Abstract
The hippocampus and prefrontal cortex are critical for learning and memory-guided behavior, but neural mechanisms underlying their coordinated operation are currently unclear. Recent evidence indicates that different network activity patterns, each marked by local field potential signatures, play distinct roles in mediating long-range interactions between these regions to support memory processing. We propose that these network patterns underlie multiple communication modes between these regions, and support different cognitive demands during ongoing behavior. Network patterns may represent a fundamental neurophysiological mechanism through which the hippocampus communicates memory-related information with other regions. Dissecting the causal roles of these network patterns in cognitive processes has the potential to delineate a coherent and dynamic functional organization across hippocampal and prefrontal networks during memory-guided behavior.
Graphical_Abstract
Introduction
The cognitive processes of learning, memory and decision making rely on neural circuits located in anatomically distinct brain regions. Networks of neurons in these regions must coordinate their activity to support these complex cognitive functions, and it is increasingly clear that cognition emerges from this coordinated activity of distributed networks. In particular, prior work indicates that long-range interactions between the hippocampus and the prefrontal cortex are important for these higher order functions. Lesion studies indicate that communication between these regions is required for goal-directed and rule-based behaviors [1–3]. However, the nature of communication between these two regions, and the physiological mechanisms that support these interactions, are still poorly understood.
The role of hippocampal - prefrontal interactions in cognition has been most clearly established in spatial memory tasks in rodents. These regions play complementary and overlapping roles in memory, with the hippocampus being critical for encoding, storage and retrieval of new memories [4], and the medial prefrontal cortex playing an integral role in long-term memory, retrieval, and working memory [5,6]. In addition, both of these regions are thought to be major components of the neural circuitry underlying planning, imagination and memory-guided decision making [7–9]. The term “functional interactions” has often been used to convey that these regions are co-active during cognitive processing, indicating coordination and communication of activity [9]. However, the neurophysiological mechanisms that enable neurons in distributed circuits to coordinate their activity, and the functional role that this coordinated activity plays in cognition, is still under investigation.
Rhythmic oscillations at distinct frequencies, seen in the electroencephalogram or local field potential (LFP) activity, are an integral feature of neural activity in many brain regions. These network activity patterns (“network patterns”) reflect organized activity of underlying neural ensembles, and are thought to support both local information processing and coordination between brain regions during cognition in diverse model systems [10–17]. In particular, phase coherence of oscillations across regions has been proposed to be a mechanism for coordination. However, current evidence remains primarily phenomenological, and a causal demonstration that coherence of network rhythms plays a specific role in cognitive processing is still lacking. Multiple network patterns have been ascribed roles in organizing local processing in the hippocampal network during behavior, including slow theta oscillations (6–12 Hz), beta (15–20 Hz) and gamma rhythms (40–100 Hz), and fast network oscillations, in particular sharp-wave ripples (SWRs, 150–250 Hz) [7,13,18–20]. Similarly, multiple rhythms are also implicated in information processing in PFC [11,14,21–24]. In the past decade, focus on hippocampal-prefrontal interactions has been primarily in the context of theta oscillations [21,25–28], but it is now increasingly clear that other network patterns also mediate interactions among these regions [7,29–31].
We review evidence indicating that different network patterns represent multiple communication modes between hippocampal-prefrontal regions by providing conduits for selective exchange of information according to current cognitive demands and internal state. These network patterns may thus play a role in globally organizing activity across hippocampal-prefrontal networks by enabling distinct mechanisms for synchronization of activity at different timescales. In order to dissect and establish the necessity of these network patterns in cognition, we suggest a multi-faceted approach: first, employing behavioral paradigms that prominently engage distinct cognitive demands; second, simultaneous physiological monitoring of ensemble activity to characterize representational similarity and coherent information processing across regions; and finally, causal perturbation techniques to selectively disrupt inter-regional coordination. We reason that this approach will delineate a coherent and dynamic functional organization of a multi-region network that is necessary for memory-guided behavior.
