Abstract
Ischemic stroke in rodents stimulates neurogenesis in the adult brain and the proliferation of newborn neurons that migrate into the penumbra zone. The present study investigated the effect of methylene blue (MB) on neurogenesis and functional recovery in a photothrombotic (PT) model of ischemic stroke in rats. PT stroke model was induced by photo-activation of Rose Bengal dye in cerebral blood flow by cold fibre light. Rats received intraperitoneal injection of either MB (0.5 mg/kg/day) from day 1 to day 5 after stroke or an equal volume of saline solution as a control. Cell proliferative marker 5-bromodeoxyuridine (BrdU) was injected twice daily (50 mg/kg) from day 2 to day 8 and animals were sacrificed on day 12 after PT induction. We report that MB significantly enhanced cell proliferation and neurogenesis, as evidenced by the increased co-localizations of BrdU/NeuN, BrdU/DCX, BrdU/MAP2 and BrdU/Ki67 in the peri-infarct zone compared with vehicle controls. MB thus effectively limited infarct volume and improved neurological deficits compared to PT control animals. The effects of MB were accompanied with an attenuated level of reactive gliosis and release of pro-inflammatory cytokines, as well as elevated levels of cytochrome c oxidase activity and ATP production in peri-infarct regions. Our study provides important information that MB has the ability to promote neurogenesis and enhance the newborn-neurons’ survival in ischemic brain repair by inhibiting microenvironmental inflammation and increasing mitochondrial function.
Keywords: Methylene blue, Neurogenesis, Inflammation, Mitochondria, Photothrombotic stroke
Introduction
Stroke is the second leading cause of death and primary cause of long term disability worldwide, imposing a substantial medical, social, and economic burden in the US and abroad (Go et al., 2014). Focal cerebral ischemia commences with the occlusion of the cerebral vasculature and the interruption of blood flow to specific regions of the brain, consequently causing localized brain damage and functional disruption that can lead to disability and death (Ma et al., 2013; Reiter et al., 2005). Tissue necrosis in the infarct core cannot be reversed or prevented; tissue damage in the surrounding penumbra, however, takes some time to mature. Focal cerebral ischemia has been reported to induce neurogenesis in the cortical peri-infarct region in both the photothrombotic stroke (PT) model (Gu et al., 2000) and the middle cerebral artery occlusion (MCAO) stroke model (Jiang et al., 2001). Unfortunately, these nascent neurons have a limited ability to survive long term, suggesting that the post-stroke local microenvironment is not conducive to this endogenous self-repair process (Arvidsson et al., 2002; Ohab et al., 2006; Thored et al., 2006). Neuroinflammation, a major facet of the brain's response to cerebral ischemia, is known to exert deleterious effects on neurogenesis (Monje et al., 2003). Suppression of this post-ischemic inflammation is therefore a promising potential therapeutic target to enhance endogenous neurogenesis after ischemic insult.
Photothrombosis-induced ischemic stroke has been widely used as an experimental model to study neuroprotective mechanisms and in vivo cellular responses. This model does not occlude a single artery as is usually the case in human stroke, rather it causes the occlusion of microvasculature in a targeted region of cortex. The PT stroke model is non-invasive and produces an infarct with a reproducible location, size, and degree of damage with the accompanying mitochondrial dysfunction, inflammation, neurodegeneration and behavioural deficits characteristic of other well validated stroke models (Demyanenko et al., 2015; Pevsner et al., 2001; Schmidt et al., 2012; Shanina et al., 2005).
Methylene blue (MB) is a well-established drug in the clinic, used classically to treat various conditions including malaria, methemoglobinemia, and cyanide poisoning (Atamna et al., 2008; Lin et al., 2012). MB receives an electron from NADH and transfers it to cytochrome c, bypassing complex I/III. This facilitates enhanced ATP production as well as decreased reactive oxygen species (ROS) output from the mitochondrial electron transport chain (Chen et al., 2003; Lu et al., 2015; Shen et al., 2013). This has made MB a promising candidate for many neurodegenerative conditions. It has been reported that low dose MB treatment attenuates behavioral, neurochemical and pathological impairments in animal models of Parkinson's disease (PD), Alzheimer's disease (AD), global cerebral ischemia (GCI) and stroke (Oz et al., 2009; Medina et al., 2011; Lu et al., 2015; Wen et al., 2011). The neurogenic property of MB in ischemic stroke, however, remains poorly understood. We tested the hypothesis that MB treatment promotes neurogenesis in the rat PT stroke model by increasing mitochondrial activity and ATP production while reducing reactive microgliosis and pro-inflammatory cytokine levels.
