Abstract
Introduction
Premature ejaculation is a common sexual disorder, which is usually underreported. Multiple treatment methodologies are in use due to the absence of an effective, universally acceptable treatment modality. The most common drug used is dapoxetine, which has adverse effects limiting its long-term use. Hence, we decided to evaluate the effectiveness of ‘on demand’ silidosin 4 mg in patients with premature ejaculation, who were dissatisfied with dapoxetine 30 mg.
Material and methods
The study included 64 patients who reported premature ejaculation who were unhappy with the treatment with ‘on demand’ dapoxetine 30 mg, either due to its adverse effects or because of its overall inefficacy. They were divided into two groups of 33 and 31 respectively by simple randomization, with Group A treated with ‘on demand’ silodosin 4 mg three hours prior to intercourse, whereas Group B was treated with placebo. Pre- and post-treatment intravaginal ejaculatory latency time (IELT), premature ejaculation profile (PEP) and clinical global impression of change (CGIC) for premature ejaculation were evaluated.
Results
Patients in Group A (silodosin 4 mg) reported statistically significant improvement (p <0.005) in intravaginal ejaculatory latency time (IELT), premature ejaculation profile (PEP) and clinical global impression of change (CGIC) for premature ejaculation, with four patients reporting uncomfortably-delayed ejaculation.
Conclusions
‘On demand’ silodosin 4 mg is an effective treatment option with very few adverse events in those patients suffering from premature ejaculation, who are dissatisfied with dapoxetine 30 mg due to its adverse effects or inefficacy.
Keywords: male sexual dysfunction, premature ejaculation, alpha blockers, selective serotonin reuptake inhibitors
INTRODUCTION
Premature ejaculation is the most prevalent sexual dysfunction among males, with a prevalence of more than 30% [1]. Given its prevalence, the onus was on us, the treating urologists, to provide these patients with optimal treatment. However, even today, a century since the first reported case [2], there are various versions in its definition, classification and treatment. Most worrying on the part of the patient is the absence of a standard, effective treatment without significant adverse effects. A range of treatment modalities has been described, varying from psychotherapy and oral medication to local applications, yet the so-called ‘gold standard’ treatment remains elusive.
Though dapoxetine 30 mg is being widely used as the treatment of premature ejaculation, there are patients who fail to comply with the treatment due to its adverse effects or inefficacy. We decided to evaluate the efficacy of ‘on demand’ silodosin 4 mg in patients who were dissatisfied with dapoxetine, as there are recent reports suggesting the efficacy of silodosin in the treatment of premature ejaculation [3, 4].
MATERIAL AND METHODS
Patients who self-reported premature ejaculation and expressed dissatisfaction with ‘on demand’ treatment with dapoxetine 30 mg were included in the study. Those satisfied with ‘on demand’ dapoxetine 30 mg were excluded. The study participants were asked to record the reason for their dissatisfaction with the treatment before their inclusion in the study. The study period was between 1 June 2014 and 31 October 2015. Approval for the proposed study was granted by the institutional ethical committee. Before inclusion in the study, written informed consent was obtained from each patient and his partner.
Sample size calculation, randomization and statistical analysis
According to available literature, the prevalence of premature ejaculation in males is reported as 30% [1]. In order to ensure the power of the study in excess of 80%, at a significance level of 95%, we calculated the minimum sample size for each (the two) group(s). This sample size calculation was performed using the formula [N = 2 ×{z1−α + z1−β/d− δ0}2 × p (1−p)]. Here N corresponds to the number of subjects in each group, p corresponds to prevalence of premature ejaculation, Zα corresponds to the standard normal deviate for a one-sided test, d corresponds to expected difference and δ0 corresponds to the clinically accepted margin of error, which is taken as 0.05. Using computer-generated simple random numbers between one and 64, the total sample was divided into two groups, Group A and Group B respectively. Patients in Group A received silodosin 4 mg three hours prior to the proposed intercourse. Patients in Group B received a placebo three hours prior to proposed intercourse. During the study, only the patients were blinded (not the investigator). Intravaginal ejaculatory latency time (IELT), premature ejaculation profile (PEP) [5] and the clinical global impression of change for premature ejaculation (CGIC) [6] were recorded in patients, prior to the initiation of the treatment and the very next day of the treatment with drug. Similarly, the premature ejaculation profile (PEP) was recorded in partners as well, as the format allows separate recording of responses from the partners. Any adverse effects reported by the patients were also recorded. Data analysis was done by applying Student’s t-test, using the Statistical Package for the Social Sciences (SPSS) trial version 17. The level of significance was assessed at a p value less than 0.05.
