Abstract
AIMS/BACKGROUND: Posterior capsular opacification is the most common postoperative complication of extracapsular cataract surgery. The purpose of this study was to attempt to inhibit this secondary cataract formation by using mitomycin (an antimitotic drug). METHODS: A solution containing mitomycin was used to perform hydrodissection (with a 5 minute pause) during extracapsular lens extraction in rabbits. This way of administration was chosen to reduce as much as possible drug diffusion into the anterior chamber. Heparin was added to the irrigating solution to avoid fibrin formation. Its ability to prevent posterior cataract opacification was also evaluated at the end of the study. The animals were sacrificed 4 or 6 months after surgery. Grading concerning two aspects of secondary cataract (proliferation and fibrosis) was obtained on gross examination. Histological analysis was subsequently performed. RESULTS: This study demonstrated that mitomycin has a significant inhibitory effect on secondary cataract formation (proliferation as well as fibrosis) in rabbits whereas heparin does not seem to have the same effectiveness. CONCLUSION: This work is a preliminary study concerning the use of mitomycin for prevention of posterior capsular opacification. It has proved its effectiveness in rabbits but more in depth studies are still necessary before its application in humans.
Full text
PDF

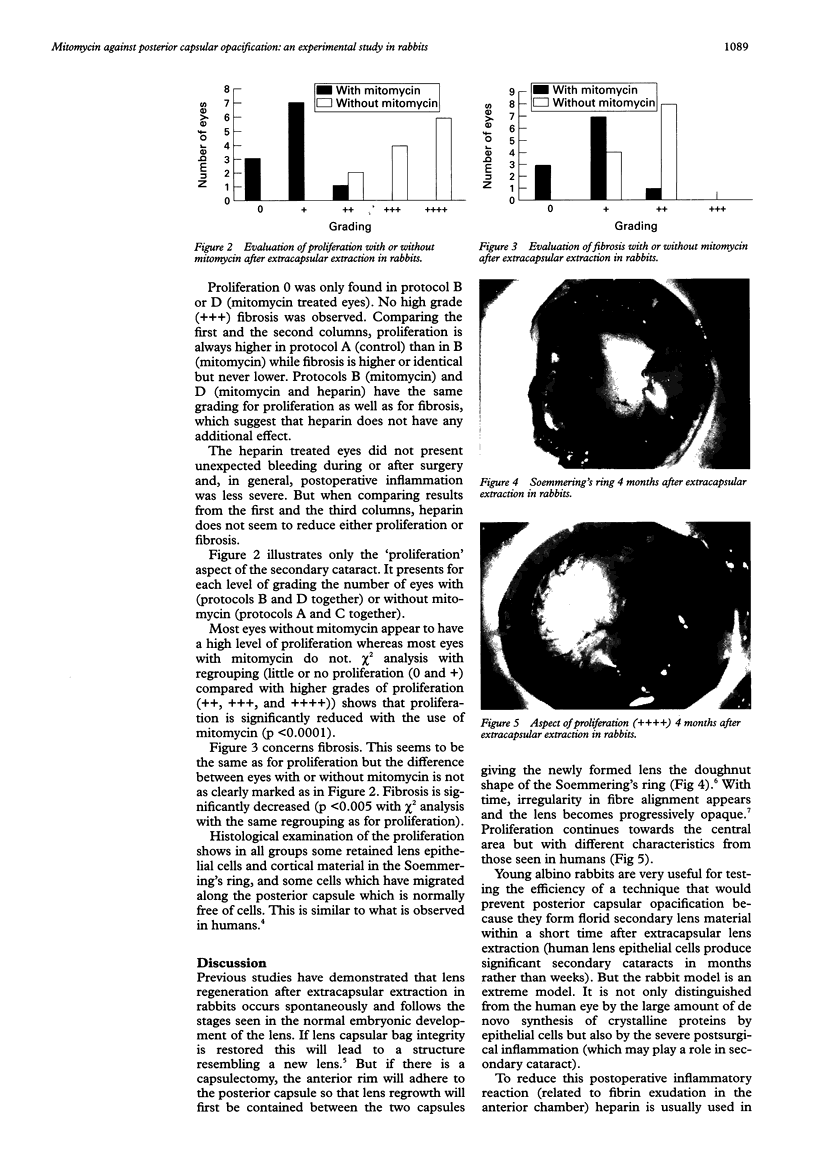


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Derick R. J., Pasquale L., Quigley H. A., Jampel H. Potential toxicity of mitomycin C. Arch Ophthalmol. 1991 Dec;109(12):1635–1635. doi: 10.1001/archopht.1991.01080120013002. [DOI] [PubMed] [Google Scholar]
- Green W. R., McDonnell P. J. Opacification of the posterior capsule. Trans Ophthalmol Soc U K. 1985;104(Pt 7):727–739. [PubMed] [Google Scholar]
- Gwon A. E., Gruber L. J., Mundwiler K. E. A histologic study of lens regeneration in aphakic rabbits. Invest Ophthalmol Vis Sci. 1990 Mar 1;31(3):540–547. [PubMed] [Google Scholar]
- Gwon A., Gruber L. J., Mantras C. Restoring lens capsule integrity enhances lens regeneration in New Zealand albino rabbits and cats. J Cataract Refract Surg. 1993 Nov;19(6):735–746. doi: 10.1016/s0886-3350(13)80343-4. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Noda S., Yamamoto Y., Setogawa T. Postoperative instillation of low-dose mitomycin C in the treatment of primary pterygium. Am J Ophthalmol. 1988 Dec 15;106(6):715–718. doi: 10.1016/0002-9394(88)90706-4. [DOI] [PubMed] [Google Scholar]