Materials and methods
Photothrombotic (PT) stroke model
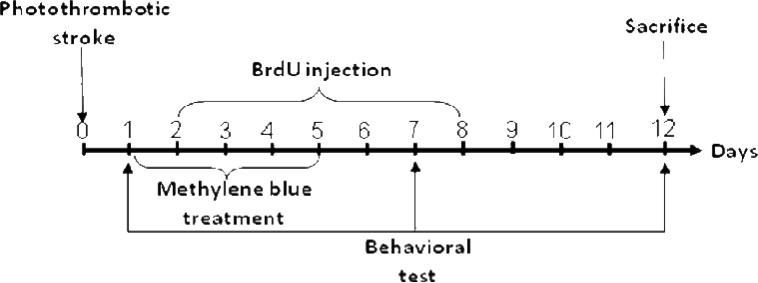
Male Sprague-Dawley rats (200-250g, Charles River Laboratories) were used for the purposes of this study. Rats were housed in propylene cages in animal housing facilities with ambient temperature set at 25 ± 2°C and a relative humidity of 45-55%. Rats were maintained on a 12 h light/dark cycle with ad libitum access to pellet chow and water. All procedures were approved by the institutional animal care committee of Augusta University. Rats were randomly divided into 3 groups (n=6-10 animals in each group): (a) Sham group without PT stroke; (b) PT stroke group, vehicle (saline) treatment; (c) PT stroke group with MB treatment. Rats were anesthetized with intraperitoneally (ip) injected sodium pentobarbital (50 mg/kg). Five minutes prior to illumination, Rose Bengal dye (0.1 mg/gram, ip) was administered. The skull was exposed and cleaned, and a fiber optic cable delivering cold light (6 mm diameter) was placed onto the skull using a stereotaxic frame. A heating pad was located under the rat at all times while mounted on the stereotaxic frame, and rectal temperature was maintained at 37 ± 0.5 °C for the duration of surgical operation. The cold light was centered 1.8 mm anterior to the bregma and 2.5 mm lateral from the midline, targeting the forelimb and hindlimb area of the right sensorimotor neocortex. The skull was illuminated for 15 min. The incision was closed using wound clips and the rats were placed on a warming pad as they recovered from anaesthesia. A control group of photo treatment without the Rose Bengal dye was also conducted, and no infarct injury was observed (data now shown). Methylene blue (0.5 mg/kg, ip) in saline was injected on days 1 through 5 after PT stroke. The experimental protocol was shown in diagram Figure 1.
Fig. 1. Schematic diagram of the experimental design.
PT model was induced on day 0, and on days 1 through 5, rats were treated with MB (0.5 mg/kg, ip). BrdU (50 mg/kg, ip) was injected two times daily on days 2 through 8. Behavioral deficits were evaluated on days 1, 7 and 12. Rats were sacrificed on day 12, and whole brains were extracted and prepared for further analysis.
5-Bromodeoxyuridine (BrdU) labelling
Proliferating cells were labelled using the thymidine analogue BrdU (5-bromodeoxyuridine, Carbosynth Ltd) dissolved in 0.9% saline. Rats were administered BrdU (50 mg/kg, ip, twice daily) on days 2 through 8 after PT stroke.
Histology examination and infarct volume assessment
Histological examination was performed as previously described (Zhang et al., 2009). Twelve days after PT stroke, rats were anesthetized with sodium pentobarbital and underwent transcardial perfusion through the ascending aorta with 0.9% saline followed by ice cold 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS). Brains were removed and postfixed overnight in 4% PFA at 4°C, and were then cryoprotected by submersion in 30% sucrose in 0.1 M PBS until they sank. Brains were then immersed in OCT cryoprotectant and frozen overnight at −80°C. Coronal brain sections (25 μm) were prepared on a cryostat (Leica RM2155, Nussloch, Germany) and sections were collected. For infarct volume assessment, every fifth section was selected and stained with 0.01 % (w/v) cresyl violet for 10 min, followed by graded ethanol dehydration. Stained sections were imaged using an AxioVision 4Ac microscope system (Carl Zeiss, Germany). The mean infarct area of each section was quantified using Image-J software (NIH). Infarct volume was expressed as the percentage of the total volume of the contralateral hemisphere per group (Vaibhav et al., 2012).
Immunofluorescence staining
Floating coronal brain sections (25 μm) were washed in 0.1 M PBS for 30 min and then blocked with 10% normal donkey serum with 0.1% Triton X-100 in PBS at room temperature for 1 h, as previously described by our laboratory (Zhang et al., 2009). Sections were then incubated with the corresponding primary antibodies at 4°C overnight. The primary antibodies used in this study were the following: rat anti-BrdU (1:200), mouse anti-NeuN (1:500, EMD Millipore, Billerica, MA), goat anti-DCX (1:50, Santa Cruz Biotechnology), goat polyclonal anti-MAP2 (1:100, Santa Cruz Biotechnology), mouse anti-Ki67 (1:200, Developmental Studies Hybridoma Bank), mouse anti-GFAP (1:200, Proteintech) and rabbit anti-Iba1(1:200, Proteintech). Sections were washed 3 times and were subsequently incubated with secondary antibodies (Alexa Fluor 568 donkey anti-mouse or rabbit, and Alexa Fluor 488 nm donkey anti-mouse or rat) at room temperature for 1 h. Sections were then mounted with a water-based mounting medium with anti-fading vectashield mounting medium for fluorescence with 4, 6- diamidino-2-phenylindole (DAPI) (H-1200; Vector Laboratories, Inc., CA, USA) and were cover slipped afterwards. Images were captured using a LSM510 Meta confocal laser microscope (Carl Zeiss).
Tissue preparation
Rats were deeply anaesthetised and sacrificed 12 days after PT stroke and whole brains were quickly removed on ice. The infarct region of the cortex was rapidly micro dissected and immediately frozen. Samples were then homogenized as previously described (Han et al., 2015). Briefly, tissue samples were homogenized in an ice cold homogenization medium consisting of 50 mM HEPES (pH 7.4), 150 mM NaCl, 12 mM β-glycerophosphate, 3 mM dithiotheitol (DTT), 2 mM sodium orthovanadate (Na3VO4), 1 mM EGTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Triton X-100 and protease and enzyme inhibitors (Thermo Scientific, Rockford, IL) in a Teflon-glass homogenizer. The homogenates were then centrifuged for 30 min at 12,000 ×g at 4 °C, and the supernatants, constituting the total protein fractions, were collected and stored at −80 °C for later use. As needed, the mitochondrial fractions and subcellular cytosolic fractions were prepared and collected. Briefly, tissue samples were homogenized in a buffer containing 10 mM HEPES (pH 7.9), 0.6% NP-40, 12 mM β-glycerophosphate with protease and enzyme inhibitors. The homogenates were centrifuged for 10 min at 800 × g. The supernatants were then centrifuged for 20 min at 17,000 ×g at 4 °C to generate cytosolic fractions (supernatant) and mitochondrial fractions (pellet). Protein concentrations were evaluated using a Modified Lowry Protein Assay (Pierce, Rockford, IL).
