Abstract
Early and accurate diagnosis of HIV is key for the reduction of transmission and initiation of patient care. The availability of a rapid nucleic acid test (NAT) for use at the point-of-care (POC) will fill a gap in HIV diagnostics, improving the diagnosis of acute infection and HIV in infants born to infected mothers. In this study, we evaluated the performance of non-instrumented nucleic acid amplification, single-use disposable (NINA-SUD) devices for the detection of HIV-1 in whole blood using reverse-transcription, loop-mediated isothermal amplification (RT-LAMP) with lyophilized reagents. The NINA-SUD heating device harnesses the heat from an exothermic chemical reaction initiated by the addition of saline to magnesium iron powder. Reproducibility was demonstrated between NINA-SUD units and comparable, if not superior, performance for detecting clinical specimens was observed as compared to the thermal cycler. The stability of the lyophilized HIV-1 RT-LAMP reagents was also demonstrated following storage at −20, 4, 25, and 30°C for up to one month. The single-use, disposable NAT minimizes hands-on time and has the potential to facilitate HIV-1 testing in resource-limited settings or at the POC.
Keywords: HIV-1, Diagnosis, Point-of-Care, RNA, Nucleic acid amplification
1. Introduction
HIV diagnostic tests continue to evolve as technology moves towards earlier detection of HIV post-infection. Early detection facilitates early initiation of antiretroviral therapy (ART), which in turn reduces the risk of disease progression, lowers the likelihood of transmission, and improves the overall quality of life for the infected individual (NIH, 2015). To date, the earliest detectable marker for HIV infection is viral RNA, which can be detected in plasma as early as 7 days post infection (Keele et al., 2008; Lee et al., 2009). One of the biggest gaps in the field of HIV diagnostics is the lack of availability of nucleic acid test (NAT) platforms for point-of-care (POC) use. In the US, there remains only one FDA-approved NAT for diagnosis of HIV-1, the APTIMA HIV-1 RNA Qualitative Assay (Hologic Inc., Marlborough, MA). The APTIMA is strictly a laboratory platform, which can limit access to nucleic acid testing and increase time to result, in cases that require a supplemental NAT result. Dried blood spots have improved accessibility to NAT testing, since samples can be collected at field sites and shipped at room temperature to a central laboratory, however, turnaround time is high and loss to care is a likely outcome (Johannessen, Troseid, and Calmy, 2009). Recently, HIV test development has shifted towards simplified, integrated, portable NAT platforms that may extend the utility of nucleic acid-based tests for POC use. Several rapid NAT platforms are in development or the early stages of commercialization, including: Cobas Liat System (Roche Molecular Systems, Branchburg, NJ), Alere q HIV-1/2 Detect (Alere Inc., Jena, Germany), SAMBA (Diagnostics for the Real World, Ltd., Sunnyvale, CA), and GeneXpert systems (Cepheid, Sunnyvale, CA) (Lee et al., 2010; Tanriverdi, Chen, and Chen, 2010). These platforms are similar in design and consist of pre-loaded test cartridges or tubes and a benchtop analyzer. None of these moderate complexity platforms are currently available for purchase in the US.
In the US or similar developed countries, POC may refer to testing clinics, community outreach centers, prisons, mobile testing units, and other testing sites, where laboratory test operation is not feasible. HIV diagnosis at the POC in resource limited settings offers additional challenges. Such areas are often where HIV prevalence is high and targeting HIV screening and early diagnosis and treatment is especially critical. In addition to cost and training considerations, key limiting factors in these areas may also include availability of clean water, refrigeration, and reliable electricity sources (Lee et al., 2010). Rapid HIV antibody-based tests have made HIV diagnosis possible in these settings, as most rapid tests are self-contained, relatively stable, and disposable. The development of a rapid NAT adds an additional level of complexity since the amplification step requires a constant heat source and temperature cycling, in the case of polymerase chain reaction (PCR)/reverse transcription PCR (RT-PCR), and cold chain storage is needed for the amplification reagents. To simplify the overall design of the NAT platform and reduce material costs, alternative sources of heat, coupled to isothermal amplification methods, have been explored for rapid NAT development. One such approach involves harnessing the heat from exothermic chemical reactions (LaBarre et al., 2011; Labarre et al., 2010). Prototype electricity-free, non-instrumented nucleic acid (NINA) heaters have been developed and demonstrated for detection of malaria and HIV (Labarre et al., 2010; Lillis et al., 2014; Sema et al., 2015; Singleton et al., 2014). NINA heaters consist of an insulated, reusable unit, which houses the chemical reactants (e.g. CaO or Mg-Fe) that provide the heat source for amplification. Depending on the optimal temperature for the specific isothermal amplification method, the design of the NINA unit is flexible and has been constructed from an insulated thermos or small soufflé cup (Curtis et al., 2012; LaBarre et al., 2011).
