Abstract
Background
Circumferential stretch on the vein wall has been suggested as a potential etiological factor in the development of varicose veins. However, the influence of vein wall stretch on vein metabolism has not yet been explored.
Aim
The aim of this study was to investigate the effect of short and prolonged mechanical stretch on the vein wall metabolism.
Methods
Circular segments of inferior vena cava from male Sprague-Dawley rats were exposed to normal 0.5 g (non-stretched) or high 2 g (stretched) tension for short 4 h or prolonged 18 h duration (5 vein segments per group). Contraction response to phenylephrine (PHE, 10−5M) and KCl (96 mM) was elicited to observe the effect of circumferential stretch on vein function. The polar and organic metabolites in vein tissue were extracted using a bilayer extraction method. Aqueous and organic extracts were analysed using nuclear magnetic resonance spectroscopy and ultra performance liquid chromatography coupled to mass spectrometry, respectively. Data acquired from both analytical platforms were analysed using mathematical modelling.
Results
Increased concentrations of valine (p= 0.02) and choline (p=0.03) metabolites and triglyceride moieties (p= 0.03) were observed in veins stretched for 18 hrs as compared to the non-stretched for 18 hrs group.
Conclusion
This study has shown that prolonged mechanical circumferential stretch (18 hours) alters the metabolic profile of rat inferior vena cava.
Introduction
Exposure to increased hydrostatic pressure in patients with environmental and genetic risk factors for venous disease is considered fundamentally responsible for disease development and progression(1–3). Mechanical stretch exerted by venous hypertension may cause damage to the valves directly or may first cause dilatation of vein walls, which then forces the valves apart causing valvular incompetence(3).
Mechanical circumferential stretch of cultured vascular smooth muscle cells has been found to regulate their proliferation, apoptosis and reorganisation of extracellular matrix (4). Zowlak et al transplanted a rabbit’s jugular vein into carotid artery circulation and noted at 1 hour intimal cell proliferation and at two weeks smooth muscle cell proliferation and vein wall thickening were observed after transplantation, suggesting that exposure to high pressure (circumferential stretch and shear stress) may be a cause for such changes (5). It has been demonstrated that prolonged stretch increases vein wall tension and up-regulates the expression of hypoxia inducible factors (HIF) −1α and −2α mRNA and proteins in a rat inferior vena cava (IVC) explant model. These factors were associated with increased matrix metalloproteinase (MMPs) expression, increased venous relaxation and hence dilatation(6).
Metabolic phenotyping of biological samples can be used to measure the alterations in small molecules or metabolites in response to diet, environmental factors, genetics and disease (7, 8) (9). Using metabolic profiling (metabonomic) approaches, it has been previously shown that an increased concentration of lactate, creatine and myo-inositol metabolites are identified in human varicose veins samples as compared to non-varicose vein controls (9), indicating that the technology is appropriate for investigating pathological changes in vein tissue. Here we apply a metabonomic approach to exploring metabolic and vein wall changes in relation to vein wall stretch to identify key metabolic pathways that may be involved in venous disease and hence potentially identify new therapeutic targets. We hypothesize that application of increased circumferential stretch (higher tension and hence pressure), will cause alterations in the metabolism of the vein wall compared to control non-stretched (lower tension; normal pressure). To understand the effect of stretch on vein metabolism, an ex-vivo rat inferior IVC organ culture model was used and vein segments were stretched for 4 and 18 hour durations. Two analytical platforms: nuclear magnetic resonance (NMR) spectroscopy and ultra performance liquid chromatography coupled to mass spectrometry (UPLC-MS) lipid profiling were used to explore metabolic perturbation in stretched and non-stretched rat IVC segments.
Methodology
Tissue Preparation and Solutions
Stretching of IVC segments and isometric contractions studies was performed at the vascular research laboratory, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA. All animal research experiments were conducted following the guidelines of the Standing Committee on Animals at Harvard Medical School and followed the Ethical Research Guidelines at the Brigham and Women's Hospital and Harvard Medical School. Krebs solution contained NaCl 120 mM, KCl 5.9 mM, NaH2PO4 1.2 mM, dextrose 11.5 mM, CaCl2 2.5 mM, MgCl2 1.2 mM bubbled with 95% O2, 5% CO2 at PH 7.4. KCl solution (96 mM) was prepared with the same equimolar substitution of NaCl with KCl. The α adrenergic agonist phenylephrine (PHE) 10−5 M was used to stimulate IVC contraction. Tissue culture medium included minimal essential medium supplemented with penicillin streptomycin and amphotericin B (Gibco/Invitrogen Grand Island, NY). Male Sprague-Dawley rats (n= 5, 12 weeks old, 250–300 g, Charles River Lab, Wilmington, MA, USA) were euthanized by inhalation of CO2. Euthanasia was judged by cessation of breathing and heart beats. The abdominal cavity was opened, and the tissue in the retroperitoneum including the inferior vena cava (IVC), aorta, kidneys, and posterior muscles and surrounding fat was rapidly excised, placed in Kreb’s solution, and the IVC carefully dissected and cleaned of all peri-adventitial tissue and nearby structures under a dissecting microscope. The IVC was portioned into four 3mm wide rings in preparation for isometric contraction experiments.
