Abstract
MicroRNAs are short non-coding RNAs that provide global regulation of gene expression at the post-transcriptional level. Such regulation has been found to play a role in stress-induced epigenetic responses in the brain. The norepinephrine transporter (NET) and glucocorticoid receptors are closely related to the homeostatic integration and regulation after stress. Our previous studies demonstrated that NET mRNA and protein levels in rats are regulated by chronic stress and by administration of corticosterone, which is mediated through glucocorticoid receptors. Whether miRNAs are intermediaries in the regulation of these proteins remains to be elucidated. The present study was undertaken to determine possible regulatory effects of miRNAs on the expression of NET and glucocorticoid receptors in the noradrenergic neuronal cell line. Using computational target prediction, we identified several candidate miRNAs potentially targeting NET and glucocorticoid receptors. Western blot results showed that overexpression of miR-181a and miR-29b significantly repressed protein levels of NET, which is accompanied by a reduced [3H] NE uptake, and glucocorticoid receptors in PC12 cells. Luciferase reporter assays verified that both miR-181a and miR-29b bind the 3’UTR of mRNA of NET and glucocorticoid receptors. Furthermore, exposure of PC12 cells to corticosterone markedly reduced the endogenous levels of miR-29b, which was not reversed by the application of glucocorticoid receptor antagonist mifepristone. These observations indicate that miR-181a and miR-29b can function as the negative regulators of NET and glucocorticoid receptor translation in vitro. This regulatory effect may be related to stress-induced upregulation of the noradrenergic phenotype, a phenomenon observed in stress models and depressive patients.
Keywords: microRNA, stress, norepinephrine transporter, glucocorticoid receptor, gene regulation, PC12 cells
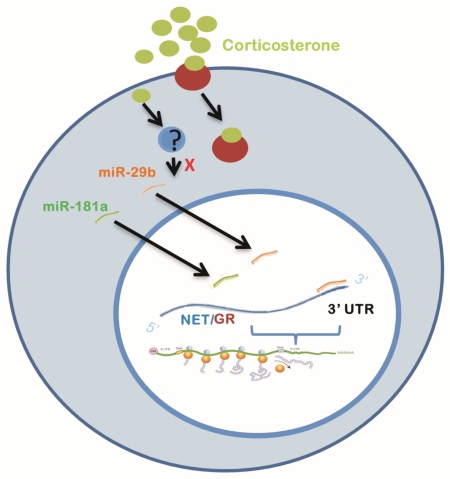
Graphical abstract
This study demonstrated that miR-29b and miR-181a, two short-non-coding RNAs that provide global regulation of gene expression, markedly repressed protein levels of norepinephrine (NE) transporter and glucocorticoid receptor (GR), as well as NE uptake by binding the 3′UTR of their mRNAs in PC12 cells. Also, exposure of cells to corticosterone significantly reduced miR-29b levels through a GR-independent way.
Introduction
MicroRNAs (miRNAs) are generally around 22-nucleotide-long noncoding RNAs that play an important role in gene-regulation (Makeyev & Maniatis 2008). They function by binding to complementary sequences on 3’ UTR regions of target mRNA molecules and to repress translation and silence gene expression (Yelamanchili et al. 2010). By this post translational regulation, miRNAs are involved in a plethora of processes, such as cell proliferation (Brennecke et al. 2003), apoptosis, differentiation, development (He & Hannon 2004), and basic cellular pathways (Mendell 2005). The human genome contains more than 500 miRNAs and each individual miRNA can usually interact with a few hundred of target mRNAs (Schratt 2009, Broderick & Zamore 2011). It is well documented that around 70% of miRNAs are expressed in brain, involved in many crucial processes in central nervous system and play crucial role in dendritic spine formation, neurite growth (Esteller 2011), neuronal development, neuronal differentiation (Yelamanchili et al. 2010), neuronal plasticity, apoptosis (Kosik 2006), dendritic arborization, and synapse formation and maturation (Schratt 2009). While it has been well known that miRNAs are essential to maintain the correct functioning mechanism in the nervous system (Esteller 2011), their dysregulation has been associated with several neurodegenerative disorders including Parkinson’s disease, Alzheimer’s diseases, Huntington disease (Saugstad 2010, Yelamanchili et al. 2010, Broderick & Zamore 2011, Esteller 2011), and a variety of psychiatric diseases such as major depression, schizophrenia and mood disorders (Beveridge et al. 2010, Dwivedi 2011b, O’Connor et al. 2012, Hansen & Obrietan 2013). Thus far, their involvement in these diseases has made it important to investigate them as the regulatory and therapeutic molecules (Wahid et al. 2010, Dwivedi 2011a). However, such kinds of studies are only beginning to emerge, in which miRNAs’ involvement in the regulation of the noradrenergic phenotypes is almost absent.
The norepinephrine (NE) transporter (NET), is a member of the Na/Cl-dependent monoamine transporter family and selectively located on the presynaptic terminals of noradrenergic neurons (Dziedzicka-Wasylewska et al. 2006). It plays a crucial role in maintaining the presynaptic and postsynaptic NE homeostasis by re-uptaking more than 90% of released NE back to presynaptic terminals (Axelrod & Kopin 1969, Amara & Kuhar 1993). Therefore, changes in NET expression would significantly influence NE levels in the synapses which will in turn highly affect noradrenergic transmission. Also, the NET is one of the main transporters serving as a target of antidepressant drugs by enhancing noradrenergic transmission (Dziedzicka-Wasylewska et al. 2006). Given NET is one of the key proteins to regulate noradrenergic transmission and its involvement in the action of certain antidepressants, abnormal NET expression and function could contribute to the development and treatment of depression. For example, NET knockout mice display significantly less depressive-like behaviors than wild type controls (Xu et al. 2000), and are more aggressive in early phases of stress and demonstrate inhibition of depressive-like behavior in chronic stress models (Haller et al. 2002). These findings suggest that depressive behavior requires functional NET (Haller et al. 2002, Haenisch et al. 2009). Hence, the regulation of NET seems to be important for the etiological exploration and therapeutic strategy of major depression. However, such regulatory studies are limited. On the other hand, as the important mediators of glucocorticoids released during stress, corticosteroid receptors play a critical role in axis and in mediating brain functions. As such, an impaired corticosteroid receptor signaling has been hypothesized as a key mechanism in the pathogenesis of depression (Holsboer 2000). Of the two types of corticosteroid receptors, is only modestly occupied during normal physiological conditions and needs higher glucocorticoid concentrations to be fully activated in the state of stress. For this reason, the GR is considered to be important in depression (De Kloet et al. 1998). Regulations of GR expression, nuclear translocation, and GR-mediated gene transcription have become an important line of research regarding the molecular mechanisms underlying development and therapy of depression.