Loop-Mediated Isothermal Amplification (LAMP) exhibits several attractive characteristics for the integration into a rapid NAT platform. LAMP or RT-LAMP (reverse transcription-LAMP) assays have been developed for the detection of a wide range of bacterial and viral targets, including HIV (Curtis, Rudolph, and Owen, 2008; Curtis, Rudolph, and Owen, 2009). LAMP is a single-step amplification reaction that requires six primers specific for eight separate sequences within the target region, contributing to the high specificity associated with the amplification method (Nagamine, Hase, and Notomi, 2002; Notomi et al., 2000). A large amount of amplicon is generated from the amplification reaction within 60 minutes or less (Mori, Hirano, and Notomi, 2006). Capitalizing on the abundant amplicon or magnesium pyrophosphate byproduct, several groups have developed LAMP detection methods that enable immediate visual detection of the amplified products. In addition to non-specific detection methods, such as measuring turbidity, use of intercalating or metal indicator fluorescent dyes, and colorimetric detection (Curtis et al., 2008; Goto et al., 2009; Mori et al., 2001; Tanner, Zhang, and Evans, 2015), sequence-specific detection methods have been developed, using fluorescent-labeled primers or probes (Curtis et al., 2009; Mori et al., 2006). LAMP/RT-LAMP is less sensitive to biological inhibitors as compared to traditional amplification methods and direct amplification from clinical specimens, such as plasma, whole blood, and oral fluid, has been demonstrated for HIV-1 (Curtis et al., 2008; Curtis et al., 2009; Liu et al., 2011).
We previously demonstrated the performance and stability of NINA heaters for amplification of HIV-1 in whole blood by RT-LAMP (Curtis et al., 2012). In moving towards a single-use, disposable NAT, significant design modifications have been made to the NINA platform in order to miniaturize the unit, minimize hands-on time, and eliminate the need for cold chain storage. The NINA-SUD (single-use disposable) heaters contain the integrated reactants and are equipped with temperature indicating labels, which alert the test user when the device has reached temperature. In this study, we evaluated the performance of the NINA-SUD heaters for amplification of HIV-1 using lyophilized RT-LAMP reagents, which contain a fluorescent probe/quencher pair for immediate visual detection of amplified products. The stability of the lyophilized RT-LAMP reagents was also evaluated following storage at varying temperatures to determine the feasibility of eliminating reagent cold chain storage.
2. Material and Methods
2.1. Linearity Panels
HIV-1 DNA and RNA linearity panels were generated from OM-10.1 cells and supernatant from 8E5 cells, respectively, as described in detail (Rudolph et al., 2015). The 104 DNA copies/mL and 105 RNA copies/mL panel members were used as DNA and RNA standards, respectively, for the lyophilized reagent stability evaluation. HIV-2 NIH-Z purified virus (Advanced Biotechnologies Inc., Columbia, MD) was included to generate a negative control. DNA was extracted from the OM-10.1 cells using a QIAamp DNA Blood Mini kit (Qiagen, Valencia, CA) and RNA was extracted from the 8E5 supernatant and NIH-Z purified virus using a QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA). Linearity panels and negative controls were stored at −80°C until use.
2.2. Clinical Specimens
Whole blood specimens were collected from an IRB-approved study in collaboration with the University of Washington (UW). Participants were recruited from the Centers for AIDS Research (CFAR) registry (AI027757), which is a database of HIV infected individuals. Inclusion criteria for study enrollment were as follows: ≥18 years old, HIV-1 seropositive, HIV-1 NAT positive, and no prior history of antiretroviral therapy (ART). Approximately 10–15 mL of whole blood were collected from a total of 31 HIV-1-infected patients. Blood samples were also collected from 50 HIV-1 seronegative patients. Specimens were collected at UW and shipped to the Centers for Disease Control and Prevention (CDC) within 24 hours of collection. HIV-1 viral load testing was performed using the Abbott RealTime HIV-1 assay (Abbott Molecular, Abbott Park, Illinois) at the time of sample collection at the UW, along with CD4 counts. Upon receipt, whole blood samples were aliquoted in 100–200μL volumes and stored at −80°C until ready for use.