Multiple network patterns mediate hippocampal-prefrontal interactions
In the hippocampus, rapid, dynamic changes in network patterns are observed in the hippocampus during memory-guided behavior [31,32] (eg. in Figure 1). Strikingly, some of these network patterns have been shown to be associated with dynamic changes in population activity in PFC. Here, PFC is used to denote the prelimbic and infralimbic regions of the medial prefrontal cortex as well as the anterior cingulate cortex. There are multiple direct and indirect connections between the hippocampus and PFC that can support these interactions. Direct connections to PFC primarily arise from the ventral and intermediate CA1 regions of the hippocampus, with a minority from dorsal CA1 [33]. Indirect connections between the hippocampus and PFC are also routed via other medial temporal lobe (MTL) areas, including the subiculum, entorhinal cortex, peri- and post-rhinal cortex [34], and also through nucleus reuniens [35,36]. Further, the dense interconnectivity between the principal hippocampal layers and throughout the longitudinal axis of the hippocampal formation [37] provides a pathway for coordination of activity throughout these structures.
Figure 1. Distinct network states in the hippocampal-prefrontal network during behavior.
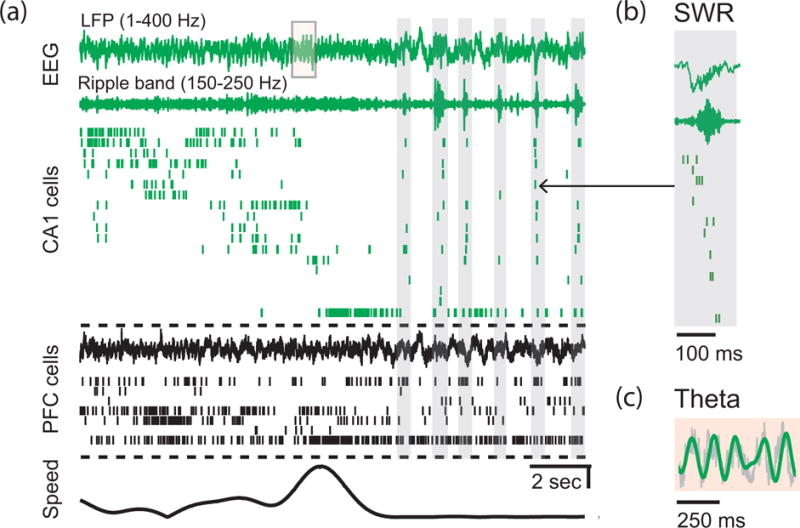
(a) Spike and local field potential (LFP) activity in CA1 region of the hippocampus (green) and PFC (black) as an animal approaches a reward well in a spatial task. From top to bottom, the plot shows respectively: broadband LFP (1–400 Hz) in CA1, ripple band filtered LFP (150–250 Hz) in CA1, raster plot with spikes from 18 CA1 place cells, broadband LFP in PFC, raster plot with spikes from 7 PFC neurons, and animal speed. As the animal runs on the track (speed scale bar: 10 cm/sec), CA1 place cells fire in a sequential order. (b) When the animal stops at the reward well, SWRs and replay activity are prominent in the hippocampus. Vertical gray rectangle backgrounds in a denote SWRs detected in CA1 LFP. (c) Theta oscillations are seen in hippocampus during running. Shaded area over CA1 LFP from a on an expanded time scale. Theta filtered LFP (6–12 Hz) is shown overlaid with the broadband LFP. Adapted from [31**] with permission from authors.
These diverse anatomical connections between the regions can support bi-directional interactions [30,38,39]. In different behavioral tasks, task selective responses of prefrontal neurons can be derived from hippocampal inputs [30], and vice versa [38], and direction of interactions can also be dependent on task phase [39]. We reason that such findings provide additional evidence that rather than regarding these regions as modular entities with discrete functions, they should be considered as part of an interacting network that sub serves joint functions necessary for memory-guided behavior. The impact of interregional dialogue and the resulting dynamics therefore needs to be considered, along with the recognition that behavioral context and internal brain state are important parameters that govern functional interactions between the regions. Coordination between these two structures could facilitate the updating of network activity during rapid transitions in behavioral state and cognitive demands that occur during learning and memory-guided behavior. In support of this notion, recent studies have provided evidence that network patterns such as theta oscillations and SWRs represent dynamical states that sub serve synchronization of ensemble activity across regions to support cognitive function.