Our previous study demonstrated that chronic social defeat significantly increased expression levels of NET in the rat brain (Chen et al. 2012). This observation is consistent with in vitro study, in which stress-relevant doses of corticosterone and corticotropin-releasing factor significantly increased mRNA and protein levels of NET, as well as the uptake of [3H]NE in SK-N-BE(2)C cells and PC12 cells (Sun et al. 2010). Interestingly, both stress- and corticosterone-induced NET upregulations are mediated through corticosteroid receptors (Sun et al. 2010, Chen et al. 2012). However, the results for the regulatory effects of stress on GR expression in the brain have been controversial. Our previous experiments showed that chronic stress increased GR expression in the locus coeruleus and raphe nucleus (unpublished data), a finding consistent with one previous report (Li et al. 2011), and exposure of cells to dexamethasone markedly increased total GR and nuclear GR levels (Zha et al. 2011). On the contrary, other laboratories reported no signifcant alteration of, or a reduced GR expression in the locus coeruleus (LC) after single-prolonged stress (Sabban et al. 2015), chronic stress (Makino et al. 2002) or chronic exposure to corticosterone (Wang et al. 2015), indicating stress-induced GR alterations in the LC are complex, though GR levels are very pronounced in the noradreenrgic cells of the LC (Sabban et al. 2015). Clearly, more investigations are required. Nevertheless, it is possible that there is a functional linkage between noradrenergic phenotypes and GR during chronic stress, as noradrenergic neurons in the LC can be a target of glucocorticoids through GR (Markey et al. 1982, Wang et al. 1998). Given that chronic stress precipitates depression (Hammen 1991) and dysregulation of the noradrenergic phenotypes and GR are related to the development of depression, clarification of the molecular mechanisms underlying their regulation is an important step to be pursued, facilitating the search for effective therapies for people with major depression and other stress-related diseases. In the present study, using a combination of in silico prediction of miRNAs binding sites, miRNAs overexpression, constructs of the 3’UTR of NET and GR genes, as well as endogenous expression investigation of miRNAs, we identified miR-181a and miR-29b act as the regulator of NET and GR.
Materials and Methods
Cell culture, miRNA transfection and drug exposure
PC12 cells (ATCC, CRL-1721, Manassas, VA, USA) were maintained in RPMI 1640 medium supplemented with 5% fetal calf serum, 10% horse serum, penicillin (final concentration, 25 U/ml) and streptomycin (final concentration 25 µg/ml, ), at 37°C in humidified air containing 5% CO2. Culture medium and supplements were obtained from the Life Technologies Corporation, ThermoFisher Scientific (Grand Island, NY). The subcultured cells, when they reached to 70-85% confluence, were transfected with miRNA mimics, mimic control, or negative control (40nM, Life Technologies, Carlsbad, CA) by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s protocol. Then the cells were incubated with the transfection complexes under normal growth conditions. Forty-eight hours after transfection, cells were harvested after washing twice with fresh, ice-cold phosphate buffer solution (PBS) and immediately lysed for isolation of RNA or protein measurements. In the separate experiments, PC12 cells were treated with 100 nM corticosterone, or 100 nM corticosterone plus 3 µM mifepristone, for 7 days. miRNAs were measured by real-time PCR after harvest. All experiments were conducted in compliance with the ARRIVE guidelines.
Construct of luciferase reporter gene plasmid and luciferase reporter assays
To confirm that the validated miRNAs target the 3’-UTR of NET, the 3’-UTR segment of NET gene was amplified by PCR from PC12 genomic DNA and cloned to the downstream of red firefly gene of pmirGlo vector. The NET 3’-UTR-bearing pmirGlo and validated miRNAs were co-transfected into PC12 cells with Lipofactamine 2000. At 48 h after transfection, cells were lysed, and luciferase reporter activity was assayed. Briefly, after washing three times with PBS, cells were lysed in 100 µl of 100 mM KPO4 buffer (pH 7.8), which contained 1 mM dithiothreitol and 0.5% (vol/vol) Triton X-100. Activity assays were performed by measurement of luminescence in a Biocounter M2500 luminometer (Lumac, Landgraaf, The Netherlands) for 20 sec immediately after addition of luciferin assay buffer (100 mM Tris acetate (pH 7.8), 10 mM MgOAc, 100 mM EDTA, 2 mM ATP (pH 7.0) and 74 µM luciferin). Luciferase activities were normalized to β-gal activity to correct for variations in transfection efficiencies. Luciferase signals of red firefly and green Renilla were measured by GloMax Microplate Multimode Reader.
Detection of NET protein levels
Protein levels of NET and GR were measured by western blotting as reported before (Sun et al. 2010). Briefly, after twice washing with ice-cold PBS, cells were lysed with lysis buffer (50 mM Tris pH7.6, 150 mM NaCl, 2 mM EDTA, 1% NP40 and SigmaFast Protease Inhibitors) and collected. The total protein concentration in the supernatant of cell preparations was measured with Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Fifty micrograms of protein from each sample were loaded onto 10% sodium lauryl sulfate-polyacrylamide gels, followed by electrophoresis. Then, proteins were transferred on Amershan Hybond-ECL membrane (GE Healthcare, NJ), which were blocked in 5% milk in Tris-buffered saline with Tween 20 (TBST) buffer (25 mM Tris, 140 mM NaCl, 3 mM KCl, pH 7.4; and 0.1% Tween 20 v/v was added before use) for 1 h at room temperature. These membranes were further incubated with primary antibodies (polyclonal antibodies against NET from rabbit, 1:330 dilution; Alpha Diagnostic Intl. Inc, San Antonio, Texas; polyclonal antibody against GR: 1:500 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA) for overnight at 4°C. On the next days, after 3 time washing with TBST buffer, these membranes were incubated with 2nd antibodies (horseradish peroxidase-conjugated anti-rabbit IgG, 1:3000; Amersham Biosciences, Little Chalfont, UK) at room temperature. Finally, membranes were treated with Enhanced Chemiluminescence Western Blotting Detection Reagents and exposed to Kodak BioMax MR Films (GE Healthcare, Marlborough, MA). The imaging software (Molecular Dynamics IQ solutions, Molecular Dynamics, Inc., Sunnyvale, CA) was used to quantify the density of bands. The β-actin as the loading control was measured by the same procedure after stripping of the membrane and incubating it with mouse anti-β-actin antibody. As usual, linear standard curve was generated from optical densities (ODs) of bands by a dilution series of total proteins prepared from cells. OD values of NET and GR signals were normalized by β-actin immunoreactivities to assess equal protein loading. Normalized values were then averaged for all replicated gels and used to calculate the relative changes of the same gel. Also, the specificity of antibodies used in these experiments was tested when each new lot of the antibody was ordered.
