Abstract
Preeclampsia is characterized by development of hypertension during pregnancy and reduced placental perfusion. Previous studies in a rat model of placental ischemia-induced hypertension demonstrated that inhibiting complement activation attenuated increased maternal blood pressure with C3a and C5a identified as the important products of complement activation. Given that in other forms of ischemia both natural IgM and antigen antibody complexes initiate complement activation, we hypothesized that placental ischemia exposes neoepitopes recognized by IgM to cause local complement activation and hypertension. Alternatively, we postulated that autoantibody to angiotensin II Type 1 receptor (AT1-AA) interacts with AT1 receptors to cause complement activation. Since complement activation occurs in kidney and placenta in preeclampsia, we used immunohistochemistry to determine IgM deposition and local complement activation in each organ (C3 deposition), and quantitative real-time polymerase chain reaction (qRT-PCR) to quantitate mRNA for endogenous regulators of complement activation CD55, CD59 and Complement receptor 1-related gene/protein y (Crry). On gestation day (GD)14.5, timed pregnant Sprague Dawley rats underwent Sham surgery or placement of clips on inferior abdominal aorta and ovarian arteries to create placental ischemia using the reduced utero-placental perfusion pressure (RUPP) model. As previously reported, RUPP surgery increased mean arterial pressure and circulating C3a on GD19.5. In placenta, IgM and C3 deposition increased, whereas mRNA for complement regulators Crry and CD59 decreased along with Crry protein in RUPP compared to Sham treated animals. In kidney, IgM deposition increased in animals subjected to RUPP vs Sham surgery without a significant change in C3 deposition and coincident with an increase in mRNA for CD55 and CD59. The AT1 receptor antagonist losartan prevents placental ischemia-induced hypertension as well as AT1-AA interaction with AT1 receptors. However, losartan did not attenuate complement activation as measured by circulating C3a or placental C3 deposition. Importantly, our studies indicate that following placental ischemia, complement activation is not due to AT1-AA but is associated with IgM deposition. These studies suggest a role for natural antibodies interacting with placental ischemia-induced neoepitopes to activate complement and contribute to hypertension.
Keywords: pregnancy-induced hypertension, preeclampsia, complement, natural antibody, C3 deposition, autoantibody
1. Introduction
Substantial evidence implicates the immune system in the pathogenesis of pregnancy-induced hypertension. Both adaptive and innate immune mechanisms are implicated in contributing to the initial abnormal spiral artery remodeling and resultant placental ischemia. In addition, aberrant immune activation occurs once the placental ischemia is established. Multiple studies demonstrated significantly enhanced systemic complement system activation in preeclamptic pregnancies compared to normal pregnancies (Derzsy et al., 2010; Regal et al., 2015a). Both the placenta and kidney also showed evidence of increased local complement activation in preeclampsia, coincident with up-regulation of message for complement regulators but no evidence of changes in regulator expression using immunohistochemistry have been reported (Burwick et al., 2014; Buurma et al., 2012; Lokki et al., 2014; Penning et al., 2015). However, the event or events that initiate complement activation in preeclampsia are unknown. Complement can be activated by multiple mechanisms and is regulated by the expression of endogenous regulators that normally limit complement activation on host surfaces. Simplistically, increased overall complement activation in preeclampsia could be the result of either increased activation of any initiation pathway and/or insufficient expression of endogenous regulators.
Complement activation by self-reactive, native IgM antibodies has been well-documented following ischemia reperfusion in many different situations and organs (Austen et al., 2004; Busche et al., 2009; Fleming et al., 2002a; Williams et al., 1999). These natural antibodies comprise up to 80% of the circulating IgM and do not require exogenous antigen for production. They are thought to recognize danger-associated molecular patterns or neoepitopes revealed following ischemia. Compared to normal pregnancy, sera from women with preeclampsia contains increased circulating IgM (Kestlerova et al., 2012) and increased IgM has been demonstrated in immune deposits in the placenta (Buurma et al., 2012). Autoantibody production, particularly agonistic IgG autoantibodies to angiotensin II Type 1 receptor (AT1-AA) (LaMarca et al., 2013), are associated with placental ischemia and preeclampsia. The Angiotensin II type 1 receptor (AT1) antagonist losartan prevents interaction of AT1-AA with the AT1 receptor, in addition to preventing interaction of angiotensin II with the receptor. Antagonism of AT1 with losartan has been demonstrated to completely prevent placental ischemia-induced hypertension (Alexander et al., 2001b; LaMarca et al., 2008) in a rat model, indicating that either angiotensin and/or the AT1-AA contribute to the increased blood pressure. Given the known capacity of natural IgM as well as antigen antibody complexes to initiate complement activation, we hypothesized that placental ischemia exposes neoepitopes that interact with IgM to cause complement activation and hypertension following placental ischemia. As an alternate hypothesis we considered that AT1-AA interaction with AT1 receptors activates complement resulting in hypertension following placental ischemia. Since complement activation has been demonstrated in kidney and placenta in preeclampsia, we considered those organs as the most likely site for complement activation, and examined local complement activation as well as the status of endogenous regulators of activation in each organ.