Application of Stretch and Isometric Contraction in Rat IVC Segments
Each IVC segment was suspended between two tungsten wire hooks, each hook had a full contact with the luminal surface of the vein segment providing a circumferential stretch, one hook was fixed to a glass rod at the bottom of the tissue bath and the other hook was connected to a Grass force displacement transducer (FT03, Astro-Med Inc, West Warwick, RI, USA). After calibration using a 2 gram weight, vein segments were stretched under a pre-determined basal tension (0.5 gram) at the beginning (non-stretched 1 hr) in a temperature controlled, water-jacketed tissue bath, filled with 50 ml Krebs solution continuously bubbled with 95% O2 and 5% CO2 at 37°C. The changes in isometric contraction were recorded on a Grass polygraph (Model 7D, Astro-Med). IVC segments of rats were then subjected to stretch and no stretch by increasing or decreasing the tension to the hook, respectively (Figure 1).
Figure 1.
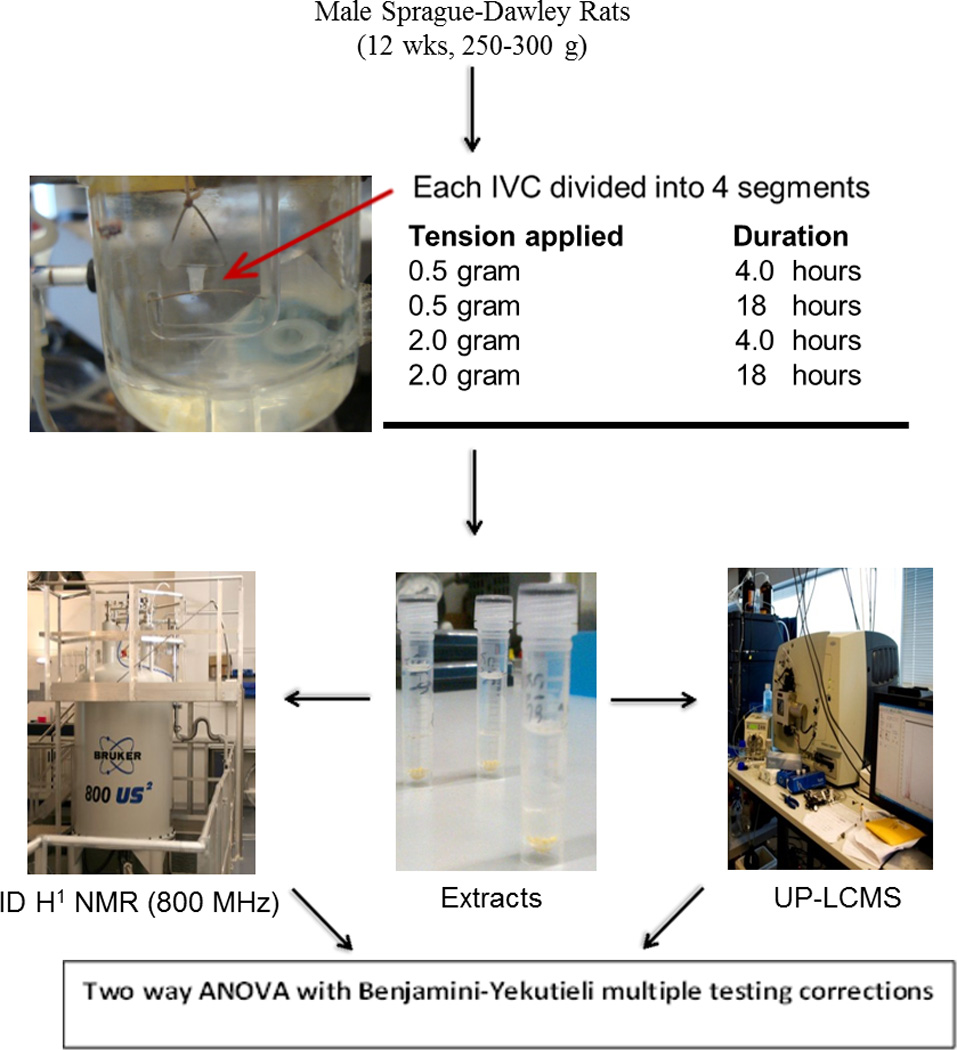
Schematic outline of the experimental design of the study. In the snapshot a small segment of rat IVC is shown stretched between the two hooks in Kreb’s solution.
Contractions were assessed using 96 mM KCl solution, as measured by positive deflection on polygraph, at the end of each experiment for each vein segment. Previous work by Raffetto et al. has demonstrated the response of contraction of rat IVC segments to 96 mM KCl membrane depolarization under the influence of different tensions (0.0625 gram to maximum of 3 gram) (10). We followed the same protocol and used 0.5 gram tension as the basal tension and 2 gram tension as the maximum load (10). The basal tension of 0.5 gram and maximum tension of 2 gram are also in concurrence with the venous pressure observed in the superficial venous system of human leg (11). For example, 0.5 gram tension is equivalent to 20.8 mmHg and 2 gram tension is equivalent to 83.4 mmHg (11).