Cytochrome C Oxidase Activity
Cytochrome c oxidase activity in the mitochondrial fractions was assessed by using an activity assay kit (ab109911; Abcam Inc) according to the manufacturer's instructions. Cytochrome c oxidase present within the protein samples oxidizes fully reduced ferro-cytochrome c to ferri-cytochrome c. Oxidation of ferro-cytochrome c was measured as a loss of absorbance at 550 nm, measured in a 96-well plate reader using a spectrophotometer (Bio-Rad Benchmark plus Microplate Spectrophotometer).
Quantification of total ATP content
The level of ATP was quantified in the total protein sample using a kit ENLITEN® rLuciferase/Luciferin reagent (FF2021, Promega, Madison, WI), as previously described by our lab (Lu et al., 2015). In brief, 30 μg of protein samples were diluted in 100 μL of reconstituted rL/L reagent buffer containing luciferase, D-luciferin, Tris-acetate buffer (pH 7.75), ethylene diamine tetra acetic acid (EDTA), magnesium acetate, bovine serum albumin (BSA), and dithiothreitol (DTT). Light emission at 10 s interval from the reaction was measured in a standard microplate luminatoer (PE Applied Biosystem). Relative light units (RLU) from “background blank” were subtracted from the sample light output in the assay. Values of ATP levels were determined using an ATP standard curve, and data were expressed as level of ATP compared to sham group.
ELISA of pro-inflammatory cytokines (TNF-α, IL-1β and IL- 6)
Indirect Enzyme-Linked-Immunosorbent Assay (ELISA) in each group were quantitatively detected as previously described (Gan and Patel., 2013). In brief, protein samples were diluted in bicarbonate/carbonate coating buffer (Sigma-Aldrich) and total volume were adjusted to 50 μL. Samples were loaded in PVC ELISA microtitre plate (Corning), sealed and incubated overnight at 4°C. Samples were discarded and the plate wells were washed three times. The remaining protein-binding sites in the coated well plate were blocked by blocking buffer (1% BSA in PBS, 0.3% solution of H2O2) for 1 hour at room temperature. Afterwards 50 μL primary antibodies of pro-inflammatory cytokines were added to well and incubated overnight at 4°C and following 3 times wash with PBST. Plate wells were then incubated with HRP-conjugated secondary antibodies for 1 hour at room temperature. Following 3 washes, samples were incubated with TMB (3, 3, 5, 5’-tetramethylbenzidine, BD Bioscience) substrate reagent for 30 min and reaction was stopped with 50 μL of sulphuric acid. The optical density was read at 450 nm on spectrophotometer (Bio-Rad).
Behavioral analysis
Behavioral tests were performed on days 1, 7 and 12 after PT. These tests included: (a) The cylinder test: The cylinder test was performed to evaluate the asymmetry of left (contralateral) paw usage on days 1, 7 and 12 after PT stroke. The rats were placed in a transparent glass cylinder (diameter: 10 cm; height: 15 cm) for 2 min. During the trial, the experimenter counted the instances in which the animal made contact with the side of the cylinder with its left or right paws, as well as both paws simultaneously. Relative contralateral paw use was calculated according to the following formula: score = (contralateral paw use/total paw use)*100. (b) The adhesive removal test: The adhesive removal test is a well-established paradigm to measure somatosensory deficits. This test was performed on days 1, 7, and 12 after PT stroke. A small rectangular adhesive strip (0.35 × 0.45 cm) was placed on the inner portion of each front paw of the animals. The animal was immediately returned to its home cage in the absence of its cagemates. Both the time taken to contact and the time take to remove the adhesive tape was recorded, with an upper time limit of 2 min. (c) The hanging wire test: A wire was stretched between two poles 60 cm above the ground. A rat was suspended by its tail, and then lowered within reach of the wire. When the rat had grasped the wire, support was gently removed, allowing the rat to hang from the wire by its front paws. A thick soft mat was placed under the apparatus to catch the rat when it dropped. Each trial was evaluated by a 5-point scale: 0, the animal falls immediately; 1, the animal hangs onto the wire by two forelimbs; 2, the animal hangs via two forelimbs and attempts to climb onto the wire; 3, The animal grasps the wire with both forelimbs and one or both hind limbs while attempting to climb onto the wire; 4, The animal grasps the wire with all four paws, as well as wrapping its tail around the wire. Each testing session included 3 trials per animal. The highest reading of three successive trials was taken for each animal (Vaibhav et al., 2013). (d) Edge beam test: Rats were habituated to the balance beam (100 cm in length, 7 cm in width, 100 cm elevated above the horizontal surface of the ground) the day prior to testing. During habituation, the animal's home cage was placed at one end of the beam and the animal was placed at three points of increasing distance from the cage, allowing the animal to cross the beam to return to its cage. During testing, the animal was placed at the far end of the beam. The time taken to initiate movement (latency) and the time taken to cross the beam (completion time) were measured, as well as the number of foot slips. Latency was defined as the time taken to advance 20 cm beyond the start point, and completion time was defined as the interval between completion and the latency time point. Each trial had an upper time limit of 5 min (Ma et al. 2013).
Statistical analysis
Quantified data are presented as mean ± SE and were analyzed using GraphPad PRISM 6.0 software. Statistical comparisons between two groups were performed using t tests. Comparisons between multiple groups were made using one-way or two way ANOVA, using Bonferroni's post hoc test or Dunnett's post hoc tests for comparisons between all groups or against control, respectively. A level of P < 0.05 was considered statistically significant.