Our studies focus on the response to placental ischemia using the Reduced Utero-placental Perfusion Pressure (RUPP) model of pregnancy-induced hypertension in the rat. Chronic placental ischemia in the RUPP model results in increased blood pressure in the mother, fetal growth restriction and complement activation as indicated by generation of C3a (Lillegard et al., 2013; Lillegard et al., 2014). As we have reported, this model also manifests other signs of preeclampsia including angiogenic imbalance with decreased vascular endothelial growth factor (VEGF) and increased soluble VEGF receptor (sFlt-1) (Bauer et al., 2013), though the validity of the VEGF and sFlt-1 assays in the rat have been questioned (Weissgerber et al., 2014). Increased protein in the urine is not routinely manifested in this model, but has been reported in the RUPP model by others (Granger et al., 2006). The diagnosis of preeclampsia has required the presence of proteinuria since the 1930s, but in 2013 the diagnosis of preeclampsia has been broadened due to the heterogeneous and systemic nature of the disease and proteinuria is not required if other signs or symptoms of systemic disease are present (ACOG, 2013). Our previous studies have demonstrated that circulating C3a increases in the RUPP rat model and inhibiting complement activation with a soluble form of an endogenous regulator, soluble CR1, inhibits the placental ischemia induced hypertension (Lillegard et al., 2013). Also, antagonism of C3a or C5a receptors significantly attenuates the placental ischemia induced hypertension (Lillegard et al., 2014). These data provide critical evidence for a role of this innate immune amplification system in hypertension following placental ischemia. Given the importance of C3a and C5a in placental ischemia induced hypertension, the goals of the present studies were to determine if placental ischemia resulted in local tissue deposition of C3 in placenta and kidney and if mRNA for endogenous complement regulators changed, regardless of the pathway of activation. In addition, our studies were designed to determine if IgM was deposited in placenta and kidney suggesting the participation of natural IgM antibody in the pathophysiology of gestational hypertension. Losartan was used to prevent AT1-AA interaction with the AT1 receptor and the effect on complement activation following placental ischemia assessed. These studies provide important insight into the potential site(s) and factors critical for complement activation resulting in placental ischemia induced hypertension.
2. Materials and Methods
2.1 Reduced Utero-placental Perfusion Pressure (RUPP) procedure
The reduced utero-placental perfusion pressure (RUPP) procedure was used to model placental ischemia induced hypertension in third trimester pregnant rat as previously described (Lillegard et al., 2013; Lillegard et al., 2014; Regal et al., 2015b). Timed pregnant Sprague Dawley dams (Crl:CD IGS, Charles River Laboratories, Portage, MI) were anesthetized with isoflurane on gestation day (GD)14.5 with the date of vaginal plug designated as GD0.5. The lower abdominal aorta was isolated and a sterile silver clip (0.203 mm ID) placed around the aorta above the iliac bifurcation. To prevent compensatory blood flow to the placenta, right and left uterine arcades were also clipped at the ovarian end, directly before the first segmental artery, using a silver clip (0.100 mm ID). Uterine perfusion pressure is reduced by approximately 40% using this procedure (Alexander et al., 2001a). For the comparison group, a Sham operation differing only in absence of the clips was also conducted. On GD18.5, the carotid artery was cannulated under isoflurane anesthesia using a 25% dextrose lock solution in sterile pyrogen free saline to maintain cannula patency. No heparin was used in the cannula due to its known ability to inhibit complement activation (Girardi et al., 2004) and affect sFlt (Searle et al., 2011). On GD19.5, mean arterial pressure (MAP) was measured from the arterial catheter in an unanesthetized restrained rat. Following measurement of blood pressure, serum, plasma and tissues were collected in necropsy as described (Alexander et al., 2001a; Gilbert et al., 2007a; Gilbert et al., 2007b; Gilbert et al., 2010). The uterus was exteriorized, the total number of viable and resorbed pups counted and the pups and placentae of the right horn weighed. A kidney and select utero-placental units were frozen in OCT for assessing deposition of C3 and IgM. The other kidney (divided into kidney cortex and medulla) and a placenta were frozen in liquid nitrogen for qRT-PCR analysis of endogenous complement regulators in RUPP and Sham animals.
2.2 Experimental Design and Treatments
For determination of C3 and IgM deposition, rats were subjected to either Sham or RUPP surgery on GD14.5 and outcomes measured on GD19.5. For determination of the role of AT1-AA in complement activation following RUPP, rats were treated with losartan in drinking water beginning 13.5 days post conception (GD13.5) with the date of vaginal plug designated as GD0.5. Losartan potassium was obtained from TCI America (Portland, OR; CAS 124750-99-8; >98% purity) and was provided in drinking water at 0.3 mg/ml from GD13.5 to necropsy. The final dose of losartan was estimated as per day consumption based on total water consumed from GD13.5 to 19.5 and the minimum and maximum weight of the animal over that time period. Rats were randomly assigned to one of four experimental groups based on surgical procedure and water treatment: 1) RUPP surgery with water ad lib (RUPP Water); 2) RUPP surgery with losartan in water (RUPP losartan); 3) sham surgery with water ad lib (Sham Water); 4) sham surgery with losartan in water (Sham losartan). The n for each outcome measured is indicated in each of the figure legends, dependent on samples available.
2.3 Complement System measurements
Rat C3a, C5a and sC5b-9
The complement activation product C3a was measured in serum by Western blot as previously described (Lillegard et al., 2013) with modification. NuPAGE Novex 10% Bis-Tris gels were used with MES SDS Running buffer. Immunodetection was modified from the original method using the secondary antibody IRDye 800CW Goat anti-Rabbit IgG (H+L) at 1/20,000 dilution and LiCor Odyssey Fc for imaging. A standard curve of rat serum activated by yeast was included on each gel. Relative amounts of C3a were expressed as C3a units/ul based on signal intensity of 1 ul of standard pool of rat serum activated by yeast. Rat C5a was measured in serum by ELISA (Cusabio Biotech Co, College Park, MD). Rat sC5b-9 was assayed in plasma using an ELISA assay purchased from Hycult (HK106, Hycult, Biotech, Uden, The Netherlands). This assay is based on the publication by Kotimaa et al (Kotimaa et al., 2014). The sC5b-9 complex is captured with mouse anti-rat C5b-9 neoepitope recognizing mAb clone 2A1 (Hycult HM3033-IA) and detected with biotinylated mouse anti-rat C6 mAb 3G11 (Hycult HM3034).
Total hemolytic complement activity
Total hemolytic complement activity was determined as previously described (Larsen et al., 2001). The inverse dilution of serum that results in 50% hemolysis of sensitized sheep erythrocytes (CH50) was determined in the presence or absence of losartan.
ELISA for Rat IgG and IgM
Total IgG and IgM concentrations were measured in rat serum using ELISA kits from Abcam (ab189578, ab157738; Cambridge, MA).