The same protocol for applying stretch and eliciting contractions to rat IVC wall was employed as previously described (10, 11). This included stimulation of IVC segments twice with 96mM KCl and then PHE 10−5 M. Each contraction was followed by a 3 minute wash in Kreb’s solution. The IVC segments were then stretched for 4 hrs. After 4 hrs of stretch, contractions were stimulated twice with 96 mM KCl followed by PHE 10−5 M and tissue was bathed with Kreb’s solution in between contractions. Two IVC segments, stretched to 0.5 gram and 2 gram tension, were then frozen at −80°C. The remaining two IVC segments were then stretched with basal tension of 0.5 gram and prolonged tension of 2 gram for 18 hrs in culture medium. At 18 hrs, contractions were assessed by stimulation with 96 mM KCl solution and PHE 10−5 M solutions again before freezing the samples at −80°C. Contractions for each sample were compared to baseline (non-stretched 1 hr) contraction. Each animal provided 4 segments of 3mm in length of IVC that were then subjected to all four tensions and periods in order to minimize inter sample variations.
For the rest of the paper samples stretched with basal tension of 0.5 gram for 4 and 18 hrs are represented with the abbreviations N4 and N18, respectively. Similarly samples stretched with increased tension of 2 gram for 4 and 18 hrs will be denoted by S4 and S18, respectively.
Metabolite extraction from tissue samples
Water/methanol (50:50) and dichloromethane/methanol (75:25) were used for extractions. A bilayer extraction protocol was applied(12). Average weight of the sample was 5.6 mg (SD 1.5), which made the weight of the sample relatively small in comparison to the sample size typically used in metabonomic studies(8, 13, 14). Please see supporting information section. Aqueous and organic extracts were dried as described before (13) and stored in a −80°C freezer pending analysis. Extracts were prepared for Nuclear magnetic resonance (NMR) spectroscopy and Ultra performance liquid chromatography –mass spectrometry (UPLC-MS) lipid profiling using well established methods (13) (14)(15).
Statistical analysis
Processed data were then analysed using Simca P+12.5 (Umetrics) and MATLAB R2009b (Mathworks™, 2009) software, the latter using in-house developed MATLAB code. The nature of untargeted analyses of complex biological matrices using NMR and UPLC-MS, results in a very data rich output with typically more variables than samples necessitating the application of a multivariate statistical approach. However, if the samples size is significantly less in comparison to the number of variables detected, a problem of multi-collinearity may arise leading to problems in analysing the data using multivariate approach.
Therefore, we applied a well-known method of reducing the high dimensionality of the data called statistical recoupling of variables (SRV) using an in-house algorithm to analyse the NMR data. This computational peak picking technique uses a correlation landscape to restore the dependency of spectral variables and integrate regions into physically and biologically meaningful segments (18). Furthermore, effect of noise on the meaningful segments of the spectra is reduced using this method as SRV minimizes the number of variables needed for the perseverance of latent variable. Univariate analysis of variance testing (1–way ANOVA) was used to analyse selected metabolites detected in the NMR and UPLC-MS data; ANOVAs were performed on a single factor with the data split into four groups (S 4hrs, S 18hrs, NS 4hrs, NS 18 hrs), and tested in a pair wise fashion. ANOVA was initially applied on the whole set of variables to account for the type I error with the presumption that each variable affects the outcome. Benjamini-Yekutieli false discovery rate correction was therefore performed on the-ANOVA statistical analyses to ensure the test statistics corresponded to the true null hypotheses(19). Only variables found to be significant after correction for multiple testing were considered to be of potential biological significance between the different experimental groups.
Spearman pairwise correlations (r) were calculated using the R programming language (2.15.2). Two-tailed tests were employed to provide the statistical significance (p-values) of correlation coefficients.
Results
Isometric Contraction in Response to 96 mM KCl and PHE10−5M
Isometric contractions in response to 96 mM KCl and PHE 10−5 M in rat IVC segments were measured using a polygraph. Compared to the initial basal contraction at 1 hr, there was a decline in contraction response to 96 mM KCl in all segments except in IVC segments exposed to increased stretched tension for 4 hrs (S4). The fold change difference from baseline contractions (1.6 fold decline) was statistically significant in rat S18 IVC segments as compared to basal contraction (Figure 2). In response to PHE 10−5 M, there was an increase in contraction at 4 hrs both in stretched and non-stretched segments. A decrease in PHE 10−5 M induced contractions was noticed in all segments at 18 hrs regardless of the tension applied indicating that there was a time dependent reduction in muscle contractility. However, statistically significant reduction in contraction (2 fold) to PHE 10−5 M was only detected in the S18 vein segments (Figure 2).
Figure 2.
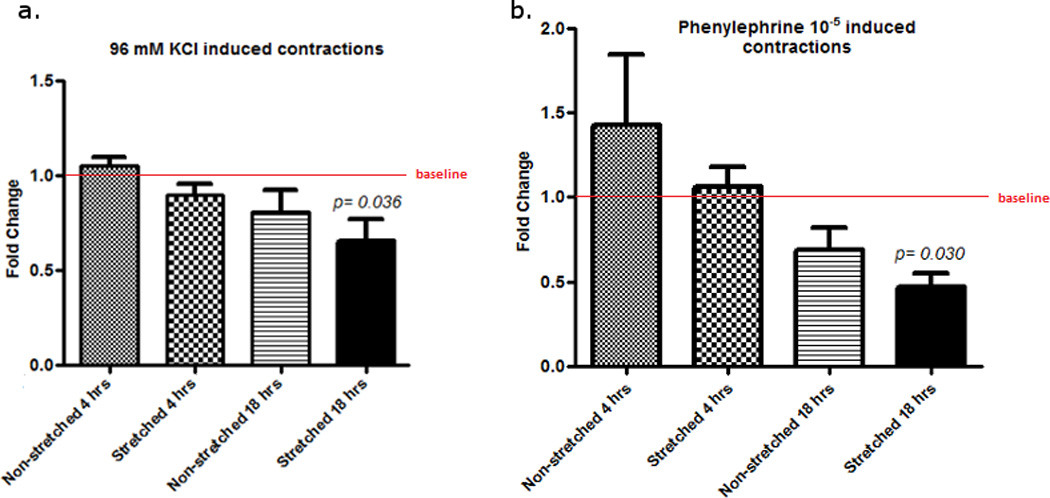
Fold change in contraction of different stretched and non-stretched rat IVC segments in response to 96 mM KCl (a) and Phenylephrine 10−5 M (b) at different time intervals. Non-stretched at 1 hr is the baseline contractions. Contraction for each sample at 4 hrs or 18 hrs was compared to baseline (non-stretched 1 hr) contraction. Error bars represent a single standard deviation.
