Abstract
Advantageous maneuvering through the environment to find food and avoid or escape danger is central to survival of most animal species. The ability to do so depends on learning and remembering different locations, especially home-base. This capacity is encoded in the brain by two systems: one using cues outside the organism (distal cues), allocentric navigation, and one using self-movement, internal cues (proximal cues), for egocentric navigation. Whereas allocentric navigation involves the hippocampus, entorhinal cortex, and surrounding structures, egocentric navigation involves the dorsal striatum and connected structures; in humans this system encodes routes and integrated paths and when over-learned, becomes procedural memory. Allocentric assessment methods have been extensively reviewed elsewhere. The purpose of this paper is to review one specific method for assessing egocentric, route-based navigation in rats: the Cincinnati Water Maze (CWM). The test is an asymmetric multiple-T maze arranged in such a way that rats must learn to find path openings along walls rather at ends in order to reach the goal. Failing to do this leads to cul-de-sacs and repeated errors. The task may be learned in the light or dark, but in the dark, wherein distal cues are eliminated, provides the best assessment of egocentric navigation. When used in conjunction with tests of other types of learning, such as allocentric navigation, the CWM provides a balanced approach to assessing the two major forms of navigational learning and memory found in mammals.
Keywords: learning and memory, egocentric learning, route-based navigation, mazes, Cincinnati water maze, swimming maze
1. INTRODUCTION
1.1 Navigational learning and memory
Navigation is the ability to find one’s way through the environment and get ‘home’ without getting lost. All mobile organisms have this capacity, i.e., the ability to leave a nest, burrow, den, or home-base of any kind and move through the environment to forage for food (hunt or graze), find water, avoid predators, locate mates, socialize, defend territory, and return safely to the home-base. This ability is essential to survival and is conserved in phyla from insects, such as ants (Wittlinger et al., 2006) and bees (Menzel et al., 1998; Henry et al., 2012), to avians, fish, and bats (Heys et al., 2013), and all terrestrial mammals (Etienne, 1992). In birds, bats, fish, and marine mammals, navigational ability operates in three dimensions and in migratory species over great distances.
1.2 Types of Navigation
There are two types of local navigation: allocentric and egocentric. Allocentric or spatial navigation is characterized by the ability to locate places using distal cues, meaning, using environmental features outside and not close to the organism. Egocentric navigation is characterized by the ability to locate places using proximal and internal cues, including limb (or wing) movements to estimate speed and heading to estimate direction, as well as optokinetic visual flow when moving past objects when there are visual cues available. Allocentric navigation requires visual or auditory cues, whereas egocentric navigation can operate in darkness, although when visual cues are absent, egocentric navigation is sometimes less accurate.
Egocentric navigation includes route-based and path integration. Both rely on internal cues, but whereas route-based navigation refers to a specific path through the environment, path integration involves vector addition. In route-based navigation, an organism follows a specific route with the order and location of turns repeated as precisely as possible, such as, straight-left-right-left-left or which direction to turn when a recognized signpost is encountered or an estimated distance is travelled. In humans, one way of assessing this is to have subjects walk around a marked circle and then have them retrace it blindfolded. Individuals with no neurological impairment do this fairly well, whereas people with striatal damage have great difficulty (Paquette et al., 2011).
A characteristic of egocentric navigation is that when routes are over-trained they become automatic and require little active attention. This form of memory extends to habits and becomes part of implicit, procedural, and episodic memory (Buzsaki and Moser, 2013). These memories are manifest as skilled behavior, such driving a car, riding a bicycle, throwing a ball, hitting a baseball, skiing, etc. and are consolidated in the prefrontal cortex as are most long-term memories (Jog et al., 1999). Allocentric navigation can also become routine, as in driving a familiar route repeatedly but it is not typically classified as implicit.
Path integration, on the other hand, is the ability to leave a starting location, move to different locations and return to the start by a different, more direct path than the outbound journey. An organism could travel from its home-base to point A, then B, and finally to C and return home traveling more directly from C to home by adding the paths together and taking the shortest route back, a process akin to triangulation (Buzsaki and Moser, 2013).
Path integration is assessed in humans in multiple ways. One way is to blindfold subjects and lead them through a large open room. After walking them in different directions, they are stopped and asked to point to where they started. Subjects with no neurological impairment show a range of errors around the point of origin but are fairly accurate. Those with damage to the hippocampus and entorhinal cortex do this task as well as controls (Shrager et al., 2008), indicating that allocentric mechanisms are not involved in this form of navigation, but subjects with striatal injury are impaired (Buzsaki and Moser, 2013). Hence, egocentric and allocentric navigation are mediated by different neural networks. Since these neural networks are important in human navigation and are closely linked to all memory systems, it is useful to be able to differentiate these systems in rodents when used as models of human memory impairment caused by a CNS perturbation. Making the distinction experimentally between these two forms of memory is not always easy however, because the systems overlap. The result is that treatments or conditions that disrupt one type of navigation sometimes affect both. This difficulty does not suggest that distinguishing between memory types is impossible, but rather that disentangling their distinct functions must be done carefully with attention to the differences and the methods used to distinguish them.
In natural environments, navigation is characterized by the range or space over which an organism moves to forage for food, find mates, hunt, or defend territory. The size of a species’ range varies widely. For some organisms it may be less than an acre; in some, a few acres; and in some, over large regions. Migratory species have very large ranges, such as when monarchs migrate from Canada to Mexico to overwinter, and bird species migrate south from Canada to the southern United States with the longest being the Arctic tern (Sterna paradisaea) that migrates from the arctic to Antarctica up to 90,000 km (56,000 mi). Among mammals, whales, elk, wildebeest, and many other species migrate long distances on a seasonal basis. Long distance navigation in birds and insects relies on systems not discussed here and involve detection of magnetic fields, orientation to the sun, olfactory cues, and other mechanisms (Barkan et al., 2016).
2. BRAIN REGIONS MEDIATING NAVIGATION IN MAMMALS
2.1 Spatial Navigation
Brain networks that mediate navigation are made up of circuits involving multiple regions. In the case of allocentric navigation, the primary regions are the hippocampus (O’Keefe and Nadel, 1978) and entorhinal cortex (Brandeis et al., 1989; McNamara and Skelton, 1993a; Moser et al., 1998; Burgess et al., 2002; Whitlock et al., 2006; Suh et al., 2011; Penner and Mizumori, 2012; Buzsaki and Moser, 2013).
2.2 Egocentric Navigation
Brain regions that mediate egocentric navigation are less well studied and as noted, ego- and allocentric networks overlap (Sherrill et al., 2013). However, it is clear that head direction cells are important for egocentric navigation. Discovered in the presubiculum, head direction cells have since been found in other regions, including the entorhinal cortex, thalamus, mammillary nucleus, retrosplenial cortex, and dorsal striatum (van Strien et al., 2009). Under some conditions ego- and allocentric systems can be dissociated, e.g., hippocampal lesions result in spatial, but not egocentric, deficits in the hidden platform version of the Morris water maze (MWM), whereas dorsal striatal lesions result in visible platform (hence, egocentric), but not spatial, deficits in the MWM (Packard and McGaugh, 1992; McDonald and White, 1994; Devan et al., 1999). When humans use virtual environments, egocentric/path integration tasks recruit neural activity in and beyond the striatum involving parts of the hippocampus and parietal cortex (Sherrill et al., 2013).
The key distinction between allocentric and egocentric navigation are the types of cues involved. Allocentric navigation depends on cues outside the organism, whereas egocentric navigation depends on cues inside or near the organism. Therefore, in developing tests for these abilities, it is necessary that a test for allocentric navigation be designed so that it has ample distal cues but is devoid of proximal cues, whereas a test assessing egocentric navigation should be designed to provide proximal cues and minimize distal cues. The latter can be done by testing egocentric navigation in a dark environment or while blindfolded. An alternate approach is to design tasks that make both types of cues available during learning, and then during recall allow the animal to select a response that reveals which system it relied on during acquisition. An example of such a task is the Star Maze, where one set of cues, if relied upon, leads animals in one direction, but where another set of cues, if relied upon, leads animals in a different direction (Rondi-Reig et al., 2006; Fouquet et al., 2013). In this test the relative reliance of the animal on one system versus the other is compared. A shift in preference caused by an experimental treatment may alter the relative proportion of animals choosing one system over the other. However, one cannot determine if a shift in preference is caused by a deficit in the non-preferred memory system or an enhancement of the other.
Regardless of the task, learning vs. performance distinctions are always critical. As with all learning and memory assessments, methods that use multiple measures of behavior to provide converging evidence are best at ensuring that results are interpreted as reflecting learning and memory and are not confounded by performance factors such as differences in motivation, thigmotaxis, motor ability, coordination, vision, lethargy, etc. In appetitive tasks, this means ensuring that animals are matched for the incentive value of the reinforcer. For shock-motivated tasks, this means equating groups for shock threshold and reactivity. For swimming tasks, this means ensuring that groups are equal in swimming ability, usually determined by measuring swim speed, but also by ensuring that subordinate task requirements are eliminated through pretraining. For example, in the MWM one should conduct training so the animals learn that the platform is the escape, that there is no escape other than the platform, and that the platform is not near the wall. If the animal is not trained in swimming tasks and the task is too difficult, the result can be behavioral ‘despair’ as seen in tasks such as the Porsolt forced swim test (FST) where animals give up searching and tread water. In the FST, giving up is the sought after behavior, but in water mazes this defeats the purpose of the test; therefore, it is important to avoid this by training in a way that minimizes frustration.
3. ASSESSING NAVIGATION IN RODENTS
3.1 Types of Tasks
Many tasks have been developed to assess allocentric navigation and they will not be reviewed here. The best known and most widely used is the MWM that we and others have reviewed (Morris, 1984; Brandeis et al., 1989; McNamara and Skelton, 1993b; Stewart and Morris, 1993; Lipp and Wolfer, 1998; D’Hooge and De Deyn, 2001; Miranda et al., 2006; Vorhees and Williams, 2006; Vorhees and Williams, 2006; Terry, 2009; Mulder et al., 2012; Vorhees and Williams, 2014a, b). Rather than a maze in the usual sense of a labyrinth, the MWM is an open pool that is featureless on the inside so as not to provide proximal cues and the goal is a hidden platform submerged below the surface that is kept in one location. Navigation in this setting requires the animal to find the platform from different start locations around the perimeter of the tank on successive trials so that no single route provides a direct path to the goal.
Another widely used test of spatial learning and memory is the radial-arm maze (RAM). Typically, the RAM is used as an appetitive test, either with all arms baited at the start of every trial to assess trial-dependent, spatial working memory, or with some arms baited on every trial and some never baited as a mixed procedure intended to assess working and spatial reference memory. Appetitive tasks have some advantages inasmuch as they use positive reinforcement, but they require more training than water mazes and require food deprivation. In addition, when used in genetic, pharmacological, or toxicological studies, it can be difficult to equalize food deprivation and reinforcement value in appetitive tasks if the treatment changes body mass, palatability of the reinforcer, or reward value.
To avoid the problems with appetitive tasks, swimming versions of the RAM have been developed that rely on negative reinforcement, i.e., radial-arm water mazes (RWM). As with the RAM, there are different versions. There are also a variety of protocols. One protocol that models the appetitive RAM uses a timed intertrial interval (ITI) to ensure that chaining does not occur (i.e., where animals always turn left, or follow some other rule rather than remembering which arms were previously visited). In this version, one arm serves as the start and the others have submerged platforms. On the first trial, the animal can enter any arm and find a platform to escape just as in the appetitive RAM where any choice leads to food and reduction of hunger. The animal is then removed for a specified length of time and the visited platform is also removed. On the second trial, if the animal remembers the arm it chose, it may revisit that arm expecting to find the platform again. Once it discovers that it is not available, it will go to another arm. Once this ‘rule’ is learned, that the platform is never in the same place on successive trials, it should not reenter arms previously entered but instead switch to a different arm on each trial. This continues until all arms have been visited. This can be adapted just like the appetitive RAM such that some arms have platforms at the start of each trial and others do not. The RWM requires about 10 days for rats or mice to become proficient (Bimonte and Denenberg, 1999; Bimonte et al., 2000). The RWM has been used in a variety of settings (French et al., 2007; Acosta et al., 2010; Mika et al., 2012), and a human virtual version has been developed (Mennenga et al., 2014). One of the things that distinguishes other mazes from the MWM is that extramaze cues are more critical in an open pool such as the MWM than in structured mazes such radial maze.
3.2 Egocentric Tests
The Cincinnati water maze (CWM), a labyrinthine maze, may be used in the light or dark, but provides the most stringent test of egocentric learning and memory when run in the dark (Vorhees et al., 2008; Vorhees et al., 2009a; Vorhees et al., 2009b). Land-based mazes can be used with blindfolding to eliminate distal cues (Maaswinkel and Whishaw, 1999), a procedure not well suited for water mazes (see below). As noted, labyrinthine mazes are suited for testing egocentric navigation since they contain proximal cues that rely on self-motion for estimating distance, direction, and signposts in order to find the goal. There are many types of mazes but the interpretation of what they show is difficult if distal cues are present. As noted, the principal solution to this is to test animals in the dark under infrared light as we have done in the CWM (Vorhees et al., 2008). Another approach is to use a circular arena with a home base from which animals exit and explore to find food in the light. In this test, the outbound journey is always done in the light, but the inbound journey back to the home base is varied. When the homebound journey is under lighted conditions, it is an allocentric and egocentric task; when the homebound journey is in the dark it is an egocentric only task (Whishaw et al., 2001). Therefore, whether tests use an open arena or labyrinth, the key feature for separating allocentric from egocentric strategies is the presence or absence of distal cues.
Before discussing the CWM in detail, there are features of water mazes in general that are worth noting. These include: (1) water is an equal-opportunity motivator. Water equalizes motivation to escape over a wide range of body weight differences (Cravens, 1974). Appetitive tasks can be problematic if a treatment causes differences in body weight, appetite, or reward salience, since under these conditions motivation is not equal across groups. (2) Swimming is impervious to substantial differences in locomotor activity. Even rats with significantly increased or decreased open-field activity typically show equal swimming speeds. This principle holds except at extremes where severe hypo- or hyperactivity may cause an animal’s swim speed to change enough that it alters the rate at which it encounters the goal by simple trial-and-error. When this happens, the mere fact of finding the platform faster allows the animal to more quickly eliminate errors and hence learn the task faster. The opposite is also true, sluggish swimming if severe enough, will slow the rate of learning. (3) Almost without exception the vast majority of Sprague-Dawley (SD), Long-Evans (LE), Wistar, and Fischer-344 rats complete swimming tasks; however, some strains of mice cannot learn the CWM (e.g., C57BL/6) and those that can learn it perform poorly (Schaefer et al., 2011); this may be because it is at or beyond their capacity, or they do not have the persistence to search long enough to find the escape (species-specific response characteristic). (4) 100% of control rats, when tested in the light, and ~95% of rats when tested in the dark, master the CWM, i.e., they improve steadily across trials and eventually become proficient. Improvement is important because flat or shallow learning curves prevent one from detecting group differences as do very steep learning curves. Both extremes present interpretational concerns about whether the test is valid; if animals cannot learn it at all or learn so quickly that the test is too easy. In essence a test of learning has to show intermediate learning. (5) Minimal training is another advantage of water mazes. Because rodents are natural swimmers, only a few trials are needed to learn that active searching leads to escape. The most effective way to introduce the task is to give a few trials in a task requiring as close to no learning as possible, such as a straight swimming channel. In the straight swimming channel, the only requirement, other than swimming, is to travel from one end to the other. Despite its simplicity, straight swimming trials before complex maze trials are essential. These trials demonstrate to the animal that there is an escape. Without this, the difficulty of complex labyrinthine mazes can cause frustration and surrender, much as seen in the FST. Straight channel swimming trials given before maze testing prevent this behavior. (6) Swimming mazes are efficient compared with other learning tests, i.e., within a few days, with relatively few trials per day, and relatively short times per trial, rats show good learning curves (not too steep and not too shallow). This is very different from appetitive mazes. Appetitive mazes may have similar numbers of trials per day but often require more days of training, more days of testing, and longer times per trial, because animals exhibit off-task behaviors (sniffing, grooming, looking, rearing, urinating, defecating) not seen in water mazes. (7) Water is as motivating on the last trial as it is on the first, whereas appetitive tasks are subject to satiation that can produce declining motivation as more rewards are obtained. Satiation effects can be controlled by limiting the number of rewards animals are allowed to earn per day and if they are kept very hungry in relation to the size of the rewards. But it should never be forgotten that appetitive tasks require food restriction to 85% of the animals’ free feeding weight or they will not perform. Lacking food deprivation, even positive reinforcement will not motivate animals enough to learn and hunger is an aversive form of motivation.