Results
MB treatment significantly ameliorates behavioral changes after PT stroke
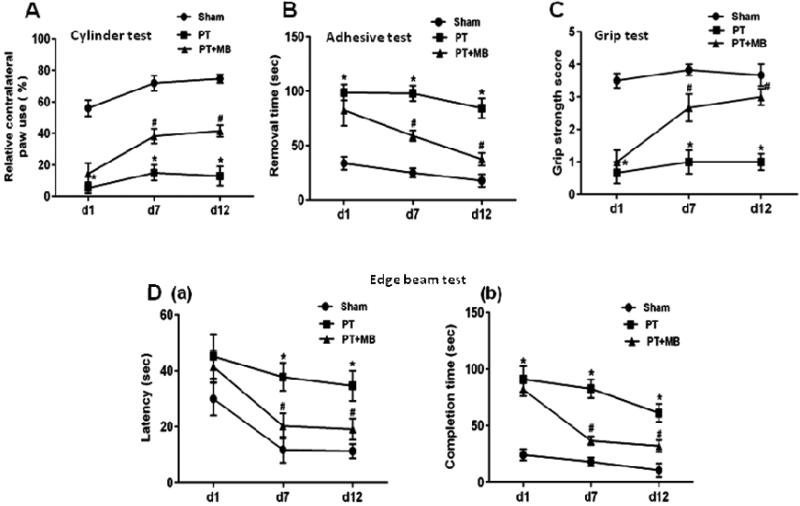
Behavioral tests were performed on days 1, 7 and 12 after PT stroke. The cylinder test was conducted to test paw usage preference as a measure of lateralized motor deficits. Relative paw use contralateral to the damaged hemisphere was significantly decreased after PT in comparison to sham animals. This preference was significantly ameliorated in the PT group treated with MB (Fig. 2A). The adhesive test was performed to test forepaw somatosensory activity. PT group animals were unable to perform the task within the given time. Treatment with MB, however, significantly decreased the time taken to remove adhesive compared to PT group animals (Fig. 2B). The grip strength test was conducted to test motor coordination. Significant deficits in grip strength and motor coordination were observed in the PT group compared to sham. A marked improvement in grip strength score was observed in the MB treatment group (Fig. 2C). The edge beam test was conducted to test sensorimotor deficits and motor coordination. PT group rats displayed discoordination while crossing the beam, including body rigidity and missed paw placement leading to slipping. The latency of initiating movement and completion time was significantly prolonged in PT group rats compared to sham. Treatment with MB significantly improves latency in initiating movement and completion time compared to the PT group (Fig. 2D).
Fig. 2.
Effect of MB on behavioral deficits in PT stroke rats. A: The cylinder test evaluates a rodent's spontaneous forelimb use. Contralateral paw use by PT group animals was significantly decreased compared to sham group rats, while treatment with MB reduced this deficit. B: Adhesive removal test investigates stimulus-directed movement after PT. Time taken to remove adhesive was prolonged in PT group compared to sham group. This effect is reversed by administration of MB. C: Grip strength test score is a measure of dexterity while hanging from a suspended wire. PT group animals scored significantly lower than sham group, while MB treated rats displayed increased motor dexterity, relative to PT. D: On the edge beam test, sham group rats were able to travel across the beam easily, whereas PT group and MB treated group rats displayed difficulties passing across the beam with paw slipping observed. Latency (a) and completion time (b) were both significantly decreased in MB post-treated group compared to PT group. Values are expressed as mean ± SE (n=10). *P < 0.05 versus sham, #P < 0.05 versus PT group.
MB treatment enhanced cortical neurogenesis in PT stroke rats
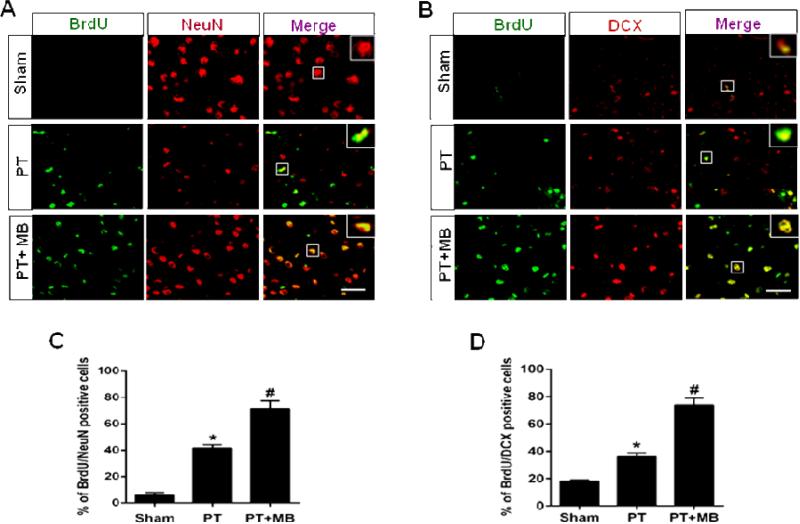
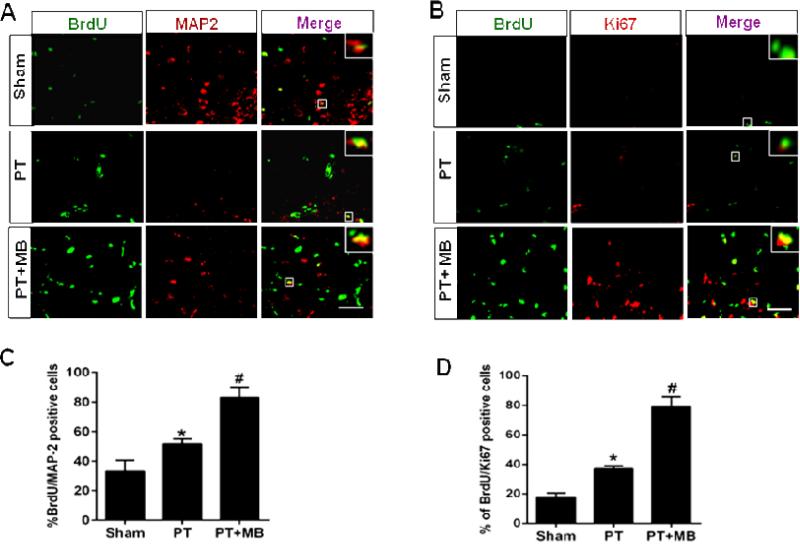
To assess the effects of MB on PT induced neurogenesis, neuronal proliferation was investigated using BrdU colocalizion with NeuN, DCX, Ki67 or MAP2. As expected, very few newly formed neurons were found in sham animals (Fig. 3A, B; Fig. 4A, B). Quantification of BrdU/NeuN, BrdU/DCX positive cells was presented in (Fig. 3C, D), and the quantification of BrdU/Ki67, BrdU/MAP2 positive cells was shown in (Fig. 4 C, D). In PT group animals, however, new neurons were present, distributed randomly throughout the cortex and concentrated in the peri-infarct region. MB group animals displayed a significantly greater population of these cells compared to the PT group, with a similar pattern of distribution. These results clearly indicate that MB administration post-stroke has the potential to support the proliferation and continued survival of neurons generated as a response to stroke.