Analysis of Aqueous Extracts of IVC tissue using NMR
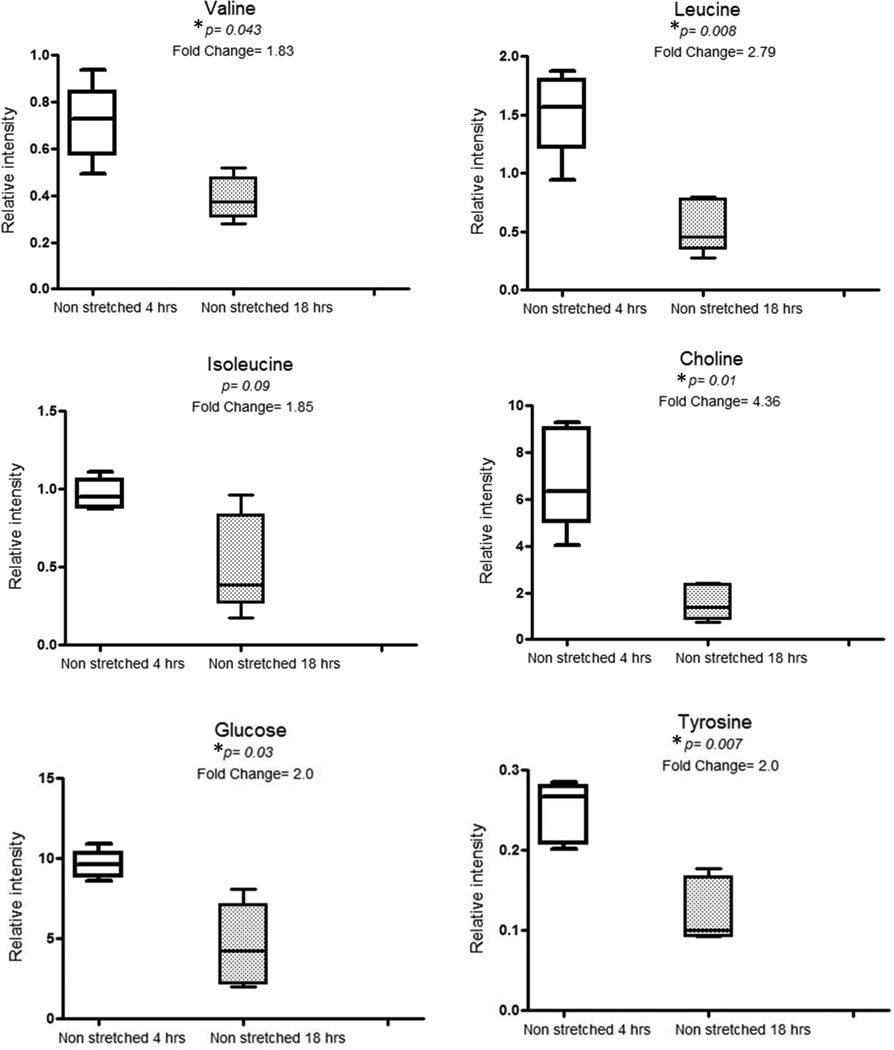
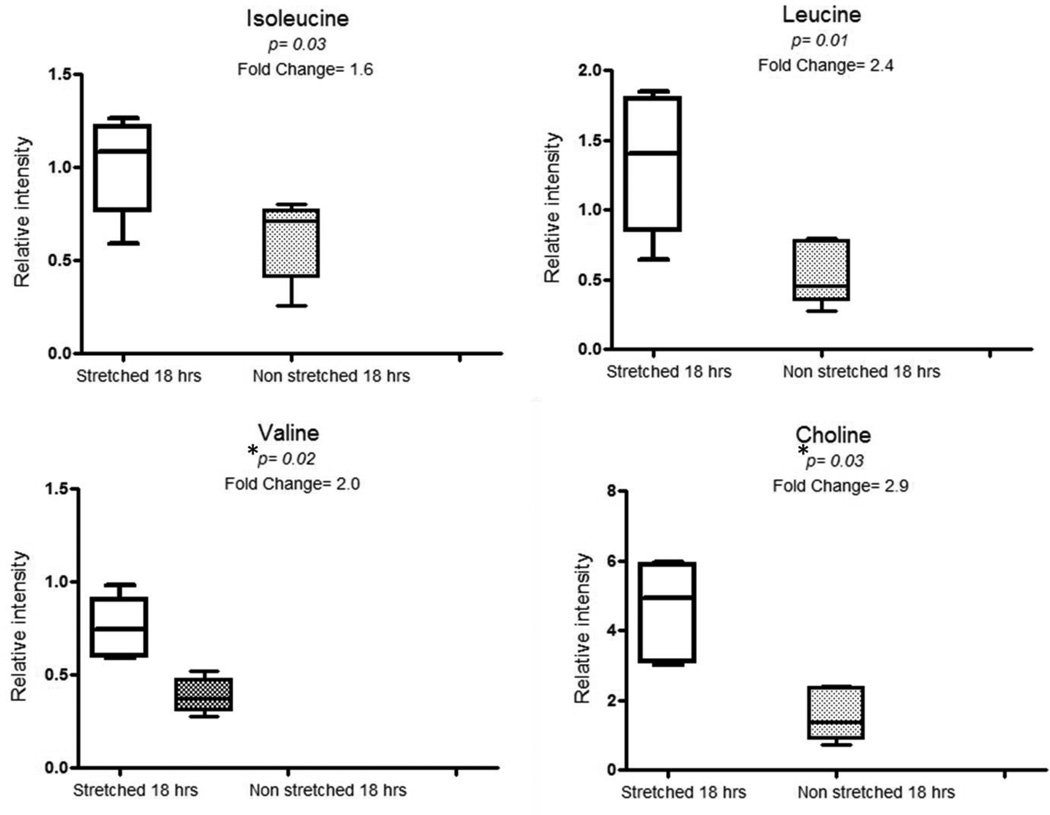
Univariate analysis using ANOVA of SRV integrated NMR peaks showed differences between samples from the S18 group and N18 groups, and between the N4 and N18 groups (Figures 3 and 4) indicating a metabolic response related both to prolonged stretching and time.
