Abstract
Introduction
Kidney stone disease is increasing worldwide with its most common location being in the lower pole. A clear strategy for effective management of these stones is essential in the light of ever increasing choice, effectiveness, and complications of different treatment options.
Material and methods
This review identifies the latest and clinically relevant publications focused on optimal management of lower pole stones.
Results
We present an up-to-date European Association of Urology and American Urological Association algorithm for lower pole stones, risks and benefits of different treatments, and changing landscape with the miniaturization of percutaneous stone treatments.
Conclusions
Available literature seems to be deficient on quality of life, patient centered decision making, and cost analysis of optimal management with no defined standard of ‘stone free rate’, all of which are critical in any surgical consultation and outcome analysis.
Keywords: lower pole stone, treatment, kidney stones, urolithiasis, ureteroscopy
INTRODUCTION
With the lifetime prevalence of stone disease estimated at up to 15% and on the rise [1], it has become even more important to formulate a clear and effective management strategy that offers high stone free rates (SFR) in as few sessions and with least invasiveness as possible. Whilst this might be possible in some stone locations, the management of lower pole stones (LPS) continues to be a subject of fierce debate; anatomical variations in the lower pole calyx pose challenges unique to this stone location [2], and with only a handful of randomised-controlled trial data comparing all current treatment options, the jury is still out.
The last couple of years have seen some significant steps forward in providing a stronger evidence base for LPS management. Of note is the systematic review and meta-analysis comparing the clinical effectiveness of the three main treatment options: extracorporeal Shockwave Lithotripsy (SWL), Retrograde Intrarenal Surgery (RIRS), and Percutaneous Nephrolithotomy (PNL) [3]. Despite providing some evidence in management of LPS, Donaldson et al. highlighted the significant clinical heterogeneity between studies; this resulted in SFR being the only comparable outcome measure. Even then, the exact definition and method of measurement of SFR varied [3].
With a lack of large prospective randomised studies and the heterogeneity highlighted by Donaldson et al., there are still many key questions awaiting answers. Most notably, there remains a strongly divided opinion over whether PNL or RIRS is better for the management of LPS >10 mm. There has not been a randomised-controlled trial evaluating the effectiveness of RIRS versus PNL for the management of LPS since the Lower Pole II study in 2003 [4]. Although highlighting a greater stone free rate for large stones (10–25 mm) at three months for PNL, the number of patients involved in the study was low (n = 28) and the results were not statistically significant (p = 0.29) [4]. Furthermore, the technological advancement and miniaturisation of instruments enjoyed by both RIRS and PNL in recent years has very much outdated this study [5]. There is also a need to directly investigate RIRS versus SWL (with and without the use of adjuvant therapies such as inversion, percussion, and diuresis) in the management of small to moderate LPS. Although Burr et al. have significantly contributed to the evidence base by recently publishing their prospective comparison of 161 procedures that found RIRS to have a stone free rate at 3 months of 92.6% versus 24.7% for SWL (p ≤0.001), they also acknowledge the need for a large prospective randomised-controlled study [6].
As a result of the need to rely on small studies and expert opinion panels, the European Association of Urology 2016 Urolithiasis guidelines and recent updates from the American Urological Association are not entirely in agreement when it comes to treatment algorithms (Figure 1 and Figure 2 respectively) [7, 8, 9]. Although they concur in the use of PNL as the first line choice for all stones >20 mm, in every other aspect the recommendations differ. This goes some way in highlighting the myriad of conflicting and inadequate evidence in this field.
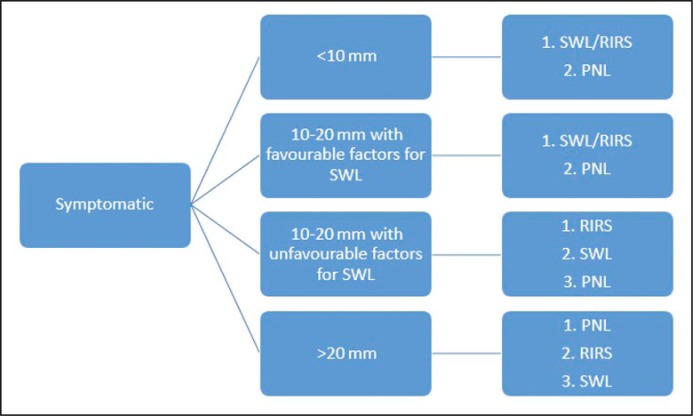
Figure 1.