Fig. 3. Effect of MB on neurogenesis in the peri-infarct cerebral cortex on day 12 after PT stroke.
BrdU was injected on days 2 through 8. Representative confocal microscopy images of BrdU (green), NeuN (red) and DCX (red) showed that treatment with MB increases the expression and colocalization of BrdU/NeuN (A) and BrdU/DCX (B) compared to PT group. Quantitative analyses of BrdU/NeuN and BrdU/DCX are shown in (C, D). Values are expressed as mean ± SE (n=6). Magnification: 40×, scale bar: 50 μm. *P < 0.05 versus sham, #P < 0.05 versus PT group.
Fig. 4. Effect of MB on the expression of BrdU/MAP2 and BrdU/Ki67 in the cortical peri-infarct region as seen 12 days after PT stroke.
Representative confocal microscopy images of the indicated markers represent that MB treatment significantly increases the expression and colocalization of BrdU/MAP2 (A) and BrdU/Ki67 (B) in the peri-infarct region cortical region following PT stroke. Quantative analyses of BrdU/MAP2 and BrdU/Ki67 are shown in (C, D). Values are expressed as mean ± SE (n=6). Magnification: 40×, scale bar: 50 μm. *P < 0.05 versus sham, #P < 0.05 versus PT group.
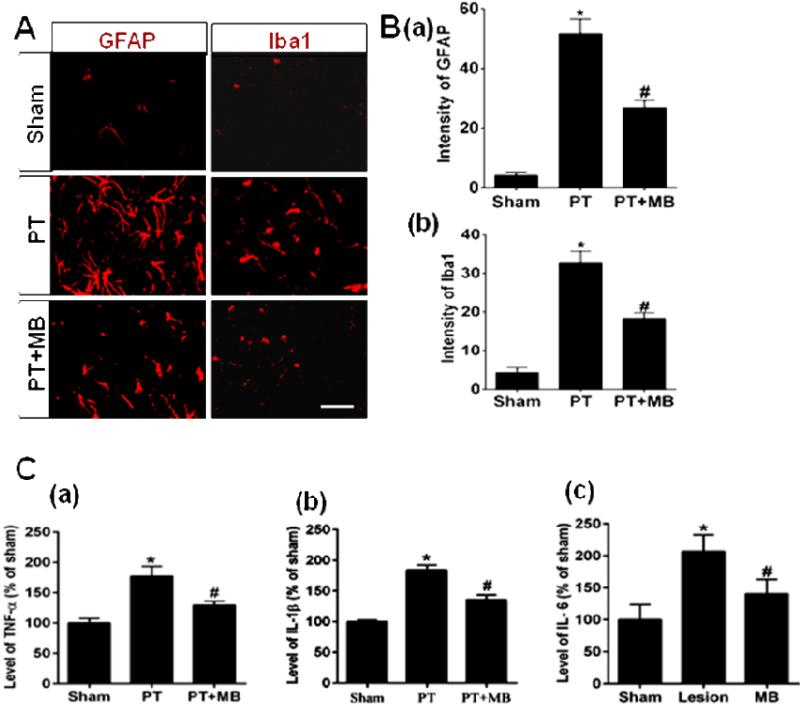
MB treatment significantly inhibits cortical PT stroke-induced gliosis and pro-inflammatory cytokine release
To determine the potential role of pro-inflammatory cytokines in MB-induced neurogenesis, we next investigated the effect of MB on reactive gliosis and pro-inflammatory cytokine levels in the infarct cortex region following PT. Confocal microscopy images of coronal sections peri-infarct and quantification in (Fig. 5A, B) indicated that staining of GFAP (a marker for reactive astrocytosis) and Iba1 (a marker for microglia activation) were negligible in the sham group. These markers were significantly elevated in the peri-infarct region in the PT group. Treatment with MB significantly decreases this elevated expression, suggesting that MB attenuated the reactive glial response to injury (Fig. 5A, B). We further measured the levels of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 via highly sensitive ELISA using cortical protein homogenates. ELISA assessment (Fig. 5C) shows robust increases in the level of these pro-inflammatory cytokines in the PT group when compared to sham. Once again, MB treatment significantly dampened this response, strongly suggesting that MB exhibits anti-inflammatory effects in the PT stroke model.
Fig. 5. Effect of MB on reactive gliosis and levels of pro-inflammatory cytokines in the infarct cortical region 12 days after PT stroke.
