory and Learning (WRAML) summary score, n=867. Exposures are: fish consumption (per serving/week), erythrocyte mercury concentration (per ng/g), erythrocyte selenium concentration (per 10 ng/ml) and plasma docosahexaenoic + eicosapentaenoic acid (DHA+EPA) concentration (per 10 mcg/ml). All estimates are adjusted for maternal race/ethnicity, parity, smoking status, education, KBIT score, and partner education; and child gestation length, birth weight for gestational age z-score, breastfeeding duration, primary language (English, other), sex, age at outcome, and HOME score. Exposures are additionally adjusted for each other, as indicated. Data from 872 mother-child pairs in the Project Viva cohort.
Results were similarly null in the subset of children whom we had included in our previous paper examining outcomes in early childhood (Table S2). KBIT verbal IQ was strongly correlated with early childhood Peabody Picture Vocabulary Test (PPVT, r=0.58) and more modestly with early childhood WRAVMA (r=0.31). In contrast, nonverbal IQ was more strongly correlated with early childhood WRAVMA (r=0.26) than with the PPVT (r=0.14). The WRAVMA drawing test administered at the two timepoints was also modestly correlated (r=0.20).
Results were also null among those with information on late pregnancy fish intake and cord plasma (Table S3). Associations of mid-pregnancy fish intake and biomarkers with dichotomous cognitive outcomes (bottom quartile vs. quartiles 2–4) were also null (data not shown). We saw an isolated association of near-delivery fish intake with lower risk of being in the lowest quartile of WRAML for those who consumed 0-<3 servings/week of fish (adjusted odds ratio 0.24, 95% CI: 0.08, 0.75), or >= 3 servings/week (adjusted odds ratio 0.24, 95% CI: 0.07, 0.79), each compared with no weekly fish servings, although the association was null for fish intake expressed as a continuous exposure (OR 1.05 per weekly serving, 95% CI: 0.81, 1.37), and also for other outcomes and the cord blood biomarkers.
Discussion
In this analysis of US mothers and children enrolled in a longitudinal cohort study, we did not observe any associations of prenatal maternal fish consumption with child cognition assessed in mid-childhood. These results are in contrast with our earlier findings in a smaller number of Project Viva participants. Among 341 pairs from the same cohort, we had previously found that, after adjustment for erythrocyte mercury, fish consumption (≥3 weekly servings vs. <1 monthly) was associated with somewhat better receptive vocabulary (2.2 points, 95% CI: −2.6, 7.0) and higher visual motor scores (6.4 points, 95% CI: 2.0, 10.8) in early childhood, whereas erythrocyte mercury was associated with lower scores on these same outcomes (−4.5, 95% CI: −9.5, −0.4 for vocabulary and −4.6, 95% CI: −8.3, −0.9 for visual motor).(Oken et al. 2008) We included these same two developmental domains in the present analysis, as well as additional domains of non-verbal intelligence and memory. Associations with all of these outcomes were null, with generally narrow confidence intervals that excluded meaningful effect sizes. Even when we restricted our analysis to the subset of children whom we had previously studied, we still observed no associations. It may be that associations present at younger ages had washed out by mid-childhood, that children learn compensatory behavioral mechanisms to improve their performance, or that our earlier findings were spurious.
Prior work, including our own, has highlighted the importance of accounting for intake of fish or highly unsaturated n-3 fattty acids when estimating associations of mercury with outcomes. For example, negative confounding might result in an underestimation of the harmful effects of mercury.(Budtz-Jorgensen et al. 2002; Choi et al. 2014; Oken et al. 2005; Oken et al. 2008) Unfortunately, many studies, including recent publications,(Lam et al. 2013; van Wijngaarden et al. 2013) have not done so.
Additionally, and highly relevant for public health guidance about fish intake, is the need to provide estimates not only for mercury concentrations, but also for fish consumption as a whole, with health outcomes.(Oken 2010) Fish consumption is generally the primary source of mercury intake, although with low fish intake or with high environmental contamination, other dietary sources may also contribute as much or even more to mercury burden.(Golding et al. 2013; Rothenberg et al. 2014) However, the nutrients in fish are not just a nuisance confounder that might mask the effects of mercury, as n-3 PUFA, vitamin D and iodine may have independent beneficial effects, and selenium might neutralize mercury’s toxicity. If the overall effect of fish consumption is neutral or beneficial, then advice to limit fish consumption would not be expected to improve public health and may even be detrimental.