Treatment algorithm for lower pole stones recommended by European Association of Urologists.
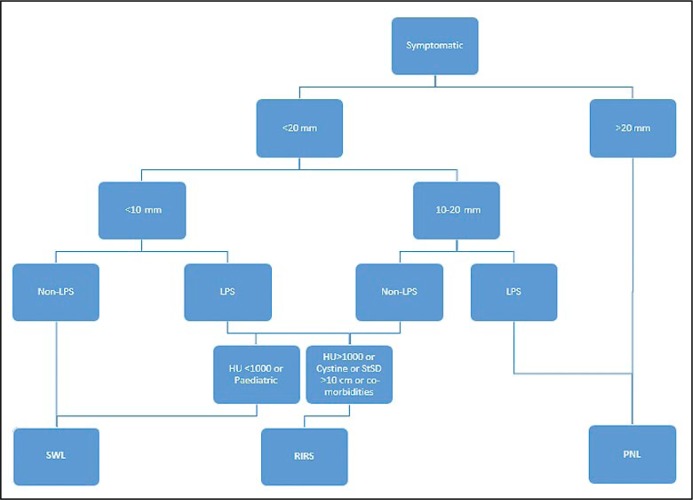
Figure 2.
Treatment algorithm for lower pole stones recommended by American Urological Association.
Bearing this in mind, we aim to collate the most current evidence to assess the challenges in LPS management, outline and compare treatment options including mini-, ultramini- and micro-PNL, and ultimately offer an evidence-based strategy for managing LPS in 2016.
Challenges in LPS management
Fundamental to the problematic nature of LPS management is the anatomy of the pelvicalyceal system. The unfavourable location of the lower pole makes for tricky spontaneous stone passage [3]. Furthermore, the impact of an acute infundibulopelvic angle, a tight infundibular width, and a long infundibular length on hindering stone clearance are, on the whole, well-established negative predictors for SFR after initial treatment with SWL [10, 11]. However, there is still a lack of consensus over both the thresholds at which these anatomical features begin to significantly inhibit stone clearance and the radiological method by which these figures might be consistently established [12]; this in itself presents a challenge to urologists in determining a patient’s suitability for SWL.
Due to the awkward anatomy of the lower pole, residual fragments are not uncommon in LPS treatment (particularly with SWL) and this forms a nidus for further stones and poses a challenge to the patient’s future management[13]. Much rides on whether these fragments are obstructive, infective, or symptomatic. Cicerello and colleagues argue that non-infective, non-obstructive, asymptomatic residual fragments <5 mm in size can be managed metabolically [14]. Their study of 34 patients with post-SWL residual fragments showed that 44.4% of patients on potassium citrate therapy were stone free at 12 months compared to 12.5% in the control group [14]. Nonetheless, residual fragments are a risk factor for further stone growth [7].
Similarly, the management of non-obstructing asymptomatic calculi is a challenge given the tricky nature of managing these in the lower pole. There has been debate over the natural history of asymptomatic renal stones in recent years in an attempt to define at what point treatment should be initiated, because most patients with small, asymptomatic lower pole stones do well with observation. A rise in healthcare screening has increased the diagnosis of asymptomatic renal calculi [15]. Some studies have suggested SWL to be a less invasive treatment option in the long term [16], but this must be balanced against cost-effectiveness considering the low SFR achieved by SWL for these stones. Equally, patient choice and previous experiences are important factors which have an impact upon how asymptomatic calculi are treated. There is certainly a need to investigate patient preference and optimal strategy for managing asymptomatic lower pole calculi [17].