2.4 Immunohistochemistry for C3, C5b-9, Crry and IgM
Placenta and kidney were flash frozen in OCT freezing medium and 8μm sections were cut and placed on slides for immunohistochemistry. Non-specific binding was blocked for 30 min with 10% donkey sera in PBS. The washed sections were incubated overnight at 4°C with goat anti-rat C3 (MP Biomedical 55713; Santa Ana, CA), rabbit anti-complement 5b-9 (EMD Millipore 204903; Darmstadt, Germany), goat anti-mouse Crry (Santa Cruz sc-25217; Dallas, TX) or rabbit anti-rat IgM (Rockland Immunochemicals 112-4107; Limerick, PA). Serial sections were incubated with appropriate isotype control antibodies. After additional washing, all tissues were incubated with fluorescently labeled secondary donkey anti-goat (C3) or rabbit (IgM) antibody (Jackson Immuno Research). Subsequent to three additional washes, the slides were mounted with ProLong Gold (Invitrogen). Images were obtained by a blinded observer using a Nikon eclipse 80i microscope equipped with a CoolSnap CF camera (Photometrics) and analyzed with Metaview software (Molecular Devices). The images from each animal were compared to isotype control to determine positive staining and scored by a blinded observer as negative (0), weakly positive (1), positive (2) or strongly positive (3).
2.5 mRNA expression of complement regulators
Prior to RNA isolation, flash frozen kidney and placenta were added to pre-chilled RNAlater-Ice (Ambion; Thermo Fisher Scientific) and allowed to thaw at −20°C for at least 16 hours. Samples were removed from RNAlater-Ice and RNA isolation completed with RNeasy Mini Prep Kit from Qiagen (Valencia, CA) with DNA digestion performed during RNA isolation using RNase-Free DNase kit from Qiagen (Valencia, CA). RNA concentration was determined using a Nanodrop spectrophotometer. Omniscript Reverse Transcription (Qiagen, Valencia, CA) and random nonamers custom synthesized by Integrated DNA Technologies (Coralville, Iowa) were used to synthesize cDNA with 1.5μg of total RNA per sample. cDNA was diluted 1:5 and real-time PCR performed in duplicate using Qiagen QuantiTect SYBR Green PCR Kit and Rotor-Gene real time PCR cycler. Custom primers (Table 1) were obtained from Integrated DNA Technologies (Coralville, Iowa). Expected PCR product size was confirmed by agarose gel electrophoresis as well as by direct sequencing (University of Minnesota Genomics Center). Equal primer efficiencies were validated as described in Applied Biosystems User Bulletin No.2. Delta-delta Ct method of relative quantification was used to determine fold change in mRNA expression compared to actin with the change in Sham animals defined as 1.
Table 1.
Primer Information for qRT-PCR in placenta and kidney
| Accession | Primer (rat) | Product (bp) | ||
|---|---|---|---|---|
| β-actin | NM 031144 | for | 5′ –CCTGGGTATGGAATCCTGTGGCAT- 3′ | 187 |
| rev | 5′ –TCTTGATCTTCATGGTGCTAGGAGCC- 3′ | |||
| CD55 | NM 022269 | for | 5′ –CACTGAAGTTAAAGTTCCAGCAACACAGC- 3′ | 181 |
| rev | 5′ –CTAGCCAGTGAGTAAGAGCATCACATGC- 3′ | |||
| CD59 | NM 012925.1 | for | 5′ –GCTGTATCCGGAAAGCAAGTCTATCAACAG- 3′ | 257 |
| rev | 5′ –GGAGGCATCGGGAGCTTAGAGATGA- 3′ | |||
| Crry | BC061736.1 | for | 5′ –GAAGGATACCGCCTCATTGGTTCC- 3′ | 265 |
| rev | 5′ –TGACCATCGATGCTGGTACAGTGTAT- 3′ |
2.6 Statistical analyses
Data were expressed as mean +/− standard error of the mean. Differences were considered significant using two tailed comparisons with p<0.05. When comparing RUPP and Sham treatments for assessing C3a, sC5b-9, C5a, IgG and IgM, a t test was used. In experiments with losartan, two way ANOVA was conducted with post hoc individual contrasts using JMP and SAS software (SAS Institute, Cary, NC). For losartan treatment the following comparisons were considered biologically relevant: RUPP Water vs Sham Water; RUPP Water vs RUPP losartan; Sham water vs Sham losartan.
3. Results
3.1 Complement activation occurs in circulation and locally in placenta
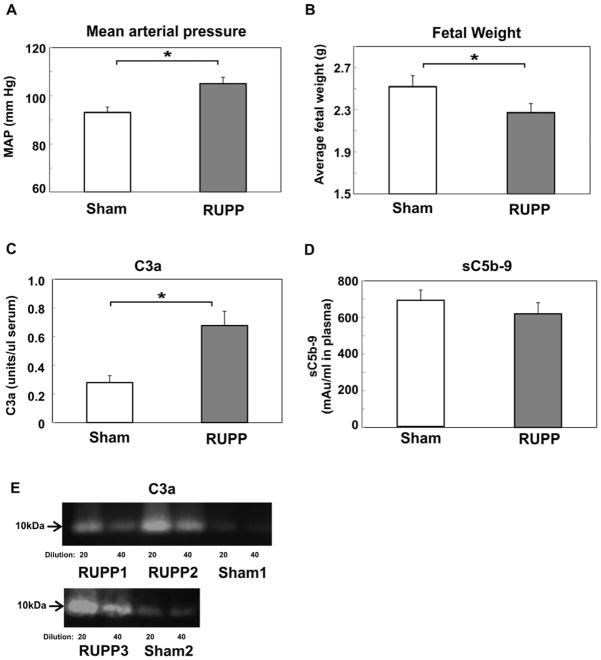
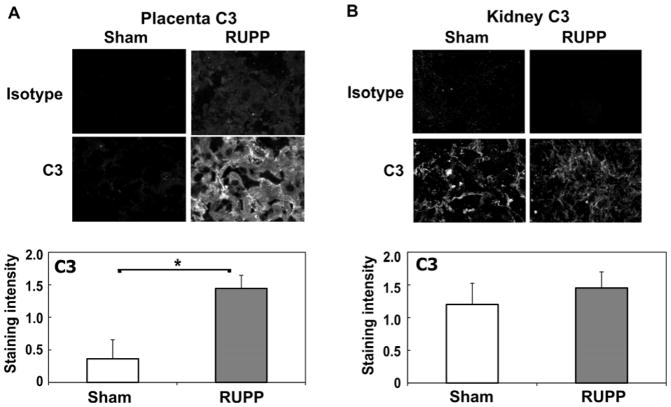
Our previous studies had demonstrated increased C3a in the circulation following placental ischemia. In addition, antagonizing either the C3a or C5a receptor attenuated the hypertension (Lillegard et al., 2013; Lillegard et al., 2014). Our continued studies reported here determined whether sC5b-9 also increased in the circulation and if complement activation could be detected locally in the placenta and kidney by deposition of C3. As seen in Figure 1A–B, placental ischemia resulted in increased mean arterial pressure and decreased fetal weight in RUPP compared to Sham animals. Circulating C3a increased whereas sC5b-9 remained constant in the same cohort of animals (Figure 1C–E). Measurement of circulating C5a by ELISA also demonstrated a significant increase in complement activation (Sham, 12.2±2.5 ug/ml; RUPP, 21.5±4.2 ug/ml; n= 7–8). Immunohistochemistry demonstrated increased C3 deposition in placenta of RUPP vs Sham animals (Figure 2A). In the kidney, although significantly brighter than isotype control, C3 was not consistently deposited in RUPP animals and was not significantly different from staining intensity in Sham kidney (Figure 2B). C5b-9 was also assessed by immunohistochemistry in placenta with no difference in staining intensity detected comparing RUPP to Sham (data not shown).