RNA isolation and Taqman-based quantitative real-time polymerase chain reaction (qPCR) analysis for miRNA
Total RNA was extracted using RNAzol reagent (Molecular Research Center, Inc., Carlsbad, CA), and was converted into cDNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems/Life technologies, Forster City, CA) according to the manufacturer’s protocol. Real-time PCR was conducted using a Taqman® microRNA assay kit (Applied Biosystems/Life technologies, Grand Island, NY). The 20µl PCR included 1 µl Taqman MicroRNA assay (20X), 1.33µl RT product, 10µl Taqman 2× Universal PCR Master mix, No AmpErase UNGa, and 7.67µl Nuclease-free water. The reactions were incubated in a 96-well plate at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The expression of miR-181a/miR-29b was normalized using U6 as the internal control. Predesigned primer/probes (Applied Biosystems) for miRNAs and rat U6 were purchased from Applied Biosystems. Measurements were normalized to U6 (ΔCt) and comparisons calculated as the inverse log of ΔΔCT to give the relative fold change in respective miRNAs/U6 snRNA levels (Livak & Schmittgen 2001). All reactions were run in triplicate, each using separate sets of samples.
Uptake of [3H]NE
[3H]NE uptake in PC12 cells was carried out using the similar procedure as described previously (Zhu & Ordway 1997). Briefly, PC12 cells treated with miRNAs 48 hours ago were washed twice with 4 ml Krebs-Ringer–HEPES buffer (KRH; in mM: NaCl 130, KCl 1.3, CaCl2 2.2, MgSO4 1.2, KH2PO4 1.2, HEPES 10, D-glucose 10, ascorbic acid 0.1 and pargyline 0.1; pH adjusted to 7.4 with HCl). Cells were then pre-incubated in 1.5 ml warm KRH buffer at 37°C for 5 min. Ninety nM [3H]NE (Perkin-Elmer Life Science, Boston, MA) were added and incubated for 5 min at 37°C to initiate uptake assays, which was removed by aspiration of incubation solutions followed by 2 rapid washes with ice-cold KRH buffer. Cells were then lysed with 1 ml 0.1% v/v Triton X-100 (in 5 mM Tris HCl, pH 7.4), and 0.5 ml of the lysate was used to measure the radioactivity in a liquid scintillation counter (Beckman LS3801, Irvine, CA, USA). The specific uptake of [3H] NE by the NET was defined as the difference between uptake in the absence of and in the presence of 100 µM desipramine. Protein concentrations in cell lysates were determined by the BCA Protein Assay.
Statistics
All data were from separate experiments. The number of replicates is showed in the figure legend. The paired Student’s t-test assay is used for the data which had only 2 groups in a comparison, such as those in Fig. 6. The analysis of variance (ANOVA) and post hoc Newman-Keuls tests were used for analysis of data when multiple treatment groups were compared. Differences between groups or treatments were recognized as statistically significant at p<0.05.
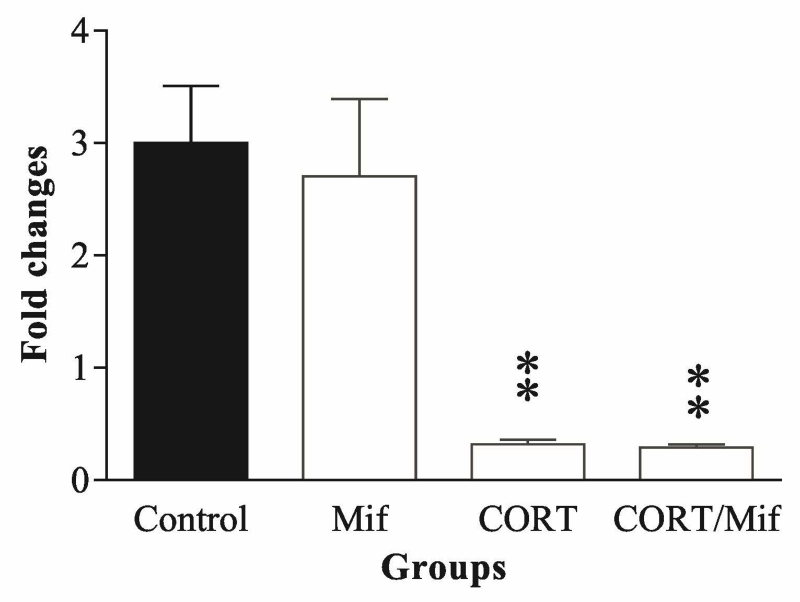
Fig. 6.
Effects of exposing PC12 cells to 100 nM corticosterone for 7 days (A), or corticosterone plus 5µM mifepristone for 7 days (B) on levels of miR-29b. The bars represent fold changes from 4 separate experiments. ** p<0.01, compared to the control. CORT: corticosterone, Mif: mifepristone.
Results
In silico prediction of candidate miRNAs targeting NET and GRs
Three leading miRNA target prediction algorithms: miRanda (http://microrna.sanger.ac.uk/sequences/) (John et al. 2004), PicTar (http://pictar.mdc-berlin.de/) (Krek et al. 2005), TargetScan (http://www.targetscan.org/) (Grimson et al. 2007), are used to identify miRNAs affecting NET and GR activities via binding to their 3’UTR site. To determine genes that were similarly identified by two or more of these algorithms, the online program Matchminer (http://discover.nci.nih.gov/matchminer/index.jsp) (Bussey et al. 2003) was also used. By these computational target prediction analyses, we identified several potential miRNAs that have complementarity to the 3’-untranslated region (UTR) of the mRNAs of NET (Table 1) and GRs (Table 2) for further experimental testing. İnterestingly, the UTRs of both NET and GR genes harbor the putative binding sites for miR-29, miR-181a, miR-9 and miR-124.