Distribution of microglia and astrocytes in the coronal section of peri-infarct region of cortex was immunostained with anti-Iba1 and anti-GFAP antibodies, respectively. A: Representative confocal microscopy images display the higher expression of GFAP and Iba1 in the peri-infarct area of cortex in PT group rats. C: The levels of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, were measured by ELISA assay. Note that post-treatment with MB significantly decreased the expression of reactive glia and the levels of pro-inflammatory cytokines. Values are expressed as mean ± SE (n=6). Magnification: 40×, scale bar: 50 μm. *P < 0.05 versus sham, #P < 0.05 versus PT group.
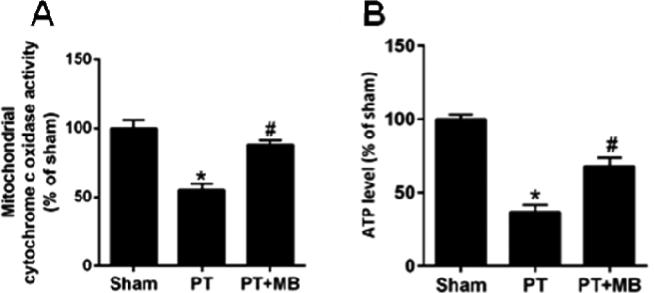
MB treatment ameliorates cortical mitochondrial dysfunction after PT stroke
Previous work has shown that MB can increase mitochondrial oxidative phosphorylation and cellular oxygen consumption, as well as preventing electron leakage and decreasing anaerobic glycolysis under pathological circumstances (Poteet et al., 2012; Wen et al., 2011). In the present study, we determined whether MB can rescue mitochondrial function after PT stroke. Our results showed that PT causes a significant decrease in mitochondrial cytochrome c oxidase activity compared to the sham group which was mitigated upon administration of MB (Fig. 6A). We further investigated whether MB can elevate cortical ATP levels which are diminished post-stroke. ATP levels were significantly decreased in PT group compared to sham group. Treatment with MB significantly enhanced the ATP level compared to PT group animals (Fig. 6B). Our results clearly suggest that MB treatment has potential to significantly restore the mitochondrial function by increasing cytochrome c oxidase activity and facilitating enhanced ATP synthesis 12 days after PT.
Fig. 6. Effect of MB on mitochondrial cytochrome c oxidase activity and ATP production 12 days after PT stroke.
Total protein samples from the infarct cortical region were subjected to a mitochondrial cytochrome c oxidase activity assay (A) and an ATP assay (B). MB post-treatment significantly increases cytochrome c oxidase activity and total ATP level compared to PT group. Values are expressed as mean ± SE (n=6). *P < 0.05 versus sham, #P < 0.05 versus PT group.
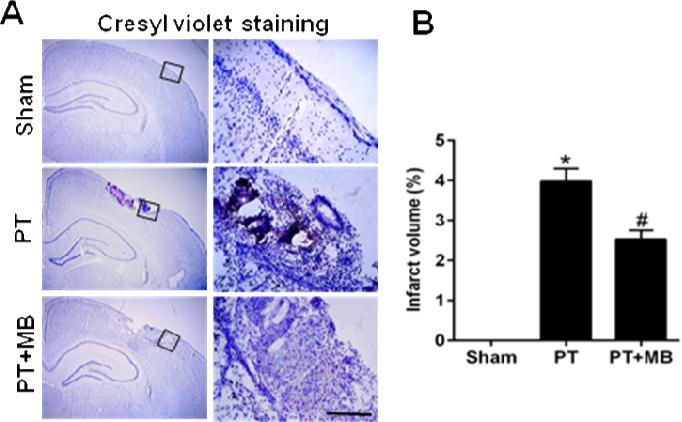
MB decreases cortical infarct size after PT stroke
Cresyl violet staining in coronal cortex sections 12 days after PT stroke showed a significant increase in infarct volume, displaying condensed pyknotic and shrunken nuclei. Treatment with MB significantly decreased the infarct volume and increased the number of surviving neurons after PT (Fig. 7A, B), suggesting that MB has the ability to promote the survival of neurons and facilitate brain repair after PT stroke
Fig. 7. Effect of MB on infarct volume in cortical region on day 12 after PT.
Cresyl violet staining shows the elevated infarct volume in the in PT group compared to sham group. Treatment with MB clearly ameliorates and reduces the infarct volume compared to PT group. Values are expressed as mean ± SE (n= 10). Magnification: 4× in left lane and 20× in right lane, scale bar: 500 μm. *P < 0.05 versus sham, #P < 0.05 versus PT group.
Discussion
In the present study, we demonstrated that the administration of MB after PT stroke significantly promoted the proliferation and survival of newborn cells in the cortical peri-infarct region. Strikingly, behavioral assessment showed that these MB-induced alterations in neurogenesis were strongly associated with ameliorated motor deficits on the adhesive test, cylinder test, hanging wire test and edge beam test. These results reinforce previous studies that show MB improves neurological outcomes in other experimental brain injury models (Shen et al., 2013; Tretter et al., 2014) by extending these findings to the PT stroke model, a clinically relevant and highly repeatable rodent stroke model that causes extensive damage in the cerebral cortex with accompanying motor deficits (Jin et al., 2001; Yan et al., 2015).
While previous evidence suggested that stroke only induced neurogenesis in the striatum, this study and others have provided evidence that cortical neurogenesis occurs both in experimental stroke models (Collin et al., 2005; Hurwitz et al., 1990; Ortega et al., 2013; Parent et al., 2002) as well as in human stroke patients (Jin et al., 2006; Macas et al., 2006). Our work revealed that newly formed neurons were randomly distributed in the peri-infarct region after PT stroke. Furthermore, MB treatment significantly increased these populations. Since a previous study has shown that MB did not stimulate neurogenesis in normal conditions (Xie et al., 2014), we believe that the neurogenic effects of effects can be attributed to its promotion of mitochondrial function and its mitigation of neuroinflammation. In these respects, we believe that post-stroke administration of MB may facilitate neural repair.
