Abstract
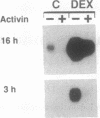
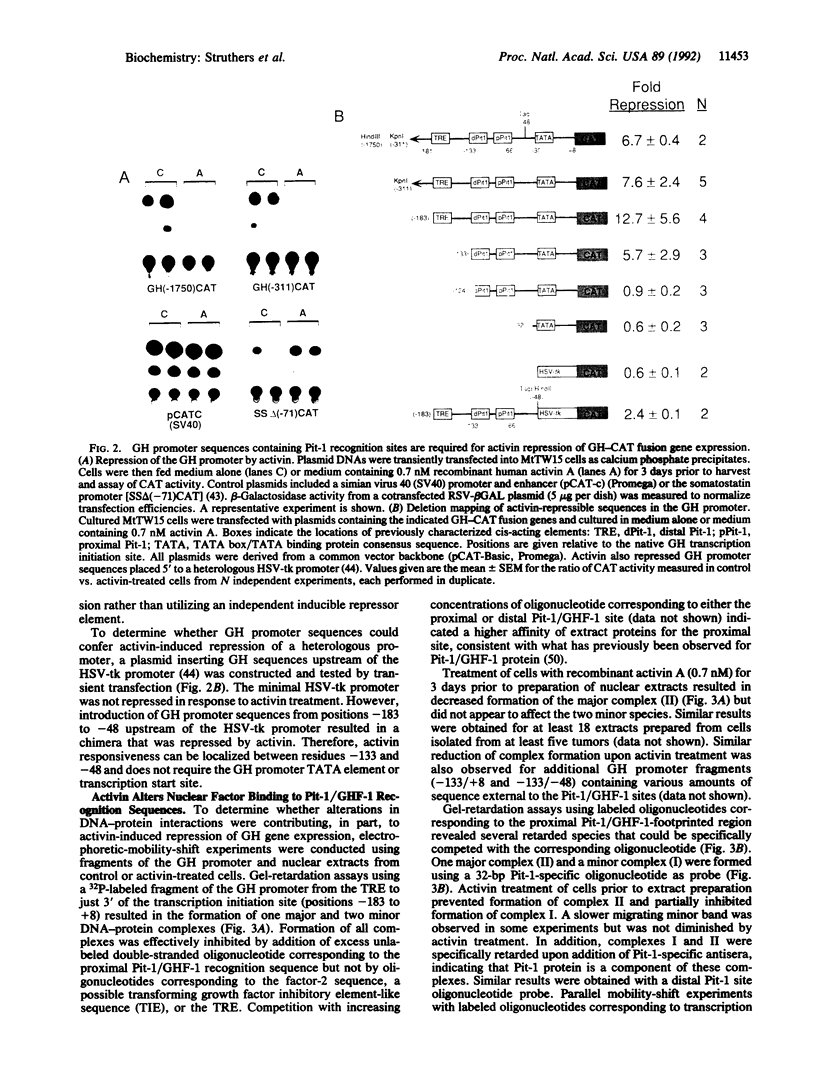
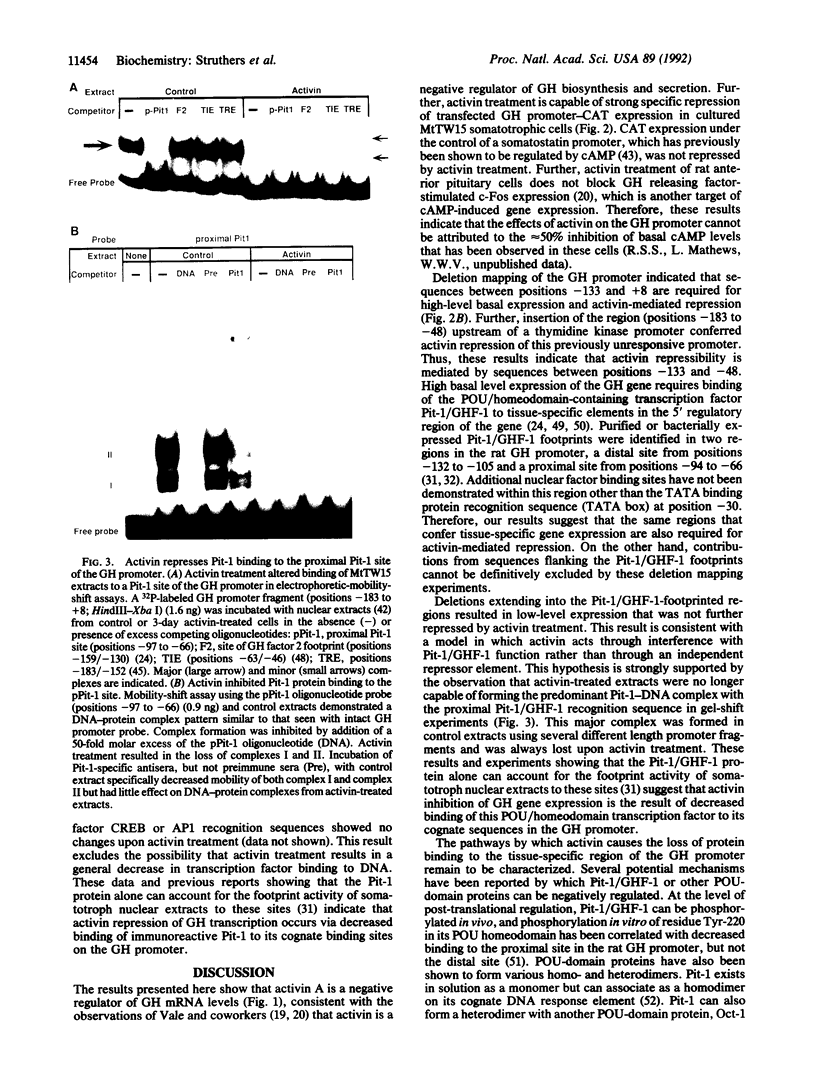
Activin A is a potent growth and differentiation factor related to transforming growth factor beta. In somatotrophs, activin suppresses the biosynthesis and secretion of growth hormone (GH) and cellular proliferation. We report here that, in MtTW15 somatotrophic tumor cells, activin decreased GH mRNA levels and inhibited expression of transfected GH promoter--chloramphenicol acetyltransferase fusion genes. Deletion mapping of nucleotide sequences mediating this inhibition led to the identification of a region that has previously been characterized as binding the pituitary-specific transcription factor Pit-1/GHF-1. Characterization of nuclear factor binding to this region demonstrated that binding of Pit-1 to the GH promoter is lost on activin treatment. These results indicate that activin-induced repression of GH biosynthesis is mediated by the loss of tissue-specific transcription factor binding to the GH promoter and suggest a possible general mechanism for other activin responses, whereby activin regulates the function of other POU- or homeodomain-containing transcription factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attisano L., Wrana J. L., Cheifetz S., Massagué J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992 Jan 10;68(1):97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- BUFFETT R. F., CLIFTON K. H., FURTH J., GADSDEN E. L. Dependent and autonomous mammotropic pituitary tumors in rats; their somatotropic features. Cancer Res. 1956 Aug;16(7):608–616. [PubMed] [Google Scholar]
- Barinaga M., Yamonoto G., Rivier C., Vale W., Evans R., Rosenfeld M. G. Transcriptional regulation of growth hormone gene expression by growth hormone-releasing factor. Nature. 1983 Nov 3;306(5938):84–85. doi: 10.1038/306084a0. [DOI] [PubMed] [Google Scholar]
- Barta A., Richards R. I., Baxter J. D., Shine J. Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4867–4871. doi: 10.1073/pnas.78.8.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikjian L. M., Corrigan A. Z., Vale W. Activin-A modulates growth hormone secretion from cultures of rat anterior pituitary cells. Endocrinology. 1990 May;126(5):2369–2376. doi: 10.1210/endo-126-5-2369. [DOI] [PubMed] [Google Scholar]
- Billestrup N., González-Manchón C., Potter E., Vale W. Inhibition of somatotroph growth and growth hormone biosynthesis by activin in vitro. Mol Endocrinol. 1990 Feb;4(2):356–362. doi: 10.1210/mend-4-2-356. [DOI] [PubMed] [Google Scholar]