Treatment options
Shockwave lithotripsy
SWL has traditionally been the first line treatment for all stones <20 mm in size due to being minimally invasive with a low morbidity, short procedural time, and causing minimal convalescence [3, 18, 19]. However, apart from anatomical considerations, there are other significant factors that can alter the success of SWL including the type of lithotripter, number of shockwaves delivered, and energy level used [10]. The aforementioned challenges of lower pole anatomy, the stone composition, and the patient’s body habitus all impact upon the ability of SWL to deliver a stone-free, fragment-free patient in a single session [18, 20]. Consequently, a large variation in reported SFR can be seen in the literature (ranging from 25 to 85%) which have unsurprisingly led to questions surrounding SWL’s use as the principal treatment option [21]. The ‘Lower Pole I’ study first highlighted this. Based on 128 patients with symptomatic LPS up to 30 mm in size, the study found a SFR at three months of 37% for SWL versus 95% for PCNL (p ≤0.001) with no significant difference in morbidity [22]. More recently, El-Nahas et al. specifically compared SWL with RIRS for LPS 10–20 mm in size, reporting a SFR at three months of 67.7% for SWL compared to 86.5% for RIRS (p = 0.038). The percentage retreatment rate was 59.7% with SWL versus 8% with RIRS (p <0.001) and the number of procedures per patient was double for SWL compared to RIRS (p <0.001) [23]. The systematic review of seven randomised-controlled trials reported a median stone free rate of 54.5% for SWL versus 96.3% for PNL and 91.7% for RIRS [3]. This group reported that, of the five randomised-controlled trials comparing RIRS with SWL, RIRS was significantly more effective for stones 10–20 mm in size but the magnitude of improvement was considerably lower for stones <10 mm [3]. Pearle et al. also highlighted that SWL is often more acceptable by patients compared to RIRS for stones <10 mm [24]. Therefore, it seems reasonable to consider using SWL as a first-line option for stones <10 mm in size taking into account the limiting factors that might reduce the efficacy of SWL. However, for stones between 10–20 mm, with technological advances and increased surgeon expertise, considerably better SFR can be achieved by other techniques with similar morbidity rates to SWL
The use of adjuvant therapies to improve stone free rates for SWL provide a compromise by improving the stone free rate of standard SWL treatment whilst being non-invasive in nature. Although some studies have called into question whether inversion, percussion, and diuresis are at all effective [25], Lee et al. published a recent systematic review comparing the effectiveness and safety of LPS treatment methods. This showed that, of the six studies comparing SWL with SWL + adjuvant therapy, SWL + adjuvant therapy had a higher stone free rate versus SWL alone in all cases [26]. Likewise, the 2013 Cochrane review included two small studies and concluded that percussion, diuresis, and inversion are likely to be safe and effective methods to increase stone clearance with SWL [27]. It is tricky to determine the level of efficacy of the different adjuvant therapies, as all were used in alternative combinations that make comparison between methods challenging. However, Cakiroglu et al. specifically analysed the effectiveness of a thirty degree inclined position during SWL in the treatment of lower pole calculi. Seven hundred and forty patients >18 years of age with solitary radiopaque calculi 4–20 mm in size were randomised and at six months. The SFR for patients who had undergone SWL + adjuvant therapy was 81% versus 73% for the control group (p = 0.015). There were no significant differences reported in the rate of complications, retreatments, or supplementary procedures. SFR was not stratified according to size and data was unavailable for stone composition, stone density, and skin-to-stone distance, but their data suggests a possible role for the use of inversion therapy with SWL treatment to improve SFR [18].
Retrograde Intrarenal Surgery
RIRS provides an alternative to SWL as a first line treatment option for stones between 10–20 mm as well as in the management of lower pole stones >15 mm [7]. As a minimally invasive intervention, RIRS can be used both to fragment the stone(s) and/or to displace the stone(s) to a more accessible location for basket removal [9]. The 2003 Lower Pole II study rated PNL to have a significantly better outcome in terms of SFR compared to RIRS (67% versus 46%, p = 0.09) [4], but this was low number and underpowered clinical trial based on all together 28 patients. Moreover, the time the trial was conducted RIRS was very much underdeveloped. Currently, this method has substantially improved; the enhanced optics, increased flexibility of holmium laser fibres, and the bidirectional 270 degree flexion capacity of the latest scopes have all helped to improve SFR for RIRS with or without the use of access sheaths [21, 28]. A recent study comparing RIRS and PNL for stones >20 mm in 109 patients with a mix of stone types demonstrated reasonably comparable but not statistically significant SFR (90.6% for RIRS versus 96.1% for PNL) [28]. A systematic review and meta-analysis of PNL versus RIRS by De et al. concurred that SFR for PNL is higher than RIRS although it also suggested that there was no difference between operative times for the two methods and RIRS led to a shorter hospital stay. The review also highlighted the drawbacks of RIRS including limited visualisation and high costs [29], although the latter is refuted by Somani et al. [30] who say that the cost is possible linked to the case load, cost of ancillary equipment, and repair of flexible ureteroscopes. Meanwhile, two recent studies comparing RIRS with SWL for LPS highlight that SFR for RIRS are still high with figures of 86.5% (p = 0.038) and 92.6% (p <0.001) being quoted. The complication rates in these studies are low and show that morbidity is similar between RIRS and SWL [6, 23]. There are other notable advantages of RIRS over SWL including its use on larger stones, in obese or pregnant patients, and in patients with coagulopathy [6, 31]. Taking everything into account, it is certainly plausible to consider RIRS as a first line option for treating LPS.