Water mazes have limitations as well as advantages. (1) One limitation is species-specific response characteristics. As noted, some inbred strains of mice tend to float rather than actively search. Another problem seen in some mice is persistent thigmotaxis in which they search the edges but not the rest of the maze. Mice also can be subject to fatigue because of their small body mass and therefore are more susceptible to hypothermia that can interfere with searching. (2) The most frequent criticism of water mazes is that they cause stress. However, all behavioral tests cause some stress; some more than others but none are stress-free. For example, handling animals, placing them in novel environments, restraint, all produce stress. In food restriction paradigms, protracted increases in corticosterone as well as behavioral changes have been observed (Pesic et al., 2010). In the MWM we showed that there are increases in corticosterone in animals after multiple days of testing, but these increases are short-lived (i.e., resolve within an hour) and, in our experiments, did not interact with the independent variable (Skelton et al., 2007). The implication of critics of water mazes is that stress is negative under all circumstances and should be avoided at all costs, never mind that these critics routinely use food deprivation that is stressful, especially for extended periods of time. It has even been suggested that swimming-induced stress may act as a confounder. According to this view, swimming-induced stress might interfere with learning or could even interact with the independent variable and result in a complex effect that is not exclusively cognitive. There is no doubt that stress could interact with the variable under study and if this occurred it might not be entirely apparent. However, such interactions are just as possible in appetitive, shock, restraint, or novel environment tasks. Furthermore, it is incorrect that stress is always negative; in reality stress is an adaptive response when it modulates arousal. The relationship between arousal and performance is an inverted U-shaped as described by the Yerkes-Dodson function (Chaby et al., 2015), and there is a direct relationship between arousal and stress (Winsky-Sommerer et al., 2005). Too little as well as too much are both counterproductive to performance, but in the mid-range, stress/arousal facilitates performance. Critics of water mazes omit from their critique that assessing learning and memory requires an appropriate level of arousal, a level that incentivizes performance. Most learning tests are validated empirically and not by direct measurement of stress/arousal markers. Using this criterion, rats show high levels of learning in the CWM and exhibit high levels of retention. Given this, it is not credible to argue that the task is too stressful to induce adequate arousal since excessive stress, by definition, would cause interference and degrade performance, something not seen in the CWM, MWM, or RWM. In fact, just the opposite is seen: highly efficient learning. Such evidence demonstrates that stress-induced interference associated with swimming is not a critical issue with water mazes.
Another factor in the use of water tasks is water temperature. Within limits, warmer water (that reduces thermal stress) slows learning. When the MWM was introduced, some thought that room temperature water was too cool and therefore too stressful, so they tested animals in warm water. If this hypothesis were true, animals would perform better in warm water than in room temperature water, but the exact opposite is seen. Warm water degrades performance. Conversely, cooler water (as long as not too cold) facilitates learning; it does not degrade learning as would be predicted based on a stress hypothesis. We find that room temperature water is highly effective for rats and mice (so long as mice are given an adequate ITI). We find that water of 20-22 °C works very well for motivation. It is worth mentioning that, as is well-known, the degree of stress is affected by locus of control over the stressor, which in water mazes, is whether escape is possible. When conditions are arranged to make escape impossible, high levels of stress are induced as in the FST and other learned helplessness procedures. When conditions are designed to make escape probable, as in the water mazes, escape is reinforcing, facilitates learning, and reduces stress after initial trials. In what follows, the focus is on rat egocentric learning and memory.
3.3 Biel Water Maze
The forerunner of the CWM was a maze designed by William Biel (Biel, 1940). The BWM is shown in Fig. 1. The path Biel used to test rats is marked S (start) and G (goal). For the sake of discussion, Biel’s method, in which rats entered the maze at point S and escaped at point G will be called path-A. Since Biel’s maze was tested under visible light, rats had access to proximal and distal cues but Biel designed the task to reduce distal cues by enclosing the maze. He did this by placing the maze in a large container. He made the maze walls 30.5 cm high, and mounted it on 73.7 cm legs for a height of 104 cm. The ceiling of the box was 122 cm high, hence, there was 18 cm of space between the top of the maze and the ceiling of the container. He mounted 9 incandescent lights on the container ceiling (Biel, 1940).
Fig. 1.
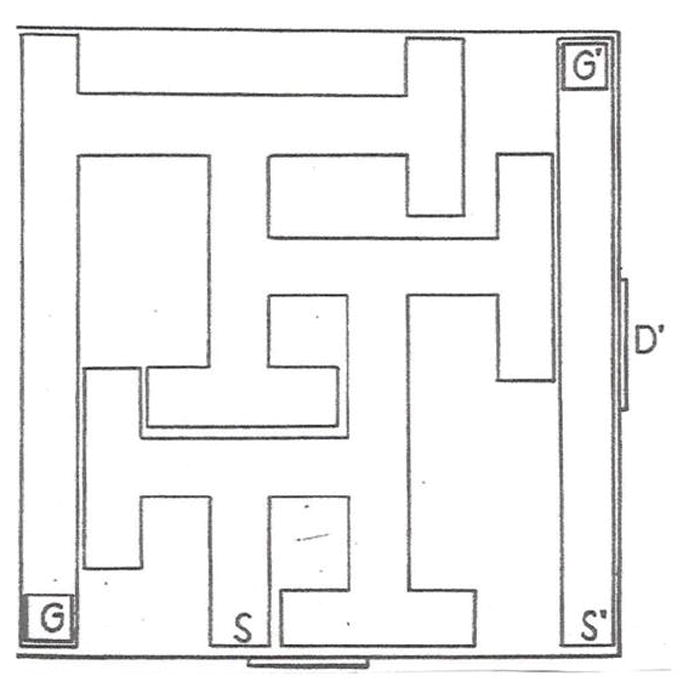
Biel Water Maze (BWM). Schematic of the BWM from Biel’s original publication (Biel, 1940).
Biel used a split-litter design. He took pairs of males and females from each of 14 different litters and divided them by age for testing. The subsets were tested starting at postnatal day (P)16, 19, 22, or 29. Not surprisingly, he found age-dependent differences in learning, with improved performance at older ages. The average number of errors on the first trial after pretraining in a straight swim channel (marked S’ and G’ in Fig. 1) was 4-5 in the youngest age group. The oldest group averaged ~3 errors on the first trial. This is noteworthy because it speaks to the relative ease of the task. If one imagines a rat navigating through the maze starting at point-S and the rat swims straight until it encounters a perpendicular wall where it is forced to turn left or right, it has a 50:50 chance of making a correct turn by chance alone. Given that the maze has 6 decision points, if a rat performed at chance it would make 3 errors on average, very close to what the oldest group did. A second aspect of the maze is that Biel gave the rats straight channel trials before putting them in the maze (see Fig. 1). He did this to acclimate them to the task and provide reinforcement for climbing the escape ramp. A third aspect is that Biel’s straight channel was separate from the maze.
After Biel published his experiment, no further use of the maze is found in the literature for 23 years (Polidora et al., 1963). When revived, it was used to assess learning in an experimental model of phenylketonuria. In a series of studies, rats given high doses of dietary phenylalanine to mimic what is seen in children with phenylketonuria, made more errors and took longer to find the escape than controls (Polidora et al., 1963; Polidora et al., 1966a, b). In the paper (Polidora et al., 1963), the maze was the same as Biel’s, but now it was used in adult rats. However, the idea of covering the maze was no longer included. Polidora et al. retained straight channel premaze testing as Biel had done. When put in the maze, instead of 1 or 2 trials per day as Biel had done, Polidora et al. gave 5 trials but in the same path (S to G; S was later renamed Point-A and G was renamed Point-B, hence swimming from A to B became Path-A and swimming the maze in reverse from B to A became known as Path-B; see Fig. 2. An indication of the ease with which the maze was learned is shown by the average number of errors made on trial-1, i.e., 4-5. Although higher than 3 errors per trial average in Biel’s P29 group, the difference in error rate may be because of age or strain differences between the two studies.
Fig. 2.
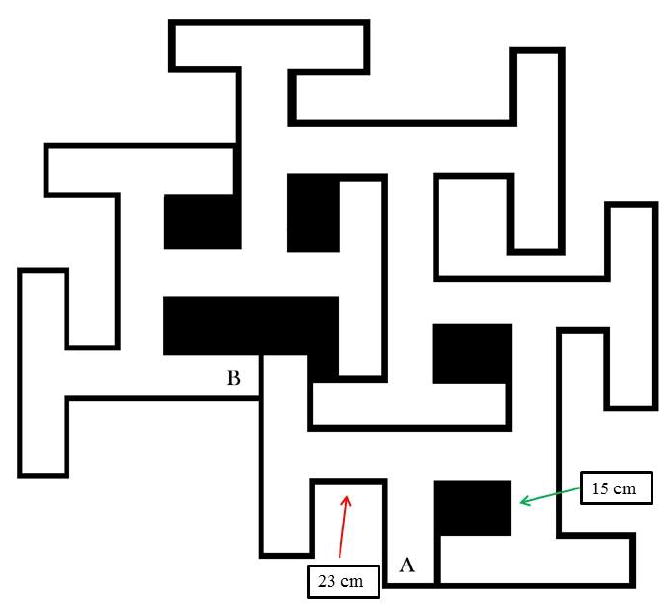
Schematic of the Cincinnati Water Maze with basic dimensions shown. Channel widths are 15 cm and T-section stems are 15 cm with one exception noted by the red arrow where one stem is 23 cm long. This permits the maze to have 9 T-shaped cul-de-sacs. The maze walls are 51 cm tall and the maze is filled to a depth of 20 cm with water.
The next use of the BWM was by the same group (Polidora et al., 1966a, b). They gave 5 trials/day in path-A, but for the first time tested animals in the reverse path, starting at point G with the goal at point S (Path-B; see Fig. 1). Polidora et al. reported that in path-A, Long-Evans control rats made an average of 8-9 errors on day-1 and <2 errors/trial by day-3. When the rats were tested in path-B, controls averaged 12 errors/trial (Polidora et al., 1966b). This was the first hint that path-A and path-B were not equally difficult despite the positive transfer that should occur from learning path-A first.
The next use was four years later (Butcher, 1970; Butcher et al., 1970). In these experiments, premaze straight channel trials were also given before maze trials. Adult Sprague-Dawley rats given hyperphenylalaninemic diets prenatally (Butcher, 1970) or at weaning (Butcher, 1970) were given 5 trials per day for 6 days, 3 days in path-A and 3 days in path-B. The average number of errors across all trials showed large experimental effects of the phenylketogenic diet compared with controls. Details of the method may be found elsewhere (Butcher, 1969). On day-1, rats received 5 trials in the long (127 cm) arm of the BWM as the straight channel pretest. On the next 3 days, rats received 5 trials per day in path-A, followed by another 3 days of 5 trials/day in path B. The trial time limit was 10 min. On day-1 of path-A, control rats averaged 25 errors1 whereas the experimental group averaged 40 errors. On path-B, controls averaged 32 errors while the experimental group averaged 150 errors. This experiment demonstrated that path-B was not only more difficult, but more difficult in a way that increased errors disproportionately from path-A. These data increased interest in the maze, but there was no understanding of what kind of learning and memory (L&M) was being measured or why path-B was more difficult than path-A. It was also used in an inbred strain of rats with hyperbilirubinemia (Butcher et al., 1971; Butcher et al., 1972b) and after prenatal acetylsalicylic acid exposure (Butcher et al., 1972a; Kimmel et al., 1974). In these experiments, rats were tested for 2 or 3 days in path-A and 2 days in path-B for 5 trials/day and total errors were found to be significantly increased in the experimental groups.
The BWM continued to be used in models of induced phenylketonuria (Butcher et al., 1977) and after prenatal hypervitaminosis A (Vorhees et al., 1978), and procedural changes were also gradually introduced. Rather than a 10 min trial limit, by 1977 the trial limit was decreased to 6 min. This was done based on fatigue noted in rats that reached the 10 min limit. Nevertheless, the maze continued to be effective at differentiating treatment-related effects of various independent variables, but there were also treatments that did not cause changes in learning on the task. One study showed that high acute doses of methylphenidate enhanced performance by increasing swim speed so much that latency and errors to find the goal were reduced (Kinney and Vorhees, 1979). No effect findings occurred after prenatal exposure to prochlorperazine, fenfluramine, or propoxyphene (Vorhees et al., 1979). The BWM procedure of 5 trials/day was later used to study the prenatal effects of naloxone (Vorhees, 1981).
The next revision to the BWM involved reducing the number of trials per day. The revised method consisted of 2 trials on day-1 and 3 trials on day-2 in path-A, followed by 3 days of 2 trials/day in path-B. This was done to further ensure that cognitive differences, not fatigue, were being measured. A number of drugs were tested using this procedure, i.e., prenatal exposure to phenytoin, phenobarbital, or trimethadione (Vorhees, 1983, 1987a), prenatal ethanol (Vorhees and Fernandez, 1986), postnatal exposure to large neutral amino acid supplements as a treatment against hyperphenylalaninemia-induced L&M deficits (McSwigan et al., 1981), and prenatal methylazoxymethanol exposure (Vorhees et al., 1984). BWM performance showed no effects from pre- and postnatal exposure to caffeine (Butcher et al., 1984), prenatal exposure to (+)-amphetamine (Vorhees, 1985a), or prenatal exposure to a low dose of methylmercury (Vorhees, 1985b).