2.3. RT-LAMP Reagents
A lyophilized preparation of the RT-LAMP amplification reagents, containing HIV-1 reverse transcriptase (RT) primers, was freeze-dried at PATH using a Millrock LD85 Lyophilizer (Millrock Technology, Inc., Kingston, NY). The RT primer sequences, along with a quencher probe, have been described elsewhere (Curtis et al., 2012; Rudolph et al., 2015). The reaction mix contained the following components: 0.2 μM each of F3 and B3 primers, 1.6 μM each of FIP and BIP primers, 0.8 μM each of LoopF and LoopB primers (LoopF contains a 5′ HEX label), 0.8 μM of the Black Hole Quencher 1 (BHQ1)-labeled quencher probe, 8 mM MgSO4, 1.4 mM dNTPs (Sigma-Aldrich, St. Louis, MO), 1X Isothermal Amplification Buffer (New England Biolabs, Ipswich, MA), 16U glycerol-free Bst 2.0 WarmStart DNA Polymerase (New England Biolabs, Ipswich, MA), 2U AMV Reverse Transcriptase (Life Technologies, Carlsbad, CA), and 5% trehalose (Life Sciences Advanced Technologies, St. Petersburg, FL). The reagents were lyophilized directly in 200μL PCR tubes (Agilent Technologies, Santa Clara, CA) and capped immediately after removing from the lyophilizer. The tubes were sealed in Vapor-Loc foil pouches (TigerPak Inc., Moonachie, NJ) with desiccant packs (Desiccare, Inc., Reno, Nevada) and stored at −20°C until ready for use.
2.4. Non-instrumented nucleic acid (NINA) heaters
Expanding upon the utility of the previously demonstrated NINA reusable heating devices, PATH (Seattle, WA) has developed a miniaturized, single-use disposable (NINA-SUD) heater prototype (Curtis et al., 2012; Sema et al., 2015; Singleton et al., 2014) (Figure 1). Mg-Fe fuel powder (Luxfer Magtech Inc., Cincinnati, OH), contained in a round pouch made from heat-sealable, hydrophilic teabag filter paper (Muslin Bag, San Diego, CA) was added to the base of each heater. To initiate the exothermic reaction, saline (2.0% NaCl in distilled water; Sigma-Aldrich, St. Louis, MO) is added to an external reservoir and delivered to the Mg-Fe fuel pouch via capillary transport in a porous, glass fiber paper media (Ahlstrom Corporation, Helsinki, Finland). The fuel pouch wicks the solution into the device, activating the exothermic reaction and subsequent temperature-buffered heat output to the PCR tubes. Steam generated by the exothermic reaction is channeled into a chamber containing cotton balls (Amazon.com, Seattle, WA) to prevent visible steam from escaping the device. Steam passing into the condensing chamber triggers the irreversible color-changing temperature indicating labels (49°C Labels, Tempil, Elk Grove Village, IL). The labels indicate whether the exothermic chemical reaction has initiated, which is not an indication of the temperature of the samples. To reduce heat loss and maximize temperature holdover time, the devices were insulated with low density polyvinyl chloride (PVC) foam (Mcmaster-Carr, Elmhurst, IL), milled to shape with a CNC 3-axis mill (Haas Automation Inc, Oxnard, CA), and fastened with double-sided acrylic tape adhesive (MBK Tape Solutions, Chatsworth, CA). The exothermic Mg-Fe oxidation reaction is thermally coupled with palmitic acid, which acts as a phase change material (PCM) on the temperature interval 58–61°C. To increase thermal conductivity of the composite and reduce the warm-up time of the system, graphene nanoparticles (Angstron Materials, Dayton, OH) were mixed (20% mass fraction) with the palmitic acid (Sigma-Aldrich, St. Louis, MO). The structural plastic components were rapid-prototyped using UV-cure photopolymer 3D printing (Objet, Rehovot, Israel). The NINA-SUD devices were designed with three sample ports to hold three 100μL PCR tubes (ThermoFisher Scientific, Waltham, MA), which allows for inclusion of a test specimen, positive control, and negative control. The NINA-SUD heaters were manufactured at PATH and shipped to the CDC for testing.