Figure 3.
Box plots showing comparisons of relative intensities of valine, leucine, isoleucine, choline, glucose and tyrosine metabolites in non-stretched 4hrs and non-stretched 18 hrs segments.
*P values are expressed as Benjamini-Yekutieli corrected values.
Figure 4.
Box plots showing comparisons of relative intensities of valine, leucine, isoleucine and choline metabolites in stretched 18hrs and non-stretched 18 hrs segments.
*P values are expressed as Benjamini-Yekutieli corrected values.
The key differential peaks responsible for variation over time i.e. between N4 and N18 groups were valine, leucine, choline, glucose and tyrosine (Figure 3). Isoleucine was found significant on metabolite on ANOVA testing but did not remain significant after application of multiple testing correction (Figure 3). The differential SRV clustered peaks observed between non-stretched and stretched IVC tissue for 18 hrs were related to valine and choline metabolites (Figure 4). Similarly isoleucine was in higher concentrations in S18 segments as compared to N18 segments (Figure 4) but the difference was not statistically significant on multiple testing correction. Table 1 shows the list of metabolites found to be significantly different between the groups following univariate analysis with Benjamini-Yekutieli multiple testing corrections. Statistical total correlation spectroscopy (STOCSY) was used to highlight peaks related on these differential metabolites on the spectra for the purpose of confirmation of structural assignments (Table 1).
Table 1.
Table showing the aqueous metabolites (acquired from SRV corrected NMR spectra) found to be significant in N4 (non-stretched 4 hr) and S18 (stretched 18hr) groups as compared to N18 (non-stretched 18 hr) group. Statistical total correlation spectroscopy (STOCSY) was used to highlight peaks correlated to differential metabolites for the purpose of assignment.
| Molecule Name |
Chemical Shift (δ) |
p value* | STOCSY assignments (δ) |
Group with increased intensities |
Groups compared |
|---|---|---|---|---|---|
| Valine | 1.04 | 0.04 | 0.98 | N4 | N4 Vs N18 |
| 2.26 | |||||
| 3.59 | |||||
| Leucine | 0.96 | 0.008 | 0.94 | N4 | N4 Vs N18 |
| 1.73 | |||||
| Choline | 3.21 | 0.01 | 3.20 | N4 | N4 Vs N18 |
| 3.50 | |||||
| 4.05 | |||||
| Glucose | 3.72 | 0.03 | 3.23 | N4 | N4 Vs N18 |
| 3.40 | |||||
| 3.51 | |||||
| 3.73 | |||||
| 3.84 | |||||
| 4.62 | |||||
| 5.22 | |||||
| Tyrosine | 6.917.19 | 0.007 | 3.02 | N4 | N4 Vs N18 |
| 3.19 | |||||
| 3.93 | |||||
| 6.89 | |||||
| 7.18 | |||||
| Valine | 0.96 | 0.02 | 0.98 | S18 | S18 Vs N18 |
| 2.26 | |||||
| 3.59 | |||||
| Choline | 3.2 | 0.03 | 3.20 | S18 | S18 Vs N18 |
| 3.50 | |||||
| 4.05 |
corrected p value (Benjamini-Yekutieli correction)
UPLC-MS-based Lipid Profiling
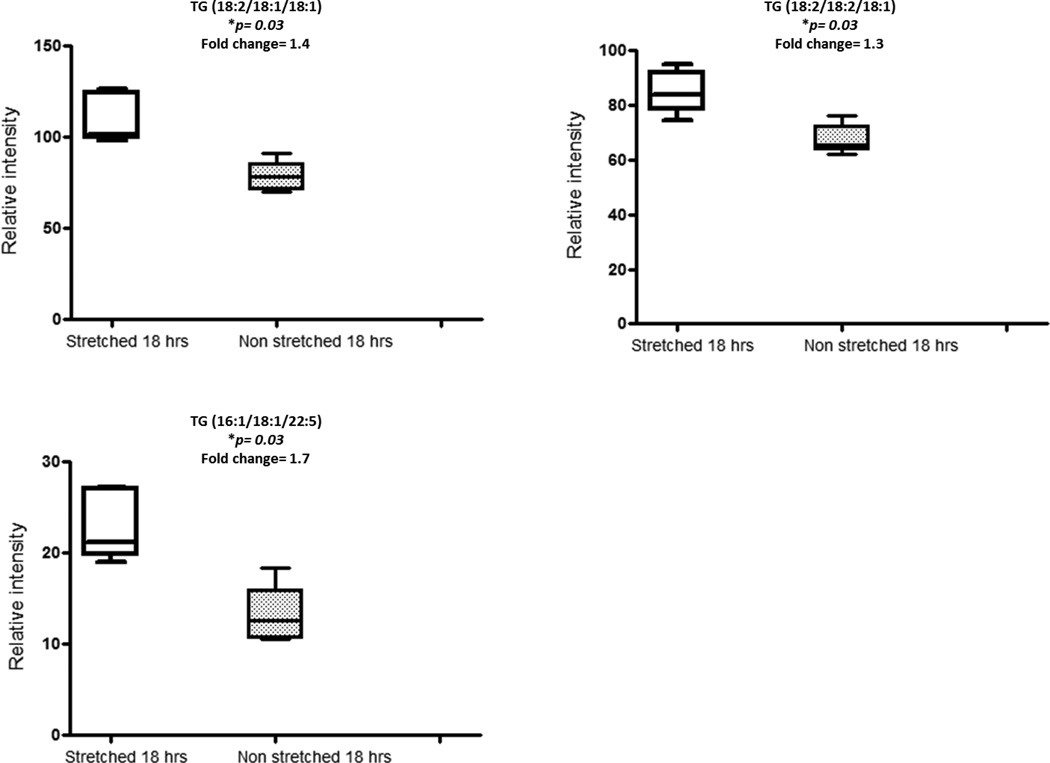