It was at this time that concerns were raised about the design of the BWM. In addition to concerns about design features, the BWM was small and large male rats found ways to prop themselves at corners with their legs in such a way that they did not have to swim but could stay perched halfway out of the water. When this occurred, the rats would spend long intervals propped in order to avoid swimming. But, of greatest importance was how rats solved path-A and path-B. Vorhees noted that most rats use a swim-straight-until-forced-to-turn strategy, i.e., until they encounter a perpendicular wall and only then did they make a decision as to which way to go next. This is the dominant pattern, although a minority try following walls, i.e., thigmotaxis. However, thigmotaxis, while it could work in theory, always fails in practice because they eventually deviate from it, and once they do they can end up actually going back toward the start. No rat has ever been found that successfully follows one wall all the way from start to finish. In path-A, the swim-until-forced-to-turn strategy is relatively successful because of the way the maze is designed as noted above, i.e., that at each junction there is a 50:50 chance of turning in the correct direction by chance. However, in path-B this strategy produces a low level of success because using it results in entering a dead-end T cul-de-sac at the end of each channel.
These concerns culminated in the invention of a new maze: The Cincinnati Water Maze (CWM). The maze is illustrated in Fig. 2. For consistency, the CWM is labeled similarly, that is, Points A and B are in the same relative positions as they are in the BWM. Both mazes share the same asymmetry and the strategy needed by rats to successfully find their way through Path-A vs. Path-B is different.
To illustrate the ease of the path-A strategy, see Fig. 3 (right) where this is shown for the CWM. Contrast this with path-B (Fig. 3, left). In path-B the rat has to learn to turn along the length of a corridor or else it will enter a dead-end T and then has to double-back until it finds the sidearm. This seems obvious to an experimenter seeing the maze from above, but rats find it difficult. Vorhees was also concerned about other features of the BWM. The BWM was small and large rats propped themselves as noted above. Furthermore, the material the mazes were made of (sheet metal) had seams that rats tried to use to climb out. Additionally, the BWM has a long arm at the goal end that appeared to act as a cue as evidenced by the fact that when rats entered this channel they visibly sped up, anticipating reaching the escape. Most salient, however, was that the maze was too easy. While path-B was more difficult than path-A, it was not very challenging for rats.
Fig. 3.
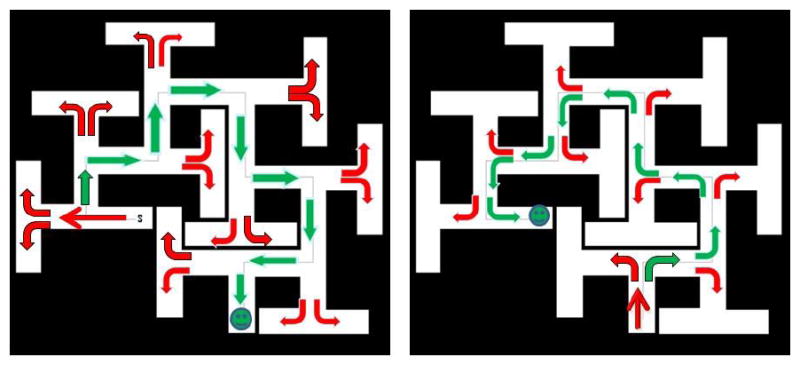
Cincinnati water maze (CWM). The CWM with arrows to denote correct (green) versus incorrect (red) turns through the maze. Right, arrows depicting turning points for path-A. Left, arrows depicted turning points for Path-B. Note that at choice points in path-A there is always a possible correct (green) or incorrect (red) choice, whereas in path-B there are only incorrect choices (red) if the rat swims to the end of the corridor. Only if a rat turns halfway down the corridor is a correct (green) turn possible in path-B.
3.4 Cincinnati Water Maze
3.4.1. Design
To summarize the design changes, recall that the BWM is a 6-unit multiple T-maze, but because it has a long arm at Point-B, it is effectively a 5-unit T-maze. By contrast, the CWM is a 9-unit multiple-T maze and has no long arm, hence the CWM is 80% more complex (Fig. 2). The CWM has wider channels (15.2 cm vs. 12.7 cm wide); this may not seem very different but it had a remarkable effect on performance as it prevented rats from being able to prop their legs against opposing corners to keep from having to swim and they could also keep themselves halfway out of the water by this maneuver. The CWM was constructed of acrylic (Plexiglas), thereby eliminating seams and making the walls impossible to climb or even attempt to climb which eliminated off-task behavior (both propping and attempts at climbing). The singular element the CWM preserved was asymmetry, i.e., for all path-A choice-points there is a 50:50 chance of making a correct turn, and for all path-B choice-points there is a zero chance of making a correct turn if made at the end of a corridor; only a turn halfway down a corridor provided access to the next part of the maze (Fig. 3). To test the new design, an experiment was conducted to compare the two mazes.
3.4.2 Early Uses
In the initial comparative experiment (Vorhees, 1987b), prenatal exposure to phenytoin was used as a positive control (3 dose levels and control). Phenytoin was used since it was already known to affect BWM performance (Vorhees, 1983, 1987a). The procedure involved 5 premaze trials in a 127 cm straight swimming channel. Then separate groups (littermates from each litter randomly assigned to one maze or the other) were tested. For the BWM the procedure was as above, i.e., 2 trials on day-1 and 3 trials on day-2 in path-A, followed by 2 trials/day for 3 days in path-B. For the CWM this was changed slightly based on concern about the greater difficulty of the maze. Therefore, rats in the CWM received 3 days, 2 trials/day in path-A and 3 days, 2 trials/day in path-B. This resulted in a slight imbalance in that rats in the BWM had a total of 11 trials while those in the CWM had 12 trials. For simplicity the data were redrawn using only the 200 mg/kg phenytoin group. For the BWM, no effects of prenatal phenytoin were found on path-A for errors (Fig. 4A) or latency whereas for the CWM the high dose phenytoin group (200 mg/kg) showed increased errors (Fig. 4B) but not latency on path-A. In the BWM for path-B, all three phenytoin groups had significantly increased errors (Fig. 4C high dose only shown). In the CWM for path-B, the 100 and 200 mg/kg (Fig. 4D) phenytoin groups had significantly increased errors and latencies but the 150 mg/kg group, although different from control this difference fell short of significance (P<0.10). In addition, the magnitude of the effects in the two mazes differed. When path-A and path-B errors as summed, the effects of the high dose phenytoin in the BWM was significant (Fig. 4E) but in the CWM the effects was much greater (Fig. 4F). For combined paths summed across days, controls made 87 errors in the BWM, whereas they made 127 errors in the CWM, nearly 50% more. The high dose phenytoin group made 145 errors in the BWM with paths combined, and 265 errors in the CWM with paths combined, greater than 80% more. This shows that path-B was not only better at detecting effects than path-A, but that the more challenging CWM path-B increased the magnitude of the differential effect of phenytoin compared with that for path-B in the BWM. Based on these data, we replaced the BWM with the CWM.
Fig. 4.
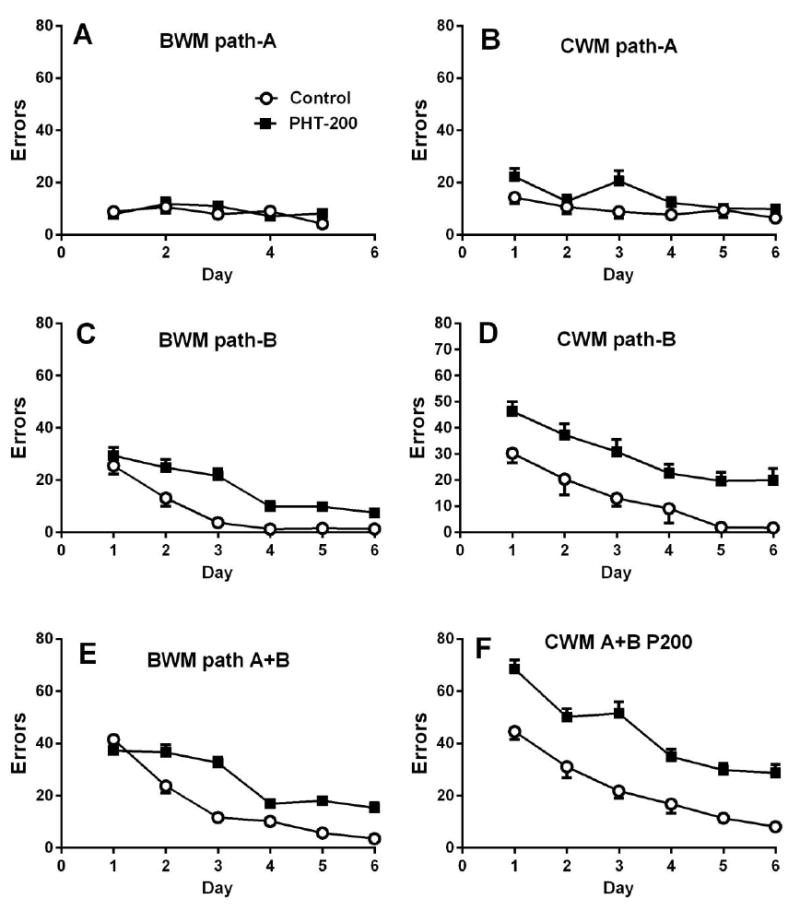
Comparison of the effects of prenatal phenytoin (PHT) on BWM vs. CWM in rats (Vorhees, 1987b). Data are Mean ± SEM errors. Note the large differences in outcome between the two mazes (ordinates are on the same scale for comparative purposes). In this experiment gravid Sprague-Dawley rats were administered 0, 100, 150, or 200 mg/kg of PHT on E7-18. For simplicity only the PHT-200 and Control groups are redrawn here. Rats all received 5 trials in a 127 cm straight water channel the day before maze trials. Significance indicators (asterisks) have been removed for this comparison. See text for which effects were significant. There were 6 trials in both mazes for path-B and in the CWM for path-A but only 5 trials in the BWM on path-A, therefore, the BWM path-A trial-6 data point is an estimate extrapolated from the slope of this group’s curve.
During the next several years, a series of experiments with different drugs given during development were evaluated in the CWM, and the method remained consistent. The only change was that the premaze straight channel was lengthened to 150 cm based on the idea that a longer channel would provide a better index of swim speed. The maze procedure was 6 trials in path-A followed by 6 trials in path-B, typically 2 trials/day. The time limit was 6 min/trial. The only variation was that most experiments gave 2 trials/day, in two experiments only, though, 1 trial/day was given, but the same total number of trials was used, i.e., 12. For the 2 trial/day experiments, significant CWM effects were found in hyperphenylalaninemic rats, and these deficits were mitigated by treatment with branched chain amino acids as an hypothesized therapeutic treatment (Vorhees and Berry, 1989). Other experiments showed large effects of prenatal sodium phenytoin on path-A and path-B errors (Weisenburger et al., 1990) and significant but not as large increases in errors after prenatal ethanol (Vorhees, 1988). An unusual finding was seen after prenatal administration of 2-methoxyethanol. In this case, increased errors in path-A were observed but not on path-B (Nelson et al., 1989). Among the experiments with 1 trial/day, the effects of prenatal phenytoin were shown on CWM errors for both path-A and path-B, but the effects were (as before) much larger for path-B (Vorhees and Minck, 1989). After prenatal valproic acid exposure, offspring showed a significant increase in CWM errors only when errors on path-A and path-B were combined (Vorhees, 1987c). The latter experiment was replicated and expanded to test the prenatal effects of valproic acid versus trans-2-ene-valproic acid, a potential therapeutic analogue that is not teratogenic as is valproic acid. The effects of valproic acid on CWM were significant on both path-A and path-B, whereas the effects of trans-2-ene-valproic acid only reached significance when path-A and path-B errors were combined and still the number of errors was less than those seen in the valproic acid group (Fisher et al., 1994). Two other experiments using the 2 trial/day method found no effects of treatment on CWM performance. One experiment was with prenatal ethanol given in a liquid diet (Vorhees, 1989), and the other was after prenatal exposure to fluoxetine (Vorhees et al., 1994b). In another experiment on the postnatal effects of hyperphenylalaninemia with and without branched chain amino acid treatment, different offspring per litter were tested in the CWM with 2 trials/day in path-A and 2 trials/day in path-B for 3 days each, but half the offspring had a 5 min/trial time limit and half had a 6 min/trial time limit. A small interaction of treatment × sex × trial × time limit on path-A was found (only in males and only on 2 of the 6 path-A trials), but no such effect was found on path-B. Overall, this suggested that the trial time limit difference had only a minor effect (Vorhees et al., 1992). Therefore, we adopted the 5 min trial limit in subsequent experiments.
The pattern of testing rats in path-A and then path-B led us to ask if both paths were really necessary. If most of the experimental differences occurred on path-B, could path-A be eliminated without changing the outcome? If the unique task demands of path-B are critical, path-A might be superfluous. In addition, we posed two other questions in this experiment: we revisited the question of the difference between the BWM and the CWM and we tested the effect of two procedural methods of dealing with trials where animals reached the time limit.
The 2-maze issue was reconsidered, because in the comparison of the two mazes described above, the mazes were physically different in terms of channel widths, the material of which the mazes were made, and the number of trials used for path-A. Hence, there were remaining comparability issues from the earlier experiment (Vorhees, 1987b), especially if one wanted to know the effect of maze complexity per se and not the overall effect of the two physically different mazes. Therefore, in this next experiment, complexity was compared in the CWM and BWM in the same apparatus by using the CWM and blocking off the Ts beyond where the BWM ended (Vorhees et al., 1991a).
Another factor investigated was escape assistance. In all water mazes, some animals fail to find the escape on some trials, usually at the beginning of the test. Failure to escape is found for the MWM and many other water (and appetitive) mazes or any test of learning. In water mazes, there have been two general approaches to this problem: (1) guide the animal to the escape (assisted escape), or (2) remove it from wherever it is when the time limit is reached (unassisted escape). In the experiment, we tested these procedures to determine if it made a difference. One may question why assisted escape is an issue? In experiments that use it, the reason is usually not explained; when explained, it is typically justified by the argument that guiding the animal to the goal helps them learn the task so they are less likely to fail the next time. This seems reasonable, but is it true? And whether true or not if it is correct, are there drawbacks to assisted escape? First, assisted escape violates a basic principle of experimentation, i.e., to minimize experimenter effects. The reason for this is that experimenters have biases, even if unknown to them that may influence outcome (Rosenthal et al., 1963). While such biases may be unintentional, the general rule is to conduct experiments as objectively as possible and part of that is to minimize experimenter intervention. Therefore, to the extent that the experimenter can be removed, it increases objectivity. For this reason having experimenters lean over the maze, reach in and guide an animal to the goal is, by definition, experimenter influence. Second, try as people might, it is impossible to guide all animals exactly the same way. If one is going to intervene, then one wants the guidance to be uniform. However, some rats reach the time limit close to the goal and others further away. This means that some rats need very little help and others need a lot. Not only are there individual differences among animals for where they are in the maze when the time limit is reached, there are often differences between groups for how many animals reach the limit. For example, animals in groups that show maze deficits need more help than controls because they fail more often. To the extent that assistance to these animals helps them, it could minimize group differences, not something one would consider desirable. In addition, no two animals respond to help the same way. If an object is held in front of an animal to guide it, some animals follow, while others do not. Furthermore, some animals lunge at the object, grasp it, and have to be pushed off, causing even more ‘intervention.’ In addition, the CWM is large and experimenters have limited reach. If the animal ends the trial far from the goal, the experimenter has to start guiding them from one side, and as they progress, change sides during which the animal swims off into cul-de-sacs before the experimenter can reach it from the other side. If the rat backtracks while the experimenter is moving, this intervention has to be repeated a second or third time. The real question then becomes what is the effect of these interventions? But we should note that not all assisted escape procedures use guidance (although most do). An alternative is to use barriers to close off sections of the maze behind the rat. As the rat progresses toward the goal, the barrier is moved to prevent backtracking. This narrows the number of remaining options until the rat finds the platform. This is the method we tested since it is the most objective.