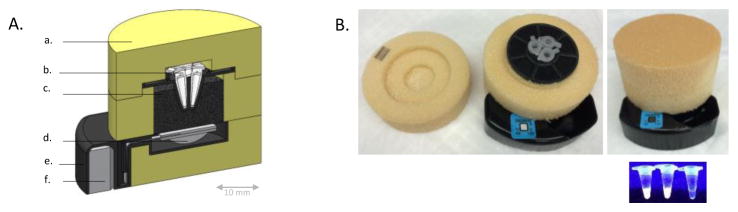
Figure 1. NINA-SUD device.
(A) A cross-sectional diagram of the NINA-SUD device. Removable foam insulation lid (a) allows insertion of 3– 100uL PCR tubes (b) into the device. An exothermic chemical reaction fuel pouch (d) delivers heat to palmitic acid PCM (c), buffering temperature to amplification specification. The reaction by-product steam is channeled into a condensing chamber (f). The PCR tube holder and support structure is made from rapid-prototyped plastic (e). (B) A photograph of the NINA-SUD device before and after initiation of the exothermic chemical reaction, along with the PCR tubes post-reaction. From left to right, the PCR tubes contain a sample, positive control, and negative control.
To ensure quality control in the manufacturing process, one device from each production lot (n=10) was tested for compliance to performance specifications (Figure 2). Additionally, PATH archived one device from each production lot as a representative for future performance analysis, if needed.
Figure 2. NINA-SUD temperature profile.
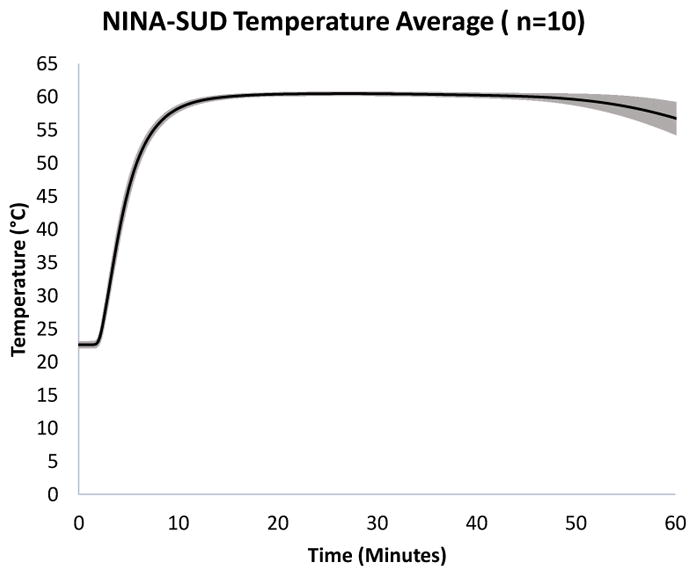
The average temperature of 10 NINA-SUD test devices (black line), recorded for 60 minutes post-initiation of chemical reactants. The shaded gray area of the plot represents the standard deviation.
2.5. NINA-SUD Operation
Prior to initiating the RT-LAMP reaction, the PCR tubes strips were removed from the foil pouches and the lyophilized reagent pellets were reconstituted with 15μL of RNase-free water containing 1.33M Betaine (Sigma-Aldrich). The reconstituted reagents were transferred to 100μL PCR tubes and 10μL of sample was added to the tube. To prevent evaporation of the reactants, 15μL mineral oil (Hologic, Inc., San Diego, CA) was overlaid on top of the reaction mix in each tube. The PCR tubes were added to the sample ports in the heater and the foam insulation lid was placed on top to close the sample compartment. To initiate the exothermic reaction, 6mL of 2.0% NaCl solution was dispensed into the reservoir on the outside of the NINA-SUD. Initiation of the exothermic chemical reaction within the NINA-SUD devices was measured indirectly by visually monitoring the color change of the temperature indicating labels.
To evaluate the performance of the NINA-SUD heaters, 15 HIV-1-positive specimens, stratified by viral load, and 5 HIV negative samples were tested. The total sample number was determined based on the availability of test materials and number of specimens with detectable viral loads. Each sample was tested in triplicate (1 replicate per device) to evaluate the consistency of the heaters. For comparison, the samples were also tested in triplicate in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA). For the baseline thermal cycler reaction, the reaction mix was prepared fresh as described (Rudolph et al., 2015). Amplification was confirmed by determining fluorescence of the reaction tubes on the UV transilluminator of a ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA).
