Abstract
Purpose
To evaluate the long-term clinical effectiveness and safety of corneal collagen cross-linking (CXL) in progressive keratoconus compared with untreated contralateral eyes.
Methods
In this retrospective study, nine eyes of nine patients with progressive keratoconus who received CXL (treatment group) and nine untreated contralateral eyes with keratoconus (control group) were included. All patients were followed for at least 5 years and assessed with best-corrected visual acuity, maximum keratometry, mean keratometry, corneal astigmatism, and corneal thickness. Clinical data were collected preoperatively and at 1, 3, 6, 12, 24, 36, 48, and 60 months, postoperatively.
Results
Mean best-corrected visual acuity improved significantly from 0.58 ± 0.37 logarithm of minimum angle of resolution preoperatively to 0.39 ± 0.29 logarithm of minimum angle of resolution at 5 years after corneal CXL (p = 0.012). There was significant flattening of the maximum keratometry and mean keratometry from preoperative values of 63.39 ± 10.89 and 50.87 ± 6.27 diopter (D) to postoperative values of 60.89 ± 11.29 and 49.54 ± 7.23 D, respectively (p = 0.038, 0.021). Corneal astigmatism decreased significantly from 7.20 ± 1.83 D preoperatively to 5.41 ± 1.79 D postoperatively (p = 0.021). The thinnest corneal thickness decreased from 434.00 ± 54.13 to 365.78 ± 71.58 µm during 1 month after treatment, then increased to 402.67 ± 52.55 µm at 5 years, which showed a statistically significant decrease compared to the baseline (p = 0.020). In the untreated contralateral eyes, mean keratometry increased significantly at 2 years compared with the baseline (p = 0.043).
Conclusions
CXL seems to be an effective and safe treatment for halting the progression of keratoconus over a long-term follow-up period of up to 5 years in progressive keratoconus.
Keywords: Cross-linking, Keratoconus, Riboflavin, Ultraviolet rays
Keratoconus is a non-inflammatory disease with progressive corneal ectasia. Corneal thinning and protrusion cause progressive myopia and irregular astigmatism, which can lead to a decrease in visual acuity. Although the exact pathogenesis of keratoconus has not been verified, a variety of genetic and mechanical factors, such as contact lens use and eye rubbing are considered possible causes [1,2,3].
Conventional treatments, such as glasses or contact lenses, help to restore visual acuity and to correct astigmatism and refractive errors, but such treatment does not inhibit the progression of keratoconus [4,5]. Intracorneal ring segment was reported to decrease mean keratometry (Kmean) and correct refractive error [6]. However, intracorneal ring segment is limited in cases where the thickness of the cornea ring insertion site is 450 µm or less. Corneal collagen cross-linking (CXL) for keratoconus using riboflavin and ultraviolet A (UV-A) with a wavelength of 370 nm was introduced by Wollensak [5] as a new treatment option to stabilize the progression of keratoconus. Ultraviolet A-riboflavin interaction generates an active oxygen species that induces covalent bonds between the amino groups of the collagen fibers. In an experimental study, the strength of the cornea increased by more than 300%, the thickness of the collagen fibers increased, and higher resistance to collagen-degrading enzymes in the crosslinking cornea was observed [7,8,9,10]. In 2003, the first clinical results of riboflavin-UVA induced CXL were reported by Wollensak et al. [11] in 23 eyes of keratoconus patients. The study showed decreased maximum keratometry (Kmax) values and an improvement in refractive error without significant ocular complications. Since then, various studies with a greater number of patients have reported an improvement in the index of keratoconus and the safety of CXL [12,13,14,15,16,17,18].
Although studies evaluating CXL have been performed, most have reported a relatively short follow-up of less than 3 years, and long-term clinical results have rarely been reported. In this study, we investigated the long-term clinical effects of CXL in Korean patients with progressive keratoconus through a comparison of the degree of keratoconus progression between CXL-treated eyes and the untreated contralateral eyes.
Materials and Methods
The nine eyes of nine patients with progressive keratoconus who received CXL (treatment group) and the nine contralateral eyes that were not treated (control group) were observed for more than 5 years.