3.4.3 Comparative Findings
In this experiment, therefore, three factors were tested: (1) maze complexity (BWM vs. CWM), (2) path order (path-A first and path-B second versus path-B first and path-A second), and (3) escape type (unassisted vs. assisted using the more objective blocking of backtracking). Gravid Sprague-Dawley rats were divided into 3 groups of 10, 13, and 11 dams treated from E7-18 with 0, 100, or 200 mg/kg phenytoin by gavage. Litter characteristics and non-CWM data from this experiment were reported separately (Weisenburger et al., 1990). Six male/female pairs within each litter were designated A-F. Pairs A and B were assigned to the complexity comparisons (pair-A to the BWM and pair-B to the CWM), pairs C and D were assigned to the path order comparison (pair-C to test order A then B, and pair-D to test order B then A), and pairs E and F were assigned to the escape comparison (pair E received assisted and pair F received unassisted escape). Only pair-A was tested in the BWM, pairs B-F the CWM. Prior to maze testing, all rats received 4 trials in a 150 × 15 cm straight water channel. In this experiment, testing was performed in standard room lighting. Rats received 2 trials/day for 6 days (12 trials total) of which 3 days (6 trials) were in path-A and 3 days (6 trials) were in path-B. Testing was staggered because of the number of rats; hence, the complexity comparison groups started on P50, the path order groups on P70, and differential escape groups on P90. Latency and errors were recorded on each trial and data analyzed by day. An additional complication was that prenatal phenytoin induces repetitive circling behavior in a percentage of offspring. Hence, the data were analyzed twice, once with all data in each group and a second time with animals exhibiting circling removed. Since circling interferes with maze performance, for simplicity, the analyses without circlers are summarized here, but the full results are available (Vorhees et al., 1991a). Analyses of complexity without circlers showed significant treatment effects on path-A errors in the high but not the low dose phenytoin group in both the BWM and CWM, and on path-B both phenytoin-treated groups showed significantly increased errors in both mazes. However, the magnitude of the differences was very different, i.e., phenytoin-treated offspring made many more errors in the CWM compared with the BWM. In addition, in the CWM the two phenytoin-treated groups showed dose-dependent differences in errors whereas this was not the case in the BWM (Fig. 5A).
Fig. 5.
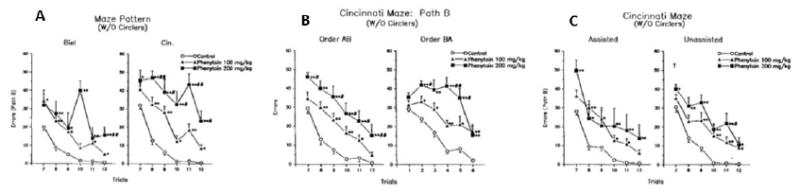
CWM errors (Mean ± SEM) in offspring of pregnant rats treated by gavage on E7-18 with phenytoin free base and tested under visible light. The experiment evaluated separate offspring from each litter in one of three factors influencing performance: (A) maze complexity (BWM vs. CWM), (B) path order (path A then B, or path B then A) where the Y-axis shows path-B errors, and (C) assistance type (assisted vs. unassisted escape). (A) Maze complexity was is compared on path-B of the Biel or Cincinnati versions but in the same apparatus: Left, BWM configuration; Right, CWM configuration. These data provide evidence that the CWM better differentiated the groups compared with the BWM. Data are mean ± SEM per trial. From (Vorhees et al., 1991a). (B) The part of the experiment compared different offspring from each litter on path order for effects on Path-B performance. Left, Path-B errors when path order was A then B; Right, Path-B errors when the path order was B then A (Vorhees et al., 1991a). (C) In this part of the experiment different offspring from each litter were used to compare the type of escape assistance used for those that reached the 5 min time limit. Left, errors using assisted escape; Right, errors using unassisted escape (Vorhees et al., 1991a).
In the comparison of path order, no significant effect for path-A was found whether given first or second (data not shown). For path-B, on the other hand, those that got path-A first showed transfer of training and had fewer errors after the first trial than those that got path-B first (Fig. 5B). While both phenytoin-treated groups showed significant dose-dependent increases in errors with either order, the separation of groups was greater when path-B was given first, except on the last trial where, for unknown reasons, the B-A order high dose group improved suddenly, but the overall pattern is clear: the effects of path-B are the critical determinant of CWM learning deficits. Giving path-A first is not only unbeneficial for differentiating groups, it also diminishes group differences.
In the comparison of escape type, tested in path-B, the assisted groups all did slightly better than the unassisted groups using the barrier blocking method, but the effect was small (barely statistically significant). But, there was no differential effect of assistance type on the effect of phenytoin treatment in impairing learning, i.e., there was no loss of discriminability among groups in the unassisted condition (Fig. 5C). Since the unassisted method is less time consuming and avoids experimenter intervention, we adopted this method. In addition, the low dose phenytoin group showed a larger difference compared with the control group in the unassisted condition supporting the view that unassisted escape on trials where the time limit is reached is the better method when effects are moderate; assisted escape makes the most difference when groups are severely impaired.
While only one positive control was used in this analysis, and the data were not perfect in all respects, the overall pattern was unambiguous: (1) The CWM is significantly more difficult than the BWM; (2) the CWM better differentiates the effects of prenatal phenytoin than does the BWM; (3) path order makes a difference, i.e., if path-A comes first, this helps rats learn path-B faster, takes more time, does not help differentiate treatment-related differences, and reduces group effects; and (4) even an objective assisted escape method (blocking retracing) has a small and not helpful effect on performance by reducing the significance of the lower dose phenytoin group effect. We concluded that when tested in the light, the best practice was to use path-B alone with unassisted escape (Vorhees et al., 1991a).
3.4.4 Additional Findings
3.4.4.1 CWM: Prenatal ultrasound
The use of prenatal ultrasound became widely used in medicine before it was systematically studied for possible adverse effects on fetal development. In the mid-1980s an NIH Consensus Panel reported that there were gaps in what was known about the safety of ultrasound in terms of teratogenesis and developmental neurotoxicity. In a collaborative project with ultrasound engineers and ultrasound physicians, we developed an ultrasound exposure system for rats (Smith et al., 1990). We used the system to test for teratogenic effects of continuous (Vorhees et al., 1991b) or pulsed ultrasound (Fisher et al., 1993) and in both cases found no teratogenicity in the CWM under standard lighting. We next used the same system to evaluate the neurobehavioral effects of prenatal continuous or pulsed wave ultrasound and again found no effects in the CWM under standard room lighting (Vorhees et al., 1994c; Fisher et al., 1996).
3.4.4.2 CWM: prenatal anticonvulsants
Another series of studies was conducted on the prenatal effects of anticonvulsants following published reports in humans of Fetal Hydantoin and Fetal Trimethadione Syndromes. We tested the effects of prenatal exposure to phenytoin, trimethadione, and phenobarbital and found a number of behavioral abnormalities in the offspring (Vorhees, 1983), including in the BWM before the CWM was invented. A follow-up experiment also used the BWM under standard room light (Vorhees, 1987a) and it was this experiment that led to the use of phenytoin as a positive control in the development of the CWM (Vorhees, 1987b). Even earlier, the BWM was used to compare the neurobehavioral teratogenic effects of phenytoin to three of its congeners, mephenytoin, ethotoin, and hydantoin. The experiment replicated the effect of phenytoin, causing large increases in path-B errors, whereas mephenytoin, ethotoin, and hydantoin had no effect on maze learning (Minck et al., 1991). Sodium phenytoin given during the same prenatal exposure period also caused marked increases in errors, this time in the CWM under standard room lighting, but at half the dose of phenytoin as in the previous studies (Vorhees et al., 1995). Hence, prenatal phenytoin proved to be a highly reliable prenatal neurobehavioral teratogen with reproducible effects on BWM and CWM learning under standard lighting. After we changed to infrared lighting, we were no longer investigating the effects of prenatal phenytoin; therefore, we have no data on how the effect of this drug might affect the more difficult infrared lighted method.
4. CWM: Egocentric versus Mixed Learning
4.1 Light versus Dark
As experience was gained with the CWM using different independent variables, and as more data were published about differences between allocentric versus egocentric navigation (Etienne and Jeffery, 2004), we wondered if there was a way to make the CWM a more specific test of egocentric navigation. As noted, the key parameter that differentiates allocentric versus egocentric learning is whether the subject uses distal cues outside itself or proximal/internal cues. Since there was already an excellent test of allocentric navigation: the MWM, and since the CWM when tested with standard lighting provides access to proximal and distal cues (visual stimuli on the walls and ceiling), we reasoned that the test could be made selective for egocentric navigation by eliminating distal cues. Initially, we used the procedure of Whishaw & Maaswinkel (1998) and made rat blindfolds. The rats were acclimated to the blindfolds for several days prior to CWM testing, however the experiment had to be discontinued. While the blindfolds stayed on while dry, when wet, the material loosened and the rats rubbed them off while swimming. We therefore tested rats in the dark. The first attempt was using a dim red light (Skelton et al., 2004; Skelton et al., 2006; Williams et al., 2007). We found that the number of errors for controls under these conditions was not much different than under standard light. Therefore, we set out to test the effects of complete darkness by testing one group of rats with infrared lighting and one with standard lighting. While only recently published (Vorhees and Williams, 2014b), the data were collected earlier (2008). The group of rats tested under infrared light first and then switched to standard light made many errors in the dark and few errors once the lights were turned on (Fig. 6) (see, (Vorhees and Williams, 2014b). By contrast, rats tested under standard lights first learned well, but when switched to infrared lighting showed a sharp increase in errors which required 3 days to overcome. These data demonstrate that if extramaze visual cues are available, rats use them along with egocentric cues to learn the maze. When extramaze cues are not available, rats can rely on internal cues alone but still learn to escape, just at a slower rate. Based on these data, we changed the procedure and used infrared lighting as our standard method. Studies published in 2008 overlap. For example, in a study of the effects of MDMA in adult rats, the maze was used with standard lighting (Skelton et al., 2008b), but in a study published the same year on the adult effects of methamphetamine (MA), the maze was used with infrared lighting (Herring et al., 2008). There was also a study on the developmental effects of MA that year (Vorhees et al., 2008) that also was done with infrared lighting. From 2009 on, all studies used infrared lighting. What follows are experiments organized by type of exposure using the CWM.
Fig. 6.
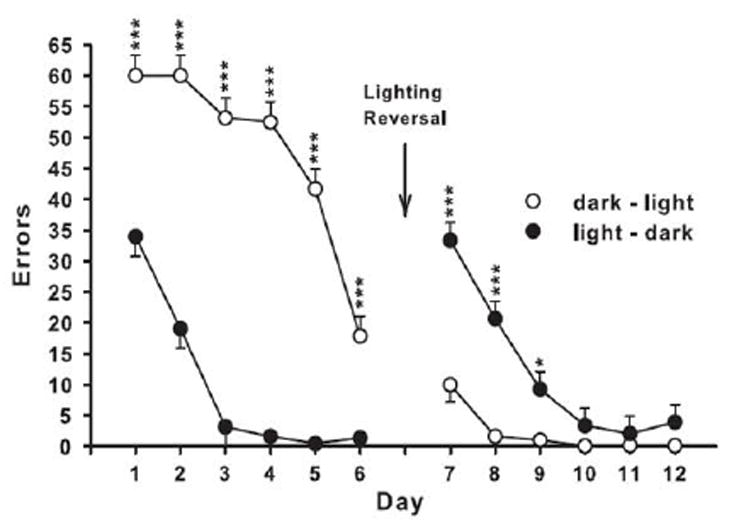
CWM data in rats tested under standard or infrared light (Mean ± SEM errors). One group of adult male Sprague-Dawley rats were tested in the dark (infrared) first then switched to standard light (dark-light group) while the other group was tested in the light first then switched to the dark (infrared; light-dark group). As can be seen, the dark-light group was slow to learn but adapted rapidly once the lights were turned on. The light-dark group learned rapidly under standard light, but were initially disrupted when switched to the dark, but rapidly improved again to asymptotic performance. From (Vorhees and Williams, 2014b).
4.2 CWM: Psychostimulants
A number of years after the CWM was introduced, the problem of MA addiction became a national concern. As the use of the drug grew it became apparent that little was known about its possible prenatal effects. In the early 1990s there were almost no data on its teratogenic or neurobehavioral teratogenic potential, nor about dose or sensitive periods. There were some data indicating it was not teratogenic; therefore, we did not conduct a standard teratology study but instead began our investigation of the drug by conducting experiments to map four exposure periods in brain development: two prenatal (E7-12 and E13-18) and two neonatal (P1-10 and P11-20) periods.
In the first postnatal experiment, Sprague-Dawley rats treated with MA from P11-20 induced an increase in CWM path-B errors but the effect fell short of significance (Vorhees et al., 1994a) when tested under standard lighting. The group treated with MA from P1-10 showed no effect. Prenatal exposure to MA on E7-12 or E13-18 induced increases in CWM errors in path-B in the MA high dose and pair-fed controls, indicating a nutritional rather than drug effect (Acuff-Smith et al., 1996).
One of the most consistent findings of learning effects was that P11-20, but not P1-10, treatment with the related substituted amphetamine (±)3,4-methylenedioxymethamphetamine (MDMA), caused increased CWM path-B errors in the offspring (Broening et al., 2001; Williams et al., 2003; Cohen et al., 2005; Skelton et al., 2006; Vorhees et al., 2007). In addition, P11-20 exposure to the related appetite suppressant, (+)-fenfluramine, also induced long-term, dose-dependent increases in CWM path-B errors in the light (Morford et al., 2002).
In older animals, it was found that P41-50 exposure to MA, but not earlier (i.e., P21-30 or P31-40) or later exposure, P51-60, increased CWM errors when tested 30 days post-treatment in the light (Vorhees et al., 2005). In adult rats, a one-day treatment regimen with MDMA also induced increased errors in the CWM tested in the light (Able et al., 2006) as did a repeated (14 day) exposure to MDMA (Skelton et al., 2008b). Also in adult rats, (±)-fenfluramine (Fig. 7) induced increased CWM errors (Williams et al., 2002), an effect we later showed could be attenuated by concurrent treatment with metyrapone (Skelton et al., 2004).
Fig. 7.
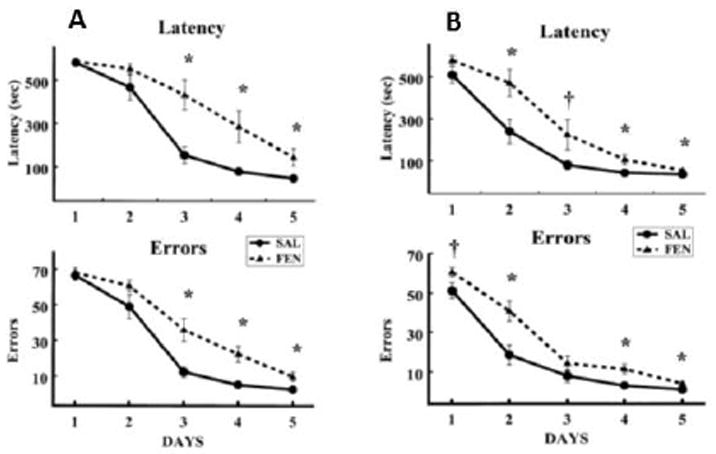
CWM data in adult rats treated with (±)-fenfluramine on a single day 2 weeks prior to testing and tested under visible light and using unassisted escape. Notice that rats reached asymptotic performance in 5 days. Data are Mean ± SEM errors or latency per day (2 trials per day). From (Williams et al., 2002).