Our study has many strengths. We assessed diet during pregnancy prospectively using validated questionnaires. We included as exposures not only fish and elongated n-3 fatty acid intake estimated by these FFQs, but also biomarkers of mercury, selenium, and fatty acids, which are all contained within fish and have been hypothesized to mediate the neurocognitive effects attributed to maternal fish consumption in prior studies. We also accounted for important non-nutritional confounders, most notably maternal intelligence and home environment. We assessed neurocognitive outcomes using validated instruments that we have previously found within this cohort to have the expected associations with other exposures such as breastfeeding(Belfort et al. 2013) and residential proximity to roadways.(Harris et al. 2015)
However, our study population included mothers who all had access to prenatal care, were relatively well educated, and most were married or living with their partner; children’s KBIT scores were on average higher than the general population mean of 100. Thus, results may not be generalizable to other populations, particularly those of lower socioeconomic status or otherwise at higher risk for developmental problems. Nevertheless, fish and DHA+EPA consumption were very similar to many populations in the US.(Nesheim and Yaktine 2007). We did not collect information on child fish intake in mid-childhood, although in our prior analysis we did not see an association of early-childhood fish intake with early-childhood cognition.(Oken et al. 2008) As we studied only one outcome domain (child development) in this analysis, we cannot comment on associations of fish intake with other outcomes, although child neurodevelopment is believed to be the endpoint most sensitive to prenatal methylmercury exposure. We were not able to account for school quality or neighborhood characteristics, which could be associated with both maternal diet and child cognitive development, and it is possible that we might have observed stronger associations if we had included them. Given the plasticity of the developing brain, and the relative higher socioeconomic status of Viva participants, our results may reflect the ability of positive environmental factors to mitigate any toxicity from prenatal mercury exposure. Alternatively, it may be that the prenatal effects of fish and methylmercury do not persist beyond early childhood, or that the tests we administered in mid-childhood were not sensitive to these effects. Finally, it may be that our earlier findings were by chance, and there is no harmful effect of the low methylmercury exposure from moderate maternal prenatal fish consumption on child neurodevelopment.
We used total mercury in erythrocytes as our measure of prenatal mercury exposure. In previous cohort studies, biomarkers of mercury exposure included maternal hair collected at or after delivery,(Crump et al. 1998; Debes et al. 2006; Myers et al. 2003; Oken et al. 2005) or umbilical cord blood,(Debes et al. 2006) In Project Viva, we did not retain cord blood erythrocytes, and we collected hair from only a small number of mothers. However, over 90 percent of the blood mercury resides in the erythrocytes,(Goyer et al. 2000) and maternal 1st and 2nd trimester blood mercury are highly correlated with maternal whole blood mercury collected at delivery, with cord blood levels,(Morrissette et al. 2004; Vahter et al. 2000) and with maternal fish consumption.(Oken et al. 2014) Thus, good evidence exists that the erythrocyte sample we used is a suitable proxy for fetal methyl mercury exposure. It is, however, difficult to translate the erythrocyte mercury concentration to a whole blood mercury level, and so to compare directly exposures in this population with other published studies that have included whole blood mercury. Given the generally low habitual fish intake in this cohort, we assume that whole blood mercury levels were generally low as is typical of levels in many US populations. In the small subset (n=135) of cohort participants for whom we had collected maternal hair at delivery, 10% had hair mercury levels above 1.2 ppm (a threshold derived from the benchmark dose),(Goyer et al. 2000; Oken et al. 2005) similar to women of childbearing age in the contemporaneous National Health and Nutrition Examination Survey (90th percentile 1.4 ppm).(Centers for Disease Control and Prevention 2001) Hair mercury was strongly correlated with erythrocyte mercury (Pearson r=0.77) in this subcohort.
We also did not collect detailed information on the exact types of fish consumed. For example, we asked a question about canned tuna intake, but albacore (white) tuna on average has substantially higher amounts of mercury than chunk light tuna; and the “dark meat fish” question included both salmon, likely to have very little mercury, and swordfish, which is one of the most highly contaminated species.(Groth 2010) Thus, we cannot characterize well the individual species that contributed to mercury intake in this population.