Percutaneous nephrolithotomy
Standard PNL consistently delivers the highest SFR of all treatment options (>90%), yet due to its invasive nature and higher risks of complications such as bleeding, need for embolization, adjacent organ injury, and urinary extravasation, as well as the higher transfusion rate and longer hospital stay, it has long been reserved for LPS >20 mm [3, 26, 28, 29]. The decreasing size of the tracts, scopes, energy sources, and retrieval devices over the past two decades have led to the development of new methods of PNL and a subsequent decrease in complication rates. This has resulted in standard PNL (tract size 26-30Fr) being replaced by Midi (20–22F), Mini- (16–18Fr), Ultramini (11–14Fr), and Micro-PNL (<10Fr) techniques in appropriate patients [32]. Mini-PNL offers decreased blood loss, hospital stay, analgesic requirements, and overall complication rates whilst maintaining similar SFR[32, 33]. Kirac et al. compared RIRS with Mini-PNL in 73 patients with LPS <15 mm where SFR were comparable (91.9% for Mini-PNL versus 91.6% for RIRS) at 24 hours. The mean theatre time was significantly lower for Mini-PNL (53.7 minutes versus 66.4 minutes for RIRS), but with a significantly longer length of hospital stay (42.6 hours versus 24.5 hours for RIRS) [34]. Further studies on greater patient numbers are required to investigate the use of Mini-PNL for LPS of all sizes. A recent case report highlighted the successful use of micro-PNL on a LPS case complicated by renal scarring from a previous partial nephrectomy [35], but to date there still exists an overall lack of high quality studies on both ultramini- and micro-PNL. Ultramini-PNL benefits from a single-step dilation process while micro-PNL’s ‘all seeing needle’ allows for perfect puncture and also causes clinically insignificant blood loss [32, 33]. With clear advantages for patient outcome in both of these techniques, particularly when tackling LPS <15 mm, it is important that multicentre studies are setup to address their use for LPS as soon as possible. Equally, the success of outpatient tubeless PNL in carefully selected patients warrants investigations into possible opportunities for this in the management of LPS [36].
A summary of the advantages and disadvantages of each treatment method, as well as the indications for each method, can be seen in Table 1.
Table 1.