Figure 1.
C3a but not sC5b-9 is significantly increased in RUPP compared to Sham animals. RUPP or Sham surgery was conducted on GD14.5 and outcomes measured on GD19.5. A. Mean arterial pressure (MAP); B. Average fetal weight; C. Circulating C3a in serum, units of C3a are relative to a standard pool of yeast activated rat serum as described in Materials and Methods; D. Circulating sC5b-9 expressed as mAu/ml in plasma as determined by ELISA. For Figures 1A–D, values represent the mean ± SE of determinations from 11–14 animals. *p<0.05 vs Sham. E. Representative near infrared images from 2 different Western blots using serum collected from 3 different RUPP and 2 different Sham animals applied to the gel at both a 1/20 and 1/40 dilution.
Figure 2.
C3 deposition is significantly increased in placenta (A) but not kidney (B) in RUPP compared to Sham animals. Immunohistochemistry was conducted as described in Materials and Methods, and staining graded by a blinded observer from 0–3, negative to strongly positive. Representative images at 200X magnification are provided. Values for staining intensity represent the mean ± SE of scores obtained from placenta and kidney from 9–11 animals. *p<0.05 vs Sham
3.2 Placental ischemia results in up-regulation of message for complement regulators in kidney but not placenta
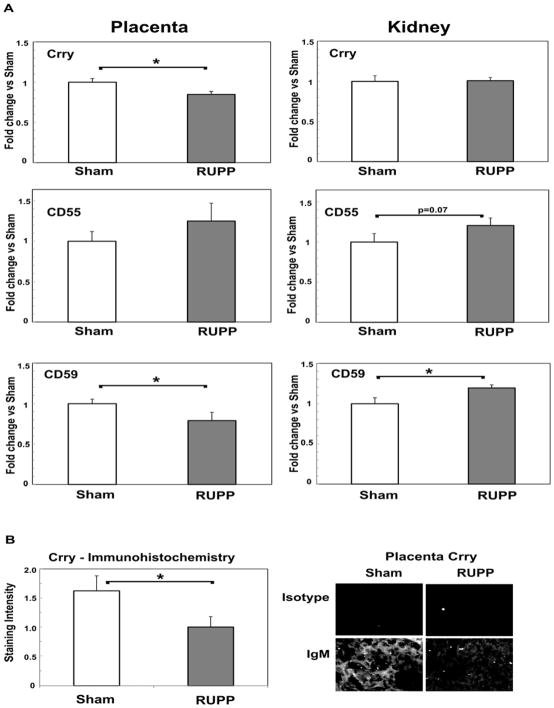
Increased complement activation in circulation and C3 deposition in tissues could be due to insufficient expression of endogenous complement regulators. Membrane bound complement regulators investigated included: 1) CD55 and Complement receptor 1-related gene/protein y (Crry) which limit complement activation through C3 and C5, and 2) CD59 which limits formation of C5b-9 membrane attack complex. Relative expression of each molecule was assessed in both kidney cortex and placenta of RUPP vs Sham animals and is shown in Figure 3A. In placenta, mRNA for Crry and CD59 significantly decreased with placental ischemia, while CD55 did not significantly change. However, in kidney cortex, Crry mRNA remained constant and CD59 mRNA significantly increased in RUPP vs. Sham with a 1.16±0.04 fold change (p<0.05). A slight increase in CD55 mRNA was noted (1.21±0.09 fold change; p= 0.07) in RUPP vs. Sham kidney cortex. Our data suggest that upregulation of complement regulators may prevent increased C3 deposition in kidney. However, in placenta, down-regulation of regulators may contribute to the net increase in local complement activation observed in RUPP vs Sham. To determine if the changes in regulators paralleled a change in translated protein, Crry in placenta was also assessed by immunohistochemistry. As seen in Figure 3B, staining intensity for Crry was significantly less in placenta of RUPP compared to Sham animals, consistent with the down-regulation of message for Crry in placenta.
Figure 3.