It is important to note that in these studies rats were tested under low-level red light. This effect, i.e., proficient learning with minimal light (red light), was confirmed in another experiment in which MDMA was given from P11-20 but in different patterns each day (4 × 10 mg/kg, 2 × 20 mg/kg, or 1 × 40 mg/kg per day), and rats were tested as adults in the CWM in Path-B. As shown in Fig. 8, even with minimal light, rats learned the maze fairly well in 5 days (Vorhees et al., 2007). Path-B errors were dramatically increased two weeks after an acute 1-day, 4-dose at 2 h interval treatment regimen with MA in adult animals when tested under infrared light (Herring et al., 2008; Herring et al., 2010) as illustrated in Fig. 9. These experiments illustrate how amounts of light that make it difficult for an experimenter to see is more than enough for rats to perform no differently than under standard lighting. Only complete darkness changes conditions enough to force rats to change their search strategy to an egocentric one and rely on internal cues to find the goal.
Fig. 8.
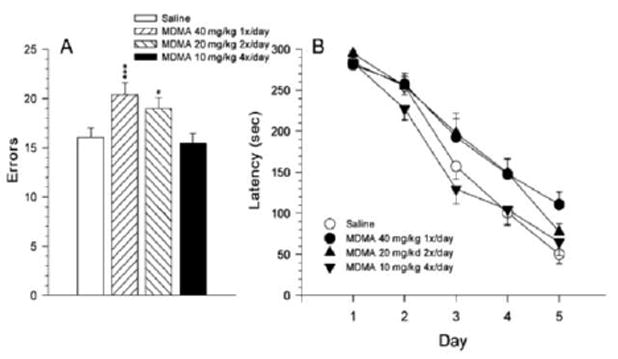
CWM errors (Mean ± SEM) in rats treated with MDMA from P11-20 with the same dose given either as a single dose of 40 mg/kg, two divided doses of 20 mg/kg, or as four divided doses of 10 mg/kg. The 2 dose group was given each dose 6 h apart and the 4 dose group was given one dose 2 h intervals per day. Rats were tested under red lighting using unassisted escape. Note how rapidly rats learn the maze even with lighting barely visible for humans. From (Vorhees et al., 2008).
Fig. 9.
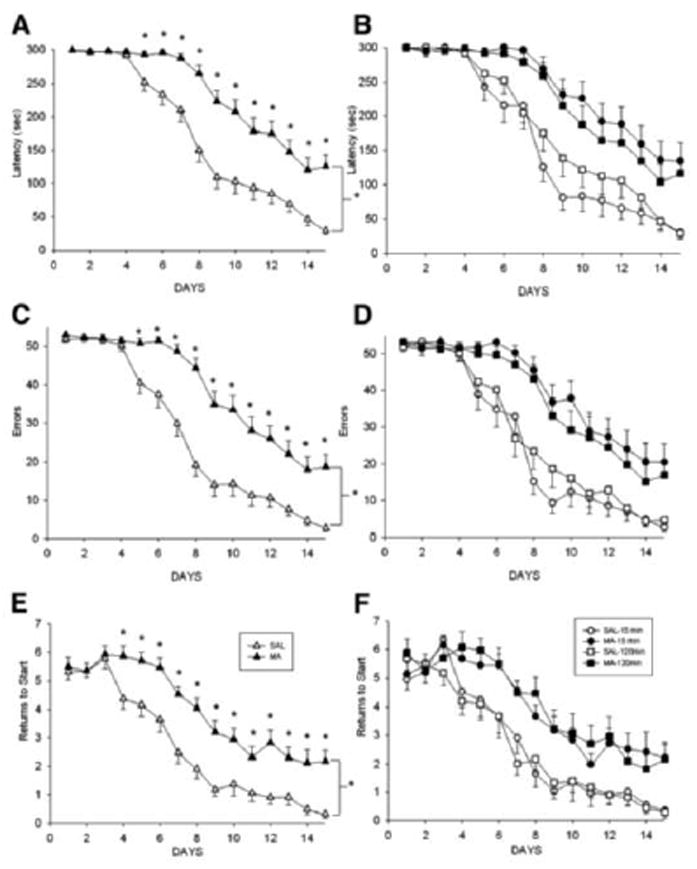
CWM data in adult rats exposed to (+)-methamphetamine on a single day in one of two dosing regimens. One MA group received 4 doses of 10 mg/kg at 2 h intervals and the other received 24 doses of 1.67 mg/kg at 0.25 h intervals such that both MA groups received the same total daily dose (40 mg/kg). Controls for each MA group were given saline on the same injection schedule as their respective MA-treated group. Left panels: The two MA and two Saline control groups combined show the overall effect of neonatal MA on maze performance. Right panels: MA and Saline groups shown separately by dosing regimen. Both groups were tested two weeks after treatment. Panels A and B show latency to escape; panels C and D show errors; and panels E and F show start returns. Data are Mean ± SEM. Rats were tested under infrared light using unassisted escape. Notice that after 15 days of testing, control rats made about 2 errors on their last day using only internal navigational cues. From (Herring et al., 2008).
To examine dose response, neonatal MA exposure (this time with a range of doses of 10, 15, 20, or 25 mg/kg) given on P11-20 four times per day spaced 2 h apart each day, with animals reared in standard or partially enriched cages (with stainless steel enclosures in each cage), produced increased CWM errors at all doses with no significant effect of enrichment when tested in the light (Vorhees et al., 2008). This is shown in Fig. 10 which also reveals another consistent finding in the CWM: sexually dimorphic learning. This is noteworthy because allocentric learning in the MWM is also sexually dimorphic wherein males learn faster than females. However, for egocentric learning, as in the CWM, females learn faster than males, which is the same pattern seen in humans.
Fig. 10.
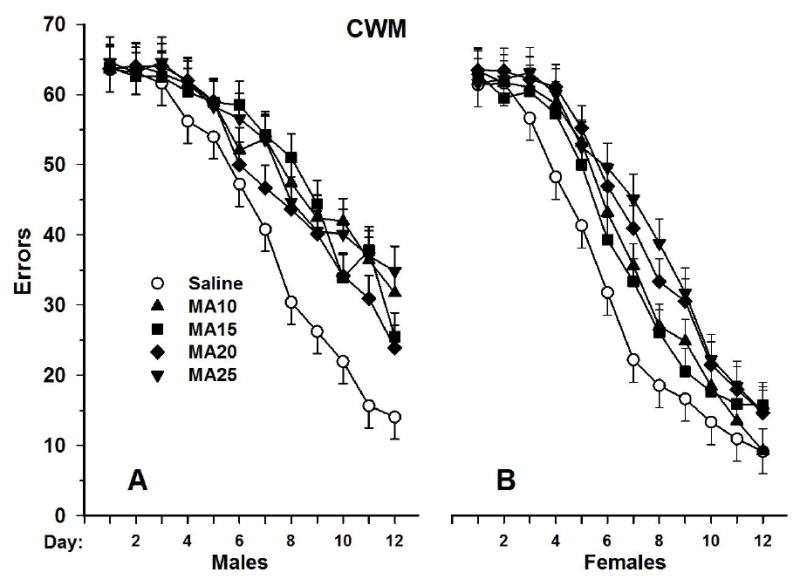
CWM errors (path B) in rats given different doses of (+)-methamphetamine 4 times daily from P11-20 and tested as adults under infrared lighting. Data are Mean ± SEM. Note how much longer it takes rats to learn the maze in what is effectively complete darkness. Also note that unlike spatial mazes such as the Morris water maze where males learn faster than females, in the CWM the opposite is observed, i.e., females learn faster than males. From (Vorhees et al., 2008).
In a related study, the critical period of neonatal MA exposure was assessed by treating litters with the drug on either P1-10, P6-15, or P11-20 with 10 or 25 mg/kg MA given 4 times/day at 2 h intervals but this time testing under infrared light. Significantly increased errors in the CWM were found in both the P6-15 and P11-20 treatment groups, but not in the P1-10 treatment group. This confirms the absence of an effect of very early postnatal MA treatment on CWM learning but reliable effects when exposure starts on P6 or P11 (Vorhees et al., 2009b), and further showed that P6-15 MA exposure induced the largest increase in errors (Vorhees et al., 2009b). Thus, P6-15 and P11-20 have stood out as a critical periods of psychostimulant exposure for the CWM.
We also tested the developmental effects of a designer psychostimulant, 5-methoxy-N,N-diisopropyltryptamine (Foxy) compared with MDMA after P11-20 exposure (administered 4 times/day at 2 h intervals). Starting on P51, 31 days after the last treatment, MDMA-treated rats, but not Foxy-exposed offspring, had significantly increased CWM errors when tested in infrared light (Skelton et al., 2009). On the other hand, short developmental exposure periods to MDMA of P1-5, P6-10, P11-15, and P16-20, while impairing MWM learning, did not affect CWM learning (Vorhees et al., 2009a); see (Skelton et al., 2008a) for fuller discussion.
In a comparison study in adult rats, nearly equal neurotoxic doses of four amphetamines were compared for their effects on CWM-learning 2 weeks after drug exposure under infrared light. Drugs that affected or preferentially affected dopamine caused significant increases in CWM errors (MA and amphetamine), whereas substituted amphetamines that primarily affect serotonin did not (MDMA and fenfluramine) (Vorhees et al., 2011).
In a developmental study similar to the critical period study described above for MA, the same three exposure periods were compared after treatment with MDMA at 10 or 15 mg/kg × 4/day at 2 h intervals on later CWM learning. As with MA, P1-10 did not significantly affect CWM performance, but P6-15 and P11-20 administration at both doses did and did so dose-dependently when tested under infrared light (Skelton et al., 2011).
In an experiment in which rats were treated from P11-20 with MDMA alone, citalopram alone, or both, all three treated groups made significantly more errors in the CWM than saline controls (Schaefer et al., 2013). The hypothesis was that citalopram, given prior to each dose of MDMA, would inhibit MDMA uptake into presynaptic terminals and attenuate the effects of MDMA on learning. However, the results showed otherwise and that citalopram itself is a developmental neurotoxin. Hence, the data revealed an unexpected effect of citalopram not previously recognized.
4.3 CWM: Other agents
Several other suspected developmental neurotoxins have also been evaluated using the CWM. An unusual effect was found with gestational and lactational exposure to chlordane that led to reduced CWM errors when tested in the light in path-B (Cassidy et al., 1994). The exposed group swam faster than controls and therefore explored the maze more rapidly. This brought them into contact with the escape platform sooner. Having once found the escape, the exposed animals were able to improve their performance more rapidly than controls. Prenatal smokeless tobacco exposure (Paulson et al., 1993), on the other hand, had no effect on CWM errors when tested in the light in path-B. Interestingly, E7-18 exposure to thalidomide (thalidomide does not induce malformations in rats), led to increased errors when tested in path-A followed by path-B in the light in the CWM (Vorhees et al., 2001).
Maternal immune activation using the viral mimic polyinosinic-polycytidylic acid (Poly IC) was also assessed. It induced a small, but significant, increase in CWM errors in the offspring as adults under infrared light (Vorhees et al., 2012), but when the Poly IC exposure was lengthened, it had no effect on CWM errors (Vorhees et al., 2015). In addition, we tested a Pde4d knockout rat. This gene is associated with depression and schizophrenia. The KO rats showed significantly increased CWM errors tested under infrared light (Schaefer et al., 2012).
5. CWM and Striatum
5.1 CWM: Striatal dopamine
The next step in our understanding of the CWM came from a series of experiments on what brain region and neurotransmitter are predominant in mediating CWM egocentric navigation. Because of our experiment showing that substituted amphetamines that preferentially affect dopamine systems and not serotonin produce long-term deficits in the CWM (Vorhees et al., 2011), we began by exploring the role of dopamine (DA) in CWM learning. In the first experiment, 6-hydroxydopamine (6-OHDA) was infused into the dorsal striatum at doses producing bilateral 80% reductions in DA. This DA reduction resulted in a significant impairment of CWM learning under infrared light in path-B. The effect was so profound that even after 9 trial blocks of 4 trials spread over 18 days (2 trials/day), the 6-OHDA treated rats showed no evidence of mastering the task or catching up to sham-treated controls (Braun et al., 2012); this is shown in Fig. 11. In a follow-up experiment, 6-OHDA-induced DA reductions were produced in subregions of the dorsal striatum. Separate groups had 6-OHDA-induced DA reductions in the dorsal medial or dorsal lateral striatum. The dorsal lateral DA reduction was 75% compared with 65% in the dorsal medial striatum, but the increase in CWM errors was less in the dorsal lateral striatum group despite the greater DA reduction. The dorsal lateral lesioned group eventually caught up with controls by the end of the test (36 trials), whereas the dorsal medial striatum lesioned group, with a slightly less severe DA reduction, never caught up to controls (Braun et al., 2015).
Fig. 11.
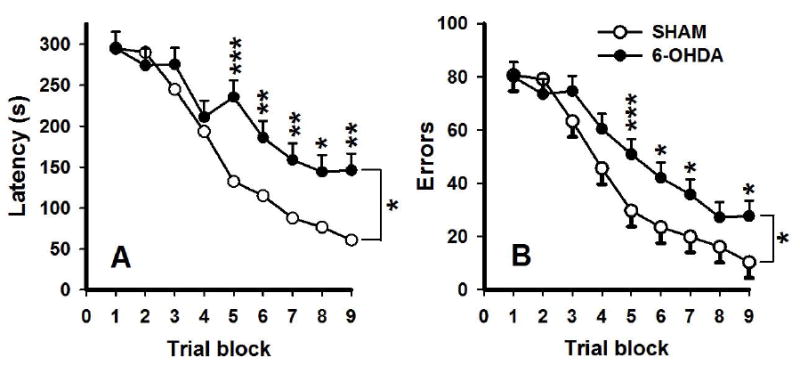
CWM errors (Mean ± SEM) in rats with bilateral intra-striatal 6-OHDA induced DA reductions. Rats were tested under infrared light with unassisted escape. Latencies are shown in the left and errors in the right. Notice that rats with dopamine reductions in the neostriatum never caught up with controls. From (Braun et al., 2012).
5.2 CWM: Nucleus accumbens and prefrontal cortex
In a third experiment, rats were given 6-OHDA injections in either the nucleus accumbens or prefrontal cortex, and substantial decreases in DA and norepinephrine were observed in both regions. The nucleus accumbens 6-OHDA-treated group showed large and lasting increases in errors in the CWM, whereas the prefrontal cortex 6-OHDA-treated group showed no change from controls (Braun et al., 2016) and is discussed further elsewhere (Vorhees and Williams, 2014a, b).