RESULTS
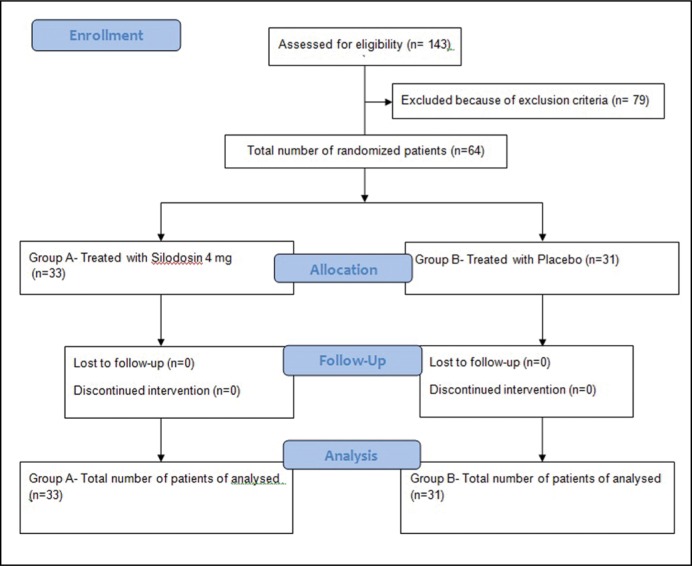
During the study period, we had a total of 143 patients subjected to treatment with ‘on demand’ dapoxetine 30 mg for self-reported premature ejaculation. Of this, 64 patients had expressed their unhappiness with the treatment. Of these 64 patients, 24 reported no improvement of premature ejaculation with dapoxetine treatment, whereas the remaining 40 reported their dissatisfaction due to adverse events, mainly dizziness. The participant flow diagram is shown in Figure 1.
Figure 1.
Participant flow diagram.
By simple randomization, the study participants were split into Group A and Group B. Group A included 33 patients and their partners. Group B included 31 patients and their partners. The average age of patients in Group A was 32.6 ±3.53 years, whereas the average age of patients in Group B was 33.1 ±3.43 years. The average age of their partners in Group A was 28.7 ±2.83 years and the average age of the partners in Group B was 28.7 ±3.14 years. Group A had 27 patients with ‘lifelong’ Premature Ejaculation and 6 patients with ‘acquired’ Premature Ejaculation. Group B consisted of 26 patients with ‘lifelong’ Premature Ejaculation and 5 patients with ‘acquired’ Premature Ejaculation. The difference between the two groups was not statistically significant.
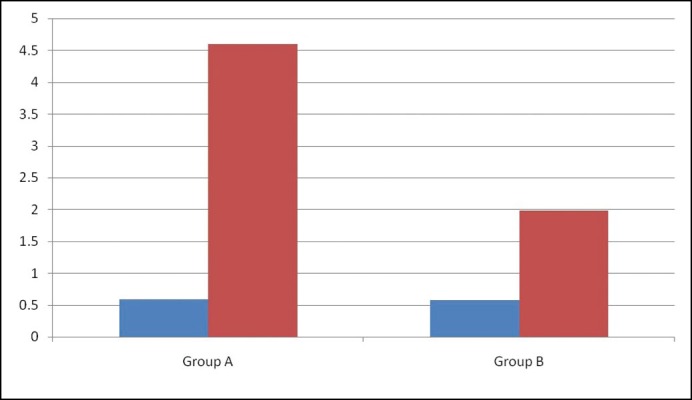
The Intravaginal ejaculatory latency time (IELT), recorded with a stopwatch operated by the partner, showed a statistically significant improvement post-treatment in the silodosin-treated group compared to placebo as shown in Figure 2.
Figure 2.
Diagram showing pre-(indicated in blue) and post-treatment (indicated in red). Intravaginal Ejaculatory Latency Time in seconds (vertical axis) in the two groups.