Hippocampal-prefrontal interactions during theta oscillations are the most well-established, with multiple studies demonstrating co-ordination of activity of neurons in the two regions during periods of high theta phase coherence [25–27,40,41] (also reviewed elsewhere [21,28]). Theta oscillations are prominent in the hippocampus during active exploration associated with place cell activity. Prefrontal neurons exhibit phase-locking to hippocampal theta, and synchronization of the theta rhythm (phase coherence) between the two regions is elevated specifically during periods of memory-guided decision making as compared to passive exploration [25–27,41] (Figure 2). Enhanced theta coherence is also seen during performance of correct vs. incorrect trials [26,27,41], and activity of theta phase-locked prefrontal neurons is correlated to task representations [27,40]. Elevated spiking correlations during these periods of high theta coherence [26] and the relationship with correct memory performance [27] indicates that ensemble spiking activity in the two regions is coordinated to support the exchange of mnemonic information.
Figure 2. Theta and SWR interactions in the hippocampal-prefrontal network during behavior.
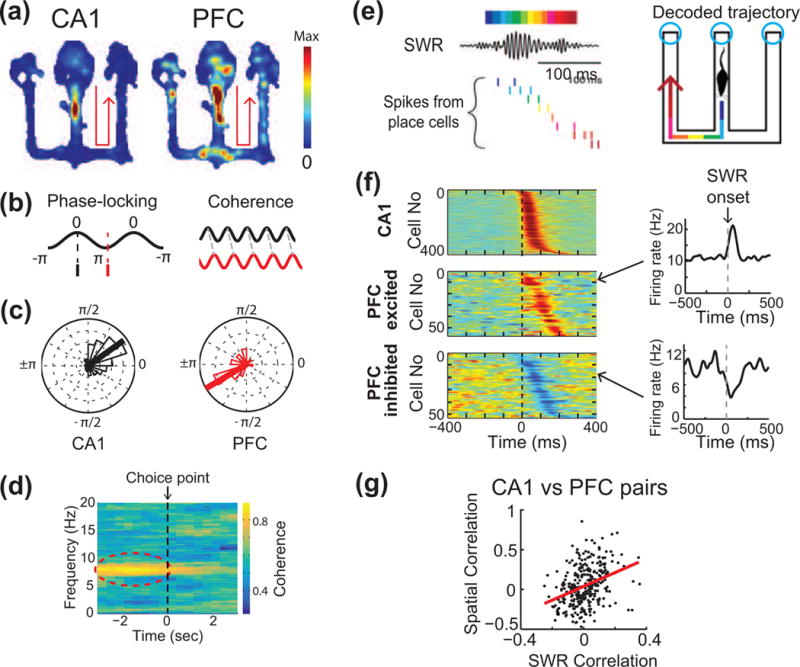
(a) Example of CA1 and PFC cells with overlapping spatial patterns of firing in a W-track spatial alternation task. Color scale indicates spiking intensity on the track. Both cells are preferentially active during outbound trajectories to right (arrows). (b) Schematic illustrating theta coupling between CA1 and PFC observed during exploratory behavior. Schematic on left illustrates phase-locking of spiking to theta oscillations; the one on right illustrates coherence, i.e. phase alignment of theta oscillations in CA1 and PFC. (c) CA1 and PFC cells are phase-locked to hippocampal theta at different characteristic phases. (d) Increase in CA1-PFC theta coherence specifically in the theta band (6–12 Hz) as animals approach the choice point in a maze. (e) Schematic illustrating replay of CA1 place cells during SWRs. Decoded CA1 activity represents spatial trajectories in the maze. SWRs are observed primarily at reward locations (cyan circles) on the W-maze. (f) Activity of CA1 and PFC cells aligned to hippocampal SWRs. PFC cells show both SWR-triggered activation and suppression of activity. (g) Reactivation of spatial information in the hippocampal-prefrontal network. CA1-PFC cell pairs with high spatial overlap are reactivated together during SWRs, while those with dissimilar patterns are suppressed. Adapted from [31**] with permission from authors.
