Abstract
Preterm labor caused by uterine contractions is a major contributor to neonatal morbidity and mortality. Treatment intended to reduce uterine contractions include tocolytic agents, such as indomethacin. Unfortunately, clinically used tocolytics are frequently inefficient and cross the placenta causing fetal side effects. Here we show for the first time in obstetrics the use of a targeted nanoparticle directed to the pregnant uterus and loaded with a tocolytic for reducing its placental passage and sustaining its efficacy. Nanoliposomes encapsulating indomethacin and decorated with clinically used oxytocin receptor antagonist were designed and evaluated in-vitro, ex-vivo and in-vivo. The proposed approach resulted in targeting uterine cells in-vitro, inhibiting uterine contractions ex-vivo, while doubling uterine drug concentration, decreasing fetal levels, and maintaining the preterm birth rate in vivo in a pregnant mouse model. This promising approach opens new horizons for drug development in obstetrics that could greatly impact preterm birth, which currently has no successful treatments.
Prematurity is a leading cause of perinatal morbidity and mortality affecting 9.6% of births in the United States1 and an estimated 15 million births worldwide2. Preterm labor is defined as regular contractions of the uterus resulting in changes in the cervix prior to 37 weeks of pregnancy3. Though there are a multitude of etiologies for preterm labor, the actual cause in any individual might sometimes be unclear. Premature newborns are at increased risk for both acute and chronic health problems, and developmental deficiencies4.
Due to maternal medical conditions or complication of pregnancy, medications are frequently essential for the health of the pregnant mother and fetus(es) requiring ongoing or episodic treatment5,6. Fetal exposure to medications most commonly occurs when free unbound drug crosses the placenta7,8,9. Targeting therapeutics to the affected tissue and minimizing the circulating free drug fraction for placental passage can open new opportunities in the field of obstetrics10.
Nanomedicine is a multidisciplinary field of research, merging notions of medicine and nanotechnology with the overall goal of accurately fine-tuning the biological processes that occur on the micron and submicron scales11,12,13. One of the great promises of nanomedicine over the traditional molecular therapeutics is the ability to vector drugs preferentially to the affected loci, thus increasing the efficacy and reducing associated adverse reactions. Nanoliposomes (liposomes, LIP) have been clinically used for over two decades to enhance drug delivery to tumor and infected loci, thereby reducing side effects of chemotherapeutic and antimicrobial therapies12,13,14,15, LIP, phospholipid based nanovesicles13,14,15, are biodegradable and generally pose no concern for toxicity.
Indomethacin (IND) is a tocolytic agent from the non-steroidal anti-inflammatory drugs (NSAID) family, which acts by reducing prostaglandin production in the maternal uterus16,17,18,19,20,21. Unfortunately, IND freely crosses the placenta22 and is associated with antenatal closure of the ductus arteriosus23,24,25, oligohydramnios26,27, necrotizing enterocolitis28, and intraventricular hemorrhage29 both in human and animal models30,31,32. Although IND is considered an effective tocolytic medication clinically available in the US to delay delivery for 48 hours to achieve corticosteroid therapy, it, like other tocolytic therapies, has never been able to improve short and long term neonatal outcomes18,33,34,35,36.
IND has a rapid onset of action and short half-life allowing its effects to be easily measured in vitro and in vivo using animal models37. The beneficial and adverse effects of IND have been shown in a multitude of studies revealing comparable effects in both pregnant women and pregnant rodents including IND’s ability to: (1) reduce uterine contractions38, (2) prolong pregnancy in the setting of preterm labor16,17,18,19,20,21, (3) cross the placenta22, and (4) cause stricture of the ductus arteriosus (DA)23,24,25,30,31,32. These inherent pharmacodynamics and pharmacokinetic properties make IND a good drug to investigate the potential for liposomes (LIP) to reduce placental passage and prevent adverse fetal effects in pregnant mice, which can then be extrapolated to humans. Our collaborative team has recently shown that untargeted LIP carrying IND (LIP-IND) reduced the placental passage of the drug to the fetus39.
Here we show for the first time in the field of obstetrics that a nanoparticle specifically designed for targeting the pregnant uterus is capable of increasing the fraction of the drug available to its intended site of action, while decreasing fetal exposure to the drug. For this purpose, we have fabricated LIP-IND-ORA, a LIP loaded with IND and decorated with a clinically available oxytocin receptor antagonist40 (ORA) on its surface, as schematically presented in Fig. 1. Oxytocin receptors are abundantly expressed on the pregnant uterus. ORA, (Atosiban®, Sigma, USA), is a clinically used tocolytic drug in Europe40. The objective of this study was to determine the ability of LIP-IND-ORA to specifically direct the delivery of IND to the pregnant uterus, inhibit uterine contractions, and reduce preterm birth.