- Hettlich H. J., Kaufmann R., Harmeyer H., Imkamp E., Kirkpatrick C. J., Mittermayer C. In vitro and in vivo evaluation of a hydrophilized silicone intraocular lens. J Cataract Refract Surg. 1992 Mar;18(2):140–146. doi: 10.1016/s0886-3350(13)80920-0. [DOI] [PubMed] [Google Scholar]
- Kappelhof J. P., Vrensen G. F., Vester C. A., Pameyer J. H., de Jong P. T., Willekens B. L. The ring of Soemmerring in the rabbit: A scanning electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 1985;223(3):111–120. doi: 10.1007/BF02148886. [DOI] [PubMed] [Google Scholar]
- Kappelhof J. P., Vrensen G. F., de Jong P. T., Pameyer J., Willekens B. L. The ring of Soemmerring in man: an ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1987;225(1):77–83. doi: 10.1007/BF02155809. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y., Suemori-Matsushita H., Yamamoto T., Kawase K. Low-dose and high-dose mitomycin trabeculectomy as an initial surgery in primary open-angle glaucoma. Ophthalmology. 1993 Nov;100(11):1624–1628. doi: 10.1016/s0161-6420(93)31426-0. [DOI] [PubMed] [Google Scholar]
- Knorr M., Wunderlich K., Steuhl K. P., Thiel H. J., Dartsch P. C. Wirkung von Heparin auf die Proliferation kultivierter boviner Linsenepithelzellen. Ophthalmologe. 1992 Aug;89(4):319–324. [PubMed] [Google Scholar]
- McDonnell P. J., Stark W. J., Green W. R. Posterior capsule opacification: a specular microscopic study. Ophthalmology. 1984 Jul;91(7):853–856. doi: 10.1016/s0161-6420(84)34226-9. [DOI] [PubMed] [Google Scholar]
- McDonnell P. J., Zarbin M. A., Green W. R. Posterior capsule opacification in pseudophakic eyes. Ophthalmology. 1983 Dec;90(12):1548–1553. doi: 10.1016/s0161-6420(83)34350-5. [DOI] [PubMed] [Google Scholar]
- Nuyts R. M., Pels E., Greve E. L. The effects of 5-fluorouracil and mitomycin C on the corneal endothelium. Curr Eye Res. 1992 Jun;11(6):565–570. doi: 10.3109/02713689209001812. [DOI] [PubMed] [Google Scholar]
- Peyman G. A., Greenberg D., Fishman G. A., Fiscella R., Thomas A. Evaluation of toxicity of intravitreal antineoplastic drugs. Ophthalmic Surg. 1984 May;15(5):411–413. [PubMed] [Google Scholar]
- Spångberg M., Kihlström I., Björklund H., Bjurström S., Lydahl E., Larsson R. Improved biocompatibility of intraocular lenses by heparin surface modification: a 12-month implantation study in monkeys. J Cataract Refract Surg. 1990 Mar;16(2):170–177. doi: 10.1016/s0886-3350(13)80726-2. [DOI] [PubMed] [Google Scholar]
- Zaturinsky B., Naveh N., Saks D., Solomon A. S. Prevention of posterior capsular opacification by cryolysis and the use of heparinized irrigating solution during extracapsular lens extraction in rabbits. Ophthalmic Surg. 1990 Jun;21(6):431–434. [PubMed] [Google Scholar]