Complement regulators Crry, CD55 and CD59 change in placenta and kidney cortex in RUPP compared to Sham animals. A. mRNA was isolated from placenta and kidney cortex and Delta-delta Ct method of relative quantification was used to determine fold change in mRNA expression compared to actin with the change in Sham animals defined as 1. Values represent the mean ± SE of fold change obtained from 4–7 animals for placenta and 7–9 animals for kidney. *p<0.05 vs Sham. B. Immunohistochemistry was conducted as described in Materials and Methods, and staining graded by a blinded observer from 0–3, negative to strongly positive. Representative images at 200X magnification are provided. Values for staining intensity represent the mean ± SE of scores obtained from placenta from 4–5 animals. *p<0.05 vs Sham
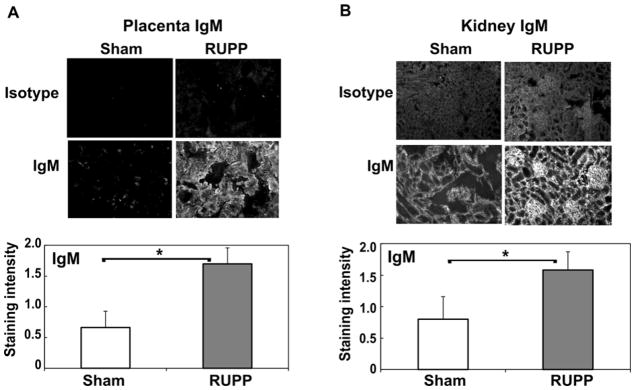
3.3 IgM deposition in kidney and placenta following placental ischemia
Complement activation can occur by multiple mechanisms with distinct stimuli initiating the cascade and amplification. Studies in ischemia reperfusion injury have implicated natural antibody in complement activation and pathology (Austen et al., 2004; Busche et al., 2009; Fleming et al., 2002b; Williams et al., 1999). Studies in preeclampsia have demonstrated increased immune deposits containing IgM in placenta of preeclamptic women compared to normal pregnancy as well as increased circulating IgM and IgG (Buurma et al., 2012; Kestlerova et al., 2012). Thus, we determined if IgM was evident in kidney and placenta of RUPP vs Sham animals. Increased IgM deposition was seen in placenta and kidney of RUPP vs Sham animals (Figure 4A–B) whereas increased C3 deposition was significantly increased only in the placenta (Figure 2). Circulating IgM did not significantly change in RUPP vs Sham animals (Sham, 339±44 ug/ml; RUPP, 338±20 ug/ml; n=10–12), nor did circulating IgG.
Figure 4.
IgM deposition is significantly increased in placenta (A) and kidney (B) in RUPP compared to Sham animals. Immunohistochemistry was conducted as described in Materials and Methods, and staining graded by a blinded observer from 0–3, negative to strongly positive. Representative images at 200X magnification are provided. Values for staining intensity represent the mean ± SE of scores obtained from placenta and kidney from 9–11 animals. *p<0.05 vs Sham
3.4 Losartan significantly inhibits the RUPP induced increase in mean arterial pressure without inhibiting complement activation
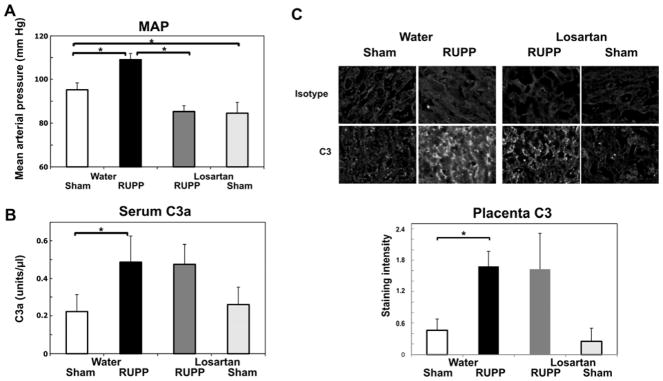
AT1-AA have been implicated in the pathophysiology in preeclampsia as well as in the RUPP model of placental ischemia induced hypertension (Harmon et al., 2016; LaMarca et al., 2011). As seen in Figure 5A, RUPP surgery significantly increased mean arterial pressure (MAP) and decreased fetal weight (data not shown). Importantly, treatment with losartan significantly inhibited the RUPP induced increase in blood pressure (Figure 5A) and did not affect the decreased fetal weight. Also consistent with studies of others (Alexander et al., 2001b; LaMarca et al., 2008), losartan significantly reduced MAP in Sham animals as well. However, losartan did not significantly prevent the RUPP induced increase in complement activation as measured by C3a (Figure 5B). To verify that losartan itself did not affect the ability of an antigen antibody complex to activate the complement cascade, the presence of losartan at 80 ug/ml was assessed in the total hemolytic complement assay. Losartan did not significantly alter the CH50 when present in the buffer during the incubation when hemolysis is occurring (data not shown). Most importantly, losartan did not inhibit C3 deposition in the placenta (Figure 5C) indicating that local complement activation was not due to AT1-AA interaction with the receptor. In addition, IgM deposition was not changed by losartan treatment in RUPP vs Sham animals (data not shown).
Figure 5.
Losartan significantly inhibits placental ischemia induced increase in mean arterial pressure (MAP) without affecting increased circulating C3a or placental C3 deposition. Values represent the mean ± SE on gestation day 19.5. *p<0.05 for the indicated comparisons. A. The increase in MAP in RUPP Water (n=10) compared to Sham Water (n=7) was significantly inhibited by administration of 0.3 mg/ml losartan in drinking water (RUPP Losartan, n=9). MAP was also significantly inhibited comparing Sham Losartan (n=7) to Sham Water. B. The increase in serum C3a in RUPP water (n=10) compared to Sham water rats (n=7) was not inhibited by administration of 0.3 mg/ml losartan in the drinking water (RUPP losartan, n=9). Losartan did not affect C3a in Sham losartan rats (n=7) compared to Sham water. Units of C3a are relative to a standard pool of yeast activated rat serum as described in Materials and Methods. C. The increase in placental C3 in RUPP water (n=11) compared to Sham water rats (n=13) was not inhibited by administration of 0.3 mg/ml losartan in the drinking water (RUPP losartan, n=4). Losartan did not affect C3 deposition in Sham losartan rats (n=4) compared to Sham water. Representative images at 200X magnification are provided.
Immunohistochemistry was conducted as described in Materials and Methods, and staining graded by a blinded observer from 0–3, negative to strongly positive.