2.6. Reagent stability evaluation
The lyophilized amplification reagents were prepared as described for the NINA-SUD heater evaluation, with the exception that the quencher probe was not included in the reagent mix. The quencher was omitted to prevent inhibition during the real-time reaction. One 8-tube strip was packaged per foil pouch. Upon receipt, the foil pouches containing the reagents strips were stored at −20°C, 4°C, 25°C (room temperature), or 30°C. The tubes were removed from storage conditions and tested on days 0, 7, 14, 21, and 28 using DNA and RNA standards. The DNA standard containing 104 DNA copies/mL and RNA standard containing 105 RNA copies/mL were tested in triplicate using a Stratagene Mx3005P real-time platform (Agilent Technologies, Santa Clara, CA). The amplification was set for 60 minutes at 60°C. The time to positive result was determined by Ct values for all standards. Each cycle corresponded to one minute of amplification time.
3. Results
3.1. Performance of NINA-SUD devices
The average temperature recording for 10 representative NINA-SUD devices (one from each production lot) is shown in Figure 2. The heating devices reached 60°C within an average of 11 minutes from the time that the reactants were initiated. The devices maintained a temperature of at least 60°C for an average of 35.8 minutes before a decline of ≥1 degree was noted.
Overall, the NINA-SUD devices exhibited comparable performance, if not superior, to the thermal cycler in detecting HIV in whole blood from HIV-1 infected individuals (Table 1). The reproducibility of the NINA-SUD devices, as measured by triplicate testing of each sample, was slightly greater as compared to the replicates tested in the thermal cycler (Table 1). In the NINA-SUD devices, all samples with a viral load (VL) ≥104 copies/mL were detected in all three replicates. Of the six samples with a VL <104 copies/mL, four were detected in all three or 2/3 replicates. The remaining two samples with a VL < 2000 copies/mL were not detected at all in the NINA-SUD devices or thermal cycler. Sample UW10 (VL=3115 copies/mL) was detected in all three replicates in the NINA-SUD devices but not detected at all in the thermal cycler. No amplification was observed with the HIV-1 negative samples in either the NINA-SUD devices or thermal cycler.
Table 1.
Evaluation of NINA-SUD devices
| Patient ID | HIV Status | Viral Load (Copies/mL) | NINA-SUDa | Thermal cyclerb | |
|---|---|---|---|---|---|
| 1 | UW5 | Positive | 230700 | 3/3 | 3/3 |
| 2 | UW17 | Positive | 161500 | 3/3 | 3/3 |
| 3 | UW29 | Positive | 128800 | 3/3 | 3/3 |
| 4 | UW15 | Positive | 57120 | 3/3 | 3/3 |
| 5 | UW24 | Positive | 50370 | 3/3 | 3/3 |
| 6 | UW13 | Positive | 39300 | 3/3 | 3/3 |
| 7 | UW16 | Positive | 28980 | 3/3 | 2/3 |
| 8 | UW2 | Positive | 22870 | 3/3 | 2/3 |
| 9 | UW31 | Positive | 11660 | 3/3 | 2/3 |
| 10 | UW7 | Positive | 5958 | 2/3 | 2/3 |
| 11 | UW25 | Positive | 4798 | 3/3 | 2/3 |
| 12 | UW18 | Positive | 3323 | 2/3 | 3/3 |
| 13 | UW10 | Positive | 3115 | 3/3 | 0/3 |
| 14 | UW12 | Positive | 1839 | 0/3 | 0/3 |
| 15 | UW23 | Positive | 1837 | 0/3 | 0/3 |
| 16 | BRH624078 | Negative | N/A | 0/3 | 0/3 |
| 17 | BRH624079 | Negative | N/A | 0/3 | 0/3 |
| 18 | BRH624080 | Negative | N/A | 0/3 | 0/3 |
| 19 | BRH624081 | Negative | N/A | 0/3 | 0/3 |
| 20 | BRH624082 | Negative | N/A | 0/3 | 0/3 |
Number positive from 3 separate NINA-SUD devices
Number positive out of 3 replicates in same thermal cycler run
3.2. Stability of Lyophilized HIV-1 RT-LAMP Reagents
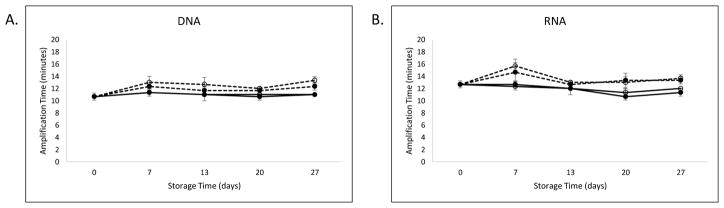
For the stability evaluation of the lyophilized RT-LAMP reagents, the average time to positivity for the amplification reaction is plotted for DNA and RNA standards relative to storage time and temperature (Figure 3). The standard for comparison at −20°C storage temperature was 10.7 minutes for DNA and 12.7 minutes for RNA at T=0. For the standard storage conditions, the amplification time remained stable for all time points, as indicated by a time to positivity of 11 minutes for DNA and 11.3 minutes for RNA after 27 days. At elevated temperatures, relative to −20°C, the amplification time was slightly increased at 25 and 30°C by day 27. The time to positivity at day 27 was 11, 12.3, and 13.3 minutes for the storage temperatures 4, 25, and 30°C, respectively. Similarly, a small increase in the amplification time was noted for RNA when the lyophilized reagents were stored at 25 and 30°C. At day 27, the time to positivity was 12, 13.3, and 13.7 minutes for the storage temperatures 4, 25, and 30°C, respectively.