The above data support the utility of the CWM and that learning in this maze is dependent on dorsal and ventral striatal DA in mediating egocentric navigation under conditions that block distal cues.
6. Comparison of CWM and MWM effects
We have conducted a few experiments in which the same drug or environmental agent was tested in both the MWM and the CWM. In adult Sprague-Dawley (SD) rats given a binge dose regimen of MA sufficient to induce a 50% or more reduction in neostriatal DA, two weeks later showed marked impairments in CWM (infrared) performance but no changes in MWM performance (Herring et al., 2008). In another experiment, adult SD rats treated with single versus repeated doses of MDMA and tested after a recovery period showed both CWM (red light) and MWM deficits (Skelton et al., 2008b). We also tested the effects of Foxy in adult SD rats in both mazes. The drug impaired CWM (red light) performance but had no effect in the MWM (Williams et al., 2007). In another experiment using Foxy, we compared the effects of this drug to that of MDMA given from P11-20, in both mazes once the animals were adults. Both drugs impaired MWM but only MDMA impaired CWM (infrared) performance, and there were no effects of Foxy on CWM (infrared) performance (Skelton et al., 2009). We also compared different developmental exposure windows using MDMA for how they affected learning in both mazes. MDMA exposure on P6-10 or P11-15 both impaired MWM performance, but treatment on P1-5 or P16-20 did not, whereas none of the four exposure periods had effects on CWM (infrared) performance (Vorhees et al., 2009a). In an experiment on the effects of a short developmental exposure window to MA we found impaired CWM (infrared) at all doses tested (10, 15, 20, and 25 mg/kg × 4/day, but effects on MWM only at the high dose of 25 mg/kg MA (Vorhees et al., 2008). In another experiment on the developmental effects of MA, we tested three overlapping exposure periods, P1-10, P6-15, and P11-20 using two doses of MA (10 or 25 mg/kg ×4/day). MA exposure from P6-15 and P11-20 impaired CWM (infrared) performance at both doses, whereas in the MWM the same exposure ages showed effects in males at both doses, but in females the effects were in the P1-10 and P11-20 age groups but not in the P6-15 age group (Vorhees et al., 2009b). We have one example where both mazes were used in a developmental model of manganese (Mn) exposure separately and in combination with iron deficiency and barren cage rearing stress. Developmental iron deficiency had little effect on performance in either maze, but Mn and barren-cage impaired CWM (infrared) and MWM performance independently and interacted only on the MWM reversal probe trial (Amos-Kroohs et al., 2016, unpublished observations). These examples show that some treatments affect performance in both mazes, some in one and not the other, and some to a greater extent in one maze versus the other. Since the mazes were designed to test different types of navigation, this is not surprising. Such findings reinforce the view that these mazes differentiate ego- from allocentric navigation function, and both can be used to understand the consequences of a given exposure. Given this, what are best practices for the CWM? In the next section we describe current procedures.
7. Design
7.1 Apparatus
If the design of the maze is analyzed carefully (Fig. 2), it will be noted that there is a small irregularity. The basic “unit” of the maze is composed of T-sections; hence, it is often referred to as a multiple-T maze. The maze was created using English measurements; therefore, for simplicity, metric conversions have been rounded to the nearest cm. In English, the basic T-unit using internal dimensions is as follows: stems 6” (15 cm); right and left cul-de-sac short walls are 9” long (23 cm); and the long back wall is 24” long (61 cm). However, in Fig. 2 note that the cul-de-sac closest to point B, the stem, is 23 cm where it should be 15 cm. This deviation has the effect of permitting 9 T-shaped cul-de-sacs before the maze wraps back on itself. The depth of the maze is 51 cm and the water depth 20 cm.
When the 23 cm stem in Fig. 2 is reduced to 15 cm it results in a slightly different design (Fig. 12). As can be seen, the revised maze design is a 10-unit multiple-T maze rather than a 9-unit maze. This moves the start location, point-S, to one of the arms of a T-unit rather than it being in a short channel of its own as in Fig. 2. In the CWM in Fig. 2 the optimal solution is the sequence of right (R) and left (L) turns as follows: RRLRRLRRL. In the maze shown in Fig. 12 the optimal solution is given by the sequence: LRRLRRLRRL when S1 is the start and RRRLRRLRRL when S2 is used as the start. The maze has also been reengineered to improve its utility and precision of its dimensions.
Fig. 12.
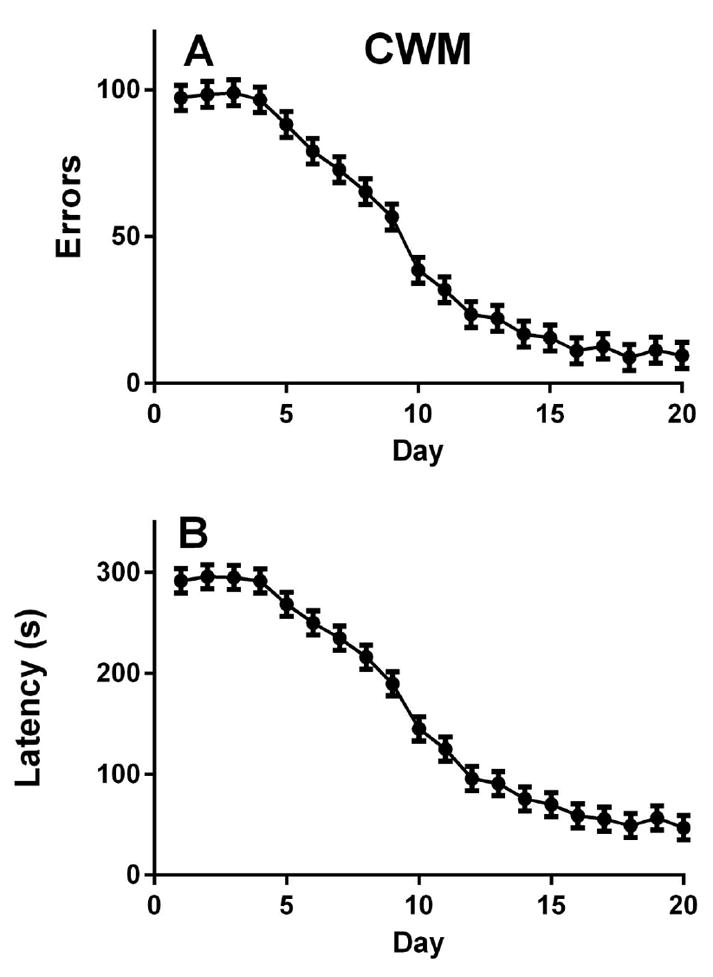
Schematic of the revised CWM with 10 T-shaped cul-de-sacs instead of 9. The revised maze has a new start location (S). The escape platform (goal; G) remains the same. The adjustment to the schematic shown in Fig. 3 where one stem was longer than all the others, once corrected, created space for an additional T where the S was in Fig. 3. The revised maze has two possible start locations, but the start location that we use is as shown.
The previous version depicted in Fig. 2 was a self-contained water-tight apparatus made of high density polypropylene (0.95 cm thick) with chemically welded seams, a flat floor with a central 5 cm (2 inch) drain opening connected to PVC pipe that was run to a floor drain ending in a shut-off valve. In one of the blind sections of the outer wall there was an overflow outlet 20 cm above the floor of the maze fitted with PVC pipe connected to the main drain line beyond the shut-off value to prevent accidental overflow and to ensure the tank was always filled to the same depth. This design proved problematic over time because high density polypropylene is pliable even though it is dense. This caused two issues. First, when the fabricator welded the seams, the heat produced during welding caused the walls to bow, but the more serious problem was the outer walls. The pressure of standing water against these walls caused them to gradually warp outward over a period of years. Repeated attempts to add reinforcement bars on the outer walls did not solve the problem but merely slowed the warping process. The bowing eventually reached the point that it created stress on the seams where the walls were attached to the floor, creating cracks that resulted in leakage.
To correct these problems, the maze in Fig. 12 was constructed differently. First, the new apparatus consists of two parts: (a) a tank and separately (b) a maze; the maze rests inside the tank. The tank is constructed of black OAH high density polyethylene, but with important differences compared with the earlier model. First, the tank walls are thicker (0.95 cm, double-walled, i.e., 0.95 × 2 = 1.9 cm) with a 5 cm (2 inch) flange at the top for increased strength. The walls are 51 cm (20 inches tall). Second, the tank has a 5 × 5 cm (2 × 2 inch) stainless steel reinforcing bar around the perimeter mounted directly beneath the flange to make the walls rigid. Third, the floor is sloped from the edge to the center with a 10 cm (4 inch) drop over this distance with the low point ending at the 5 cm (2 inch) drain opening in the center. As before the central opening is attached to PVC drain pipe extending to the floor drain with a shut-off valve at the end. The overflow is in the tank wall and located 25 cm (10 inches) above the floor measured at the sidewall. The inner walls of the maze itself are constructed of 0.95 cm Type I Eurogray PVC with chemically welded seams. Because PVC is “truer” than polypropylene and reasonably heat resistant when chemically welded, it does not warp. The maze is welded in six sections with stainless steel screws joining the sections. The screw heads are not visible to the rat even if the lights are on as they are located inside sections on the outside of the channels. Each section is bolted to a PVC subfloor. This permits the maze to be disassembled should the need arise. The subfloor is perforated with 1.9 cm holes spaced regularly throughout to permit water and waste to flow through to the main sloping floor and flow down to the drain. The subfloor is supported by a series of 5 cm (2 inch) diameter stanchions. The escape platform at the goal has a vertical section that extends to the top edge of the maze with a short horizontal lip that extends over the edge of the maze so that it hooks at a standard depth. The bottom of the platform support is a horizontal 15 × 15 cm platform submerged 1.5 cm below the water surface with a textured surface.
7.2 Procedures
A day or so before maze testing, rats are given 4 consecutive trials in a separate straight water channel with a submerged platform at one end located in a different room than the maze. The channel is 244 cm long, 15 cm wide, and 51 cm deep filled with water to a depth of 20 cm. Each trial is timed. Rats are placed at one end facing the end wall and allowed to find the platform for up to 2 min. This premaze assessment is run under standard light. If treatment effects are found on these trials they may temper interpretations of any effects found in the maze.
For the maze itself, we fill it with water at least a day before testing to allow it to equilibrate to room temperature before testing. Water temperature is recorded daily and typically is between 20-23 °C and water is changed every 1-2 days. We test during the light cycle, and we have not investigated dark cycle performance. Rats are placed in the maze at a Start position as shown in Fig. 12 (we currently use S1) and they are allowed up to 5 min to find the escape. Errors and latency are recorded on each trial. Errors are of three types: stem, arm, and start returns. These are defined below in Section 7.3. Error types are not differentiated when analyzing the data. Rats are given 2 trials/day for 5-6 days when tested under standard light. Sprague-Dawley (SD) and Long Evans (LE) rats reach asymptotic performance under these conditions on day 5 or 6, making 2-4 errors by the end and taking 10-20 s (LE data unpublished observations). Rats failing to find the escape on early trials within 5 min are removed from wherever they are when the time limit is reached (unassisted escape). If the rat finds the escape in under 5 min on trial-1, they are immediately given trial-2. If they fail to find the platform on trial-1, they are removed at the 5 min mark and placed in a holding cage and given a minimum of 5 min rest before being given trial-2 of the day. Alternatively, rats may be tested in rotation, i.e., a squad of 6-12 rats may all be given trial-1 in succession and then all given trial-2 in the same order. Back-to-back vs. rotational testing has little effect on adult SD, LE, or Fischer-344 rats. If a treatment causes severe body weight reductions or visible neurological signs, then rotational testing has the benefit of reducing fatigue, but this only makes a difference if the experimental group is small or less fit than controls.
If tested under infrared light, infrared LED lights are mounted around the maze. The door is sealed tightly with weather stripping (even tiny amounts of light leaking in the room dramatically change performance in the maze by acting as a beacon to which rats readily orient). A light-sensitive video or closed circuit CCD camera is mounted above the maze and attached to a video monitor located in an adjoining room. Groups of rats (6-12) are placed in the room away from the maze and allowed at least 5 min for adaptation to the dark before being given the first trial. Each rat is placed in the maze at the start and a timer started. The experimenter exits the room as quickly as possible, shutting the door tightly. From the next room, errors and elapsed time are scored via the monitor. Under infrared lighting, rats are tested for 2 trials/day for 15-20 days (we commonly use 18 days). Under these conditions many rats reach the time limit on the first several days of testing. Occasionally, one or two rats in a study never learn to find the escape. These rats are removed from data analyses. The number of animals that never learn are analyzed to determine if the treatment increased this number or if it is equally divided among groups. Under infrared lighting, asymptotic performance in controls is typically 10 errors (Fig. 13, top) with latencies of 20-25 s on the last day (Fig. 13, bottom).
Fig. 13.
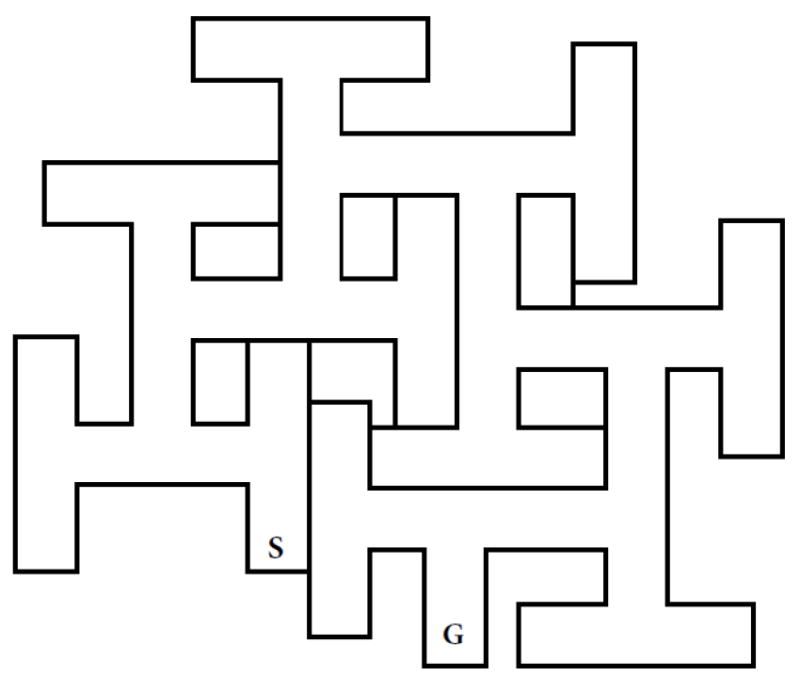
CWM errors and latency under infrared light in control male SD rats. The control group was taken from an experiment on developmental exposure to manganese from P4-28 given every other day and/or injected bilaterally with Vehicle or 6-hydroxydopamine (6-OHDA) as adults (unpublished). Data are Mean ± SEM. Notice that controls needed 18-20 days (2 trials/day) to reach asymptotic performance. In this experiment, average errors and latencies were correlated 0.87 with one another (Bailey et al., unpublished observations).
Hence, the maze can be run under standard or infrared light and provide useful information either way, however, our data indicate that the test is more sensitive when used under infrared light. Moreover, the interpretation of the data is more straightforward when used under infrared light since the test is a more specific assessment of egocentric L&M rather than being a blend of egocentric and allocentric L&M.