Pairwise group comparisons (for all pair combinations) of UPLC-MS-based lipid profiling data of rat IVC segments acquired in ESI+ mode was performed using ANOVA. A range of features were found to be significant (p <0.05). However, all of the metabolic features found to be significant according to the ANOVA test did not hold up to multiple testing correction with the exception of triglyceride (TG) moieties [TG (18:2/18:1/18:1), TG(18:2/18:2/18:1), TG(16:1/18:1/22:5)] which were significant (corrected p values for each were 0.03) for comparison of the stretched and non-stretched tissue after 18 hrs (S18 vs N18; Table 2 and Figure 5). Time of stretching did not impact on the biochemical composition of the IVC segments, as demonstrated by the comparison of N4 and S4 against N18 and S18 and no statistically significant differences were found between these groups. For ESI-, no statistical difference was observed between any of the group comparisons. The three detected TGs demonstrated high positive pairwise correlation coefficients: TG (18:2/18:1/18:1) with TG(18:2/18:2/18:1) demonstrated r=0.90, TG (18:2/18:1/18:1) with TG(16:1/18:1/22:5) r=0.79 and TG(18:2/18:2/18:1) with TG(16:1/18:1/22:5) r=0.82 (p < 2.3x10−5).
Table 2.
Details of Triglycerides moieties (UPLC-MS ESI+ mode data) found to be higher in stretched for 18 hrs as compared to non-stretched for 18 hrs group.
| Metabolite Name |
TG (18:2/18:1/18:1) | TG(18:2/18:2/18:1) | TG(16:1/18:1/22:5) |
|---|---|---|---|
|
Mol Formula as Detected |
C57H106NO6+ [M+NH4]+ |
C57H104NO6+ [M+NH4]+ |
C59H104NO6+ [M+NH4]+ |
|
Retention time (min) |
15.44 | 15.20 | 15.08 |
|
m/z (found) |
900.8038 | 899.7938* | 922.7900 |
|
m/z (theor) |
900.8020 | 899.7897* | 922.7864 |
| Δppm | 2 | 5 | 4 |
|
Groups Compared |
N18 vs S18 | N18 vs S18 | N18 vs S18 |
| Intensities | Higher in S18 | Higher in S18 | Higher in S18 |
|
p-value (corrected) |
0.03 | 0.03 | 0.03 |
TG Triglycerides
Represents the m/z of the secondary isotope.
Figure 5.
Box plots showing comparisons of relative intensities of triglycerides (TG) moieties in stretched 18hrs and non-stretched 18 hrs segments.
*P values are expressed as Benjamini-Yekutieli corrected values.
Discussion
This study comprehensively characterized metabolic changes of rat IVC segments in an ex vivo organ bath. 1H NMR analysis of aqueous extracts of rat IVC segments revealed an interesting motif with relative depletion in the concentrations of some important metabolites over time. Valine, leucine, isoleucine, choline, tyrosine, and glucose were higher in N4 segments as compared to N18. Although multiple testing corrections ruled out isoleucine as significant with the p value of 0.09 after correction (figure 3), these findings indicated that branched chain amino acids, released from breakdown of muscle proteins in high energy state, were depleted over time in N18 group. The other possible effect is that with time comparing N4 vs. N18, that the metabolites (amino acids) decrease due to unstimulated tissue (i.e. lack of tension). However, once tissue are stimulated with increased tension and time (S18 vs. N18), that increased metabolic activity and demands are placed on the 2 g stretched tissue, indicating that the tension applied (therefore increased pressure) is critical factor that determines the metabolic function of the tissue response, and that duration of exposure to the tension is a secondary determinant of metabolic function. A similar explanation could apply to the observed depletion of glucose over time in N18 group. When the stretched group was compared to the non-stretched group at 18 h (S18 Vs N18), most metabolites were observed to be significant using univariate ANOVA analysis, of which only choline and valine retained significance after multiple testing correction (figure 4).