Figure 3. Lyophilized RT-LAMP reagent stability.
The total amplification time until positivity is plotted for DNA (A) and RNA (B) over days the lyophilized reagents were stored at temperatures of −20°C (solid line, closed circle), 4°C (solid line, open circle), 25°C (dashed line, closed circle), and 30°C (dashed line, open circle).
4. Discussion
Rapid tests have transformed the field of HIV diagnostics, facilitating HIV screening at the POC, where resources are often limited. Rapid POC HIV testing considerably reduces turnaround time, providing immediate knowledge of a preliminary positive test result and improved linkage to care. HIV antibodies become detectable by most antibody-based assays within 3–6 weeks post-infection (Branson, 2007; Butto et al., 2010). In acute or early infection, antibody tests may yield false-negative test results if an individual is tested prior to or during early seroconversion. The availability of a cost-effective, rapid NAT could potentially improve detection of acute infection at the POC and reduce the need for follow-up laboratory testing when supplemental NAT testing is required. The primary goal of the current study was to demonstrate the performance of a prototype single-use, electricity-free heating device for the detection of HIV-1 by RT-LAMP. Previously, it has been demonstrated that the HIV-1 RT-LAMP assay can reduce the window of detection between infection and seroconversion (Rudolph et al., 2015). The results from this study demonstrate a step forward towards the development of a single-use, rapid NAT that may supplement rapid antibody testing at the POC.
The simplified NINA-SUD device is made from low-cost, fully incinerable materials, estimated to cost less than $1.00 USD to manufacture at scale in the current embodiment. Since electricity or batteries are not required and the NINA-SUD materials are non-toxic, disposal of the device and its components poses no hazardous risk. The temperature indicator labels and integrated chemical reactants are inexpensive yet valuable modifications to the device design, further reducing hands-on time. The device operation requires minimal training and alerts the test user when the heating device has reached the indicated temperature, ensuring the validity of the run. This study, along with previous NINA prototype evaluations, demonstrates the flexibility of the housing materials for the exothermic chemical heating device, especially when coupled to isothermal amplification methods (Curtis et al., 2012; Lillis et al., 2014; Sema et al., 2015). Future modifications to the NINA-SUD design intend to reduce the size of the unit, eliminate the need for PCR tubes, and integrate the ambient temperature-stable, lyophilized amplification reagents. An additional important modification to the RT-LAMP assay was the use of an ambient temperature-stable lyophilized reaction mix, containing a fluorescent-labeled primer and quencher pair, which eliminates the need to open the tube after the amplification reaction is complete and reduces the risk of amplicon contamination. For convenience, a transilluminator was used to visualize the reaction tubes in this study, however, a handheld UV light or portable, battery-operated fluorescent reader can be used at field sites.