Keratoconus was diagnosed with the presence of conical corneal protrusion, Kmax >47.0 diopter (D) by keratometry, stromal thinning of the cornea, the presence of Vogt's striae (a vertical fine, whitish line near Descemet's membrane), and characteristic findings of central corneal opacity. Progressive keratoconus was defined based on a previous report by Wittig-Silva et al. [17]; an increase of >1.0 D in the Kmax, an increase of >1.0 D in refractive astigmatism, an increase of >1.0 D in spherical equivalent refraction, or a decrease of >5% in central corneal thickness within the last year. The staging of keratoconus was based on the Amsler-Krumeich keratoconus classification.
Logarithm of minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA), Kmax, Kmean, the thinnest corneal thickness, and corneal astigmatism using Pentacam (Oculus, Wetzlar, Germany) were measured at 1, 3, 6, 12, 24, 36, 48, and 60 months after the procedure including baseline reference values. Pentacam was repeatedly measured three times or more at each visit. According to the instrument's "Examination Quality Specification," the qualitative evaluation including eye movement, improper palpebral fissure and the decentration, the examination were repeated until they met criteria. The anterior segment, lens, vitreous and retina were evaluated with slit lamp examination. These exams were performed in both eyes.
CXL was performed following the standard protocol reported by Wollensak et al. [11]. Under topical anesthesia with 0.5% proparacaine hydrochloride, a portion of corneal epithelium 7 mm in diameter was removed by a laser-assisted subepithelial keratomileusis epithelial peeler followed by the application of 0.1% riboflavin solution (10 mg riboflavin-5-phosphate in 10 mL dextran-T-500 20% solution) onto the cornea every 5 minutes for 30 minutes. Before UV irradiation, the surgeon confirmed that the riboflavin was fully absorbed into the corneal stroma by slit-lamp inspection and by the presence of a riboflavin flare in the anterior chamber. The cornea was exposed to UV light with a wavelength of 370 nm and an irradiance of 3 mW/cm2 for a total time of 30 minutes using a UV-X System (Peschke Meditrade, Hunenberg, Switzerland); this