Table 1.
miRNAs are predicted to target mRNA transcript coding for NET.
| miRNA | Spices | Position on SLC6A2-3’UTR |
Sequential pairing |
Conservation (%) |
|---|---|---|---|---|
| miR-29b | Rno | 878-884 | AGCACCAUU | 100 |
| miR-181a | Rno | 712-718 | ACAUUCA | 100 |
| miR-9 | Rno | 121-128 | ACCAAAGA | 100 |
| miR-124 | Rno | 729-735 | AAGGCAC | 100 |
| miR- 146a | Rno | 725-731 | CCCUAGGGA | 100 |
| miR-377 | Rno | 1095-1101 | GAGGUUG | 100 |
Table 2.
miRNAs are predicted to target mRNA transcript coding for the glucocorticoid receptor.
| miRNA | Species | Position on Nr3c1-3’UTR |
Sequential pairing |
Conservation (%) |
|---|---|---|---|---|
| miR-29b | Rno | 1450-1457 | AGCACCAUU | 100 |
| miR-181a | Rno | 2177-2183 | ACAUUCA | 100 |
| miR-9 | Rno | 1304-1310 | UCGACAU | 100 |
| miR-124 | Rno | 20-26 | AAGGCAC | 100 |
| miR-129 | Rno | 1532-1539 | AGCCCUU | 100 |
| miR-384 | Rno | 436-442 | GUAAAC | 100 |
MiR-181a and miR-29b down-regulate proteins of NET and GRs in PC12 cells
MiRNAs show high ability to bind to the 3’UTR of mRNAs of target genes; thereby they repress their translation and ultimately lead to reduction of protein levels. Therefore, all mimics of the miRNAs targeting the 3’UTR of NET and GRs, as well as the mimic control and negative control were transfected into PC12 cells. Forty eight hours after transfection, cells were harvested. Protein levels of NET and GRs were assayed by western blotting. As shown in Figs. 1 and 2, the overexpression of miR-181a and miR-29b dramatically downregulated NET (F3, 19=4.75, p<0.05) and GR (F3, 19=4.36, p<0.05) protein levels, as compared to those of the mimic control and negative control. In contrast, transfection with respective miR-9, miR-124, miR-146a, miR-377, miR-129 or miR-383 did not reduce NET or glucocorticoid protein levels. These results indicate that both miR-181a and miR-29b are potentially relevant miRNAs to NET and GRs.
Fig. 1.
Overexpression of miR-29b and miR-181a reduced NET expression in PC12 cells. A: Western blot measurements show NET protein levels after transfection of cells with mimics of mR-181a and miR-29b. Each bar in the quantitative anlysis (the lower panel of A) represents data obtained from 5 separate experiments. B: Similar measurements show NET protein levels after transfection of cells with mimics of miR-9, miR-124, miR-146a and miR-377. Con: mimic control; N.con: negative control. * p<0.05, ** p<0.01, as compared to the corresponding mimic control.
Fig. 2.
Overexpression of miR-29b and miR-181a reduced GR expression in PC12 cells. A: Western blot measurements show GR protein levels after transfection of cells with mimics of mR-181a and miR-29b. Each bar in the quantitative anlysis (the lower panel of A) represents data obtained from 5 separate experiments. B: Similar measurements show GR protein levels after transfection of cells with mimics of miR-9, miR-124, miR-129 and miR-383. Con: control; N.con: negative control. * p<0.05, as compared to the control.
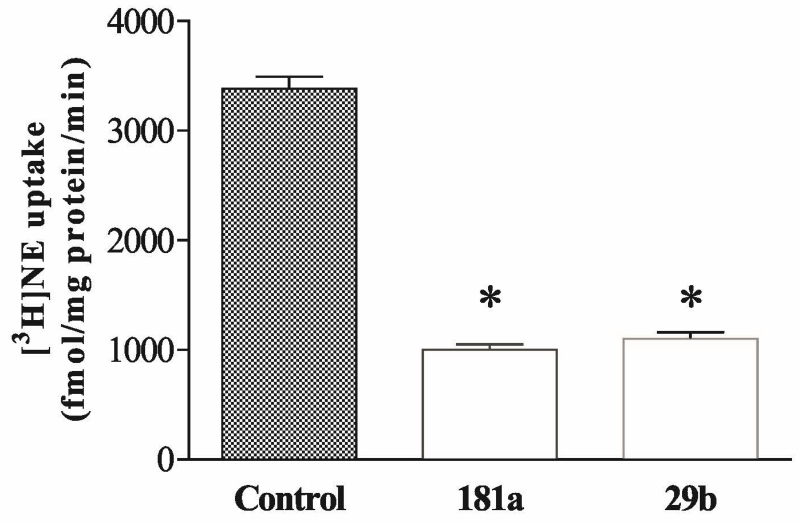
Overexpression of miRNAs 181a and 29b inhibited [3H] NE uptake in PC12 cells
The uptake of [3H] NE assay was performed to evaluate whether over-expression of miR-181a or miR-29b was associated with a change of the ability of cells to transport NE. PC12 cells were transfected with mimics of miR-181a or miR-29b for 48 hours. The uptake assays showed that both miRNAs significantly reduced the uptake of [3H] NE (F2,14=6.27, p<0.01; Figure 3).
Fig. 3.
Overexpression of miR-181a and miR-29b reduced [3H]NE uptake in PC12 cells. Cells were exposed to 100 nM corticosterone for 7 days. [3H]NE uptake assays were performed and each bar represents data obtained from 5 separate experiments. * p<0.01, compared to the control.
MiR-181a and miR-29b decreased the gene expression by direct binding to 3’UTR of the NET and GR
To prove that downregulation of NET and GR protein levels is indeed caused by a direct interaction of miR181a and miR-29b with the 3’UTR of NET and GR mRNA, a putative target sites on 3’UTR of NET was identified by the computational analysis. As illustrated from Fig. 4, the NET 3’UTR fragments containing putative miRNA binding sites for miR-181a were cloned to multiple cloning site at downstream of firefly luciferase gene that is under control of the cytomegalovirus (CMV) promoter in pmirGLO reporter plasmid. Cloning orientation was verified by diagnostic cuts followed by sequencing. PC12 cells were then co-transfected with 100 µM of either miR-181a or miR-29b, and 100µg/L pmirGLO-NET3’UTR plasmid, or empty pmirGLO (as the control). Forty-eight hours after transfection, luciferase reporter assays from cell preparations were performed and results revealed that both miR-181a and miR-29b significantly reduced luciferase activity (F2,14=3.98, p<0.05, Fig. 5A), indicating that NET is direct target of miR-181a and miR-29b. The same procedure was also used for GR gene and a similar result validated GR similarly is the direct target of miR-181a and miR-29b (Fig. 5B). These results verify that miR181a and miR-29b downregulated the gene expression of the NET and GR by binding to the 3’UTR of NET or GR mRNAs.