Hippocampal SWRs represent another prominent network state associated with hippocampal-cortical interactions. SWRs are high-frequency (150–250 Hz) transient oscillations (~100 ms) seen prominently during slow-wave sleep, and in the awake state during consummatory behaviors and periods of low movement speed [7,13,19]. These highly synchronized events are associated with fast time-scale replay of place cell sequences representing trajectory events [7,42–46]. Hippocampal reactivation during sleep has long been proposed to be a part of a neocortical dialogue for consolidation of memory related information [47]. Indeed, interactions with cortical regions, specifically enhanced activity correlations and coordination of LFP patterns, have been observed during sleep SWRs [48–52]. In contrast, the role of awake SWRs has only begun to be elucidated recently (differences between awake and sleep SWRs are also reviewed elsewhere [53]), with evidence suggesting a role of awake SWRs in memory consolidation, memory retrieval to guide future actions, as well as planning [7,46,54,55]. Hippocampal replay during awake SWRs comprises reactivation of place cell sequences in forward and reverse temporal order, as well as novel shortcut sequences, suggestive of a diverse role in reinforcement learning, planning and prospective decision making [7,42–46]. Critically, awake SWRs have been shown to be necessary for spatial learning [56], establishing that awake hippocampal replay is required for memory processes. But until recently, whether or how awake hippocampal replay engages other brain regions, in particular PFC, was still unclear. It has now been shown that awake SWRs are associated with coherent reactivation of behaviorally concordant information in the hippocampal-PFC network (Figure 2). This strong coordination results in structured reactivation of representations related to ongoing experience, and also reflects reactivation of coordinated hippocampal-cortical activity during theta oscillations [31]. SWRs and theta oscillations mark distinct network states during distinguishable behavioral epochs, and are associated with unique modes of hippocampal information processing. Thus, this body of evidence establishes that theta oscillations and awake SWRs represent distinct mechanisms for communication between these distant brain structures.
Another prominent rhythm, gamma oscillations, plays a role in local processing in both regions. In the hippocampus, slow (40–60 Hz) and fast (80–120 Hz) gamma are associated with different phases of theta oscillations, and are thought to mediate internally driven representations arising from CA3 and externally driven representations via entorhinal cortex, respectively [32,57]. Outside of theta states, slow gamma is also linked to SWR replay, possibly influencing attractor states that govern replay events [58–60]. In PFC, gamma oscillations are prominently linked to cognitive processes, including attentional processing, rule representation, and flexible rule switching [11,14,23,61]. Critically, there is evidence indicating the existence of hippocampal-cortical gamma interactions: PFC gamma oscillations are coordinated with hippocampal theta [29], and perturbing ventral hippocampal connections to PFC selectively impairs PFC gamma oscillations in a memory task [30]. Thus, activity during gamma oscillations must be coordinated between the regions, although this still remains to be shown.
In addition to these three network patterns, other oscillations have also been proposed to play a role in governing hippocampal-prefrontal interactions, including beta oscillations [16,20], and 4 Hz rhythms [62]. Hence, converging lines of evidence indicate a highly dynamic, multiple-scale organization comprised of network patterns that can engage coordination between the hippocampus and prefrontal cortex. The precise roles of these interactions driven by different network patterns, namely, how they are differentially engaged during different behaviors, how memory-related information is processed and communicated during these patterns, and what cognitive processes they support, are still being investigated.
What is the role of network patterns in memory-guided behavior?
There are some clues regarding the specific role of interactions during these different network states in memory processes. The most well-studied are theta interactions, but critical questions regarding their role still remain. Although theta coherence is seen specifically during periods of memory-guided decision making and is elevated during correct memory performance [26,27,41], whether representations in the two regions are coherent and depict similar task selective activity during periods of high theta coherence is still not clear. If periods of phase coherence are indeed markers of behaviorally relevant coupling, we would expect that hippocampal and prefrontal representation will also be coherent, in analogous fashion to gamma synchronization in cortical areas [14]. Hippocampal place cell sequences within individual theta oscillations (termed “theta sequences”) represent ongoing behavioral experiences, including future goal representation and deliberation during periods of vicarious trial and error [63–66], but whether ongoing PFC activity is aligned with these sequences during periods of high theta coherence is yet to be explored. Since theta coherence emerges after behavioral learning [27], rhythmic and representational coherence could underlie information exchange related to current location and learnt rules, although the causal role of this coherence in memory has also yet to be demonstrated. Intriguingly, inactivating direct connections from the hippocampus to PFC impairs gamma synchrony, but not theta synchrony, in spatial memory tasks [30]. In contrast, theta synchrony is affected during anxiety-related behaviors [67]. This indicates that theta and gamma synchronization may play different roles in cognition and moreover differentially depend on behavioral context.