corresponds to an exposure of 5.4 J/cm2 to the cornea. Patients were instructed to fix their eyes on the center of the diode such that the UV-A rays were focused on the center of the cornea. During the UV irradiation, the cornea was rinsed with riboflavin solution every 5 minutes. After the treatment, ofloxacin ointment (Ocuflox; Samil Pharmaceutical, Seoul, Korea) and a treatment contact lens were applied until the epithelium was completely healed. Topical moxifloxacin (Vigamox; Alcon, Fort Worth, TX, USA) and 0.01% fluorometholone (Ocumetholone; Samil Pharmaceutical) were instilled every 6 hours during the first 2 weeks and then tapered off by 4 weeks.
Statistical analysis between the preoperative and postoperative values was performed using the Wilcoxon signed-ranks test of PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA). The independent sample t-test was used to compare the difference between the treatment group and the control group. A p-value less than 0.05 was considered to be statistically significant.
Results
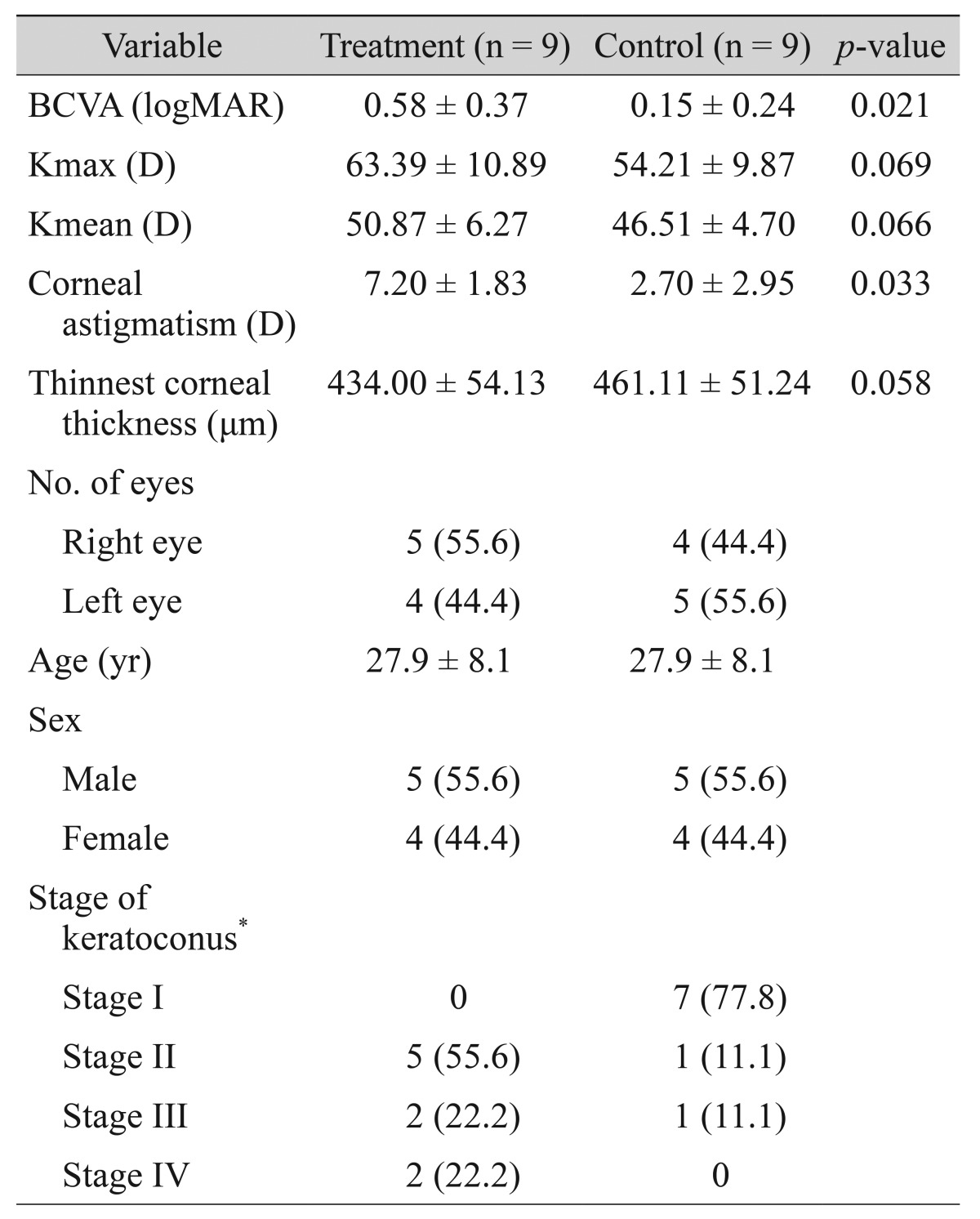
A total of 18 eyes of nine patients were examined. The mean age was 27.9 ± 8.1 years (range, 13 to 43) and there were five men and four women (Table 1). Surgical results in BCVA, Kmax and Kmean, corneal astigmatism and corneal thickness are shown in Fig. 1A-1E.
Table 1. Baseline demographic and clinical characteristics of the eyes in the treatment and control groups.
Values are presented as mean ± standard deviation or number (%).
BCVA = best-corrected visual acuity; logMAR = logarithm of minimum angle of resolution; Kmax = maximum keratometry; D = diopter; Kmean = mean keratometry.
*Amsler-Krumeich classification.
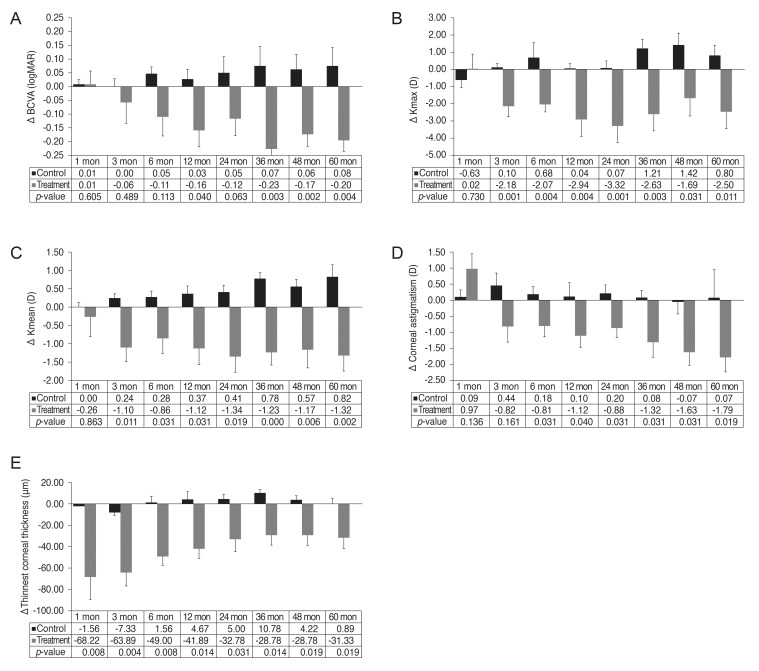
Fig. 1. Comparison of the mean change relative to baseline in best-corrected visual acuity (BCVA) (A), maximum keratometry (Kmax) (B), mean keratometry (Kmean) (C), corneal astigmatism (D), and thinnest corneal thickness (E) at each time point in the treatment and control groups. At each time point, the bar represents the mean change from baseline, and the p-value is calculated using the independent sample t-tests between the control and the treatment groups.