Conclusion
Among a cohort of Massachusetts women with generally moderate fish intake, we did not find evidence for either a harmful or a beneficial effect of maternal fish consumption during pregnancy on offspring cognition in mid-childhood. We also saw no evidence for associations of prenatal mercury with cognitive outcomes, nor any change in the effect estimate for mercury after adjustment for selenium or DHA+EPA. It is possible that the effects of prenatal exposure to nutrients and contaminants in fish are overshadowed by childhood experiences, including external factors such as education as well as perhaps the child’s own diet. These findings, within the context of the larger body of research on this topic, suggest that moderate maternal fish consumption during pregnancy is not associated with harm for child neurocognitive function.
Supplementary Material
Highlights.
Fish contains beneficial nutrients as well as methylmercury, a neurotoxicant
We previously found that higher maternal fish intake predicted better cognition
We did not see an association of prenatal fish intake with mid-childhood cognition
Results were also null for prenatal selenium, fatty acids, and mercury levels
Acknowledgments
Sources of financial support:
This work was supported by the National Institutes of Health (grant numbers K24 HD069408, R01 ES016314, R37 HD034568, R01 HL064925, R01ES013744, P30ES023515, P30ES000002, and the Intramural Program of NIAAA). The funding sources had no involvement in the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit the paper for publication.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- DHA
docosahexaenoic acid 3
- EPA
eicosapentaenoic acid
- FFQ
food frequency questionnaire
- HOME
Home Observation Measurement of the Environment
- IQR
inter quartile range
- KBIT
Kaufman Brief Intelligence Test
- OR
odds ratio
- ppm
parts per million (mcg/g)
- WRAML
Wide Range Assessment of Memory and Learning
- WRAVMA
Wide Range Assessment of Visual Motor Abilities
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests declaration:
None of the authors has any conflict of interest to report.
References
- Adams W, Sheslow D. Wide range assessment of visual motor abilities. Wilmington, DE: Wide Range, Inc; 1995. [Google Scholar]
- Al-Saleh I, Al-Rouqi R, Obsum CA, Shinwari N, Mashhour A, Billedo G, et al. Mercury (hg) and oxidative stress status in healthy mothers and its effect on birth anthropometric measures. Int J Hyg Environ Health. 2014;217:567–585. doi: 10.1016/j.ijheh.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Belfort MB, Rifas-Shiman SL, Kleinman KP, Guthrie LB, Bellinger DC, Taveras EM, et al. Infant feeding and childhood cognition at ages 3 and 7 years: Effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013;167:836–844. doi: 10.1001/jamapediatrics.2013.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1:2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Blood and hair mercury levels in young children and women of childbearing age - united states, 1999. JAMA. 2001;285:1436–1437. [PubMed] [Google Scholar]
- Chin CE, Ledesma HM, Cirino PT, Sevcik RA, Morris RD, Frijters JC, et al. Relation between kaufman brief intelligence test and wisc-iii scores of children with rd. J Learn Disabil. 2001;34:2–8. doi: 10.1177/002221940103400101. [DOI] [PubMed] [Google Scholar]
- Choi AL, Mogensen UB, Bjerve KS, Debes F, Weihe P, Grandjean P, et al. Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicol Teratol. 2014;42:85–92. doi: 10.1016/j.ntt.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: Benchmark analysis of a new zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol. 2004;14:754–762. doi: 10.1016/j.annepidem.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. [Last accessed July 17, 2007];Consumer advisory: An important message for pregnant women and women of childbearing age who may become pregnant about the risks of mercury in fish. 2001 Available at: http://vmcfsanfdagov/~dms/admehghtml.