Optimal management of LPS in 2016. Comparison of advantages and disadvantages of the various treatment options for lower pole stones. As there is a lack of good quality data comparing Mini-, Ultramini-, and Micro-PNL versus other treatment methods for lower pole stones, these have not been included as separate entities
| Treatment Method | Advantages | Disadvantages | Indications |
|---|---|---|---|
| SWL | Non-invasive Few risks and low morbidity Short procedural time Can be done in an Outpatient setting with minimal anaesthetic required |
Poor stone free rate for lower pole stones Poor efficacy with stones >1000HU Contraindicated in patients with coagulopathy, large stones, and patients who are pregnant or obese High set-up cost |
First line treatment option for lower pole stones <10 mm where no unfavourable factors1 are present |
| SWL + Adjuvant Therapy2 | As above, except stone free rates are higher | As above | As above. The use of adjuvant therapy adds little to no extra cost but is shown to improve stone free rates. Adjuvant therapy is therefore recommended where possible |
| RIRS | Minimally invasive Minimal hospital stay required High stone free rates Suitable for all stone compositions Morbidity comparable to SWL |
Limited visualization Small size of fragment required for removal Reliance on high-cost disposable instrumentation |
First line treatment option for lower pole stones <20 mm Indicated where SWL has failed. Second line treatment for stones >20 mm |
| PNL | Highest stone free rates Suitable for all types of stones and in all locations; Variable (usually short) procedural time Mini-, ultramini-, and micro-PNL with smaller tract sizes can help to minimise morbidity |
Invasive Morbidity higher than other treatment options Significant blood loss (in some cases) Longer hospitalisation Serious complications such as urosepsis, pneumothorax and colonic injury are possible; Contra-indicated in patients with bleeding diatheses |
Indicated for stones >15 mm. Further research is required to determine the use of mini-, ultramini- and micro-PNL for stones 10-20 mm |
shockwave-resistant stones, acute infundibulopelvic angle, long lower pole calyx, narrow infundibulum
inversion, percussion, diuresis
Conclusions and future direction
Lower pole stones make up an estimated 25–35% of all kidney stones [11]. Despite this, there is a lack of consensus over the optimal management plan. SWL with and without adjuvant therapies, RIRS, and PNL (including mini-, ultramini- and micro-PNL) are all possible options, each with their respective merits and flaws. The use of SWL with adjuvant therapies has, to date, recorded better SFR than SWL alone [27]. The systematic review from Aberdeen highlighted only a minor difference in risk ratios in favour of RIRS for LPS <10 mm, thus supporting a trial of SWL (preferably with at least one adjuvant therapy) for stones <10 mm [3]. A recent comparison of SWL versus RIRS for LPS of all sizes sided in favour of RIRS as a first line treatment considering the statistically significant, far superior SFR and comparable morbidity rates [6]. Both are reasonable first line treatments and much depends on the individual surgeons’ skill, available equipment, experience with each modality, and patient preference, as well as factors that might reduce the efficacy of SWL. For larger stones, RIRS and PNL are both possibilities. PNL has the consistently higher SFR but at the expense of greater morbidity and longer hospital stay. Again, patient and surgeon preference has a considerable impact on decision making as well as anatomical/stone factors that might make one more suitable than the other.
The advent of refined PNL techniques undoubtedly spells a new era in LPS management, and data from prospective randomised trials is needed in order to establish how and where these techniques fit into management algorithms. Similarly, there is a critical need to add to the evidence base with more long term data and randomised-controlled trials comparing a host of qualitative and quantitative outcomes for all available treatment methods in the context of LPS.
At present, there is limited data surrounding patient-centred outcomes and cost-benefit analysis, and again this needs addressing in respect to the contemporary treatment options. Finally, there is a lack of consensus over the measurement of outcomes, most notably SFR, and this makes comparison of treatment methods a greater challenge. Somani et al. have published a system of categorisation for SFR [37]. Future development of care pathways will certainly benefit if outcomes work to a pre-determined standard.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Long LO, Park S. Update on Nephrolithiasis Management. Minerva Urol Nefrol. 2007;59:317–325. [PubMed] [Google Scholar]