Fig. 4.
Schematic shows the process to the analysis of the miR-181a and miR-29b binding site in 3’UTR region of NET mRNAs and clone 3’UTR of NET into pmirGlo vector to measure the luciferase activity.
Fig. 5.
Confirmation of direct binding of miR-181a and miR-29b to 3’UTR region of NET (A) and GR (B) mRNAs by luciferase assay. The bars represent relative activity of luciferase normalized by renilla from 5 separate experiments. * p<0.05, compared to the control.
Corticosterone represses the expression levels of endogenous miR-29b
The investigation of the effect of corticosterone on expression of endogenous miR-181a and miR-29b was performed. PC12 cells were exposed to 100 nM corticosterone for 7 days, after which q-PCR from cell preparations showed that corticosterone treatment significantly reduced miR-29b in PC12 cells (Fig. 6A, p<0.01). In a separate experiment, PC12 cells were exposed to 100 nM corticosterone or 100 nM corticosterone plus 5 µM mifepristone (RU486), a GR antagonist (Cadepond et al. 1997) for 7 days. The measurement results showed that mifepristone neither affects the expression of miR-29b, nor influences corticosterone-induced repression of miR-29b expression (Fig. 6), indicating that alteration of endogenous miR-29b caused by corticosterone is GR-independent. However, although it was reported that miR-181a is expressed in PC 12 cells (Hamada et al. 2012, Liu et al. 2013), our preliminary study using real-time PCR could not prove it. Therefore, PC12 cells were transfected with miR181a mimic and then exposed to 100 nM corticosterone for 7 days. Real time PCR measurements showed that in this transfected PC12 cells corticosterone treatment did not cause any significant alteration in the extraneously introduced miR-181a level (data not shown).
Discussion
In the present study we used in silico analysis to predict several miRNAs that may commonly bind mRNAs of NET and GR, leading to reduction of their activities. Our experimental results showed that overexpression of either miR-181a or miR-29b significantly reduced protein levels of NET and GR in PC12 cells, and this downregulation of NET is accompanied by a functional decrease in NET activity as indicated by a reduction of [3H]NE uptaking. Further experiments verified that both miR-181a and miR-29b are able to bind the predicted seed region in the 3’UTR of NET and GR, and exposure of cells to corticosterone markedly repressed the expression of endogenous miR-29b, which was not blocked by application of GR antagonists. These findings reveal that the expression of NET and GR can be commonly regulated by miR-181a and miR-29b, and that endogenous miR-29b is also downregulated by stress hormones. This observation indicates that these two miRNAs may serve as the intermediaries in stress-induced upregulation of the noradrenergic phenotypes, and may play an important role in the development and treatments of depression (Goddard et al. 2010).
As a recently discovered class of noncoding RNA, miRNAs have been implicated in diverse biological process and may act as modulators of several brain processes. Among these miRNAs, miR-181 family is expressed in mature neurons across most vertebrates (Miska et al. 2004, Smith et al. 2010), especially with a high concentration in the brain (Chen et al. 2004, Saba et al. 2012b), indicating its potential regulatory roles in the central nervous system. Recent studies demonstrated that as a multifunctional miRNA, miR-181a has been found to target the Bcl-2 family (Ouyang et al. 2012a), a protein that can protect from cerebral ischemia (Lawrence et al. 1996); the heat shock protein GRP78 (Ouyang et al. 2012b), transcription factor CREB1 (Liu et al. 2013), and glutamate receptor 2 subunit (GluA2) (Saba et al. 2012a). As such, it is potentially involved in neuronal protection against cerebral ischemia (Ouyang et al. 2012b, Ouyang et al. 2012a), drug addiction-related synaptic changes in the hippocampus (Saba et al. 2012b), the memory performance associated with epilepsy (Liu et al. 2015), and in Parkinson’s disease (Ding et al. 2016). On the other hand, miR-29 family is also involved in translational repression of a wide range of target genes. It has been reported that members of the miR-29 family behave as epi-miRNAs by down-regulating important drivers of the epigenetic machinery, and many studies found that one function of miR-29 family is related to the cancer epigenetic (Amodio et al. 2015). However, increased expression of miR-29b was found in the brain of aged mice (Fenn et al. 2013), and its expression was significantly down-regulated in the blood serum of Alzheimer’s disease patients (Geekiyanage et al. 2012) and after stroke (Khanna et al. 2013). miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis (Kole et al. 2011) and regulates ethanol-induced neuronal apoptosis in the developing cerebellum (Qi et al. 2014). Our present study indicates that both miR-181a and miR-29b repress protein levels of NET and GR by directly targeting 3’UTR of these two genes. Furthermore, exposure of cells to corticosterone reduced the endogenous levels of miR-29b. Based on our knowledge, this is the first study reporting the involvement of both miRNAs in the regulation of NET and GR, which adds a new evidence for the notion that miRNAs are important regulators for neuronal homeostasis.