Despite the reduction in size and complexity of the NINA-SUD devices, the design modifications were not at the detriment of the assay performance. The reproducibility between devices and overall performance for amplifying clinical specimens was similar to the reusable, thermos-style prototype (Curtis et al., 2012). Although the temperature of the NINA-SUD devices began to decline from the optimal 60°C reaction temperature prior to the completion of the 60 minute amplification step, initial validation studies indicated that the majority of all amplification reactions occur within 30 minutes (data not shown). Importantly, all clinical specimens were detected within the reported limit of detection of the assay for RNA, which is 104 copies/mL (Rudolph et al., 2015). Since the RT-LAMP reaction can amplify from RNA, as well as DNA targets, the contribution from proviral DNA cannot be excluded and may explain amplification of specimens with a VL <104 RNA copies/mL. In this study, the NINA-SUD devices appeared to perform slightly better as compared to a thermal cycler. A direct comparison between the lyophilized RT-LAMP reagents and freshly prepared reagent mix showed comparable results (data not shown); therefore, it is not expected that the performance discrepancy was related to the amplification reagents. One plausible explanation for these findings is the slight temperature deviations or fluctuations in the heaters may be favorable for the RT-LAMP reaction, especially if slightly higher or lower reaction temperatures are more favorable for certain patient sequences.
In this study, we demonstrated the stability of the lyophilized HIV-1 RT-LAMP reagents and fluorescent label when stored at room and elevated temperatures, which validates the feasibility for POC use, where refrigeration might not be available. With the final version of the rapid NAT, extensive stability studies will be needed to fully characterize the shelf-life and storage conditions of the reaction materials. The next steps towards development of the NINA-SUD are designed toward the incorporation of a whole blood sample preparation step into the device and inclusion of RT-LAMP primers capable of amplifying group M HIV-1 sequences. In the current study, subtype B reverse transcriptase-specific primers were used for convenience as the focus of our laboratory is HIV diagnostics in the US and; therefore, US-derived standards are readily available. Conserved RT-LAMP primers, targeting a region within the HIV-1 integrase gene, have also been developed for detection of subtype B infections (Rudolph et al., 2015). Validation of the HIV-1 INT RT-LAMP primers, optimized for detection of group M, is currently underway.
A limitation of the NINA-SUD evaluation was the relatively small number of clinical specimens from HIV-1 infected individuals. The goal of this IRB-approved study was to obtain whole blood from recently diagnosed HIV-1 infected participants, however, low enrollment expectations dictated a broader recruiting mechanism, which included primarily chronic cases of HIV infection. Given that most previously diagnosed, HIV-infected individuals in the US are receiving antiretrovirals, it is challenging to obtain whole blood samples from a large number of study participants who are not virally suppressed. The projected enrollment of HIV positive study participants was at least 50, however, only 31 participants enrolled during the study period. Of the 31 study subjects, approximately 1/3 exhibited undetectable plasma viral loads or levels under 1000 RNA copies/mL and an additional seven subjects had viral loads under 104 RNA copies/mL. Future validation studies may require a revised recruiting mechanism or expansion to study sites outside of the US.
5. Conclusions
The NINA-SUD device in conjunction with the HIV-1 RT-LAMP assay is a promising rapid, cost-effective NAT platform that requires minimal training and hands-on time. The single-use device has the potential to supplement rapid serologic testing at the POC and increase the number of individuals that are aware of their HIV status.
Highlights.
A rapid, single-use nucleic acid amplification device (NINA-SUD) was evaluated.
An exothermic chemical reaction provides the heat source for the NINA-SUD devices.
We demonstrated amplification from whole blood by HIV-1 RT-LAMP in the NINA-SUD.
Reproducibility was observed between NINA-SUD units and a thermal cycler.
Stability of the lyophilized HIV-1 RT-LAMP reagents was demonstrated.