- Billestrup N., Swanson L. W., Vale W. Growth hormone-releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6854–6857. doi: 10.1073/pnas.83.18.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Theill L. E., Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991 Jul 12;253(5016):197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Chen R. P., Ingraham H. A., Treacy M. N., Albert V. R., Wilson L., Rosenfeld M. G. Autoregulation of pit-1 gene expression mediated by two cis-active promoter elements. Nature. 1990 Aug 9;346(6284):583–586. doi: 10.1038/346583a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DePaolo L. V., Bicsak T. A., Erickson G. F., Shimasaki S., Ling N. Follistatin and activin: a potential intrinsic regulatory system within diverse tissues. Proc Soc Exp Biol Med. 1991 Oct;198(1):500–512. doi: 10.3181/00379727-198-43286a. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Birnberg N. C., Rosenfeld M. G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flug F., Copp R. P., Casanova J., Horowitz Z. D., Janocko L., Plotnick M., Samuels H. H. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem. 1987 May 5;262(13):6373–6382. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Flynn S. E., Voss J. W., Albert V. R., Kapiloff M. S., Wilson L., Rosenfeld M. G. The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell. 1990 Jun 15;61(6):1021–1033. doi: 10.1016/0092-8674(90)90067-o. [DOI] [PubMed] [Google Scholar]
- Kapiloff M. S., Farkash Y., Wegner M., Rosenfeld M. G. Variable effects of phosphorylation of Pit-1 dictated by the DNA response elements. Science. 1991 Aug 16;253(5021):786–789. doi: 10.1126/science.1652153. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B., Matrisian L. M. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990 Apr 20;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Brent G. A., Warne R. L., Larsen P. R., Moore D. D. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5670–5674. doi: 10.1073/pnas.84.16.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Esch F., Denoroy L., Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7217–7221. doi: 10.1073/pnas.82.21.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N., Ying S. Y., Ueno N., Shimasaki S., Esch F., Hotta M., Guillemin R. Pituitary FSH is released by a heterodimer of the beta-subunits from the two forms of inhibin. Nature. 1986 Jun 19;321(6072):779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- Lira S. A., Crenshaw E. B., 3rd, Glass C. K., Swanson L. W., Rosenfeld M. G. Identification of rat growth hormone genomic sequences targeting pituitary expression in transgenic mice. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4755–4759. doi: 10.1073/pnas.85.13.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W., Kintner C. R. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992 Mar 27;255(5052):1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Hammer R. E., Swanson L. W., Brinster R. L., Rosenfeld M. G., Evans R. M. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Mol Endocrinol. 1988 Jul;2(7):606–612. doi: 10.1210/mend-2-7-606. [DOI] [PubMed] [Google Scholar]