Glucocorticoids are the prominent mediators of cellular stress on neuronal function and behavior, and are also known to structurally alter brain cytoarchitecture (Hunsberger et al. 2009). It has been shown that treatment of cells with glucocorticoids activates gene transcription, a primary mechanism for increased expression of the target genes (Eberwine et al., 1984; 1987; Karin, 1998). As a nuclear receptor, the GR not only mediates stressful effects of glucocorticoids, but also can act as a ligand-dependent transcription factor to influence gene expression (Aoyagi & Archer 2011). Our previous study showed that glucocorticoids bind to GR and activate another transcription factor c/EBP-β to transactivate the transcription of NET (Zha et al. 2011). Based on the biological characteristics of miRNAs, their regulatory effects may also be involved in this regulation. First, miRNAs influence complex gene networks and cellular processes more quickly, relatively specifically, and effectively (Kosik 2006, Soifer et al. 2007, Hansen & Obrietan 2013, Wang et al. 2014). Second, miRNAs can modulate stress response and associated neuronal function (Leung & Sharp 2010). The psychological stress significantly alters the expression of miRNAs in the brain (Zucchi et al. 2013). For example, the expression of numerous miRNAs in the hippocampus, amygdala (Meerson et al. 2010), thymus (Belkaya et al. 2011) and frontal cortex (Rinaldi et al. 2010, Babenko et al. 2012) of experimental animals was differentially altered after stress. Among these altered miRNA levels caused by stress, miR-181 family is one of most stress-responsive miRNAs. In mice injected with lipopolysaccharide, an infection stress inducer, miR-181d exhibited a 15-fold decrease while miR-181a and miR-181b are reduced 2- and 6-fold, respectively in the thymic tissue (Belkaya et al. 2011). Downregulated miR-181a increases the survival of astrocytes from ischemia-like injury following glucose deprivation (Ouyang et al. 2012a). As mentioned above, in our previous studies chronic stress and glucocorticoid treatments significantly increased the expression of NET (Sun et al. 2010, Zha et al. 2011, Chen et al. 2012), and GR (Zha et al. 2011), which is consistent with the reports from other laboratories (Froger et al. 2004, Li et al. 2011, Guidotti et al. 2013). In the present study, neither did we identify the expression of miR-181a in PC12 cells, nor did we find any significantly expressional change of miR-181a after treatment with corticosterone in PC12 cells transfected with miR-181a mimics. Currently we do not have satisfactory explanation for failing to detect endogenous miR-181a in PC12 cells, with the only possible reason that its expression is lower than the detectable level in the present technical conditions of our laboratory. However, exposure of cells to corticosterone significantly reduced expression of endogenous miR-29b, indicating that this miRNA is negatively regulated by glucocorticoids. Together with the observation that miR-29b significantly represses protein levels of NET (Fig. 1), it suggests that this miRNA may be involved in the upregulation of NET caused by treatment with glucocorticoids. As an increased expression of NET may allow a homeostatic stress response to evolve into a pathological stress (Goddard et al. 2010), our current observation may be of pivotal importance for study regarding possible correlation between stress and development of depression. However, more studies are required to seek the direct evidence for that there is a relationship between the effect of corticosterone on mirR-29b and the up-regulation of NET expression by corticosterone.
To further explore whether GR is involved in the negative regulation of corticosterone on endogenous miR-29b, GR antagonist mifepristone was administered in the experiment. The results show that blockage of GR cannot reverse the repressive effect of corticosterone on miR-29b (Fig. 6B), indicating the effect of corticosterone on miR-29b in PC12 cells is GR-independent, which is in conflict with the only other report that mifepristone blocked glucocorticoid-induced increase of miR-29c in rat aortic smooth muscle cells (Chuang et al. 2015). While use of different cell lines may be an explanation for this discrepancy, different action mechanisms of glucocorticoids can be accounted for. It is known that glucocorticoids act through both genomic and non-genomic mechanisms. Generally, the genomic effect of glucocorticoids can be achieved by binding to GRs, and activation of GRs in turn regulates gene expression (Rousseau et al. 1972). However, glucocorticoids can exert many important effects on the nervous systems through non-genomic pathways, by which the effect of glucocorticoids might be mediated through nonspecific interactions with intracellular machinery, rather than through GR signaling (Cato et al. 2002, Song & Buttgereit 2006), although the underlying mechanisms remain to be elucidated (Makara & Haller 2001). The non-genomic effects of glucocorticoids can be found in the inhibition of ion channels (Qiu et al. 1998, Lovell et al. 2004), CRF-induced ACTH secretion (Hinz & Hirschelmann 2000), activation of MAPKs pathways (Xiao et al. 2005), reduction of gonadotropin-releasing hormone (GnRH) pulse (Wagenmaker et al. 2009), catecholamine release in chromaffin cells (Park et al. 2008), and upregulation of SP-B mRNA (Tillis et al. 2011). Given the study about miRNAs is still an emerging field, the non-genomic mechanism underlying the regulation of corticosterone on miR-29b in the present study can be a possibility.
It is noteworthy that expression and function of NET is closely related to the action mechanisms for some types of antidepressants with characteristics of tricyclic structures or specific inhibition of norepinephrine reuptake. Also, the therapeutic action of some antidepressants has been thought to be mediated, at least in part, by changing GR functional status, and blocking the GR represents a potential treatment strategy for depression (Anacker et al. 2011). Likewise, miRNAs may have similar activity as accumulating evidence reveals that they may be critical for the pathophysiology of depressive disorders by taking part in monoamine signaling (Dwivedi 2011b, Millan 2011) and psychotropic drug effectiveness (Baudry et al. 2010, Hansen & Obrietan 2013). It has been reported that antidepressant treatment significantly increased blood levels of 28 miRNAs (Bocchio-Chiavetto et al. 2013). In animal study, treatment with antidepressants ketamine and electroconvulsive shock therapy also increased miRNA expression in the hippocampus of stress animals (O’Connor et al. 2013). Interestingly, miR-29b is one of these miRNAs increased by antidepressant treatments. The present study identifies two miRNAs that commonly repress NET and GR expression, which suggests that miRNAs may be a possible mechanism regulating the protein levels involved in antidepressant actions.
In conclusion, our data suggest that stress and stress-released glucocorticoids may downregulate expression levels of miR-29b in the brain, which erase or reduce their normal repressive effects on NET and GR, and in turn increase the expression of NET and GR, as observed in our previous studies. Further elucidation of their roles in vivo may contribute to the investigation of mechanisms related to stress and antidepressant action. Furthermore, the data from present study provide in vitro evidence for an interaction between miRNA and NET. Downregulation of NET expression may be more effective than pharmaceutical treatments because of miRNA’s high potential as mentioned above. The novel miRNAs, mir181a and mir29b, can help us to understand mechanism of interaction between miRNAs and monoamine transporters and to look for possibilities of using them for treatment of psychiatric and neurological disorders. However, the understanding of miR-181a and miR-29b is still fragmented. More investigations are warranted.
Acknowledgements
This work is supported by NIH grant MH080323.
Abbreviation
- GR
glucocorticoid receptors
- HPA
hypothalamic-pituitary-adrenocortical
- KRH
Krebs-Ringer–HEPES buffer
- LC
locus coeruleus
- miRNAs
microRNAs
- NE
norepinephrine
- NET
norepinephrine transporter
- ODs
optical densities
- PBS
phosphate-buffer saline
- q-PCR
quantitative real-time polymerase chain reaction
Footnotes
ARRIVE guidelines have been followed:
-
=>if No, skip complete sentence
-
=>if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
-
=>if ‘none’, insert “The authors have no conflict of interest to declare.”