In addition to theta, various findings suggest that coupling between regions during SWRs may have a distinct role in memory-guided behavior. Hippocampal reactivation during awake SWRs is known to contribute to learning [56], with a proposed role in memory consolidation and retrieval [7,54]. Critically, awake SWRs are up-regulated by both novelty and reward, suggesting a role in memory formation, possibly by establishing spatial cognitive maps and linking behavioral history with reward [42,45,56,68,69]. In addition, SWRs continue to occur after learning, and have thus been proposed to also play a role in planning and prospective decision making [46,55]. These various functions also involve the prefrontal cortex, and the recent finding of coordinated reactivation in hippocampal-prefrontal ensembles during SWRs points towards specific mechanisms for these functions [31]. Since both hippocampal SWRs and theta sequences have been implicated in future memory-guided choices, it will be crucial to investigate how the two interaction modes, coordinated hippocampal-PFC SWR reactivation and theta coherence, change over the course of learning, as well as their variability during trial-by-trial performance of memory-guided decisions. This may potentially reveal crucial relationships between these network patterns on different timescales.
The role of hippocampal-PFC gamma interactions in memory processes is still not clear and requires further elaboration. How the distinctive place cell sequences seen during slow and fast gamma rhythms in CA1 [70] relate to activity beyond the hippocampus remains unknown. The finding that hippocampal gamma rhythms are modulated by theta oscillations [32,57], and PFC gamma is influenced by hippocampal theta [29], suggests the existence of a fine temporal relationship between hippocampal and prefrontal gamma activity. In addition, the fact that hippocampal SWR replay is correlated with periods of slow gamma coherence [58–60] raises the possibility that coordinated reactivation of behaviorally relevant information in hippocampal-prefrontal networks may be influenced by gamma. It has also been recently shown that inactivating direct ventral hippocampal inputs to PFC during memory-guided behavior impairs gamma oscillations and task representations in PFC, and selectively affects memory encoding [30]. Hence, current evidence suggests a functionally important coordination of gamma rhythms between the two regions, which requires further investigation.
Linking network patterns and cognitive processes
Dissecting the roles of these diverse network patterns in cognition presents a significant challenge. In order to understand the influence of separate physiological patterns on cognition, examining a diverse array of behavior tasks will be necessary [4,20,23,26,27,30,56]. Investigating interactions during the learning and performance stages of complex tasks that manifest distinctive cognitive demands, such as encoding, retrieval, novelty, and flexibility, can reveal the specific network pattern(s) that are prevalent during these periods. Diverse behavioral tasks that accentuate particular cognitive processes, such as working memory or cue-guided retrieval, can provide the opportunity to unambiguously link network patterns to cognition. Common elements of different tasks that consistently engage the same network patterns for coordination of multi-regional activity can also provide similar evidence. Furthermore, this would also enable investigation of information represented in the joint ensemble activity in distant regions using decoding of activity during particular task phases that are associated with specific cognitive processes.
Simultaneous physiological monitoring in multiple regions will be necessary to further understand the role of interactions. Are there linked representations in distant populations during these network interactions that encode information regarding specific experiences? Task-relevant representations within the hippocampal and prefrontal cortex have been found to be inter-dependent [30,38], indicating that neural ensembles in these regions may act in concerted fashion to support memory-guided behavior. These early findings delineate physiological interactions that may serve to promote the emergence of joint representations of similar experiences. In addition, during SWRs, there is coordinated reactivation in the hippocampus and PFC, but whether this represents retrospective or prospective information, and how this reactivation depends on learning and behavioral state has to be elucidated. Simultaneous physiological monitoring during task phases that require cognitive elements linked to reactivation, such as retrieval, working memory and planning, will enable us to answer these questions.
Finally, interventional approaches with requisite temporal and spatial specificity must be utilized to perturb communication between brain regions during specific physiological patterns. Recent studies have shown that disrupting anatomical connections between the hippocampus and PFC leads to deficits in spatial navigation and memory [30,38], but in order to link network patterns to certain aspects of cognition, real-time detection of these patterns must be coupled with temporally precise methods of perturbing network activity. Combining these two methods would result in a closed loop feedback paradigm, which can detect, for example, incidences of SWR events, specific oscillation phase, or bouts of high coherence between two interacting regions (Figure 3). The feasibility of such an approach has been demonstrated for network patterns within the hippocampus [56,71]. A more stringent procedure would be to perturb oscillatory coherence without disrupting the overall activity of the cells. This would require independent methods such as perturbing neuromodulation to change oscillatory frequency in a particular region, which still represents a major technical challenge.
Figure 3.