Visual acuity
The BCVA improved from 0.58 ± 0.37 to 0.42 ± 0.32 logMAR in the treatment group at 1 year after CXL (p = 0.043), which was steadily maintained for up to 5 years compared with the baseline. In contrast, the untreated eyes demonstrated deterioration of the BCVA from 0.15 ± 0.24 to 0.22 ± 0.24 logMAR at 5 years, which was not statistically significant (p = 0.279) (Table 2). The improvement of visual acuity was significantly higher in the treatment group than in the control group from year 1 to year 5 (Fig. 1A).
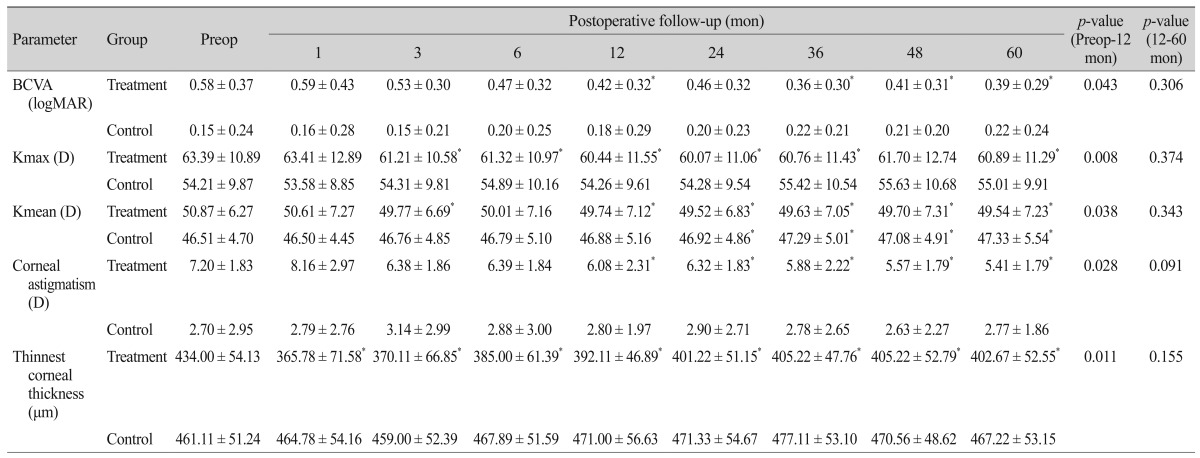
Table 2. Clinical characteristics of eyes in the treatment and control groups compared with baseline measurements.
Values are presented as mean ± standard deviation.
Preop = preoperative; BCVA = best-corrected visual acuity; logMAR = logarithm of minimum angle of resolution; Kmax = maximum keratometry; D = diopter; Kmean = mean keratometry.
*p < 0.05, compared with the baseline.