The dose of losartan in drinking water was estimated by calculating the average ml consumed/day. The highest body weight from GD13.5 to necropsy was used to determine the lowest estimated mg/kg delivered. In the losartan experiment, sham and RUPP animals consumed similar amounts of water (135.8±6.8 vs 132.4±7.4ml/kg/day water; Sham to RUPP) and consumption was not significantly affected by losartan. The estimated losartan dose based on the greatest weight of the animal over the time period of exposure was 40.8±3.5 mg/kg/day and 48.6±2.1 mg/kg/day in RUPP and Sham animals, respectively. Thus, exposure to losartan in drinking water at 0.3 mg/ml delivered more than sufficient dose to achieve at least 30 mg/kg/day reported as effective in RUPP by LaMarca et al (LaMarca et al., 2008).
Discussion
A pathophysiological role for complement activation in preeclampsia is evidenced by enhanced complement activation in preeclamptic pregnancies compared to normal pregnancy, detected both before and after development of preeclamptic symptoms (Derzsy et al., 2010; Lynch et al., 2008; Lynch et al., 2011). In addition, hypertension resulting from placental ischemia in the rat is attenuated following inhibition of complement activation with a demonstrated role for complement activation products C3a and C5a (Lillegard et al., 2013; Lillegard et al., 2014). More importantly and consistent with these findings is the fact that anti-C5 antibody eculizumab was reported to extend a preeclamptic pregnancy by more than two weeks (Burwick and Feinberg, 2013). In addition, eculizumab treatment in individuals with disorders in complement regulation such as atypical hemolytic uremic syndrome (Tsai and Kuo, 2016) or paroxysmal nocturnal hemoglobinuria (Kelly et al., 2010) reduces the risk of adverse pregnancy outcomes.
Both the events that initiate increased complement activation in preeclampsia as well as the important pathway(s) of activation are unknown. Increased activation could be due to insufficient regulation as well as by increased activation. In this study, we considered possible mechanisms by which antigens combining with self-reactive antibodies could increase complement activation following placental ischemia. Given the general concept that neoepitopes are exposed following ischemia, we considered that autoreactive IgM antibodies reacted with the as yet unidentified neoepitopes resulting in local IgM and C3 deposition. In addition, we considered a specific self-reactive antibody that has been identified in the RUPP model, the AT1-AA, as potentially responsible for complement activation after interacting with the AT1 receptor. Considering IgM deposition as a stimulus for complement activation, our studies clearly demonstrated IgM deposited in kidney and placenta following placental ischemia along with local C3 deposition in the placenta. Our studies do not provide any insight on the important neoepitope(s) potentially responsible for increased IgM deposition in the placenta and/or kidney following placental ischemia. However, studies of others in ischemia reperfusion injury provide the basis for continued studies to consider the importance of such molecules as non-muscle myosin, β2 glycoprotein or annexin IV as potential sites of IgM binding following placental ischemia (Fleming, 2012; Kulik et al., 2009; Zhang et al., 2006).
Following up on this observed complement activation in placenta but not kidney, we noted that message for complement regulators CD55 and CD59 increased in kidney but not placenta following ischemia, consistent with control of C3 deposition in kidney, but not placenta. In fact, message for regulators Crry and CD59 decreased in placenta, consistent with increased C3 deposition in placenta. We demonstrated using immunohistochemistry that Crry decreased in placenta of RUPP vs Sham, consistent with the decrease in mRNA for Crry in placenta. Future studies with additional tissue will more extensively characterize expression of complement regulators by immunohistochemistry.
Our studies do not directly address the pathway(s) of complement activation involved in generation of C3a/C5a leading to hypertension. The fact that IgM deposition is increased suggests that classical pathway activation may be involved. However, there is also evidence that as a glycosylated molecule IgM may lead to MBL binding and involvement of the lectin pathway (Arnold et al., 2005; McMullen et al., 2006). The fact that circulating sC5b-9 and C5b-9 placental deposition does not increase in RUPP vs Sham suggests that widespread systemic activation through C9 does not occur. Since C3a and/or C5a receptor antagonists attenuate placental ischemia induced hypertension similarly to sCR1, the terminal complement complex is not likely critical for placental ischemia induced hypertension. Our future studies will address the important pathway(s) involved in complement activation to determine the therapeutic intervention most likely to attenuate the placental ischemia induced hypertension
Since the seminal publication by Wallakut et al (Wallukat et al., 1999) describing the IgG3 antibody with agonist activity for the angiotensin type 1 receptor (AT1), multiple studies have demonstrated an increase in autoantibodies to the angiotensin receptor (AT1-AA) in preeclampsia (LaMarca et al., 2011). Studies in the RUPP model in the rat have provided evidence that these autoantibodies are important for development of the hypertension following placental ischemia (LaMarca et al., 2009; LaMarca et al., 2011; LaMarca et al., 2008). Additional studies have demonstrated that administration of AT1-AA mimics symptoms of preeclampsia in a mouse model (Zhou et al., 2008) associated with deposition of complement in kidney and placenta (Wang et al., 2012). These studies demonstrated that both the blood pressure increase and the complement deposition were prevented by losartan providing evidence that complement activation occurs in conjunction with symptoms of preeclampsia when autoantibody is the inciting stimuli. Using a C3a receptor antagonist Wang et al (Wang et al., 2012) also provided evidence for complement activation playing an important role in the preeclamptic symptoms. In our studies using the RUPP model and the more clinically relevant stimuli of placental ischemia, losartan prevented the increase in blood pressure as previously reported (LaMarca et al., 2008), but did not significantly change the increase in C3a or the C3 deposition in the placenta. These data indicate that complement activation following placental ischemia is not the result of AT1-AA interaction with the AT1 receptor. Local complement activation following placental ischemia is associated with IgM deposition so future investigations will focus on determining if IgM deposition or production of natural IgM is critical for placental ischemia induced hypertension.
There is certainly strong evidence of excessive complement activation in circulation during preeclamptic pregnancies compared to normal pregnancies with decreased C3, increased C3a/C3 ratio, increased C5a and sC5b-9 concentrations (Derzsy et al., 2010; Soto et al., 2010) and increased complement activation products also found in urine (Burwick et al., 2013). Also, 8–18% of women with preeclampsia have mutations in complement regulatory proteins (Salmon et al., 2011). Using immunohistochemistry, Lokki et al reported no differences in complement regulators in human placenta of preeclamptic pregnancies compared to normal pregnancy. The study by Buurma et al reported increased mRNA for complement regulators CD55 and CD59 but protein expression was not determined. Both of these studies also demonstrated increased complement activation in the placenta. Presumably, the increased complement activation results in up-regulation of complement regulators to limit the potential of complement mediated damage in mother and fetus.