7.3 Scoring
Errors are defined by an imaginary line at the base of each T and at the end of each arm of a T. An error occurs whenever a rat passes one of these imaginary lines with its head and forelimbs. Since rats typically hold their bodies at an angle as they swim, once their heads and forelimbs cross one of these lines their body does too, even if the rats turn around and exits the area their bodies cross the plane demarcating an error. Hence, errors are entering any stem or arm of a T or returning to the start arm once having left it.
For example, if a rat enters a stem, that counts as one error; if it proceeds to the end of the T and turns without entering one of the arms, that constitutes an error even if they do not cross the line for entry in an arm. If they enter the right or left arm of a T, that is not an additional error since they made an error by entering the crossing arm of the T. But, if they enter an arm and then cross to the other arm, that is another error. Hence, a rat entering the stem makes one error, if it enters the crossing arm of a T-section, that is a second error whether it enters an arm or not, but if it enters the right arm, it is still only 2 errors. If it crosses to the left arm, it has made 3 errors. Repetitive crossing back and forth between the arms is counted as additional errors. On early trials, rats cross back-and-forth in the crossing arms frequently and make many repetitive errors. As rats learn the maze they start eliminating this behavior; one of the first errors they eliminate are of repetitive arm entries. When tested in the light, once rats see the end of a T, they will start to enter the crossing arm and then abruptly make a U-turn and leave without going into an arm. This sudden turning before entry into the arm is rarely seen in rats tested under infrared light when visual cues of the wall are absent.
Rats are natural swimmers and >99% swim with ease the moment they are put in water. Sinking is extremely rare. Over many years of testing many rats, sinking has been seen on only a few occasions. If a treatment causes disorientation, as with prenatal phenytoin or hyperbilirubinemia, rolling or sinking can occur. If a treatment makes rats weak such that they cannot swim for 5 min, they will start swimming vertically with just their noses above water and tread water. In the latter case, vertical swimmers should be watched carefully to ensure they do not become exhausted and sink. When vertical swimming is seen or if one observes periodic sinking, such rats should be removed from the test. In our experience one should see no more than one rat having difficulty every 5 years when the maze is used almost continuously. An even rarer event is when a rat goes into shock. This is so rare that most people will never see it. Such rats apparently go into cardiac arrest and cannot be resuscitated. We have seen this 4 or 5 times in 35 years in SD rats; the frequency in other strains is unknown.
7.4 Data Analysis
We analyze CWM data by day, hence we average the data for the two trials per day and use day as a repeated measure factor in ANOVA models. We analyze both errors and latency. A small percentage of animals on a given trial may stop active searching and remain in one part of the maze and tread water for long intervals. They may do this on one or several trials, most typically on early trials under infrared light. It is uncommon to see this when testing under standard light, but it can occur. It usually does not cause the rat to fail on all trials. In cases where it is seen repeatedly, we recommend removing such animals from the dataset before analyzing the data.
The number of errors rats make varies. An actively searching rat on early trials may make 60-100 errors, whereas a rat that gives up and treads water may make 15-20 errors. Because of this, before analyzing the data, we correct for treading water by determining the maximum error score across all animals in the experiment. We identify the animal in the experiment that made the most errors and assign that score to any animal that reached the time limit. This is basically the same as for animals reaching the time limit since they all receive a latency of 5 min. Once rats adopt a non-searching strategy, it may persist and distort the data inasmuch as experimental animals can have 5 min trials but fewer errors than controls. Using a ‘corrected’ error score on 5 min trials prevents this problem.
Errors show fairly high correlations to latency. This is not surprising since the more errors a rat makes the longer it takes to reach the goal. But the correlation is not unity. The correlation in a recent experiment with 4 groups and 20 days of testing under infrared light showed a correlation coefficient between errors and latency of r = 0.88 (Bailey et al. 2016, unpublished data). When errors are corrected for animals that stop searching on those trials where the time limit is reached, the correlation rises to r = 0.98. This is not surprising since for the first several days both latency and errors for most of the animals is at a ceiling that compresses the range of possible values. If, however, one divides the data and determines the correlation between errors and latency for the first vs. the second 10 days of testing, then the correlation for the first 10 days is r = 0.74 whereas it is r = 0.86 for the second 10 days. The latter is more meaningful since it represents the middle part of the learning curve and has a broader range of values, but both serve to demonstrate that although errors and latency are correlated, they provide some non-overlapping information.
Occasionally, one can find a significant effect on one measure but not the other. When this occurs the one that is not significant typically shows a trend in the same direction but falls short of significance at the standard cutoff of p < 0.05. When this occurs we prefer to make note of the fact that the trend in the non-significant measure is consistent with the one that is significant. However, a large disparity between errors and latency should be a source of concern. In such a case, one should investigate to ensure that those testing the animals are counting errors correctly.
Finally, the time limit used for each trial, while necessary, as it is in most behavioral tests, creates censored data. There are formal methods for dealing with censored data. An alternative is to look for a break-point in the data and analyze the data from the day the control group begins to show improvement separately from the first few days when all animals are reaching the time limit. This method provides a clear picture of whether the experimental group is showing impaired learning.
8. Conclusions
There are a number of excellent tests available for measuring spatial/allocentric learning and memory. These include the MWM, RAM, RWM, Barnes maze, cheeseboard, and others. By comparison there are very few established tests of egocentric learning and memory and many that have been used are confounded by the presence of spatial cues. The CWM, when tested under infrared lighting is an efficient, well-characterized test with a wealth of data supporting it as a measure of egocentric navigation. The experimental data in young and adult rodents exposed to a wide range of drugs and chemicals provide empirical validation for the maze’s utility. In addition, we recently added brain region-specific and neurotransmitter data supporting its construct validity as a striatally- and dopaminergically-mediated test of egocentric navigation. These data add to the empirical validation of the method. These data do not imply that other neurotransmitters or other brain regions are not involved in this form of learning but rather that striatum and nucleus accumbens DA play a major role. Undoubtedly there are other neurotransmitters involved as is the case with all complex learning and memory, but the striatal-DA connection provides a first approximation for understanding how CWM egocentric navigation is mediated. Hence, for practical and theoretical reasons (Vorhees and Williams, 2014a) and for a range of applications, including basic neurotoxicity and regulatory studies, the CWM demonstrates value (Vorhees and Williams, 2014b) and assesses a type of learning and memory not measured by other methods, such as tests of allocentric navigation, active or passive avoidance, recognition memory, or schedule-controlled operant methods. It is this unique aspect of the test that makes it a singular contribution to the assessment of adverse exposure effects.
HIGHLIGHTS.
Reference memory-based navigation is essential for survival in most species
Striatal-dependent egocentric navigation relies on internal and proximal cues
Hippocampus/entorhinal cortex-dependent allocentric navigation relies on distal cues
Cincinnati water maze (CWM) is a test of route-based egocentric navigation
Development, supporting evidence, and recommended methods for the CWM are reviewed
Acknowledgments
The authors wish to thank Drs. Jenna Sprowles and Sarah Jablonski for feedback on a draft of this manuscript. Many of those who contributed to the research reviewed herein were support by NIH T32 ES007051 over the years this review spans.
Footnotes
Note the disparity in the number of errors reported by Polidora et al. vs. Butcher. It is not known why these are different but it appears that Polidora et al. counted entry into a T as one error even if the rats then entered arms of the T, whereas Butcher scored each arm entry separately. In addition, there appears to be a possible difference in the way “entry” was defined (head vs. whole body).
Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Able JA, Gudelsky GA, Vorhees CV, Williams MT. 3,4- Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biol Psychiatry. 2006;59:219–1226. doi: 10.1016/j.biopsych.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer LP, Braden BB, Nonnenmacher S, Mennenga SE, Bimonte-Nelson HA. The cognitive effects of conjugated equine estrogens depend on whether menopause etiology is transitional or surgical. Endocrinology. 2010;151:795–3804. doi: 10.1210/en.2010-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal d-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicol Teratol. 1996;18:99–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Barkan S, Roll U, Yom-Tov Y, Wassenaar LI, Barnea A. Possible linkage between neuronal recruitment and flight distance in migratory birds. Sci Rep. 2016;6:21983. doi: 10.1038/srep21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel WC. Early age differences in maze performance in the albino rat. J Genet Psychol. 1940;56:439–453. [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Braun AA, Graham DL, Schaefer TL, Vorhees CV, Williams MT. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol Learn Mem. 2012;97:402–408. doi: 10.1016/j.nlm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, Amos-Kroohs RM, Gutierrez A, Lundren KH, Seroogy KB, Vorhees CV, Williams MT. 6-Hydroxydopamine reductions in the nucleus accumbens, but not the medial prefrontal cortex, impair Cincinnati water maze egocentric and Morris water maze allocentric navigation in male Sprague-Dawley rats. Neurotox Res. 2016 doi: 10.1007/s12640-016-9616-6. [DOI] [PubMed] [Google Scholar]
- Braun AA, Amos-Kroohs RM, Gutierrez A, Lundgren KH, Seroogy KB, Skelton MR, Vorhees CV, Williams MT. Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiol Learn Mem. 2015;118:55–63. doi: 10.1016/j.nlm.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy) induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Butcher RE. Behavioral effects of maternal phenylketonuria in rats. University of Cincinnati; 1969. pp. 1–46. [Google Scholar]
- Butcher RE. Learning impairment associated with maternal phenylketonuria. Nature. 1970;226:555–556. doi: 10.1038/226555a0. [DOI] [PubMed] [Google Scholar]
- Butcher RE, Vorhees CV, Berry HK. A learning impairment associated with induced phenylketonuria. Life Sci. 1970;9:1261–1268. doi: 10.1016/0024-3205(70)90266-3. [DOI] [PubMed] [Google Scholar]
- Butcher RE, Stutz RM, Berry HK. Behavioral abnormalities in rats with neonatal jaundice. Am J Ment Retard. 1971;75:755–759. [PubMed] [Google Scholar]
- Butcher RE, Vorhees CV, Kimmel CA. Learning impairment from maternal salicylate treatment in rats. Nature. 1972a;236:211–212. doi: 10.1038/newbio236211a0. [DOI] [PubMed] [Google Scholar]
- Butcher RE, Vorhees CV, Wootten V. Behavioral and physical development of rats chronically exposed to caffeinated fluids. Fundam Appl Toxicol. 1984;4:1–13. doi: 10.1016/0272-0590(84)90214-8. [DOI] [PubMed] [Google Scholar]
- Butcher RE, Vorhees CV, Kindt CW, Keenan WJ. An experimental evaluation of phototherapy for hyperbiliruninemia in the Gunn rat. Dev Psychobiol. 1972b;16:83–109. doi: 10.1001/archpedi.1972.02110120100011. [DOI] [PubMed] [Google Scholar]
- Butcher RE, Vorhees CV, Kindt CW, Kazmaier-Novak KJ, Berry HK. Induced PKU in rats: Effects of age and melatonin treatment. Pharmacol Biochem Behav. 1977;7:129–133. doi: 10.1016/0091-3057(77)90196-4. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RA, Vorhees CV, Minnema DJ, Hastings L. The effects of chlordane exposure during pre- and postnatal periods at environmentally relevant levels on sex steriod-mediated behaviors and functions in the rat. Toxicol Appl Pharmacol. 1994 doi: 10.1006/taap.1994.1123. [DOI] [PubMed] [Google Scholar]
- Chaby LE, Sheriff MJ, Hirrlinger AM, Braithwaite VA. Can we understand how developmental stress enhances performance under future threat with the Yerkes-Dodson law? Commun Integr Biol. 2015;8:e1029689. doi: 10.1080/19420889.2015.1029689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (Ecstasy) in rats: Interaction with exposure in adulthood. Synapse. 2005;57:148–159. doi: 10.1002/syn.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens RW. Effects of maternal undernutrition on offspring behavior: Incentive value of a food reward and ability to escape from water. Dev Psychobiol. 1974;7:61–69. doi: 10.1002/dev.420070110. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Etienne AS. Navigation of a small mammal by dead reckoning and local cues. Curr Dir Psychol Sci. 1992;1:48–52. [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Fisher JE, Acuff-Smith KD, Schilling MA, Nau H, Vorhees CV. Trans-2-ene-valproic acid is less behaviorally teratogenic than an equivalent dose of valproic acid in rats. Teratology. 1994;49:479–486. doi: 10.1002/tera.1420490608. [DOI] [PubMed] [Google Scholar]
- Fisher JE, Acuff-Smith KD, Schilling MA, Vorhees CV, Meyer RA, Smith NB, O’Brien WD., Jr Teratologic evaluation of rats prenatally exposed to pulsed-wave ultrasound. Teratology. 1993;49:150–155. doi: 10.1002/tera.1420490211. [DOI] [PubMed] [Google Scholar]
- Fisher JE, Jr, Acuff-Smith KD, Schilling MA, Meyer RA, Smith NB, Moran MS, O’Brien WD, Jr, Vorhees CV. Behavioral effects of prenatal exposure to pulsed-wave ultrasound in unanesthetized rats. Teratology. 1996;54:65–72. doi: 10.1002/(SICI)1096-9926(199606)54:2<65::AID-TERA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Fouquet C, Babayan BM, Watilliaux A, Bontempi B, Tobin C, Rondi-Reig L. Complementary roles of the hippocampus and the dorsomedial striatum during spatial and sequence-based navigation behavior. PLoS ONE. 2013;8:e67232. doi: 10.1371/journal.pone.0067232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French KL, Bimonte-Nelson HA, Granholm AC. Galantamine effects on memory, spatial cue utilization, and neurotrophic factors in aged female rats. Cell Transplant. 2007;16:197–205. doi: 10.3727/000000007783464759. [DOI] [PubMed] [Google Scholar]
- Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Herring NR, Gudelsky GA, Vorhees CV, Williams MT. (+)-Methamphetamine-induced monoamine reductions and impaired egocentric learning in adrenalectomized rats is independent of hyperthermia. Synapse. 2010;64:773–785. doi: 10.1002/syn.20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, MacLeod KM, Moss CF, Hasselmo ME. Bat and rat neurons differ in theta-frequency resonance despite similar coding of space. Science. 2013;340:363–367. doi: 10.1126/science.1233831. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kimmel CA, Butcher RE, Vorhees CV, Schumacher RJ. Metal-salt potentiation of salicylate-induced teratogenesis and behavioral changes in rats. Teratology. 1974;10:293–300. doi: 10.1002/tera.1420100312. [DOI] [PubMed] [Google Scholar]
- Kinney L, Vorhees CV. A comparison of methylphenidate induced active avoidance and water maze performance facilitation. Pharmacol Biochem Behav. 1979;10:437–439. doi: 10.1016/0091-3057(79)90210-7. [DOI] [PubMed] [Google Scholar]