The premature ejaculation profile consisted of four questions related to satisfaction with sexual intercourse, control over ejaculation, ejaculation-related distress and ejaculation-related difficulty in interpersonal relationships respectively. The first two questions i.e. satisfaction with sexual intercourse and control over ejaculation were assessed according to a five-point, ascending scale, with one representing very poor and five representing very good. The last two questions, i.e. ejaculation-related distress and ejaculation-related difficulty in interpersonal relationships were assessed in descending order, with five representing extreme and one representing negligible or nil. The patient’s ejaculatory control and associated distress could be scaled according the partner’s perception as well. The average scores in both groups are shown in Table 1. It clearly shows statistically significant improvement (p <0.05) in the silodosin-treated group in all four subsets of Premature Ejaculation Profile in both patients and their partners.
Table 1.
Average scores in two groups (pre and post treatment) in different Premature Ejaculation Profile subsets
| PEP subsets | Group A (Average score) | Group B (Average score) | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Partners | Patients | Partners | |||||
| Pre Rx* | Post Rx* | Pre Rx* | Post Rx* | Pre Rx* | Post Rx* | Pre Rx* | Post Rx* | |
| Satisfaction with intercourse (Scale: 1 – very poor, 2 – poor, 3 – fair, 4 – good, 5 – very good) | 1.49 | 4.27 | 1.73 | 4.49 | 1.48 | 2.77 | 1.55 | 3.29 |
| Control over ejaculation (Scale: 1 – very poor, 2 – poor, 3 – fair, 4 –good, 5 – very good) | 1.27 | 4.55 | 1.27 | 4.64 | 1.32 | 2.81 | 1.23 | 2.94 |
| Ejaculation related distress (Scale: 1 – not at all, 2 – a little bit, 3 – moderate, 4 - quite a bit, 5 – extreme) | 4.61 | 1.24 | 4.82 | 1.36 | 4.58 | 3.68 | 4.74 | 3.32 |
| Ejaculation related difficulty in interpersonal relationship (Scale: 1 – not at all, 2 – a little bit, 3 – moderate, 4 - quite a bit, 5 – extreme) | 4.58 | 1.12 | 4.36 | 1.09 | 4.52 | 3.74 | 4.52 | 3.26 |
Rx – treatment
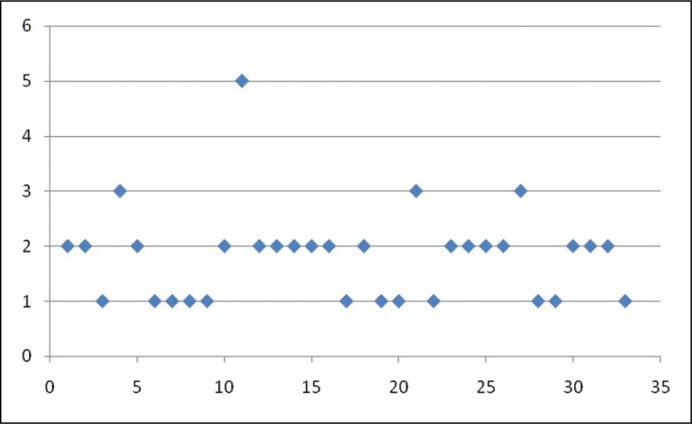
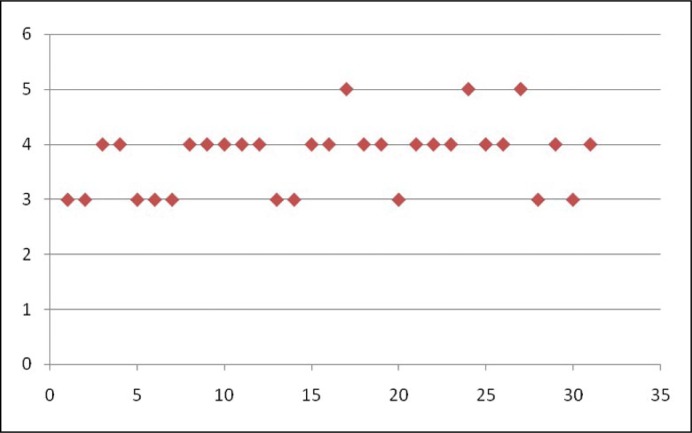
The patients were asked to rate the effect of treatment on premature ejaculation using a seven-point response scale to measure Clinical Global Impression of Change for premature ejaculation. The seven points correspond to very much improved, much improved, minimally improved, no change, minimally worse, much worse and very much worse respectively. The silodosin-treated group reported an average score of 1.82 ±0.85 (corresponding to much improved), whereas the placebo treated group reported an average score of 3.8 ±0.63 (corresponding to almost no improvement). The scatter diagrams showing the clinical global impression of change for premature ejaculation (CGIC) ratings by patients in the two groups are shown in Figure 3 and 4 respectively.