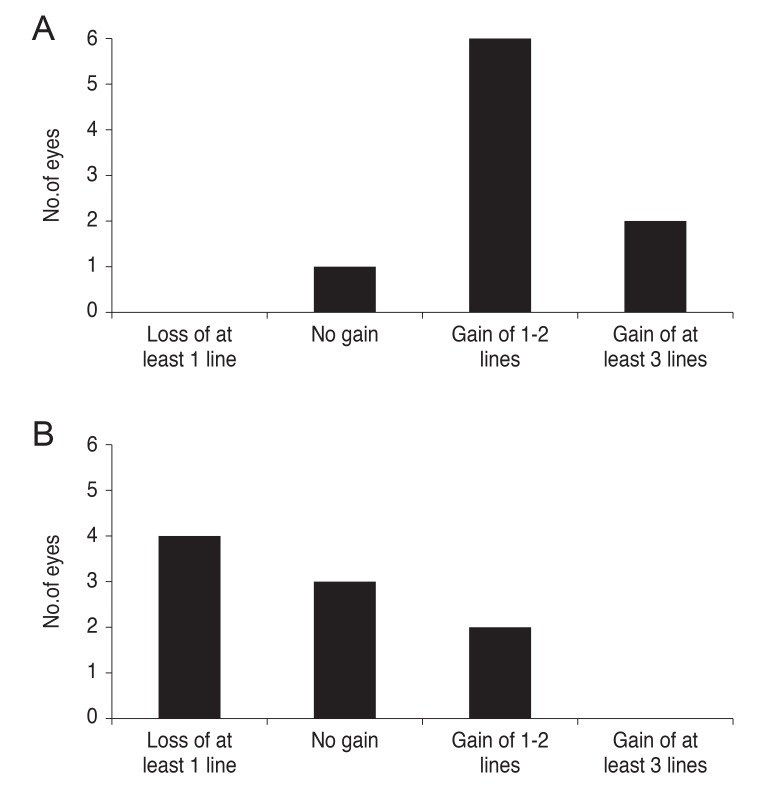
The BCVA was improved in eight of nine eyes (88.9%) in the treatment group at 5 years. Two eyes showed at least three lines of BCVA improvement. In contrast, the control group demonstrated BCVA improvement in only two of nine eyes (12.1%) and the BCVA decreased in four eyes (24.2%) (Fig. 2A and 2B).
Fig. 2. Bar graphs demonstrating best-corrected visual acuity changes (lines) 5 years after corneal cross-linking in keratoconic patients (A) and in the control group (B).
Topographic results
In the treatment group, Kmax decreased significantly from 63.39 ± 10.89 D preoperatively to 61.21 ± 10.58 D after 3 months (p = 0.011) and 60.89 ± 11.29 D after 5 years (p = 0.038), as compared with the baseline. Kmean decreased significantly from 50.87 ± 6.27 D preoperatively to 49.77 ± 6.69 D after 3 months (p = 0.033) and 49.54 ± 7.23 D at 5 years (p = 0.021). In the control group, Kmax increased by 0.8 D after 5 years from 54.21 ± 9.87 to 55.01 ± 9.91 D, which was not statistically significant (p = 0.192). However, Kmean increased significantly by 0.4 D after 2 years from 46.51 ± 4.70 D preoperatively to 46.92 ± 4.86 D (p = 0.043). At 5 years, Kmean increased significantly by 0.8 to 47.33 ± 5.54 D (p = 0.028) (Table 2).
The improvement of Kmax and Kmean was more significant in treated eyes compared to untreated eyes at 3 months (p = 0.001 and p = 0.011, respectively) which remained until 5 years (p = 0.011 and p = 0.002, respectively) (Fig. 1B and 1C).
Corneal astigmatism
At 1 month postoperatively, corneal astigmatism increased from 7.20 ± 1.83 D preoperatively to 8.16 ± 2.97 D with no statistical significance (p = 0.086). Corneal astigmatism subsequently decreased to 6.08 ± 2.31 and 5.41 ± 1.79 D at 1 and 5 years respectively (p = 0.028 and p = 0.021, respectively). In the control group, corneal astigmatism increased from 2.70 ± 2.95 D preoperatively to 2.77 ± 1.86 D at 5 years (p = 0.500) (Table 2). The improvement of the corneal astigmatism was more significant in the treated eyes compared to the untreated eyes at 6 months (p = 0.031), which remained until 5 years (p = 0.019) (Fig. 1D).
Corneal thickness measurements
In the treated eyes, the thinnest corneal thickness decreased significantly from 434.00 ± 54.13 µm preoperatively to 365.78 ± 71.58 µm at 1 month (p = 0.008). After 1 month, corneal thickness showed a tendency to gradually increase to 402.67 ± 52.55 µm at 5 years, but showed a statistically significant decrease compared with the baseline (p = 0.020). In the untreated eyes, the thinnest corneal thickness changed from 461.11 ± 51.24 µm at baseline to 467.22± 53.15 µm at 5 years (p = 0.236) (Table 2). The decrease of the thinnest corneal thickness was more significant in the treated eyes for up to 5 years compared with the untreated eyes (Fig. 1E).