Acknowledgments
The authors would like to acknowledge Michalina Montano at the University of Washington for coordinating the enrollment of study subjects through CFAR, the sample and data collection from study participants, and shipment of samples to the CDC. This research was funded in part by a 2013 developmental grant from the University of Washington Center for AIDS Research (CFAR), an NIH funded program under award number AI027757 which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK). Funding for the research and development of the NINA-SUD was provided under the NIH grant R01EB012641 to PATH supported by the NIBIB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Branson BM. State of the art for diagnosis of HIV infection. Clin Infect Dis. 2007;45(Suppl 4):S221–5. doi: 10.1086/522541. [DOI] [PubMed] [Google Scholar]
- Butto S, Suligoi B, Fanales-Belasio E, Raimondo M. Laboratory diagnostics for HIV infection. Ann Ist Super Sanita. 2010;46:24–33. doi: 10.4415/ANN_10_01_04. [DOI] [PubMed] [Google Scholar]
- Curtis KA, Rudolph DL, Nejad I, Singleton J, Beddoe A, Weigl B, LaBarre P, Owen SM. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PloS one. 2012;7:e31432. doi: 10.1371/journal.pone.0031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KA, Rudolph DL, Owen SM. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP) Journal of virological methods. 2008;151:264–70. doi: 10.1016/j.jviromet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Curtis KA, Rudolph DL, Owen SM. Sequence-specific detection method for reverse transcription, loop-mediated isothermal amplification of HIV-1. Journal of medical virology. 2009;81:966–72. doi: 10.1002/jmv.21490. [DOI] [PubMed] [Google Scholar]
- Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–72. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- Johannessen A, Troseid M, Calmy A. Dried blood spots can expand access to virological monitoring of HIV treatment in resource-limited settings. J Antimicrob Chemother. 2009;64:1126–9. doi: 10.1093/jac/dkp353. [DOI] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarre P, Boyle D, Hawkins K, Weigl B. Instrument-free nucleic acid amplification assays for global health settings. Proceedings of SPIE--the International Society for Optical Engineering 8029. 2011 doi: 10.1117/12.882868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarre P, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Weigl B. Non-instrumented nucleic acid amplification (NINA): instrument-free molecular malaria diagnostics for low-resource settings. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society Annual Conference; 2010; 2010. pp. 1097–9. [DOI] [PubMed] [Google Scholar]
- Lee HH, Dineva MA, Chua YL, Ritchie AV, Ushiro-Lumb I, Wisniewski CA. Simple amplification-based assay: a nucleic acid-based point-of-care platform for HIV-1 testing. The Journal of infectious diseases. 2010;201(Suppl 1):S65–72. doi: 10.1086/650385. [DOI] [PubMed] [Google Scholar]
- Lee HY, Giorgi EE, Keele BF, Gaschen B, Athreya GS, Salazar-Gonzalez JF, Pham KT, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Hahn BH, Shaw GM, Korber BT, Bhattacharya T, Perelson AS. Modeling sequence evolution in acute HIV-1 infection. Journal of theoretical biology. 2009;261:341–60. doi: 10.1016/j.jtbi.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis L, Lehman D, Singhal MC, Cantera J, Singleton J, Labarre P, Toyama A, Piepenburg O, Parker M, Wood R, Overbaugh J, Boyle DS. Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PloS one. 2014;9:e108189. doi: 10.1371/journal.pone.0108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Geva E, Mauk M, Qiu X, Abrams WR, Malamud D, Curtis K, Owen SM, Bau HH. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst. 2011;136:2069–76. doi: 10.1039/c1an00007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Hirano T, Notomi T. Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC biotechnology. 2006;6:3. doi: 10.1186/1472-6750-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun. 2001;289:150–4. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Hase T, Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Molecular and cellular probes. 2002;16:223–9. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- NIH. [accessed 07.24.15];Starting Antiretroviral Treatment Early Improves Outcomes for HIV-Infected Individuals. 2015 http://www.niaid.nih.gov/news/newsreleases/2015/Pages/START.aspx.
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph DL, Sullivan V, Owen SM, Curtis KA. Detection of Acute HIV-1 Infection by RT-LAMP. PloS one. 2015;10:e0126609. doi: 10.1371/journal.pone.0126609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sema M, Alemu A, Bayih AG, Getie S, Getnet G, Guelig D, Burton R, LaBarre P, Pillai DR. Evaluation of non-instrumented nucleic acid amplification by loop-mediated isothermal amplification (NINA-LAMP) for the diagnosis of malaria in Northwest Ethiopia. Malaria journal. 2015;14:44. doi: 10.1186/s12936-015-0559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton J, Osborn JL, Lillis L, Hawkins K, Guelig D, Price W, Johns R, Ebels K, Boyle D, Weigl B, LaBarre P. Electricity-free amplification and detection for molecular point-of-care diagnosis of HIV-1. PloS one. 2014;9:e113693. doi: 10.1371/journal.pone.0113693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner NA, Zhang Y, Evans TC., Jr Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. Biotechniques. 2015;58:59–68. doi: 10.2144/000114253. [DOI] [PubMed] [Google Scholar]
- Tanriverdi S, Chen L, Chen S. A rapid and automated sample-to-result HIV load test for near-patient application. The Journal of infectious diseases. 2010;201(Suppl 1):S52–8. doi: 10.1086/650387. [DOI] [PubMed] [Google Scholar]