- 2.Juan YS, Chuang SM, Wu WJ, Shen JT, Wang CJ, Huang CH. Impact of lower pole anatomy on stone clearance after shock wave lithotripsy. Kaohsung J Med Sci. 2005;21:385–364. doi: 10.1016/S1607-551X(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 3.Donaldson JF, Lardas M, Scrimgeour D, et al. Systematic review and meta-analysis of the clinical effectiveness of shock wave lithotripsy, retrograde intrarenal surgery, and percu-taneous nephrolithotomy for lower-pole renal stones. Eur Urol. 2015;67:612–616. doi: 10.1016/j.eururo.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 4.Kuo RL, Lingeman JE, Leveillee RJ, et al. Lower Pole II – initial results from a comparison of shock wave lithotripsy, ureteroscopy and percutaneous nephrolithotomy for lower pole nephrolithiaisis. J Urol. 2003;169:486. [Google Scholar]
- 5.Patterson JM, Rukin NJ. Lower-pole stones: do we finally have more answers than questions? Eur Urol. 2015;67:617–618. doi: 10.1016/j.eururo.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Burr J, Ishii H, Simmonds N, Somani BK. Is flexible ureterorenoscopy and laser lithotripsy the new gold standard for lower pole renal stones when compared to shock wave lithotripsy: Comparative outcomes from a University hospital over similar time period. Cent European J Urol. 2015;68:183–186. doi: 10.5173/ceju.2015.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Türk C, Petřík A, Sarica K, Seitz C, Skolarikos S, Straub M, Knoll T. EAU Guidelines on Interventional Treament for Urolithiasis. Eur Urol. 2015;69:475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Flannigan RK, Harriman DI, Paterson RF, Chew BH. Where does Shock Wave Lithotripsy fit in 2015? Technology, patient selection and techniques. American Urological Association Update Series. 2015;34 [Google Scholar]
- 9.Kaplan AG, Lipkin ME. Ureteroscopic Management of Renal Calculi. American Urological Association Update Series. 2015;34 [Google Scholar]
- 10.Ullah A, Zubair M, Khan N, Malik A. Frequency and Factors Effecting Non-clearance of Lower Pole Renal Stones. J Ajub Med Coll Abbottabad. 2015;27:384–387. [PubMed] [Google Scholar]
- 11.Gurocak S, Kupeli B, Acar C, Tan MO, Karaoglan U, Bozkirli I. The impact of pelvicaliceal features on problematic lower pole stone clearance in different age groups. Int Urol Nephrol. 2008;40:31–37. doi: 10.1007/s11255-007-9220-z. [DOI] [PubMed] [Google Scholar]
- 12.Manikandan R, Gall Z, Gunendran T, Neilson D, Adeyoju A. Do anatomic factors pose a significant risk in the formation of lower pole stones? Urology. 2007;69:620–624. doi: 10.1016/j.urology.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Danuser H, Muller R, Descoeudres B, Dobry E, Studer UE. Extracorporeal shock wave lithotripsy of lower calyx calculi: how much is treatment outcome influenced by the anatomy of the collecting system? Eur Urol. 2007;52:539–546. doi: 10.1016/j.eururo.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 14.Cicerello E, Merlo F, Maccatrozzo L. Management of Clinically Insignificant Residual Fragments following Shock Wave Lithotripsy. Adv Urol. 2012:320104. doi: 10.1155/2012/320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang HW, Lee SK, Kim WT, et al. Natural history of asymptomatic renal stones and prediction of stone related events. J Urol. 2013;189:1740–1746. doi: 10.1016/j.juro.2012.11.113. [DOI] [PubMed] [Google Scholar]
- 16.Osman MM, Alfano Y, Kamp S, et al. 5-year-follow-up of patients with clinically insignificant residual fragments after extracorporeal shockwave lithotripsy. Eur Urol. 2005;47:860–864. doi: 10.1016/j.eururo.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Sarkissian C, Noble M, Li J, Monga M. Patient decision making for asymptomatic renal calculi: balancing benefit and risk. Urology. 2013;81:236–240. doi: 10.1016/j.urology.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Cakiroglu B, Sinanoglu O, Tas T, Hazar IA, Balci MB. The effect of inclined position on stone free rates in patients with lower caliceal stones during SWL session. Arch Ital Urol Androl. 2015;87:38–40. doi: 10.4081/aiua.2015.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Honey J. Treating lower pole renal stones: in defence of shock wave lithotripsy. Can Urol Assoc J. 2008;2:625–627. doi: 10.5489/cuaj.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preminger GM. Management of lower pole renal calculi: shock wave lithotripsy versus percutaneous nephrolithotomy versus flexible ureteroscopy. Urol Res. 2006;34:108–111. doi: 10.1007/s00240-005-0020-6. [DOI] [PubMed] [Google Scholar]