- Lipp HP, Wolfer DP. Genetically modified mice and cognition. Curr Opin Neurobiol. 1998;8:272–280. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res. 1999;99:143–152. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Rev. 1993a;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res Brain Res Rev. 1993b;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- McSwigan JD, Vorhees CV, Brunner RL, Butcher RE, Berry HK. Amelioration of maze deficits from induced hyperphenylalaninemia in adult rats using valine, isoleucine, and leucine. Behav Neural Biol. 1981;33:378–384. [Google Scholar]
- Mennenga SE, Baxter LC, Grunfeld IS, Brewer GA, Aiken LS, Engler-Chiurazzi EB, Camp BW, Acosta JI, Braden BB, Schaefer KR, Gerson JE, Lavery CN, Tsang CW, Hewitt LT, Kingston ML, Koebele SV, Patten KJ, Ball BH, McBeath MK, Bimonte-Nelson HA. Navigating to new frontiers in behavioral neuroscience: traditional neuropsychological tests predict human performance on a rodent-inspired radial-arm maze. Front Behav Neurosci. 2014;8:294. doi: 10.3389/fnbeh.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R, Geiger K, Joerges J, Muller U, Chittka L. Bees travel novel homeward routes by integrating separately acquired vector memories. Anim Behav. 1998;55:139–152. doi: 10.1006/anbe.1997.0574. [DOI] [PubMed] [Google Scholar]
- Mika A, Mazur GJ, Hoffman AN, Talboom JS, Bimonte-Nelson HA, Sanabria F, Conrad CD. Chronic stress impairs prefrontal cortex-dependent response inhibition and spatial working memory. Behav Neurosci. 2012;126:605–619. doi: 10.1037/a0029642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minck DR, Acuff-Smith KD, Vorhees CV. Comparison of the behavioral teratogenic potential of phenytoin, mephenytoin, ethotoin, and hydantoin in rats. Teratology. 1991;43:279–293. doi: 10.1002/tera.1420430402. [DOI] [PubMed] [Google Scholar]
- Miranda R, Blanco E, Begega A, Rubio S, Arias JL. Hippocampal and caudate metabolic activity associated with different navigational strategies. Behav Neurosci. 2006;120:641–650. doi: 10.1037/0735-7044.120.3.641. [DOI] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with d-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser M-B, Morris RGM. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Mulder E, Davis A, Gawley L, Bowen A, Einarson A. Negative impact of non-evidence-based information received by women taking antidepressants during pregnancy from health care providers and others. J Obstet Gynaecol Can. 2012;34:66–71. doi: 10.1016/S1701-2163(16)35136-2. [DOI] [PubMed] [Google Scholar]
- Nelson BK, Vorhees CV, Scott WJ, Jr, Hastings L. Effects of 2-methoxyethanol on fetal development, postnatal behavior, and embryonic intracellular pH of rats. Neurotoxicol Teratol. 1989;11:273–284. doi: 10.1016/0892-0362(89)90070-6. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- Paquette C, Franzen E, Jones GM, Horak FB. Walking in circles: navigation deficits from Parkinson’s disease but not from cerebellar ataxia. Neuroscience. 2011;190:177–183. doi: 10.1016/j.neuroscience.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson RB, Shanfeld J, Vorhees CV, Sweazy A, Gagni S, Smith AR, Paulson JO. Behavioral effects of prenatally administered smokeless tobacco on rat offspring. Neurotoxicol Teratol. 1993;15:183–192. doi: 10.1016/0892-0362(93)90014-f. [DOI] [PubMed] [Google Scholar]
- Penner MR, Mizumori SJ. Neural systems analysis of decision making during goal-directed navigation. Prog Neurobiol. 2012;96:96–135. doi: 10.1016/j.pneurobio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Pesic V, Marinkovic P, Janac B, Ignjatovic S, Popic J, Kanazir S, Ruzdijic S. Changes of behavioral parameters during long-term food restriction in middle-aged Wistar rats. Physiol Behav. 2010;101:672–678. doi: 10.1016/j.physbeh.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Polidora VJ, Boggs DE, Waisman HA. A behavioral deficit associated with phenylketonuria in rats. Proc Soc Exp Biol Med. 1963;113:817–820. doi: 10.3181/00379727-113-28500. [DOI] [PubMed] [Google Scholar]
- Polidora VJ, Cunningham RF, Waisman HA. Phenylketonuria in rats: Reversibility of behavioral deficit. Science. 1966a;151:219–221. doi: 10.1126/science.151.3707.219. [DOI] [PubMed] [Google Scholar]
- Polidora VJ, Cunningham RF, Waisman HA. Dosage parameters of a behavioral deficit associated with phenylketonuria in rats. J Comp Physiol Psychol. 1966b;61:436–441. doi: 10.1037/h0023257. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci. 2006;26:4071–4081. doi: 10.1523/JNEUROSCI.3408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Persinger GW, Kline LV, Mulry RC. The Role of the Research Assistant in the Mediation of Experimenter Bias. J Pers. 1963;31:313–335. doi: 10.1111/j.1467-6494.1963.tb01302.x. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Lingrel JB, Moseley AE, Vorhees CV, Williams MT. Targeted mutations in the Na,K-ATPase alpha 2 isoform confer ouabain resistance and result in abnormal behavior in mice. Synapse. 2011;65:520–531. doi: 10.1002/syn.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Braun AA, Amos-Kroohs RM, Williams MT, Ostertag E, Vorhees CV. A new model of Pde4d deficiency: genetic knock-down of PDE4D enzyme in rats produces an antidepressant phenotype without spatial cognitive effects. Genes Brain Behav. 2012;11:614–622. doi: 10.1111/j.1601-183X.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Grace CE, Braun AA, Amos-Kroohs RM, Graham DL, Skelton MR, Williams MT, Vorhees CV. Cognitive impairments from developmental exposure to serotonergic drugs: citalopram and MDMA. Int J Neuropsychopharmacol. 2013:1–12. doi: 10.1017/S1461145712001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J Neurosci. 2013;33:19304–19313. doi: 10.1523/JNEUROSCI.1825-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Neural basis of the cognitive map: path integration does not require hippocampus or entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:12034–12038. doi: 10.1073/pnas.0805414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11-20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 2006;189:307–318. doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Vorhees CV. Developmental effects of 3,4-methylenedioxymethamphetamine: a review. Behav Pharmacol. 2008a;19:91–111. doi: 10.1097/FBP.0b013e3282f62c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Blankenmeyer TL, Gudelsky GA, Brown-Stritthold CA, Vorhees CV, Williams MT. Metyrapone attenuates the sequential learning deficits but not monoamine depletions following d,l-fenfluramine administration to adult rats. Synapse. 2004;54:214–222. doi: 10.1002/syn.20077. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Schaefer TL, Herring NR, Grace CE, Vorhees CV, Williams MT. Comparison of the developmental effects of 5-methoxy-N,N-diisopropyltryptamine (Foxy) to (+/-)-3,4-methylenedioxymethamphetamine (ecstasy) in rats. Psychopharmacology (Berl) 2009;204:287–297. doi: 10.1007/s00213-009-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Able JA, Grace CE, Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. (+/-)-3,4-Methylenedioxymethamphetamine treatment in adult rats impairs path integration learning: A comparison of single vs once per week treatment for 5 weeks. Neuropharmacology. 2008b;55:1121–1130. doi: 10.1016/j.neuropharm.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Burns LN, Amos-Kroohs RM, Williams MT, Vorhees CV. Distinct periods of developmental sensitivity to the effects of 3,4-(+/-)-methylenedioxymethamphetamine (MDMA) on behaviour and monoamines in rats. Int J Neuropsychopharmacol. 2011;15:811–824. doi: 10.1017/S1461145711000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NB, Vorhees CV, Meyer RA, O’Brien WD., Jr . IEEE 1990 Ultrasonic Symposium Proceedings. New York: Institute of Electrical and Electronics Engineers; 1990. An automated ultrasonic exposure system to assess the effects of in utero diagnostic ultrasound; pp. 1385–1388. [Google Scholar]
- Stewart CA, Morris RGM. The watermaze. In: Sahgal A, editor. Behavioural Neuroscience, A Practical Approach. I. Oxford: IRL Press at Oxford University Press; 1993. pp. 107–122. [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Terry AV., Jr . Spatial Navigation (Water Maze) Tasks. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2. Boca Raton (FL): 2009. [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Effects of prenatal naloxone exposure on postnatal behavioral development of rats. Neurobehav Toxicol Teratol. 1981;3:295–301. [PubMed] [Google Scholar]
- Vorhees CV. Fetal anticonvulsant syndrome in rats: Dose- and period-response relationships of prenatal diphenylhydantoin, trimethadione and phenobarbital exposure on the structural and functional development of the offspring. J Pharmacol Exp Ther. 1983;227:274–287. [PubMed] [Google Scholar]
- Vorhees CV. Behavioral effects of prenatal d-amphetamine in rats: A parallel trial to the Collaborative Behavioral Teratology Study. Neurobehav Toxicol Teratol. 1985a;7:709–716. [PubMed] [Google Scholar]
- Vorhees CV. Behavioral effects of prenatal methylmercury in rats: A parallel trial to the Collaborative Behavioral Teratology Study. Neurobehav Toxicol Teratol. 1985b;7:717–725. [PubMed] [Google Scholar]
- Vorhees CV. Fetal hydantoin syndrome in rats: Dose-effect relationships of prenatal phenytoin on postnatal development and behavior. Teratology. 1987a;35:287–303. doi: 10.1002/tera.1420350302. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987b;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Behavioral teratogenicity of valproic acid: Selective effects on behavior after prenatal exposure in rats. Psychopharmacology (Berl) 1987c;92:73–179. doi: 10.1007/BF00177911. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maternal age as a factor in determining the reproductive and behavioral outcome of rats prenatally exposed to ethanol. Neurotoxicol Teratol. 1988;10:23–34. doi: 10.1016/0892-0362(88)90063-3. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. A fostering/crossfostering analysis of the effects of prenatal ethanol exposure in a liquid diet on offspring development and behavior in rats. Neurotoxicol Teratol. 1989;11:115–120. doi: 10.1016/0892-0362(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Fernandez K. Effects of short-term prenatal alcohol exposure on maze, activity, and olfactory orientation performance in rats. Neurobehav Toxicol Teratol. 1986;8:23–28. [PubMed] [Google Scholar]
- Vorhees CV, Berry HK. Branched chain amino acids improve complex maze learning in rat offspring prenatally exposed to hyperphenylalaninemia: Implications for maternal phenylketonuria. Pediatr Res. 1989;25:568–572. doi: 10.1203/00006450-198906000-00002. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Minck DR. Long-term effects of prenatal phenytoin exposure on offspring behavior in rats. Neurotoxicol Teratol. 1989;11:295–305. doi: 10.1016/0892-0362(89)90072-x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR J. 2014a;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol Teratol. 2014b;45:75–90. doi: 10.1016/j.ntt.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Brunner RL, Butcher RE. Psychotropic drugs as behavioral teratogens. Science. 1979;205:1220–1225. doi: 10.1126/science.472738. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Minck DR. Neurobehavioral teratogenic effects of thalidomide in rats. Neurotoxicol Teratol. 2001;23:255–264. doi: 10.1016/s0892-0362(01)00140-4. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Williams MT. Developmental effects of +/-3,4-methylenedioxymethamphetamine on spatial versus path integration learning: Effects of dose distribution. Synapse. 2007;61:488–499. doi: 10.1002/syn.20379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Brunner RL, McDaniel CR, Butcher RE. The relationship of gestational age to vitamin A induced postnatal dysfunction. Teratology. 1978;17:271–275. doi: 10.1002/tera.1420170305. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Fernandez K, Dumas RM, Haddad RK. Pervasive hyperactivity and long-term learning impairments in rats with induced micrencephaly from prenatal exposure to methylazoxymethanol. Dev Brain Res. 1984;15:1–10. doi: 10.1016/0165-3806(84)90134-2. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991a;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, Moran MS. Prenatal exposure to sodium phenytoin in rats induces complex maze learning deficits comparable to those induced by exposure to phenytoin acid at half the dose. Neurotoxicol Teratol. 1995;17:627–632. doi: 10.1016/0892-0362(95)02005-5. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Weisenburger WP, Minck DR, Berry HK. Branched chain amino acids improve radial-arm maze acquisition and water maze forced-choice learning in rat offspring exposed in utero to hyperphenylalaninemia. Neurotoxicol Teratol. 1992;14:35–41. doi: 10.1016/0892-0362(92)90026-7. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology (Berl) 1994a;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Weisenburger WP, Meyer RA, Smith NB, O’Brien WD., Jr A teratologic evaluation of continuous wave, daily ultrasound exposure in unanesthetized pregnant rats. Teratology. 1991b;44:667–674. doi: 10.1002/tera.1420440609. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, Fisher JE, Moran MS, Buelke-Sam J. A developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in rats. Fund Appl Toxicol. 1994b;23:194–205. doi: 10.1006/faat.1994.1098. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Schaefer TL, Skelton MR, Grace CE, Herring NR, Williams MT. (+/-)3,4-Methylenedioxymethamphetamine (MDMA) dose-dependently impairs spatial learning in the morris water maze after exposure of rats to different five-day intervals from birth to postnatal day twenty. Dev Neurosci. 2009a;31:107–120. doi: 10.1159/000207499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Acuff-Smith KD, Schilling MA, Fisher JE, Meyer RA, Smith NB, O’Brien WD., Jr Behavioral teratologic effects of prenatal exposure to continuous-wave ultrasound in unanesthetized rats. Teratology. 1994c;50:238–249. doi: 10.1002/tera.1420500309. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci. 2009b;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Braun AA, Schaefer TL, Skelton MR, Richtand NM, Williams MT. Prenatal immune challenge in rats: altered responses to dopaminergic and glutamatergic agents, prepulse inhibition of acoustic startle, and reduced route-based learning as a function of maternal body weight gain after prenatal exposure to poly IC. Synapse. 2012;66:725–737. doi: 10.1002/syn.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Braun AA, Schaefer TL, Skelton MR, Richtand NM, Williams MT. Prenatal immune challenge in rats: effects of polyinosinic-polycytidylic acid on spatial learning, prepulse inhibition, conditioned fear, and responses to MK-801 and amphetamine. Neurotoxicol Teratol. 2015;47:54–65. doi: 10.1016/j.ntt.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/--methylenedioxymethamphetamine, (+)-amphetamine and +/--fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2011;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41-50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21-30 or P31-40) or adult rats (P51-60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Weisenburger WP, Minck DR, Acuff KD, Vorhees CV. Dose-response effects of prenatal phenytoin exposure in rats: Effects on early locomotion, maze learning, and memory as a function of phenytoin-induced circling behavior. Neurotoxicol Teratol. 1990;12:145–152. doi: 10.1016/0892-0362(90)90127-x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, McCrea AE, Wood SL, Vorhees CV. Administration of d,l-fenfluramine produces learning deficits in the Cincinnati water maze but not the Morris water maze: Relationship to adrenal cortical output. Neurotoxicol Teratol. 2002;24:1–14. doi: 10.1016/s0892-0362(02)00318-5. [DOI] [PubMed] [Google Scholar]
- Williams MT, Herring NR, Schaefer TL, Skelton MR, Campbell NG, Lipton JW, McCrea AE, Vorhees CV. Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘foxy’) to adult rats: a new drug of abuse. Neuropsychopharmacology. 2007;32:1404–1420. doi: 10.1038/sj.npp.1301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects, or injection stress. Brain Res. 2003;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 2005;32:285–294. doi: 10.1385/MN:32:3:285. [DOI] [PubMed] [Google Scholar]
- Wittlinger M, Wehner R, Wolf H. The ant odometer: stepping on stilts and stumps. Science. 2006;312:1965–1967. doi: 10.1126/science.1126912. [DOI] [PubMed] [Google Scholar]