Figure 3.
Scatter diagram showing Clinical Global Impression of Change for premature ejaculation in Group A patients (x axis represents patients and y axis represents Clinical Global Impression of Change score).
Figure 4.
Scatter diagram showing Clinical Global Impression of Change for premature ejaculation in Group B patients (x axis represents patients and y axis represents Clinical Global Impression of Change score).
Surprisingly, very few adverse events were reported by the silodosin-treated group. This included reduced quantity of ejaculate as reported by 26 out of 33 and uncomfortably delayed ejaculation by four. However, none of the patients discontinued treatment and all reported normal orgasm.
DISCUSSION
Premature ejaculation is defined as a male sexual dysfunction characterized by (i) ejaculation which always or nearly always occurs prior to or within about one minute of vaginal penetration from the first sexual experience or a clinically significant bothersome reduction in latency time often to about 3 minutes or less (ii) the inability to delay ejaculation on all or nearly all vaginal penetrations; and (iii) negative personal consequences, such as, distress, bother, frustration, and/or the avoidance of sexual intimacy [7]. Though there are many treatments prescribed for premature ejaculation, none, thus far, has been able to provide most satisfactory treatment to the patient. At present, selective serotonin reuptake inhibitors (SSRIs), specifically dapoxetine, forms the mainstay of pharmacotherapy due to the delay it causes in ejaculation by inhibition of the ejaculatory reflex through inhibitory descending pathways from higher centres [8]. But these are associated with significant adverse effects, including psychiatric and neurologic complications along with unwanted sexual side effects [9]. These effects are less prominent when ‘on demand’ treatment is given; however, adverse effects such as dizziness, loss of libido and nausea still appear even on ‘on demand’ treatment with dapoxetine, making it less than optimal drug for the patient [10]. We have had many such patients who desired pharmacotherapy with a drug other than dapoxetine, which prompted our search for a better, more tolerable drug.
Alpha blockers have long been established in the management of lower urinary tract symptoms. However, their use is associated with patient-reported sexual side effects, mainly retrograde ejaculation and anejaculation. The basis of these effects was reported to be their action on seminal vesicles, which have abundant alpha 1a adrenergic receptors [11]. Silodosin is a highly selective antagonist for this receptor and has been proved effective in the treatment of premature ejaculation at a dosage of 8 mg by Masciovecchio S et al. [1] Hence, we decided to study its effectiveness in those patients dissatisfied with dapoxetine treatment. The dose had to be halved in view of the high incidence of anejaculation at a dose of 8 mg, as reported by Masciovecchio S et al. [1]. Sato Y et al. [3] reported reduced incidence of anejaculation at a 4 mg dose.
We found significant improvement in intravaginal ejaculatory latency time (IELT), which is in line with the reports of Masciovecchio S et al. [1] and Sato Y et al. [3] Both the patients and their partners reported better satisfaction with intercourse following silodosin treatment as compared to placebo. Control over ejaculation was also reported to be good in the silodosin group, with very little ejaculation-related distress. In our study, only four patients reported uncomfortably-delayed ejaculation, and reported minimally-worse to worse on the CGIC scale, following treatment with silodosin. Though other studies have reported an incidence of anejaculation in about 20–25% of patients, none of our patients reported anejaculation [1, 3]. Though Sato Y et al. have reported some discomfort while achieving orgasm in more than 80% of studied patients, none of our patients reported any discomfort during orgasm [3]. A separate study is required to evaluate the efficacy of a lower dose of silodosin in those patients who reported uncomfortably-delayed ejaculation.
The safety of alpha blockers in humans has been well-established for more than three decades by their use in patients with lower urinary tract symptoms (LUTS) [12]. However, using their major adverse event to our advantage was a novel idea floated by Masciovecchio S et al. and Sato Y et al. [1, 3]. As there is a significant subset of patients who are dissatisfied with present standard pharmacotherapy with dapoxetine, mainly due to its adverse effects, the search continues for a safer and better alternative. We are the first to try silodosin in this group of patients, who have either failed therapy with dapoxetine or were unhappy due to its adverse effects.