-
=>otherwise insert info unless it is already included
References
- Amara SG, Kuhar MJ. Neurotransmitter Transporters: Recent Progress. Annual Review of Neuroscience. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Amodio N, Rossi M, Raimondi L, Pitari MR, Botta C, Tagliaferri P, Tassone P. miR-29s: a family of epi-miRNAs with therapeutic implications in hematologic malignancies. Oncotarget. 2015;6:12837–12861. doi: 10.18632/oncotarget.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi S, Archer TK. Differential glucocorticoid receptor-mediated transcription mechanisms. J Biol Chem. 2011;286:4610–4619. doi: 10.1074/jbc.M110.195040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod J, Kopin IJ. The uptake, storage, release and metabolism of noradrenaline in sympathetic nerves. Prog Brain Res. 1969;31:21–32. doi: 10.1016/S0079-6123(08)63224-0. [DOI] [PubMed] [Google Scholar]
- Babenko O, Golubov A, Ilnytskyy Y, Kovalchuk I, Metz GA. Genomic and Epigenomic Responses to Chronic Stress Involve miRNA-Mediated Programming. PloS one. 2012;7:e29441. doi: 10.1371/journal.pone.0029441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Belkaya S, Silge RL, Hoover AR, Medeiros JJ, Eitson JL, Becker AM, de la Morena MT, Bassel-Duby RS, van Oers NS. Dynamic modulation of thymic microRNAs in response to stress. PloS one. 2011;6:e27580. doi: 10.1371/journal.pone.0027580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Molecular Psychiatry. 2010;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam Encodes a Developmentally Regulated microRNA that Controls Cell Proliferation and Regulates the Proapoptotic Gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Broderick JA, Zamore PD. microRNA Therapeutics. Gene therapy. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey KJ, Kane D, Sunshine M, Narasimhan S, Nishizuka S, Reinhold WC, Zeeberg B, Ajay W, Weinstein JN. MatchMiner: a tool for batch navigation among gene and gene product identifiers. Genome biology. 2003;4:R27. doi: 10.1186/gb-2003-4-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Science’s STKE : signal transduction knowledge environment. 2002;2002:re9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen P, Fan Y, Li Y, Sun Z, Bissette G, Zhu MY. Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int. 2012;60:9–20. doi: 10.1016/j.neuint.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TD, Pearce WJ, Khorram O. miR-29c induction contributes to downregulation of vascular extracellular matrix proteins by glucocorticoids. Am J Physiol Cell Physiol. 2015;309:C117–125. doi: 10.1152/ajpcell.00254.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Ding H, Huang Z, Chen M, Wang C, Chen X, Chen J, Zhang J. Identification of a panel of five serum miRNAs as a biomarker for Parkinson’s disease. Parkinsonism & related disorders. 2016;22:68–73. doi: 10.1016/j.parkreldis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. Journal of Chemical Neuroanatomy. 2011a;42:142–156. doi: 10.1016/j.jchemneu.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y. Evidence demonstrating role of microRNAs in the etiopathology of major depression. J Chem Neuroanat. 2011b;42:142–156. doi: 10.1016/j.jchemneu.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Gorecka A, Kusmider M, Drozdowska E, Rogoz Z, Siwanowicz J, Caron MG, Bonisch H. Effect of Antidepressant Drugs in Mice Lacking the Norepinephrine Transporter. Neuropsychopharmacology. 2006;31:2424–2432. doi: 10.1038/sj.npp.1301064. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Smith KM, Lovett-Racke AE, Guerau-de-Arellano M, Whitacre CC, Godbout JP. Increased micro-RNA 29b in the aged brain correlates with the reduction of insulin-like growth factor-1 and fractalkine ligand. Neurobiology of Aging. 2013;34:2748–2758. doi: 10.1016/j.neurobiolaging.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, et al. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. J Neurosci. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: Non-invasive biomarkers for Alzheimer’s disease. Experimental Neurology. 2012;235:491–496. doi: 10.1016/j.expneurol.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology. 2013;38:616–627. doi: 10.1038/npp.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Bilkei-Gorzo A, Caron MG, Bonisch H. Knockout of the norepinephrine transporter and pharmacologically diverse antidepressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem. 2009;111:403–416. doi: 10.1111/j.1471-4159.2009.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Rodriguiz RM, Caron MG, Wetsel WC, Liposits Z. Behavioral responses to social stress in noradrenaline transporter knockout mice: effects on social behavior and depression. Brain Res Bull. 2002;58:279–284. doi: 10.1016/s0361-9230(02)00789-x. [DOI] [PubMed] [Google Scholar]
- Hamada N, Fujita Y, Kojima T, Kitamoto A, Akao Y, Nozawa Y, Ito M. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int. 2012;60:743–750. doi: 10.1016/j.neuint.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hansen KF, Obrietan K. MicroRNA as therapeutic targets for treatment of depression. Neuropsychiatric Disease and Treatment. 2013;9:1011–1021. doi: 10.2147/NDT.S34811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharmaceutical research. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Austin DR, Chen G, Manji HK. MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuromolecular medicine. 2009;11:173–182. doi: 10.1007/s12017-009-8070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Rink C, Ghoorkhanian R, et al. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. Journal of Cerebral Blood Flow & Metabolism. 2013;33:1197–1206. doi: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes & Development. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Ho DY, Sun GH, Steinberg GK, Sapolsky RM. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci. 1996;16:486–496. doi: 10.1523/JNEUROSCI.16-02-00486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Han F, Shi Y. Expression of locus coeruleus mineralocorticoid receptor and glucocorticoid receptor in rats under single-prolonged stress. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2011;32:625–631. doi: 10.1007/s10072-011-0597-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu Y, Huang Q, Zou D, Qin W, Chen Z. Grouping Pentylenetetrazol-Induced Epileptic Rats According to Memory Impairment and MicroRNA Expression Profiles in the Hippocampus. PloS one. 2015;10:e0126123. doi: 10.1371/journal.pone.0126123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhao Z, Yang F, Gao Y, Song J, Wan Y. microRNA-181a is involved in insulin-like growth factor-1-mediated regulation of the transcription factor CREB1. J Neurochem. 2013;126:771–780. doi: 10.1111/jnc.12370. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovell PV, King JT, McCobb DP. Acute modulation of adrenal chromaffin cell BK channel gating and cell excitability by glucocorticoids. J Neurophysiol. 2004;91:561–570. doi: 10.1152/jn.01101.2002. [DOI] [PubMed] [Google Scholar]