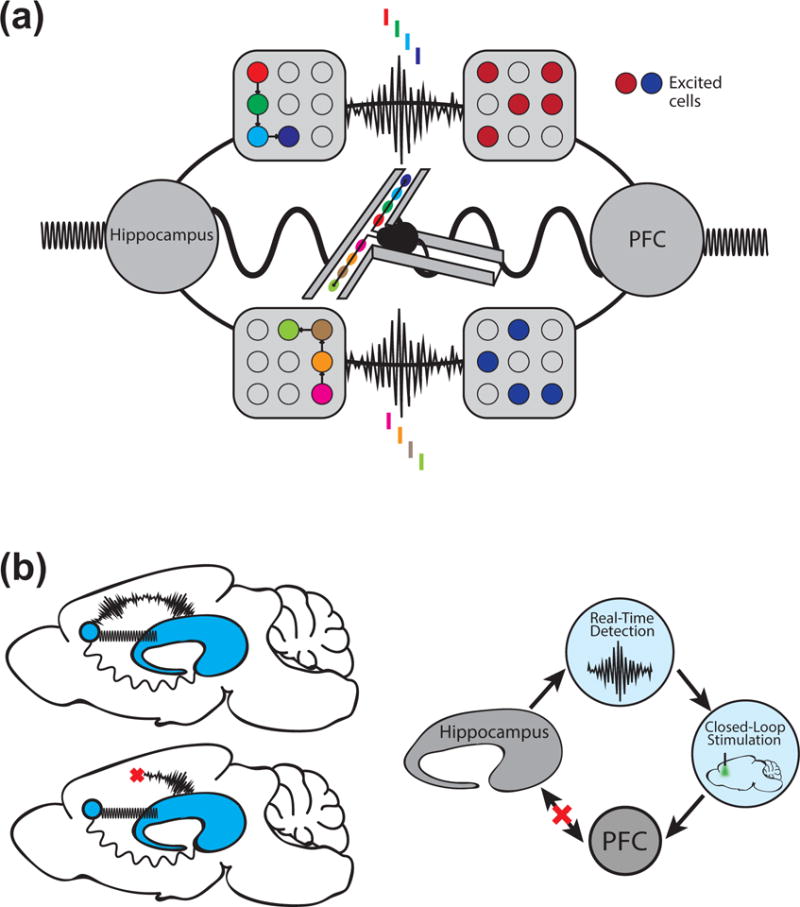
Different network patterns can represent different modes of communication between brain regions. (a) Schematic illustrating that multiple network activity patterns mediate hippocampal-prefrontal interactions during memory-guided behavior. Theta interactions, associated with place cell activity in the hippocampus, are prominent as animals traverse locations in spatial mazes. Awake SWRs are associated with coordinated reactivation of ongoing experience in hippocampal-prefrontal ensembles. During SWRs, PFC cells with representations related to concurrently reactivated hippocampal cells are preferentially excited, while PFC cells with unrelated representations are inhibited. Gamma synchrony also mediates hippocampal-prefrontal interactions, and may play a role in organizing theta and SWR interactions. (b) A closed-loop feedback strategy that couples real-time detection to optogenetic perturbation can be used to selectively perturb communication during specific activity patterns (illustrated for SWRs).
Summary
It has become increasingly clear that we must transition from approaching brain regions as modular entities, and instead consider the influence and role of joint inter-regional activity, particularly in the generation of complex behaviors. As we described here, the role of the hippocampus in learning and memory is well-established, but the mechanisms through which it communicates with other regions to engender multi-region mnemonic representations and enable memory-guided behavior is poorly understood. It appears likely that bidirectional flow of memory related information between the hippocampus and PFC supports memory formation, contextual memory retrieval, and memory-guided decisions. The ubiquitous presence of ensemble network activity patterns in these regions and the dynamic transitions between these states during behavior suggests that a mechanism where neural populations switch on and off their activity for sub serving certain functions is too simplistic. Understanding the role of prominent physiological patterns in these regions can reveal organizational principles through which the brain utilizes specific activity patterns for local information processing in the context of long-range communication across regions. Multiple experimental and analytical approaches must be combined for this goal, and can potentially serve as a template to study long-range interaction in other systems. Further, dissecting the functional role of multiple modes of hippocampal-prefrontal coordination in memory-guided behavior also has the potential to provide a foundation to link specific impairments in long-range co-ordination to cognitive deficits. Since the hippocampus and PFC are implicated in many neurological disorders, this could provide novel insight into the pathophysiology of these disorders.
Highlights.