Safety evaluations
All patients showed transient corneal edema in the healing process of the epithelial cells, with all corneal edema disappearing within 7 days. Although mild corneal haze occurred in one case, it disappeared 8 months after CXL.
Discussion
The name keratoconus is derived from the Greek word for cornea "kerato" and cone-shaped "conus" and was first described by Nottingham in 1854 [19]. In general, keratoconus is a non-inflammatory eye disease characterized by an asymmetric, bilateral, progressive corneal ectasia causing a cone-like bulge. The protruding cornea can cause high myopia and irregular astigmatism resulting in substantial distortion of vision [20]. A conservative treatment option for keratoconus is the fitting of rigid contact lenses or eyeglasses to improve the quality of vision. Alternatively, more invasive methods include intracorneal ring segment implants and lamellar keratoplasty or penetrating keratoplasty in case of advanced keratoconus. With the exception of keratoplasty, these treatments cannot stop the progression of keratoconus because they focus on the correction of the refraction rather than the pathological mechanism of stromal instability stemming from the collagen abnormalities. In contrast, CXL induces biomechanical stability of the cornea through a formation of covalent bonds between the collagen fibrils using UV-A and riboflavin to stop the progression of the disease. CXL fundamentally addresses the pathophysiology of keratoconus, that is, the instability of the corneal stroma due to collagen abnormalities [21].
Many studies have reported that CXL improves visual acuity, reducing the corneal curvature and inhibiting disease progression. Previously, Hassan et al. [22] reported that CXL inhibits the progression of keratoconus, demonstrating no significant aggravation in corneal curvature, astigmatism, and visual acuity after 3 years compared to the baseline in 38 eyes. Poli et al. [23] reported the results of CXL in 45 eyes with keratoconus observed for 36 months, in which visual acuity improved significantly over 3 years, as compared to the control group, where the Kmax value significantly deteriorated after 6 months with no further significant variation after CXL. In a Korean study, a decrease in the Kmax value and corneal astigmatism and improvement of the BCVA at 1 year after CXL was reported by Lee and Jin [24]. Noh et al. [25] showed that the Kmean value and corneal astigmatism improved significantly at 1 year after CXL in 12 eyes.
In this study, we analyzed the 5-year results of CXL in nine eyes of patients with progressive keratoconus through a comparison of visual acuity, keratometry, and corneal thickness between the CXL-treated eyes and the untreated contralateral eyes.
In the treatment group, BCVA gradually recovered and showed a significant improvement at 1 year as compared to the baseline. Improved vision was steadily maintained for up to 5 years, with the exception of a slight decline in improvement at 2 years (Table 2). The improvement in visual acuity is caused by the recovery of corneal symmetry, with a reduction in the difference between the superior and inferior corneal hemi-meridians. Caporossi et al. [12] documented an improvement in corneal symmetry for up to 4 years after CXL. In the control eyes, the vision gradually deteriorated, but a statistically significant difference was not shown. This can be explained by the slow progression of the clinical course of the keratoconus, and visual acuity is considered to be a less sensitive indicator than other parameters [26,27]. In the control group, two eyes showed visual gains during the follow-up period without changes in the other clinical indicators, including Kmax, Kmean, or astigmatism. Although the visual gain was not explainable by keratometry, long-term neural compensation could result in improved visual performance in the keratoconus patients, as suggested by Sabesan and Yoon [28] in their study of adaptive optics.
In this study, eight eyes of nine patients (88.9%) in the treatment group gained one or more lines of BCVA without visual deterioration during 5 years of follow-up (Fig. 2). However, in the control eyes, BCVA improvement was observed only in two of nine eyes (22.2%). This suggests that CXL inhibits the progression of keratoconus and improves visual acuity. In a previous report, the BCVA was reported to improve by at least one line in 58% of patients at 3 years after treatment [4].
Kmax steadily decreased during the 5 years of follow-up, with temporal deterioration at 1 month. Kmean decreased significantly 3 months after treatment and then remained stable during the 5 years of follow-up (Fig. 2). In a previous study, Caporossi et al. [12] documented a decrease in Kmean by an average of -2.24 D at 3 years, and Grewal et al. [13] reported a decrease in Kmax of -2.57 D at 3 years. Similarly, we observed decreases in Kmax of -2.63 D at 3 years and -2.50 D at 5 years after CXL. Based on these results, the effects of CXL on keratometric values were found to be maintained without progression up until 5 years after treatment.