Complement regulators in the rat and mouse differ from the human (Lesher and Song, 2010). In contrast to human where CD46 is expressed on multiple cell types, in adult rat, CD46 is limited to expression in spermatozoa (Mizuno et al., 2004). In addition to CD55 and CD59, rat and mouse utilize Crry, a membrane bound regulator with both decay accelerating and cofactor activity against C3 convertase. Crry is expressed in the placenta and in endothelium, mesangium and tubules in the kidney. CD55 in the rodent kidney is expressed in podocytes and endothelial cells (Lesher and Song, 2010).
Our previous studies indicated that antagonism of the C3a or C5a receptor attenuated hypertension following placental ischemia, similarly to the attenuation seen with sCR1 administration to inhibit complement activation (Lillegard et al., 2013; Lillegard et al., 2014). These data suggested that the terminal complement complex was not important for the increase in high blood pressure. In the circulation, our previous studies demonstrated an increase in C3a following placental ischemia (Lillegard et al., 2013), and our present study also indicates an increase in C5a, but no increase in the formation of the terminal complement complex sC5b-9. These data suggest a feedback mechanism is present that adequately limits circulating C5b-9 formation but is insufficient to limit excessive C3a and/or C5a production. Endogenous regulators are sufficient to prevent widespread activation of complement with sC5b-9 production, but activation through C5 is not sufficiently limited following placental ischemia.
In summary, complement activation clearly occurs following placental ischemia and contributes to hypertension (Lillegard et al., 2013; Lillegard et al., 2014). Endogenous regulators control complement activation in the kidney, but not placenta. Our studies aimed at determining the mechanism of complement activation have ruled out autoantibodies to the angiotensin II Type 1 receptor as important and provide new evidence that complement activation is associated with IgM deposition. Thus, continuing studies will be focused on determining if neoepitopes and IgM interaction are critical for complement activation and the resultant hypertension. Gaining a better understanding of the mechanisms of complement activation in preeclampsia and gestational hypertension will enable the pursuit of specific immune therapeutics for improving maternal and fetal outcomes in many complicated pregnancies.
HIGHLIGHTS.
Placental ischemia activates complement locally in placenta but not kidney
Placental complement activation is consistent with decreased complement regulators
Autoantibody to angiotensin II Type1 receptor does not activate complement
IgM deposition suggests a role for natural antibody in complement activation
Acknowledgments
The authors acknowledge the excellent technical assistance of Barbara Elmquist, Jon Opacich, and Jacob Wilcox. This work was supported by NIH R15 HL109843 (JFR, JSG, SDF).
Abbreviations
- AT1
Angiotensin II type 1 receptor
- AT1-AA
agonistic IgG autoantibodies to angiotensin II Type 1 receptor
- GD
gestation day
- MAP
mean arterial pressure
- qRT-PCR
quantitative real-time polymerase chain reaction
- RUPP
reduced utero-placental perfusion pressure
- Crry
Complement receptor 1-related gene/protein y
Footnotes
Disclosures or Conflict of Interest
The authors have no conflicts to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jean F Regal, Email: jregal@d.umn.edu.
Megan E Strehlke, Email: streh036@d.umn.edu.
Jenna M Peterson, Email: jenna-peterson-1@uiowa.edu.
Cameron R Wing, Email: wing0155@umn.edu.
Jordan E Parker, Email: j.elizabeth.parker@gmail.com.
Noel Fernando Nieto, Email: nieto@ksu.edu.
Lynne T Bemis, Email: ltbemis@d.umn.edu.
Jeffrey S Gilbert, Email: jgilbert@d.umn.edu.
Sherry D Fleming, Email: sdflemin@ksu.edu.
References
- ACOG. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001a;37:1191–5. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001b;37:485–9. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Suter DM, Radcliffe CM, Harvey DJ, Dwek RA, Rudd PM, Sim RB. Human serum IgM glycosylation: identification of glycoforms that can bind to mannan-binding lectin. J Biol Chem. 2005;280:29080–7. doi: 10.1074/jbc.M504528200. [DOI] [PubMed] [Google Scholar]
- Austen WG, Jr, Zhang M, Chan R, Friend D, Hechtman HB, Carroll MC, Moore FD., Jr Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery. 2004;136:401–6. doi: 10.1016/j.surg.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Bauer AJ, Banek CT, Needham K, Gillham H, Capoccia S, Regal JF, Gilbert JS. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension. 2013;61:1103–10. doi: 10.1161/HYPERTENSIONAHA.111.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwick RM, Easter SR, Dawood HY, Yamamoto HS, Fichorova RN, Feinberg BB. Complement activation and kidney injury molecule-1-associated proximal tubule injury in severe preeclampsia. Hypertension. 2014;64:833–8. doi: 10.1161/HYPERTENSIONAHA.114.03456. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34:201–3. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. Urinary excretion of c5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. 2013;62:1040–5. doi: 10.1161/HYPERTENSIONAHA.113.01420. [DOI] [PubMed] [Google Scholar]
- Busche MN, Pavlov V, Takahashi K, Stahl GL. Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am J Physiol Heart Circ Physiol. 2009;297:H1853–9. doi: 10.1152/ajpheart.00049.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ. Preeclampsia is characterized by placental complement dysregulation. Hypertension. 2012;60:1332–7. doi: 10.1161/HYPERTENSIONAHA.112.194324. [DOI] [PubMed] [Google Scholar]