Hippocampal-prefrontal interactions are mediated by distinct network activity patterns.
Theta oscillations, sharp-wave ripples, and gamma oscillations represent multiple modes of communication.
These patterns may be critical for coordination of activity during memory-guided decision making.
A causal approach is needed to dissect the role of long-range interaction modes in memory and cognition.
Acknowledgments
This work was supported by NIH Grant R00 MH100284, a Sloan Research Fellowship in Neuroscience (Alfred P. Sloan Foundation), and a NARSAD Young Investigator grant (Brain and Behavior Foundation) to S.P.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of special interest
** of outstanding interest
- 1.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GW, Cai JX. Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats. Neurobiol Learn Mem. 2008;90:365–373. doi: 10.1016/j.nlm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93:415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection. New York: Oxford University Press; 2001. [Google Scholar]
- 5.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 6.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JY, Frank LM. Hippocampal-cortical interaction in decision making. Neurobiol Learn Mem. 2015;117:34–41. doi: 10.1016/j.nlm.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 10.Buzsaki G, Freeman W. Editorial overview: brain rhythms and dynamic coordination. Curr Opin Neurobiol. 2015;31:v–ix. doi: 10.1016/j.conb.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 13.Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17:239–249. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris AZ, Gordon JA. Long-range neural synchrony in behavior. Annu Rev Neurosci. 2015;38:171–194. doi: 10.1146/annurev-neuro-071714-034111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brincat SL, Miller EK. Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci. 2015;18:576–581. doi: 10.1038/nn.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fries P. Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 19.Buzsaki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rangel LM, Rueckemann JW, Riviere PD, Keefe KR, Porter BS, Heimbuch IS, Budlong CH, Eichenbaum H. Rhythmic coordination of hippocampal neurons during associative memory processing. Elife. 2016;5 doi: 10.7554/eLife.09849. * This study reveals that principal cells and interneurons in the hippocampus engage multiple rhythmic oscillations, and that this relationship is influenced by task performance to different degrees depending on the underlying rhythm. For instance, interneurons showed significant phase locking to the beta rhythm during correct choices, while theta phase locking occurred during both correct and incorrect choices. This suggests that rhythmic coordination within the hippocampus in distinctive frequency bands serves different functions during behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Miller EK, Buschman TJ. Cortical circuits for the control of attention. Curr Opin Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/−) mice. Neuron. 2015;85:1332–1343. doi: 10.1016/j.neuron.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundqvist M, Rose J, Herman P, Brincat SL, Buschman TJ, Miller EK. Gamma and Beta Bursts Underlie Working Memory. Neuron. 2016 doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. * In this study, the authors show that hippocampal-PFC theta coherence during performance of a rule-based task results in dynamic reoganization of PFC cell ensembles. Theta coherence emerges with learning, and PFC cell assemblies synchronize to specific phases of hippocampal theta. [DOI] [PubMed] [Google Scholar]
- 28.Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. ** Here, the authors investigated the interaction between hippocampal theta and neocortical gamma oscillations. Results show that gamma frequency oscillations locally generated in the neocortex are modulated by hippocampal theta, suggesting a mechanism through which information can be selectively routed between cortex and the hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. ** This study demonstrates the importance of a direct hippocampal projection to PFC in a spatial working memory task. The authors show that disrupting ventral hippocampal connections to PFC impairs PFC task selectivity and gamma synchrony. This perturbation specifically affects encoding of task-relevant information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadhav SP, Rothschild G, Roumis DK, Frank LM. Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events. Neuron. 2016 doi: 10.1016/j.neuron.2016.02.010. ** In this study, the authors examined whether awake SWRs mediate hippocampal-prefrontal interactions during spatial learning. Prefrontal cortical neurons were found to indeed reactivate during SWRs. Reactivation is coordinated across hippocampal and PFC populations, and distinct PFC populations exhibit excitation and inhibition during reactivation. These results establish that awake SWRs mark times of strong coordination between the hippocampus and PFC that reflects structured reactivation of representations related to ongoing experience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemere C, Carr MF, Karlsson MP, Frank LM. Rapid and continuous modulation of hippocampal network state during exploration of new places. PLoS One. 2013;8:e73114. doi: 10.1371/journal.pone.0073114. * This study investigates the role novelty plays in influencing hippocampal dynamics. During exploration in a novel environment, there is a dynamic relationship between CA3 input to CA1 and movement speed of the animal. Overall, the findings suggest that this graded and continuous modulation of CA1 by CA3 input may allow for processing of different information depending on the behavioral state of the animal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delatour B, Witter MP. Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. Eur J Neurosci. 2002;15:1400–1407. doi: 10.1046/j.1460-9568.2002.01973.x. [DOI] [PubMed] [Google Scholar]