Corneal astigmatism increased at 1 month after treatment compared to the baseline, and then decreased significantly from 1 year onward and remained consistent through 5 years (Fig. 1D). In previous studies, there was an initial aggravation of keratoconus because it took time for the CXL to take effect in the process of corneal epithelial healing [15,16]. Less initial improvements in visual acuity can be similarly explained.
The thinnest corneal region showed the greatest decrease at 1 month after treatment, which was maintained until 3 months, after which corneal thickness increased gradually, but showed a significant decrease at 5 years as compared to the baseline (Fig. 1E). The reduction of corneal thickness after CXL has been reported in previous studies [13,14,15]. The mechanism of initial corneal thinning has been associated with anatomic and structural changes such as compression of collagen fibrils, changes in corneal hydration, and keratocyte apoptosis. It is known that corneal thickness recovers after 3 months through re-epithelialization and epithelial remodeling [29]. The restoration of corneal thickness back to the baseline has been reported to occur starting at 1 year after surgery [13,14]. Wittig-Silva et al. [18] documented that corneal thickness was mostly recovered at 12 months and decreased by 19.52 µm at 36 months [18]. In this study, corneal thickness recovered steadily for the first 2 years and decreased by 32.7 µm at 2 years and by 31.3 µm at 5 years. During the 5-year follow-up period of this study, the pattern of corneal thickness recovery slowed after 3 years, in contrast to previous studies that reported that corneal thickness steadily recovered for 3 years. Thus, we suggest that longer follow-up periods were required after CXL in patients with thin corneas. The corneal thickness of the control eyes did not show any statistically significant differences when compared to the baseline, unlike other clinical indicators including keratometric values. We suspect that most of the control eyes in this study were in the early stages of keratoconus, and the initial changes in corneal thickness were not significantly greater than the keratometric values, which were sensitive parameters of initial corneal changes.
One year after treatment, all keratoconus indices were significantly improved compared to the baseline and this improvement was maintained without statistically significant changes for 5 years (Table 2). This suggests that the effectiveness of CXL stabilized at 1 year after treatment and remained consistent through the 5 years of follow-up.
In the untreated eyes, the BCVA decreased and the Kmax, Kmean, and corneal astigmatism gradually increased over time. In particular, the Kmean significantly increased by 0.4 D at 2 years and by 0.8 D at 5 years, indicating the progression of keratoconus. Although the Kmax increased by 0.8 D from 54.21 ± 9.87 to 55.01 ± 9.91 D at 5 years, there was no statistically significant difference from the baseline. A previous study reported increases in Kmax of 0.14 D at 3 months, 1.28 D at 1 year, and 1.75 D at 3 years in patients with untreated keratoconus [18,29]. In this study, the changes in the corneal curvature of the untreated group were not larger than those of a previous study. However, considering that the untreated eyes were initially diagnosed as non-progressive keratoconus, the keratoconus was aggravated over time compared to the treated eyes, suggesting that prompt and aggressive treatment such as with CXL can be a potential therapeutic option to halt disease progression, even in non-progressive keratoconus.
We analyzed corneal edema and stromal haze to evaluate the corneal toxicity of CXL. Corneal edema temporally occurred after CXL and disappeared within 1 week. In the treatment group, one of nine eyes (11.1%) developed mild stromal haze, which regressed within 1 month. A previous study reported stromal haze in 9.8% of treated eyes after CXL, a similar prevalence to that in our study [12].
The limitations of this study include the small number of treated eyes and the retrospective analysis without randomized controls.