With the massive energy demands of the brain, mitochondrial function plays a vital role in neuronal survival. The interruption of cerebral blood flow during stroke limits the delivery of oxygen and glucose, disrupting oxidative phosphorylation within the mitochondria (Szeto, 2008). This derailment of the electron transfer chain results in the loss of the mitochondrial membrane potential, leading to decreased ATP production, production of damaging free radicals, and the release of apoptotic signals (Bolanos and Almeida, 2006). While ATP production is essential throughout the brain, it is especially important in differentiating neural progenitor cells. The elevated energy demands of these cells are reflected in their observed upregulation of mitochondrial proteins during differentiation (Cordeau-Lossouarn et al., 1991; Vayssiere et al., 1992). This elevated energy damage is needed for growth, migration, and cytoskeletal remodeling (Bernstein and Bamburg, 2003). In our study, we found that PT stroke significantly decreased cortical ATP levels as well as complex IV activity. MB treatment ameliorated these deficits, and commensurately increased the number of surviving nascent neurons. These results implicate mitochondrial function as a key contributor to maintenance of neurogenesis in the injured brain.
Neuro-inflammation is involved in the pathogenesis of several neurological disorders such as stroke and epilepsy (Sieber et al., 2011; Vezzani et al., 2011), conditions known to stimulate neurogenesis as well (Arvidsson et al., 2002; Parent et al., 1997). The exact impact of inflammation on neurogenesis, however, is not fully understood (Das and Basu, 2008). A primary component of neuroinflamation is microglial activation, a result of which is the production and secretion of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 (Gebicke-Haerter, 2001; Liu and Hong, 2003; Pocock and Liddle, 2001). Accumulation of these pro-inflammatory cytokines is deleterious to neurons, contributing to neurodegeneration (Cagnin et al., 2006; Minghetti, 2005) and potentially stifling neurogenesis (Green et al., 2012; Keohane et al., 2010; Koo and Duman, 2008; Monje et al., 2003). Over-expression of IL-6 within the brain is known to reduce proliferation, survival and differentiation of new cells (Vallieres et al., 2002), while TNF-α has been found to reduce cell proliferation within the granular layer following systemic administration (Seguin et al., 2009). A recent study has shown that MB treatment attenuated the expression of pro-inflammatory cytokines and activated microglia in the hippocampus after TBI (Fenn et al., 2015). In accordance with these findings, we demonstrated a significant increase of reactive gliosis and pro-inflammatory cytokines after PT stroke which was significantly mitigated by MB administration. This, in combination with our other results, corroborates our hypothesis that the limited capacity for neurogenesis after stroke may be, in part, a result of the unfavorable microenvironment presented by neuroinflammation.
Conclusions
In conclusion, the present study demonstrates that MB administered post-stroke can promote neurogenesis and improve behavioral outcomes in the rat PT model. We found that MB mitigated post-stroke mitochondrial dysfunction and ATP output. Furthermore, we found MB treatment decreased reactive gliosis and accompanying release of pro-inflammatory factors. We believe that the amelioration of inflammation and improved mitochondrial function are responsible for the observed increase in neurogenesis. This, along with its proven safety record in the clinic, position MB as a promising therapy for stroke. Furthermore, MB should be investigated in the context of other neurodegenerative conditions associated with neuroinflammation and mitochondrial dysfunction. This and other studies shine a new light on an old drug.
● Methylene blue ameliorates sensorimotor deficits after photothrombotic (PT) stroke.
● Methylene blue stimulates neurogenesis in the peri-infarct region after PT stroke.
● Methylene blue reduces neuroinflammation in the peri-infarct region after PT stroke.
● Methylene blue treatment promotes mitochondrial activity and ATP production.
● Methylene blue treatment reduces infarct volume after PT stroke.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke (NS086929) and the American Heart Association Grant-in-Aid (15GRNT25240004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The author declares that there is no conflict of interest.