- 35.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 36.Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 38.Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB. A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature. 2015;522:50–55. doi: 10.1038/nature14396. ** This study elucidates a possible role for indirect projections from PFC to the hippocampus via the nucleus reuniens. The authors reveal that goal-related trajectories are represented in distinct structures in this circuit, and selectivity of hippocampal representations depend on the PFC input via nucleus reuniens. [DOI] [PubMed] [Google Scholar]
- 39.Place R, Farovik A, Brockmann M, Eichenbaum H. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci. 2016 doi: 10.1038/nn.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- 41.Remondes M, Wilson MA. Cingulate-hippocampus coherence and trajectory coding in a sequential choice task. Neuron. 2013;80:1277–1289. doi: 10.1016/j.neuron.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson MP, Frank LM. Awake replay of remote experiences in the hippocampus. Nat Neurosci. 2009;12:913–918. doi: 10.1038/nn.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–79. doi: 10.1038/nature12112. * This study showed that hippocampal replay events are biased to represent trajectories toward upcoming rewarded locations in a spatial maze. Temporal replay of place cell sequences during hippocampal sharp wave ripples can thus play a role in guiding future decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 48.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 49.Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 51.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 53.Roumis DK, Frank LM. Hippocampal sharp-wave ripples in waking and sleeping states. Curr Opin Neurobiol. 2015;35:6–12. doi: 10.1016/j.conb.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Singer AC, Carr MF, Karlsson MP, Frank LM. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron. 2013;77:1163–1173. doi: 10.1016/j.neuron.2013.01.027. * This study proposes a role for awake sharp-wave ripples during memory-guided decision making. Here, the authors show that increased coordinated activity of hippocampal place cells during sharp wave ripple reactivation is predictive of future decisions during the initial stage of learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. ** This study specifically disrupted sharp wave ripples during learning of a spatial working memory task, while place cell activity was left intact. Animals with SWR disruption exhibited specific deficits in learning and task performance, demonstrating the necessity of sharp wave ripples for hippocampal memory processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 58.Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75:700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfeiffer BE, Foster DJ. PLACE CELLS. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science. 2015;349:180–183. doi: 10.1126/science.aaa9633. [DOI] [PubMed] [Google Scholar]
- 60.Remondes M, Wilson MA. Slow-gamma Rhythms Coordinate Cingulate Cortical Responses to Hippocampal Sharp-Wave Ripples during Wakefulness. Cell Rep. 2015;13:1327–1335. doi: 10.1016/j.celrep.2015.10.005. * Here, the authors show that neurons in the cingulate cortex are modulated by hippocampal sharp wave ripples and slow gamma oscillations. The degree to which the cingulate neurons respond to sharp wave ripples is influenced by the strength of modulation by hippocampal slow gamma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Fujisawa S, Buzsaki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wikenheiser AM, Redish AD. Hippocampal theta sequences reflect current goals. Nat Neurosci. 2015;18:289–294. doi: 10.1038/nn.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foster DJ, Wilson MA. Hippocampal theta sequences. Hippocampus. 2007;17:1093–1099. doi: 10.1002/hipo.20345. [DOI] [PubMed] [Google Scholar]
- 66.Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–159. doi: 10.1038/nrn.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA. Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron. 2016;89:857–866. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng S, Frank LM. New experiences enhance coordinated neural activity in the hippocampus. Neuron. 2008;57:303–313. doi: 10.1016/j.neuron.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64:910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C, Bieri KW, Hsiao YT, Colgin LL. Spatial Sequence Coding Differs during Slow and Fast Gamma Rhythms in the Hippocampus. Neuron. 2016;89:398–408. doi: 10.1016/j.neuron.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siegle JH, Wilson MA. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. Elife. 2014;3:e03061. doi: 10.7554/eLife.03061. ** Here, the authors studied the effect of phase specific manipulation of theta oscillations in the hippocampus during learning of a spatial navigation task. Their results suggest that encoding and retrieval of task-relevant information takes place at specific phases of theta. [DOI] [PMC free article] [PubMed] [Google Scholar]