- Makara GB, Haller J. Non-genomic effects of glucocorticoids in the neural system. Evidence, mechanisms and implications. Prog Neurobiol. 2001;65:367–390. doi: 10.1016/s0301-0082(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Makeyev EV, Maniatis T. Multilevel Regulation of Gene Expression by MicroRNAs. Science (New York, N.Y.) 2008;319:1789–1790. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res. 2002;943:216–223. doi: 10.1016/s0006-8993(02)02647-1. [DOI] [PubMed] [Google Scholar]
- Markey KA, Towle AC, Sze PY. Glucocorticoid influence on tyrosine hydroxylase activity in mouse locus coeruleus during postnatal development. Endocrinology. 1982;111:1519–1523. doi: 10.1210/endo-111-5-1519. [DOI] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci. 2010;40:47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JT. MicroRNAs: Critical Regulators of Development, Cellular Physiology and Malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- Millan MJ. MicroRNA in the regulation and expression of serotonergic transmission in the brain and other tissues. Current opinion in pharmacology. 2011;11:11–22. doi: 10.1016/j.coph.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome biology. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry. 2012;17:359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- O’Connor RM, Grenham S, Dinan TG, Cryan JF. microRNAs as novel antidepressant targets: converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int J Neuropsychopharmacol. 2013;16:1885–1892. doi: 10.1017/S1461145713000448. [DOI] [PubMed] [Google Scholar]
- Ouyang Y-B, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012a;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis. 2012b;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Ha Choi Y, Park CH, Kim KT. Nongenomic glucocorticoid effects on activity-dependent potentiation of catecholamine release in chromaffin cells. Endocrinology. 2008;149:4921–4927. doi: 10.1210/en.2007-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, Chen G. MicroRNA-29b Regulates Ethanol-induced Neuronal Apoptosis in the Developing Cerebellum through SP1/RAX/PKR Cascade. The Journal of Biological Chemistry. 2014;289:10201–10210. doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Lou LG, Huang XY, Lou SJ, Pei G, Chen YZ. Nongenomic mechanisms of glucocorticoid inhibition of nicotine-induced calcium influx in PC12 cells: involvement of protein kinase C. Endocrinology. 1998;139:5103–5108. doi: 10.1210/endo.139.12.6376. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Vincenti S, De Vito F, Bozzoni I, Oliverio A, Presutti C, Fragapane P, Mele A. Stress induces region specific alterations in microRNAs expression in mice. Behav Brain Res. 2010;208:265–269. doi: 10.1016/j.bbr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Rousseau GG, Baxter JD, Tomkins GM. Glucocorticoid receptors: relations between steroid binding and biological effects. Journal of molecular biology. 1972;67:99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Saba R, Storchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, Schratt GM. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Mol Cell Biol. 2012a;32:619–632. doi: 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba R, Störchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, Schratt GM. Dopamine-Regulated MicroRNA MiR-181a Controls GluA2 Surface Expression in Hippocampal Neurons. Molecular and Cellular Biology. 2012b;32:619–632. doi: 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban EL, Laukova M, Alaluf LG, Olsson E, Serova LI. Locus coeruleus response to single-prolonged stress and early intervention with intranasal neuropeptide Y. J Neurochem. 2015;135:975–986. doi: 10.1111/jnc.13347. [DOI] [PubMed] [Google Scholar]
- Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G. microRNAs at the synapse. Nat Rev Neurosci. 2009;10:842–849. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- Smith B, Treadwell J, Zhang D, Ly D, McKinnell I, Walker PR, Sikorska M. Large-scale expression analysis reveals distinct microRNA profiles at different stages of human neurodevelopment. PloS one. 2010;5:e11109. doi: 10.1371/journal.pone.0011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soifer HS, Rossi JJ, Saetrom P. MicroRNAs in Disease and Potential Therapeutic Applications. Mol Ther. 2007;15:2070–2079. doi: 10.1038/sj.mt.6300311. [DOI] [PubMed] [Google Scholar]
- Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol. 2006;246:142–146. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Sun Z, Fan Y, Zha Q, Zhu MY. Corticosterone up-regulates expression and function of norepinephrine transporter in SK-N-BE(2)C cells. J Neurochem. 2010;113:105–116. doi: 10.1111/j.1471-4159.2010.06587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillis CC, Huang HW, Bi W, Pan S, Bruce SR, Alcorn JL. Glucocorticoid regulation of human pulmonary surfactant protein-B (SP-B) mRNA stability is independent of activated glucocorticoid receptor. Am J Physiol Lung Cell Mol Physiol. 2011;300:L940–950. doi: 10.1152/ajplung.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150:762–769. doi: 10.1210/en.2008-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2010;1803:1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Wang JIE, Wang Y, Yang J, Huang Y. microRNAs as novel biomarkers of schizophrenia (Review) Experimental and Therapeutic Medicine. 2014;8:1671–1676. doi: 10.3892/etm.2014.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Kitayama I, Nomura J. Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci. 1998;62:2083–2092. doi: 10.1016/s0024-3205(98)00183-0. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Zhang XQ, Cui XY, et al. Glucocorticoid receptors in the locus coeruleus mediate sleep disorders caused by repeated corticosterone treatment. Scientific reports. 2015;5:9442. doi: 10.1038/srep09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Qi A, Chen Y. Cultured embryonic hippocampal neurons deficient in glucocorticoid (GC) receptor: a novel model for studying nongenomic effects of GC in the neural system. Endocrinology. 2005;146:4036–4041. doi: 10.1210/en.2004-1652. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]
- Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell Death & Disease. 2010;1:e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Q, Wang Y, Fan Y, Zhu MY. Dexamethasone-induced up-regulation of the human norepinephrine transporter involves the glucocorticoid receptor and increased binding of C/EBP-beta to the proximal promoter of norepinephrine transporter. J Neurochem. 2011;119:654–663. doi: 10.1111/j.1471-4159.2011.07448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Ordway GA. Down-regulation of norepinephrine transporters on PC12 cells by transporter inhibitors. J Neurochem. 1997;68:134–141. doi: 10.1046/j.1471-4159.1997.68010134.x. [DOI] [PubMed] [Google Scholar]
- Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, Kovalchuk I, Kovalchuk O, Metz GA. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PloS one. 2013;8:e56967. doi: 10.1371/journal.pone.0056967. [DOI] [PMC free article] [PubMed] [Google Scholar]