- 21.Wright AE, Rukin NJ, Somani BK, Smith RD. An update on lower pole stone management for 2015. UROMA 15 Synopsis. 2015 [Google Scholar]
- 22.Albala D, Assinos D, Clayman R. Lower Pole I - A prospective randomised trial of extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy for lower pole nephrolithiasis - initial results. J Urol. 2001;166:2072–2080. doi: 10.1016/s0022-5347(05)65508-5. [DOI] [PubMed] [Google Scholar]
- 23.El-Nahas AR, Ibrahim HM, Youssef RF, Sheir KZ. Flexible ureterorenoscopy versus extracorporeal shock wave lithotripsy for treatment of lower pole stones of 10-20 mm. BJU Int. 2012;110:898–902. doi: 10.1111/j.1464-410X.2012.10961.x. [DOI] [PubMed] [Google Scholar]
- 24.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:2005–2009. doi: 10.1097/01.ju.0000158458.51706.56. [DOI] [PubMed] [Google Scholar]
- 25.Albanis S, Ather HM, Papatsoris AG, et al. Inversion, hydration and diuresis during extracorporeal shock wave lithotripsy: does it improve the stone-free rate for lower pole stone clearance? Urol Int. 2009;83:211–216. doi: 10.1159/000230026. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, Chaiyakunapruk N, Chong HY, Liong ML. Comparative effectiveness and safety of various treatment procedures for lower pole renal calculi: a systematic review and network meta-analysis. BJU Int. 2015;116:252–264. doi: 10.1111/bju.12983. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Li Q, Wei Q, Liu Z, Xu Y. Percussion, diuresis and inversion therapy for the passage of lower pole kidney stones following shock wave lithotripsy. Cochrane Database Syst Rev. 2013:CD008569. doi: 10.1002/14651858.CD008569.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Koyuncu H, Yencilek F, Kalkan M, Bastug Y, Yencilek E, Ozdemir AT. Intrarenal Surgery vs Percutaneous Nephrolithotomy in the Management of Lower Pole Stones Greater than 2 cm. Int Braz J Urol. 2015;41:245–251. doi: 10.1590/S1677-5538.IBJU.2015.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De S, Autorino R, Kim FJ, et al. Percutaneous nephrolithotomy versus retrograde intrarenal surgery: a systematic review and meta-analysis. Eur Urol. 2015;67:125–137. doi: 10.1016/j.eururo.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Somani BK, Robertson A, Kata SG. Decreasing the cost of flexible ureterorenoscopic procedures. Urology. 2011;78:528–530. doi: 10.1016/j.urology.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 31.Ishii H, Aboumarzouk O, Somani BK. Current status of ureteroscopy for stone disease in pregnancy. Urolithiasis. 2014;42:1–7. doi: 10.1007/s00240-013-0635-y. [DOI] [PubMed] [Google Scholar]
- 32.Schilling D, Hüsch T, Bader M, Herrmann TR, Nagele U. Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Nomenclature in PCNL or The Tower of Babel: a proposal for a uniform terminology. World J Urol. 2015;33:1905–1907. doi: 10.1007/s00345-015-1506-7. [DOI] [PubMed] [Google Scholar]
- 33.Wells H, Rukin N, Wright A, Somani BK. Outcome-Based Comparison of Percutaneous Procedures for Urinary Lithiasis with Calibre of Instrumentation less than 12Fr. Curr Urol Rep. 2015;16:53. doi: 10.1007/s11934-015-0528-4. [DOI] [PubMed] [Google Scholar]
- 34.Kirac M, Bozkurt OF, Tunc L, Guneri C, Unsal A, Biri H. Comparison of retrograde intrarenal surgery and mini-percutaneous nephrolithotomy in management of lower-pole renal stones with a diameter of smaller than 15 mm. Urolithiasis. 2013;41:241–246. doi: 10.1007/s00240-013-0552-0. [DOI] [PubMed] [Google Scholar]
- 35.Karatag T, Buldu I, Kaynar M, Taskapu H, Tekinarslan E, Istanbulluoglu MO. Treatment of symptomatic lower pole stones of a kidney with partial nephrectomy using micropercutaneous nephrolithotomy technique. Case Rep Urol. 2015:456714. doi: 10.1155/2015/456714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beiko D, Lee L. Outpatient tubeless percutaneous nephrolithotomy: the initial case series. Can Urol Assoc J. 2010;4:86–90. doi: 10.5489/cuaj.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somani BK, Desai M, Traxer O, Lahme S. Stone-free rate (SFR): a new proposal for defining levels of SFR. Urolithiasis. 2014;42:95. doi: 10.1007/s00240-013-0630-3. [DOI] [PubMed] [Google Scholar]