Though premature ejaculation remains the most common sexual dysfunction in males, the number of patients seeking help from their treating physician for this complaint are considerably lower [13]. Similar was the case in our study, wherein the sample size became quite small as we had picked only that subset of patients self-reporting premature ejaculation who were dissatisfied with dapoxetine treatment. We acknowledge that this reduces the statistical power of the study for generalization of its findings. However, the use of silodosin as second-line treatment for premature ejaculation is a novel idea and our study supports such an approach. A larger, multicentre study, in this regard, is likely to provide stronger evidence in this aspect.
CONCLUSIONS
Silodosin, a drug that has already been proved safe for human use by its safety profile in the treatment of LUTS, is a safe and better-tolerated alternative to dapoxetine in the management of premature ejaculation.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Masciovecchio S, Saldutto P, Del Rosso A, et al. The daily therapy with silodosin can have a role in the treatment of ‘life long’ premature ejaculation. J Androl Sci. 2011;18:48–51. [Google Scholar]
- 2.Serefoglu EC, Saitz TR, Trost L, Hellstrom WJG. Premature ejaculation: do we have effective therapy? Transl Androl Urol. 2013;2:45–53. doi: 10.3978/j.issn.2223-4683.2013.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato Y, Tanda H, Nakajima H, et al. Silodosin and its potential for treating premature ejaculation: A preliminary report. Int J Urol. 2012;19:268–272. doi: 10.1111/j.1442-2042.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- 4.Akin Y, Gulmez H, Ates M, Bozkurt A, Nuhoglu B. Comparison of alpha blockers in the treatment of premature ejaculation: A pilot clinical trial. Iran Red Crescent Med. J. 2013;15:e13805. doi: 10.5812/ircmj.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick DL, Giuliano F, Ho KF, Gagnon DD, McNulty P, Rothman M. The Premature Ejaculation Profile: validation of self-reported outcome measures for research and practice. BJU Int. 2009;103:358–364. doi: 10.1111/j.1464-410X.2008.08041.x. [DOI] [PubMed] [Google Scholar]
- 6.Althof SE, Brock GB, Rosen RC, et al. Validity of the patient reported Clinical Global Impression of Change as a measure of treatment response in men with premature ejaculation. J Sex Med. 2010;7:2243–2252. doi: 10.1111/j.1743-6109.2010.01793.x. [DOI] [PubMed] [Google Scholar]
- 7.Serefoglu EC, McMahon CG, Waldinger MD, et al. An evidence- based unified definition of lifelong and acquired premature ejaculation: report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. Sex Med. 2014;2:41–59. doi: 10.1002/sm2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano F, Clement P. Serotonin and premature ejaculation: from physiology to patient management. Eur Urol. 2006;50:454–466. doi: 10.1016/j.eururo.2006.05.055. [DOI] [PubMed] [Google Scholar]
- 9.Rosen Rc, Lane RM, Menza M. Effects of SSRIs on sexual function: a critical review. J Clin Psychopharmacol. 1999;19:67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Montague DK, Jarrow J, Broderick GA, et al. AUA guideline on the pharmacologic management of premature ejaculation. J Urol. 2004;172:290–294. doi: 10.1097/01.ju.0000132159.61156.ea. [DOI] [PubMed] [Google Scholar]
- 11.Hisaue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T. Ejaculatory disorder caused by alpha 1-adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol. 2006;13:1311–1316. doi: 10.1111/j.1442-2042.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 12.Caine M, Perlbert S, Meretyk S. A placebo-controlled double-blind study of the effect of phenoxybenzamine in benign prostatic obstruction. Br J Urol. 1978;50:551–554. doi: 10.1111/j.1464-410x.1978.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 13.Serefoglu EC, Yaman O, Cayan S, et al. Prevalence of the complaint of ejaculating prematurely and the four premature ejaculation syndromes: results from the Turkish Society of Andrology Sexual Health Survey. J Sex Med. 2011;8:540–548. doi: 10.1111/j.1743-6109.2010.02095.x. [DOI] [PubMed] [Google Scholar]