- Derzsy Z, Prohaszka Z, Rigo J, Jr, Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–6. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Fleming SD. Naturally occurring autoantibodies mediate ischemia/reperfusion-induced tissue injury. Adv Exp Med Biol. 2012;750:174–85. doi: 10.1007/978-1-4614-3461-0_13. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Shea-Donohue T, Guthridge JM, Kulik L, Waldschmidt TJ, Gipson MG, Tsokos GC, Holers VM. Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J Immunol. 2002a;169:2126–33. doi: 10.4049/jimmunol.169.4.2126. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Starnes BW, Kiang JG, Stojadinovic A, Tsokos GC, Shea-Donohue T. Heat stress protection against mesenteric I/R-induced alterations in intestinal mucosa in rats. J Appl Physiol (1985) 2002b;92:2600–7. doi: 10.1152/japplphysiol.01008.2001. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Dukes M, LaMarca B, Cockrell K, Babcock S, Granger J. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in pregnant rats. Am J Hypertens. 2007a;20:686–91. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007b;50:1142–7. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension. 2010;55:380–5. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nature medicine. 2004;10:1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–92. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Jr, Wallace K, LaMarca B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 2016;130:409–19. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Arnold L, Richards S, Hill A, Bomken C, Hanley J, Loughney A, Beauchamp J, Khursigara G, Rother RP, Chalmers E, Fyfe A, Fitzsimons E, Nakamura R, Gaya A, Risitano AM, Schubert J, Norfolk D, Simpson N, Hillmen P. The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. British journal of haematology. 2010;149:446–50. doi: 10.1111/j.1365-2141.2010.08099.x. [DOI] [PubMed] [Google Scholar]
- Kestlerova A, Feyereisl J, Frisova V, Mechurova A, Sula K, Zima T, Belacek J, Madar J. Immunological and biochemical markers in preeclampsia. Journal of reproductive immunology. 2012;96:90–4. doi: 10.1016/j.jri.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kotimaa J, van der Pol P, Leijtens S, Klar-Mohammad N, Schilders G, Daha MR, Rutjes H, van Kooten C. Functional assessment of rat complement pathway activities and quantification of soluble C5b-9 in an experimental model of renal ischemia/reperfusion injury. J Immunol Methods. 2014;412:14–23. doi: 10.1016/j.jim.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GC, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. Journal of Immunology. 2009;182:5363–73. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology (Bethesda) 2013;28:225–33. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–9. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Wallace K, Granger J. Role of angiotensin II type I receptor agonistic autoantibodies (AT1-AA) in preeclampsia. Curr Opin Pharmacol. 2011;11:175–9. doi: 10.1016/j.coph.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–72. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CP, Regal RR, Regal JF. Trimellitic anhydride-induced allergic response in the guinea pig lung involves antibody-dependent and -independent complement system activation. J Pharmacol Exp Ther. 2001;296:284–92. [PubMed] [Google Scholar]
- Lesher AM, Song WC. Review: Complement and its regulatory proteins in kidney diseases. Nephrology (Carlton) 2010;15:663–75. doi: 10.1111/j.1440-1797.2010.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol. 2013;56:91–7. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard KE, Loeks-Johnson AC, Opacich JW, Peterson JM, Bauer AJ, Elmquist BJ, Regal RR, Gilbert JS, Regal JF. Differential effects of complement activation products c3a and c5a on cardiovascular function in hypertensive pregnant rats. J Pharmacol Exp Ther. 2014;351:344–51. doi: 10.1124/jpet.114.218123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokki AI, Heikkinen-Eloranta J, Jarva H, Saisto T, Lokki ML, Laivuori H, Meri S. Complement activation and regulation in preeclamptic placenta. Frontiers in immunology. 2014;5 doi: 10.3389/fimmu.2014.00312. Article 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354, e1–8. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–66. doi: 10.1016/j.imbio.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Harris CL, Johnson PM, Morgan BP. Rat membrane cofactor protein (MCP; CD46) is expressed only in the acrosome of developing and mature spermatozoa and mediates binding to immobilized activated C3. Biology of reproduction. 2004;71:1374–83. doi: 10.1095/biolreprod.104.030114. [DOI] [PubMed] [Google Scholar]
- Penning M, Chua JS, van Kooten C, Zandbergen M, Buurma A, Schutte J, Bruijn JA, Khankin EV, Bloemenkamp K, Karumanchi SA, Baelde H. Classical Complement Pathway Activation in the Kidneys of Women With Preeclampsia. Hypertension. 2015;66:117–25. doi: 10.1161/HYPERTENSIONAHA.115.05484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal JF, Gilbert JS, Burwick RM. The complement system and adverse pregnancy outcomes. Mol Immunol. 2015a;67:56–70. doi: 10.1016/j.molimm.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal JF, Lillegard KE, Bauer AJ, Elmquist BJ, Loeks-Johnson AC, Gilbert JS. Neutrophil Depletion Attenuates Placental Ischemia-Induced Hypertension in the Rat. PloS one. 2015b;10:e0132063. doi: 10.1371/journal.pone.0132063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS medicine. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle J, Mockel M, Gwosc S, Datwyler SA, Qadri F, Albert GI, Holert F, Isbruch A, Klug L, Muller DN, Dechend R, Muller R, Vollert JO, Slagman A, Mueller C, Herse F. Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro--brief report. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2972–4. doi: 10.1161/ATVBAHA.111.237784. [DOI] [PubMed] [Google Scholar]
- Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, Kim YM, Hong JS, Goncalves LF, Yeo L, Mazor M, Hassan SS, Kusanovic JP. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23:646–57. doi: 10.3109/14767050903301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HM, Kuo E. From Gestational Hypertension and Preeclampsia to Atypical Hemolytic Uremic Syndrome. Obstet Gynecol. 2016 doi: 10.1097/AOG.0000000000001340. [DOI] [PubMed] [Google Scholar]
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, Kellems RE, Xia Y. Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension. 2012;60:712–21. doi: 10.1161/HYPERTENSIONAHA.112.191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissgerber TL, McConico A, Knudsen BE, Butters KA, Hayman SR, White WM, Milic N, Miller VM, Garovic VD. Methodological differences account for inconsistencies in reported free VEGF concentrations in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2014;306:R796–803. doi: 10.1152/ajpregu.00544.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, Carroll MC, Hechtman HB. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86:938–42. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- Zhang M, Michael LH, Grosjean SA, Kelly RA, Carroll MC, Entman ML. The role of natural IgM in myocardial ischemia-reperfusion injury. Journal of Molecular and Cellular Cardiology. 2006:62–67. doi: 10.1016/j.yjmcc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nature medicine. 2008;14:855–62. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]