Choline is a constituent of the cell membrane and is a precursor molecule to the two common phospholipids also present in the cell membrane: phosphatidylcholines (PC) and sphingomyelins (SM) (20). The main functions of choline involve cell membrane integrity and cell membrane signalling (20). The presence of increased choline in IVC tissue stretched for 18 hrs (S18) compared to tissue held under normal tension for the same length of time (N18) may be due to increased metabolism of cell membrane phospholipids under the influence of stretch releasing choline metabolite, an important constituent of its structural integrity and cell proliferation. This may also explain why there is a progressively decreased contraction of IVC segments especially those subjected to S18. This occurs with both receptor dependent (PHE) and independent (KCl) contractile agonists, indicating that IVC reduced contraction under S18 conditions is likely a downstream event from the agonist-receptor interaction (10).
Based on UPLC-MS analysis of the organic IVC tissue extracts, prolonged stretch resulted in a significant change between tissues held under normal and increased tension for 18 hrs in the intensities of TG moieties. Three TGs [TG (18:2/18:1/18:1), TG (18:2/18:2/18:1), TG(16:1/18:1/22:5)] were detected in higher intensities in the stretched vein segments for 18 hrs (Figure 5). The high pairwise correlation between these TGs is indicative of an origin of common biological response. Triglycerides are the major constituents of very low density lipoproteins (VLDL) and chylomicrons and play a key role in metabolism by releasing fatty acids in high metabolic states. Triglyceride rich VLDL and chylomicrons induce vascular cell adhesion molecule-1 (VCAM-1) expression on the vessel wall (21). Increased expression of VCAM-1 and intercellular adhesion molecule-1 have been reported in varicose veins wall (22). This evidence strongly suggests that high pressure may induce an inflammatory response, which may have a role in varicose veins disease progression. A high pressure induced inflammation is also present in aneurysmal and atherosclerotic diseases and is considered an important aetiological factor for such diseases (23). Previously, we have characterized the metabolic profile of intact human varicose veins tissue biopsies using magic angle spinning 1H NMR spectroscopy and identified increased concentration of lactate, creatine and myo-inositol metabolites in varicose veins as compared to non-varicose veins control. Here, we observed a different set of differential metabolites (valine, choline and triglycerides) in rat IVC segments exposed to 18 hrs stretch as compared to non-stretched 18 hrs. It may not be accurate to compare metabolic changes under static stretch for 18 hrs to chronic changes occurring over a long period of repetitive stretch as observed in varicose veins patients.
The study has several limitations, the main one being the low number of samples per group (n-5). Given the fact that untargeted metabolic profiling can generate a wealth of features corresponding to metabolites but also noise, the inclusion of more samples may have served to reduce the experimental noise and identify further discriminatory metabolites. Nevertheless, the data pre-processing and analysis approaches used in this study, helped in reducing the noise or artefacts in this relatively small study size (14, 18). Univarate approaches with multiple testing corrections are a robust alternative especially when dealing with laboratory animal studies where inter-sample heterogeneity is expected to be low. This is an ex vivo study so there may be a time effect on physiological and metabolic activity in vein segments. However both N18 and S18 (non stretched and stretched for 18 hrs segments) remained in organ bath for 18 hrs and there were statistically significant metabolic differences between the two groups purely based on the observation that one group was exposed to 2 gram of tension/ stretch for 18 hrs and the other groups was exposed to 0.5 gram of tension for 18 hrs. Future studies may need to consider in-vivo modelling to diminish changes related to time, and to determine the metabolites expressed in the venous system under conditions of prolonged venous hypertension. Another limitation which should be addressed in planning future studies examining metabolic perturbation of rat IVC segments in response to stretch is inclusion of a group sampled at zero hours (IVC harvested fresh from rat) before applying any stretch. Metabolic profile of these freshly frozen rat IVC segments will provide the baseline metabolic activity as well as the ability to determine variation due to stretch or otherwise, through time. This was not included in this study as our main focus was the comparison of stretched to non-stretched vein tissue. Rat vena cava does not have the same biomechanical properties as human leg veins and caval veins also have less muscular tissue (24). Moreover, this is an in-vitro study and it is likely true that in-vivo and result would differ, and hence an in-vivo model of venous hypertension may clarify these findings. A true representative model of chronic venous disease and hypertension is lacking, and ex-vivo experimentation allows for controlled conditions to test the hypothesis. A one to one inference to human venous system is not possible, but these data allow for the extraction of data without significant confounding variables (age, medical comorbidities, medications), and set the foundation for future experiments in human venous tissue. Understanding the metabolic profiles during different experimental conditions which reflect increased venous tension and hence pressure allows for a better understanding of possible mechanism which may be important in varicose vein pathophysiology.