In conclusion, the 5-year data from our study demonstrated that CXL improves visual acuity and inhibits the progression of keratoconus. All clinical indicators improved at 1 year after treatment and our study found that the effect of CXL remained stable up to 5 years. In contrast to previous studies, the recovery of corneal thickness slowed after 3 years, suggesting that cautious long-term follow-up of corneal thickness is required after CXL treatment, especially in patients with thin corneas. Though this study provides important data on the long-term stability of CXL with UVA in Korean patients, prospective studies with larger numbers of patients are required in the future.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Rabinowitz YS, Garbus J, McDonnell PJ. Computer-assisted corneal topography in family members of patients with keratoconus. Arch Ophthalmol. 1990;108:365–371. doi: 10.1001/archopht.1990.01070050063032. [DOI] [PubMed] [Google Scholar]
- 2.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 3.Espandar L, Meyer J. Keratoconus: overview and update on treatment. Middle East Afr J Ophthalmol. 2010;17:15–20. doi: 10.4103/0974-9233.61212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17:356–360. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Lee TH, Lee KH. Intracorneal ring segment implantation for the management of keratoconus: short-term safety and efficacy. J Korean Ophthalmol Soc. 2009;50:1505–1509. [Google Scholar]
- 7.Mencucci R, Marini M, Paladini I, et al. Effects of riboflavin/UVA corneal cross-linking on keratocytes and collagen fibres in human cornea. Clin Experiment Ophthalmol. 2010;38:49–56. doi: 10.1111/j.1442-9071.2010.02207.x. [DOI] [PubMed] [Google Scholar]
- 8.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 9.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23:503–507. doi: 10.1097/01.ico.0000105827.85025.7f. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 12.Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. 2010;149:585–593. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Grewal DS, Brar GS, Jain R, et al. Corneal collagen cross-linking using riboflavin and ultraviolet-A light for keratoconus: one-year analysis using Scheimpflug imaging. J Cataract Refract Surg. 2009;35:425–432. doi: 10.1016/j.jcrs.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 14.Koller T, Iseli HP, Hafezi F, et al. Scheimpflug imaging of corneas after collagen cross-linking. Cornea. 2009;28:510–515. doi: 10.1097/ICO.0b013e3181915943. [DOI] [PubMed] [Google Scholar]
- 15.Vinciguerra P, Albe E, Trazza S, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009;116:369–378. doi: 10.1016/j.ophtha.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Vinciguerra P, Albe E, Trazza S, et al. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009;127:1258–1265. doi: 10.1001/archophthalmol.2009.205. [DOI] [PubMed] [Google Scholar]
- 17.Wittig-Silva C, Whiting M, Lamoureux E, et al. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008;24:S720–S725. doi: 10.3928/1081597X-20080901-15. [DOI] [PubMed] [Google Scholar]
- 18.Wittig-Silva C, Chan E, Islam FM, et al. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;121:812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Nottingham J, editor. Practical observations on conical cornea: and on the short sight, and other defects of vision connected with it. London: John Churchill; 1854. pp. 23–26. [Google Scholar]
- 20.Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33:157–166. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Tomkins O, Garzozi HJ. Collagen cross-linking: strengthening the unstable cornea. Clin Ophthalmol. 2008;2:863–867. doi: 10.2147/opth.s2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan Z, Szalai E, Modis L, Jr, et al. Assessment of corneal topography indices after collagen crosslinking for keratoconus. Eur J Ophthalmol. 2013;23:635–640. doi: 10.5301/ejo.5000249. [DOI] [PubMed] [Google Scholar]
- 23.Poli M, Cornut PL, Balmitgere T, et al. Prospective study of corneal collagen cross-linking efficacy and tolerance in the treatment of keratoconus and corneal ectasia: 3-year results. Cornea. 2013;32:583–590. doi: 10.1097/ICO.0b013e31825e8414. [DOI] [PubMed] [Google Scholar]
- 24.Lee P, Jin KH. Clinical results of riboflavin and ultraviolet-A -induced corneal cross-linking for progressive keratoconus in Korean patients. J Korean Ophthalmol Soc. 2011;52:23–28. [Google Scholar]
- 25.Noh SJ, Ahn JM, Han KE, Seo KY. Changes in corneal keratometry readings after corneal collagen cross-linking using alcohol in keratoconus patients. J Korean Ophthalmol Soc. 2012;53:1591–1596. [Google Scholar]
- 26.Zadnik K, Mannis MJ, Johnson CA, Rich D. Rapid contrast sensitivity assessment in keratoconus. Am J Optom Physiol Opt. 1987;64:693–697. doi: 10.1097/00006324-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Kanellopoulos AJ, Asimellis G. Revisiting keratoconus diagnosis and progression classification based on evaluation of corneal asymmetry indices, derived from Scheimpflug imaging in keratoconic and suspect cases. Clin Ophthalmol. 2013;7:1539–1548. doi: 10.2147/OPTH.S44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol Vis Sci. 2010;51:3835–3839. doi: 10.1167/iovs.09-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:691–700. doi: 10.1016/j.jcrs.2010.10.052. [DOI] [PubMed] [Google Scholar]