- Frankenburg WK, Coons CE. Home screening questionnaire: Its validity in assessing home environment. J Pediatr. 1986;108:624–626. doi: 10.1016/s0022-3476(86)80853-8. [DOI] [PubMed] [Google Scholar]
- Golding J, Steer CD, Hibbeln JR, Emmett PM, Lowery T, Jones R. Dietary predictors of maternal prenatal blood mercury levels in the alspac birth cohort study. Environ Health Perspect. 2013;121:1214–1218. doi: 10.1289/ehp.1206115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer R, Aposhian V, Arab L, Bellinger D, Burbacher T, Burke T, et al. Toxicological effects of methylmercury. Washington, D.C: National Academy Press; 2000. [Google Scholar]
- Groth E., 3rd Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the united states: Implications for risk communication. Environ Res. 2010;110:226–236. doi: 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the project viva cohort (massachusetts, USA) Environ Health Perspect. 2015;123:1072–1078. doi: 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (alspac study): An observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- Lam HS, Kwok KM, Chan PH, So HK, Li AM, Ng PC, et al. Long term neurocognitive impact of low dose prenatal methylmercury exposure in hong kong. Environ Int. 2013;54:59–64. doi: 10.1016/j.envint.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, et al. Relation between cord blood mercury levels and early child development in a world trade center cohort. Environ Health Perspect. 2008;116:1085–1091. doi: 10.1289/ehp.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Salem N, Jr, Wells EM, Zhou W, Loewke JD, Brown JA, et al. Automated high-throughput fatty acid analysis of umbilical cord serum and application to an epidemiological study. Lipids. 2012;47:527–539. doi: 10.1007/s11745-012-3661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissette J, Takser L, St-Amour G, Smargiassi A, Lafond J, Mergler D. Temporal variation of blood and hair mercury levels in pregnancy in relation to fish consumption history in a population living along the st. Lawrence river. Environ Res. 2004;95:363–374. doi: 10.1016/j.envres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. Prenatal methylmercury exposure from ocean fish consumption in the seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Nesheim M, Yaktine A, editors. Seafood choices: Balancing benefits and risks. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- Oken E, Kleinman KP, Berland WE, Simon SR, Rich-Edwards JW, Gillman MW. Decline in fish consumption among pregnant women after a national mercury advisory. Obstet Gynecol. 2003;102:346–351. doi: 10.1016/S0029-7844(03)00484-8. PMCID: PMC1989666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a us pregnancy cohort. Am J Epidemiol. 2004;160:774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, et al. Maternal fish consumption, hair mercury, and infant cognition in a u.S. Cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a us cohort. Am J Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E. Fish intake and mercury levels: Only part of the picture. J Pediatr. 2010;157:10–12. doi: 10.1016/j.jpeds.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Oken E, Guthrie LB, Bloomingdale A, Gillman MW, Olsen SF, Amarasiriwardena CJ, et al. Assessment of dietary fish consumption in pregnancy: Comparing one-, four- and thirty-six-item questionnaires. Public Health Nutr. 2014;17:1949–1959. doi: 10.1017/S1368980013001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: Project viva. Int J Epidemiol. 2015;44:37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston NV, Raymond LJ. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- Rothenberg SE, Windham-Myers L, Creswell JE. Rice methylmercury exposure and mitigation: A comprehensive review. Environ Res. 2014;133:407–423. doi: 10.1016/j.envres.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshack JP, Ward MB. Mercury advisories and household health trade-offs. J Health Econ. 2010;29:674–685. doi: 10.1016/j.jhealeco.2010.05.001. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture Agricultural Research Service. Usda nutrient database for standard reference, release 13. 1999 Nutrient Data Laboratory Home Page, Available at: http://wwwarsusdagov/main/site_mainhtm?modecode=12354500.
- US Department of Health and Human Services. [Accessed January 11, 2013];What you need to know about mercury in fish and shellfish. 2004 Mar; 2004. In: Available at: http://waterepagov/scitech/swguidance/fishshellfish/outreach/advice_indexcfm.
- US Food and Drug Administration. Fish: What pregnant women and parents should know. 2014 http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm393070.htm Last Updated Feb 24, 2015.
- Vahter M, Akesson A, Lind B, Bjors U, Schutz A, Berglund M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ Res. 2000;84:186–194. doi: 10.1006/enrs.2000.4098. [DOI] [PubMed] [Google Scholar]
- Valent F, Mariuz M, Bin M, Little D, Mazej D, Tognin V, et al. Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: A prospective cohort study in italy. Journal of epidemiology / Japan Epidemiological Association. 2013;23:360–370. doi: 10.2188/jea.JE20120168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Thurston SW, Myers GJ, Strain JJ, Weiss B, Zarcone T, et al. Prenatal methyl mercury exposure in relation to neurodevelopment and behavior at 19 years of age in the seychelles child development study. Neurotoxicol Teratol. 2013;39:19–25. doi: 10.1016/j.ntt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.