References
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A. Modulation of astroglial energy metabolism by nitric oxide. Antioxid Redox Signal. 2006;8:955–965. doi: 10.1089/ars.2006.8.955. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neurodegenerative dementia. Acta Neurol Scand Suppl. 2006;185:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- Collin T, Arvidsson A, Kokaia Z, Lindvall O. Quantitative analysis of the generation of different striatal neuronal subtypes in the adult brain following excitotoxic injury. Exp Neurol. 2005;195:71–80. doi: 10.1016/j.expneurol.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Cordeau-Lossouarn L, Vayssiere JL, Larcher JC, Gros F, Croizat B. Mitochondrial maturation during neuronal differentiation in vivo and in vitro. Biol Cell. 1991;71:57–65. doi: 10.1016/0248-4900(91)90051-n. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Demyanenko SV, Panchenko SN, Uzdensky AB. Expression of neuronal and signaling proteins in penumbra around a photothrombotic infarction core in rat cerebral cortex. Biochemistry (Mosc) 2015;80:790–799. doi: 10.1134/S0006297915060152. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, Eiferman DS, Godbout JP. Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J Neurotrauma. 2015;32:127–138. doi: 10.1089/neu.2014.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SD, Patel KR. Enzyme immunoassay and enzyme-linked immunosorbent assay. Invest Dermatol. 2013;133(9):e12. doi: 10.1038/jid.2013.287. doi: 10.1038/jid.2013.287. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ. Microglia in neurodegeneration: molecular aspects. Microsc Res Tech. 2001;54:47–58. doi: 10.1002/jemt.1120. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane AK, Sullivan AM, O'Keeffe GW, Nolan YM. A role for interleukin-1beta in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49:311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Gu W, Brannstrom T, Wester P. Cortical neurogenesis in adult rats after reversible photothrombotic stroke. J Cereb Blood Flow Metab. 2000;20:1166–1173. doi: 10.1097/00004647-200008000-00002. [DOI] [PubMed] [Google Scholar]
- Han D, Scott EL, Dong Y, Raz L, Wang R, Zhang Q. Attenuation of mitochondrial and nuclear p38alpha signaling: a novel mechanism of estrogen neuroprotection in cerebral ischemia. Mol Cell Endocrinol. 2015;400:21–31. doi: 10.1016/j.mce.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Hurwitz A, Hernandez ER, Resnick CE, Packman JN, Payne DW, Adashi EY. Basic fibroblast growth factor inhibits gonadotropin-supported ovarian androgen biosynthesis: mechanism(s) and site(s) of action. Endocrinology. 1990;126:3089–3095. doi: 10.1210/endo-126-6-3089. [DOI] [PubMed] [Google Scholar]
- Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke. 2001;32:1201–1207. doi: 10.1161/01.str.32.5.1201. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43:127–135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Poteet E, Du F, Gourav RC, Liu R, Wen Y, Bresnen A, Huang S, Fox PT, Yang SH, Duong TQ. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One. 2012;7:e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q. Neuroprotective and Functional Improvement Effects of Methylene Blue in Global Cerebral Ischemia. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9455-0. (DOI 10.1007/s12035-015-9455-00) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Feng Q, Ma J, Feng Z, Zhan M, Ouyang L, Mu S, Liu B, Jiang Z, Jia Y, Li Y, Lei W. Melatonin ameliorates injury and specific responses of ischemic striatal neurons in rats. J Histochem Cytochem. 2013;61:591–605. doi: 10.1369/0022155413492159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DX, Caccamo A, Oddo S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FJ, Jolkkonen J, Mahy N, Rodriguez MJ. Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2013;33:356–364. doi: 10.1038/jcbfm.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer's disease. Biochem Pharmacol. 2009;78:927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner PH, Eichenbaum JW, Miller DC, Pivawer G, Eichenbaum KD, Stern A, Zakian KL, Koutcher JA. A photothrombotic model of small early ischemic infarcts in the rat brain with histologic and MRI correlation. J Pharmacol Toxicol Methods. 2001;45:227–233. doi: 10.1016/s1056-8719(01)00153-8. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Liddle AC. Microglial signalling cascades in neurodegenerative disease. Prog Brain Res. 2001;132:555–565. doi: 10.1016/S0079-6123(01)32103-9. [DOI] [PubMed] [Google Scholar]
- Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, Wen Y, Yang SH. Neuroprotective actions of methylene blue and its derivatives. PLoS One. 2012;7:e48279. doi: 10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Leon J, Kilic U, Kilic E. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp Biol Med (Maywood) 2005;230:104–117. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hoppen M, Strecker JK, Diederich K, Schabitz WR, Schilling M, Minnerup J. Photochemically induced ischemic stroke in rats. Exp Transl Stroke Med. 2012;4:13. doi: 10.1186/2040-7378-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin JA, Brennan J, Mangano E, Hayley S. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat. 2009;5:5–14. [PMC free article] [PubMed] [Google Scholar]
- Shanina EV, Redecker C, Reinecke S, Schallert T, Witte OW. Long-term effects of sequential cortical infarcts on scar size, brain volume and cognitive function. Behav Brain Res. 2005;158:69–77. doi: 10.1016/j.bbr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Shen Q, Du F, Huang S, Rodriguez P, Watts LT, Duong TQ. Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS One. 2013;8:e79833. doi: 10.1371/journal.pone.0079833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PLoS One. 2011;6:e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tretter L, Horvath G, Holgyesi A, Essek F, Adam-Vizi V. Enhanced hydrogen peroxide generation accompanies the beneficial bioenergetic effects of methylene blue in isolated brain mitochondria. Free Radic Biol Med. 2014;77:317–330. doi: 10.1016/j.freeradbiomed.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Vaibhav K, Shrivastava P, Javed H, Khan A, Ahmed ME, Tabassum R, Khan MM, Khuwaja G, Islam F, Siddiqui MS, Safhi MM. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-kappaB in middle cerebral artery occlusion rat model. Mol Cell Biochem. 2012;367:73–84. doi: 10.1007/s11010-012-1321-z. [DOI] [PubMed] [Google Scholar]
- Vaibhav K, Shrivastava P, Khan A, Javed H, Tabassum R, Ahmed ME, Khan MB, Moshahid Khan M, Islam F, Ahmad S, Siddiqui MS, Safhi MM. Azadirachta indica mitigates behavioral impairments, oxidative damage, histological alterations and apoptosis in focal cerebral ischemia-reperfusion model of rats. Neurol Sci. 2013;34:1321–1330. doi: 10.1007/s10072-012-1238-z. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssiere JL, Cordeau-Lossouarn L, Larcher JC, Basseville M, Gros F, Croizat B. Participation of the mitochondrial genome in the differentiation of neuroblastoma cells. In Vitro Cell Dev Biol. 1992;28A:763–772. doi: 10.1007/BF02631065. [DOI] [PubMed] [Google Scholar]
- Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Choudhury GR, Wang J, Park Y, Liu R, Yuan F, Zhang CL, Yorio T, Jin K, Yang SH. Methylene blue promotes quiescence of rat neural progenitor cells. Front Cell Neurosci. 2014;8:315. doi: 10.3389/fncel.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G, Dai Z, Xuan Y, Wu R. Early metabolic changes following ischemia onset in rats: an in vivo diffusion-weighted imaging and 1H-magnetic resonance spectroscopy study at 7.0 T. Mol Med Rep. 2015;11:4109–4114. doi: 10.3892/mmr.2015.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]