Conclusion
This is the first metabolic profiling study to investigate early metabolic changes occurring in an ex-vivo tissue culture stretch model. For mapping the comprehensive metabolic fluctuations under stretch stimuli, future studies should measure metabolic response to repetitive stretch and relaxation for different duration applied to a larger sample size. Although the results are influenced by experimental limitations, they nevertheless indicate that valine, choline and triglyceride metabolic pathways are affected under venous stretch.
Supplementary Material
Statement.
This study comprehensively characterized metabolic changes of rat IVC segments stretched in an ex vivo organ bath. Further research into identifying the causes for increased metabolites in conditions representing venous hypertension, will allow for identifying mechanisms for each metabolite and possible therapeutic targets that may be beneficial in reducing the progression and recurrence of varicose veins.
Acknowledgments
Authors acknowledge Graham Dixon Charitable Trust, European Venous Forum and Imperial College London Healthcare NHS Charitable Trust for their funding in carrying out this research project.
PAV acknowledges the Royal Society of Chemistry for funding his PhD studentship.
Footnotes
Conflict of Interests
None
References
- 1.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111(18):2398–2409. doi: 10.1161/01.CIR.0000164199.72440.08. [DOI] [PubMed] [Google Scholar]
- 2.Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96(11):1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- 3.Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23(2):85–98. doi: 10.1258/phleb.2007.007027. [DOI] [PubMed] [Google Scholar]
- 4.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40(5):947–960. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987;5(1):126–136. [PubMed] [Google Scholar]
- 6.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, et al. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg. 2010 doi: 10.1016/j.jvs.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson JK, Lindon JC, Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 8.Vorkas PA, Shalhoub J, Isaac G, Want EJ, Nicholson JK, Holmes E, et al. Metabolic phenotyping of atherosclerotic plaques reveals latent associations between free cholesterol and ceramide metabolism in atherogenesis. J Proteome Res. 2015;14(3):1389–1399. doi: 10.1021/pr5009898. [DOI] [PubMed] [Google Scholar]
- 9.Anwar MA, Shalhoub J, Vorkas PA, Lim CS, Want EJ, Nicholson JK, et al. In-vitro Identification of Distinctive Metabolic Signatures of Intact Varicose Vein Tissue via Magic Angle Spinning Nuclear Magnetic Resonance Spectroscopy. Eur J Vasc Endovasc Surg. 2012;44(4):442–450. doi: 10.1016/j.ejvs.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Raffetto JD, Qiao X, Koledova VV, Khalil RA. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J Vasc Surg. 2008;48(2):447–456. doi: 10.1016/j.jvs.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim CS, Qiao X, Reslan OM, Xia Y, Raffetto JD, Paleolog E, et al. Prolonged mechanical stretch is associated with upregulation of hypoxia-inducible factors and reduced contraction in rat inferior vena cava. J Vasc Surg. 2011;53(3):764–773. doi: 10.1016/j.jvs.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, Southam AD, Hines A, Viant MR. High-throughput tissue extraction protocol for NMR- and MS-based metabolomics. Analytical biochemistry. 2008;372(2):204–212. doi: 10.1016/j.ab.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Anwar MA, Vorkas PA, Li JV, Shalhoub J, Want EJ, Davies AH, et al. Optimization of metabolite extraction of human vein tissue for ultra performance liquid chromatography-mass spectrometry and nuclear magnetic resonance-based untargeted metabolic profiling. The Analyst. 2015;140(22):7586–7597. doi: 10.1039/c5an01041a. [DOI] [PubMed] [Google Scholar]
- 14.Vorkas PA, Isaac G, Anwar MA, Davies AH, Want EJ, Nicholson JK, et al. Untargeted UPLC-MS profiling pipeline to expand tissue metabolome coverage: application to cardiovascular disease. Anal Chem. 2015;87(8):4184–4193. doi: 10.1021/ac503775m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2(11):2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 16.Dieterle F, Ross A, Schlotterbeck G, Senn H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal Chem. 2006;78(13):4281–4290. doi: 10.1021/ac051632c. [DOI] [PubMed] [Google Scholar]
- 17.Gika HG, Theodoridis GA, Wingate JE, Wilson ID. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine. J Proteome Res. 2007;6(8):3291–3303. doi: 10.1021/pr070183p. [DOI] [PubMed] [Google Scholar]
- 18.Blaise BJ, Shintu L, Elena B, Emsley L, Dumas ME, Toulhoat P. Statistical recoupling prior to significance testing in nuclear magnetic resonance based metabonomics. Anal Chem. 2009;81(15):6242–6251. doi: 10.1021/ac9007754. [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Yoav YD. The control of the false discovery rate in multiple testing under dependency. Ann Statist. 2001;29(4):1165–1188. [Google Scholar]
- 20.Hollenbeck CB. An introduction to the nutrition and metabolism of choline. Central nervous system agents in medicinal chemistry. 2012;12(2):100–113. doi: 10.2174/187152412800792689. [DOI] [PubMed] [Google Scholar]
- 21.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107(10):1209–1210. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aunapuu M, Arend A. Histopathological changes and expression of adhesion molecules and laminin in varicose veins. Vasa. 2005;34(3):170–175. doi: 10.1024/0301-1526.34.3.170. [DOI] [PubMed] [Google Scholar]
- 23.Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49(6):463–478. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- 24.Lee YU, Naito Y, Kurobe H, Breuer CK, Humphrey JD. Biaxial mechanical properties of the inferior vena cava in C57BL/6 and CB-17 SCID/bg mice. J Biomech. 2013;46(13):2277–2282. doi: 10.1016/j.jbiomech.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.