- McCormick A., Brady H., Theill L. E., Karin M. Regulation of the pituitary-specific homeobox gene GHF1 by cell-autonomous and environmental cues. Nature. 1990 Jun 28;345(6278):829–832. doi: 10.1038/345829a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Meunier H., Rivier C., Evans R. M., Vale W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci U S A. 1988 Jan;85(1):247–251. doi: 10.1073/pnas.85.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Hasegawa Y., Fukuda M., Nomura M., Igarashi M., Kangawa K., Matsuo H. Isolation of porcine follicular fluid inhibin of 32K daltons. Biochem Biophys Res Commun. 1985 Jun 14;129(2):396–403. doi: 10.1016/0006-291x(85)90164-0. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Norman M. F., Lavin T. N., Baxter J. D., West B. L. The rat growth hormone gene contains multiple thyroid response elements. J Biol Chem. 1989 Jul 15;264(20):12063–12073. [PubMed] [Google Scholar]
- Rivier J., Spiess J., McClintock R., Vaughan J., Vale W. Purification and partial characterization of inhibin from porcine follicular fluid. Biochem Biophys Res Commun. 1985 Nov 27;133(1):120–127. doi: 10.1016/0006-291x(85)91849-2. [DOI] [PubMed] [Google Scholar]
- Roberts V., Meunier H., Vaughan J., Rivier J., Rivier C., Vale W., Sawchenko P. Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology. 1989 Jan;124(1):552–554. doi: 10.1210/endo-124-1-552. [DOI] [PubMed] [Google Scholar]
- Robertson D. M., Foulds L. M., Leversha L., Morgan F. J., Hearn M. T., Burger H. G., Wettenhall R. E., de Kretser D. M. Isolation of inhibin from bovine follicular fluid. Biochem Biophys Res Commun. 1985 Jan 16;126(1):220–226. doi: 10.1016/0006-291x(85)90594-7. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Kimura H., LaCorbiere M., Vaughan J., Karr D., Fischer W. H. Activin is a nerve cell survival molecule. Nature. 1990 Apr 26;344(6269):868–870. doi: 10.1038/344868a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Struthers R. S., Vale W. W., Arias C., Sawchenko P. E., Montminy M. R. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature. 1991 Apr 18;350(6319):622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Treacy M. N., He X., Rosenfeld M. G. I-POU: a POU-domain protein that inhibits neuron-specific gene activation. Nature. 1991 Apr 18;350(6319):577–584. doi: 10.1038/350577a0. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Vale W., Vaughan J., Yamamoto G., Bruhn T., Douglas C., Dalton D., Rivier C., Rivier J. Assay of corticotropin releasing factor. Methods Enzymol. 1983;103:565–577. doi: 10.1016/s0076-6879(83)03040-2. [DOI] [PubMed] [Google Scholar]
- Voss J. W., Wilson L., Rosenfeld M. G. POU-domain proteins Pit-1 and Oct-1 interact to form a heteromeric complex and can cooperate to induce expression of the prolactin promoter. Genes Dev. 1991 Jul;5(7):1309–1320. doi: 10.1101/gad.5.7.1309. [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Van Raaij A. J., van Zoelent E. J., van Nimmen K., Koster C. H., Snoek G. T., Durston A. J., Huylebroeck D. Activin-like factor from a Xenopus laevis cell line responsible for mesoderm induction. Nature. 1990 Jun 21;345(6277):732–734. doi: 10.1038/345732a0. [DOI] [PubMed] [Google Scholar]