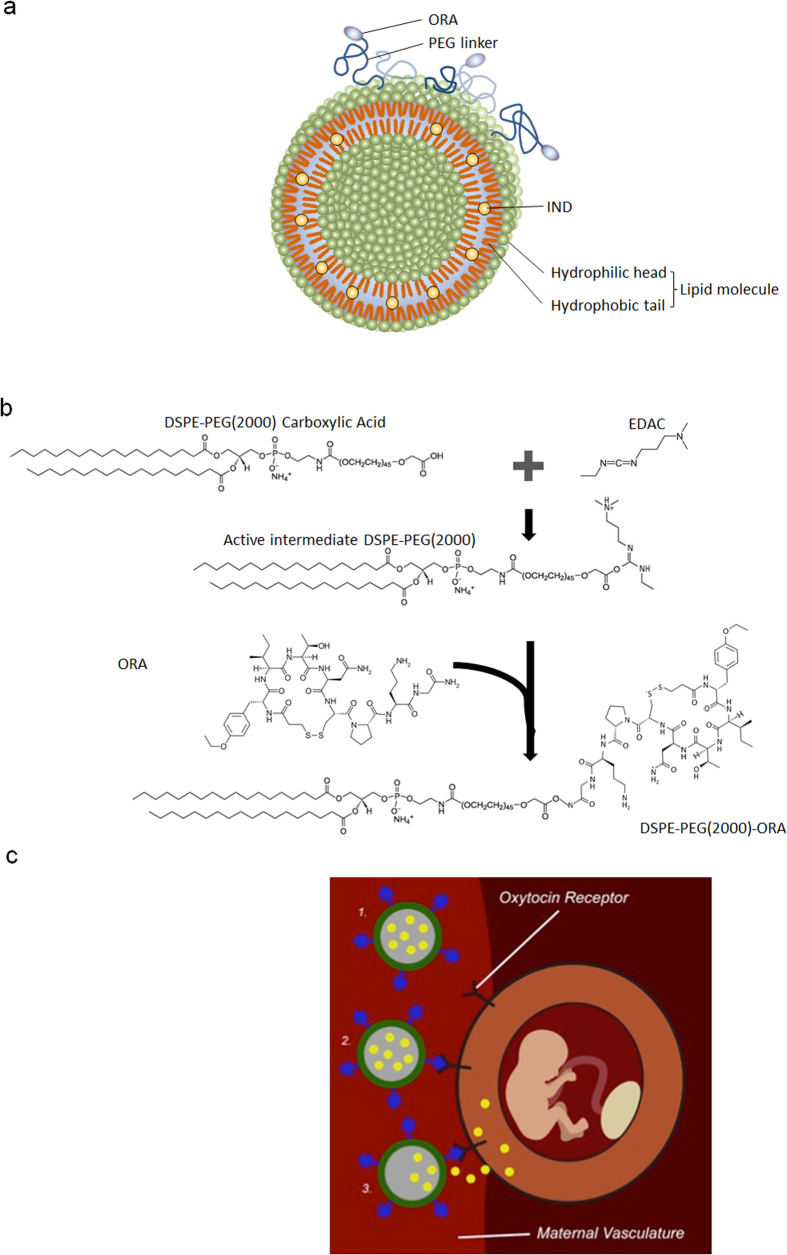
Figure 1. Schematic presentation of the LIP-IND-ORA design.
(a) Illustration of LIP-IND-ORA structure and (b) Schematic of ORA conjugation to the LIP membrane. (c) Schematics of the LIP-IND-ORA mechanism of action: (1–2) binding to the oxytocin receptor expressed on the pregnant uterus and directing IND (yellow) specifically to the uterus (3) thereby improving the tocolytic efficacy of indomethacin while reducing its placental passage.
Results
Liposome design and fabrication
To achieve active targeting of the LIP-IND system to the uterus, we have conjugated clinically used ORA to the liposome’s surface. For this purpose, the liposomes were engineered to include phospholipids with a spacer and carboxylic group (PEG-DSPE), which can react with the amino group of the ORA using a post-insertion technique. Various concentrations of the constituents were tested. Among the evaluated systems, 3% PEG-DSPE was found to be the most efficient in ORA conjugation (51.8% conjugation efficiency as normalized to the molar concentration of PEG-DSPE). Total ORA density was calculated to be ~1983 molecules/liposome. The resulting LIP-IND-ORA nanoliposomes are 124.2 ± 0.7 nm in size and possess negative zeta potential of −21.2 ± 0.4 mV. Molar ratio of ORA was 1.5% and IND was 10% of total phospholipid molecules in the liposome. LIP-IND-ORA also had a 93% IND loading efficiency meaning for 0.45 mg IND/mL liposome used in the production, 0.42 mg were incorporated into the liposomes. The loading rate was 3.69% (36.9 μg IND loaded per mg of LIP-IND-ORA). The addition of ORA to LIP-IND did not alter the drug encapsulation and fluorescent properties of the nanoparticles.
Targeting efficiency in vitro
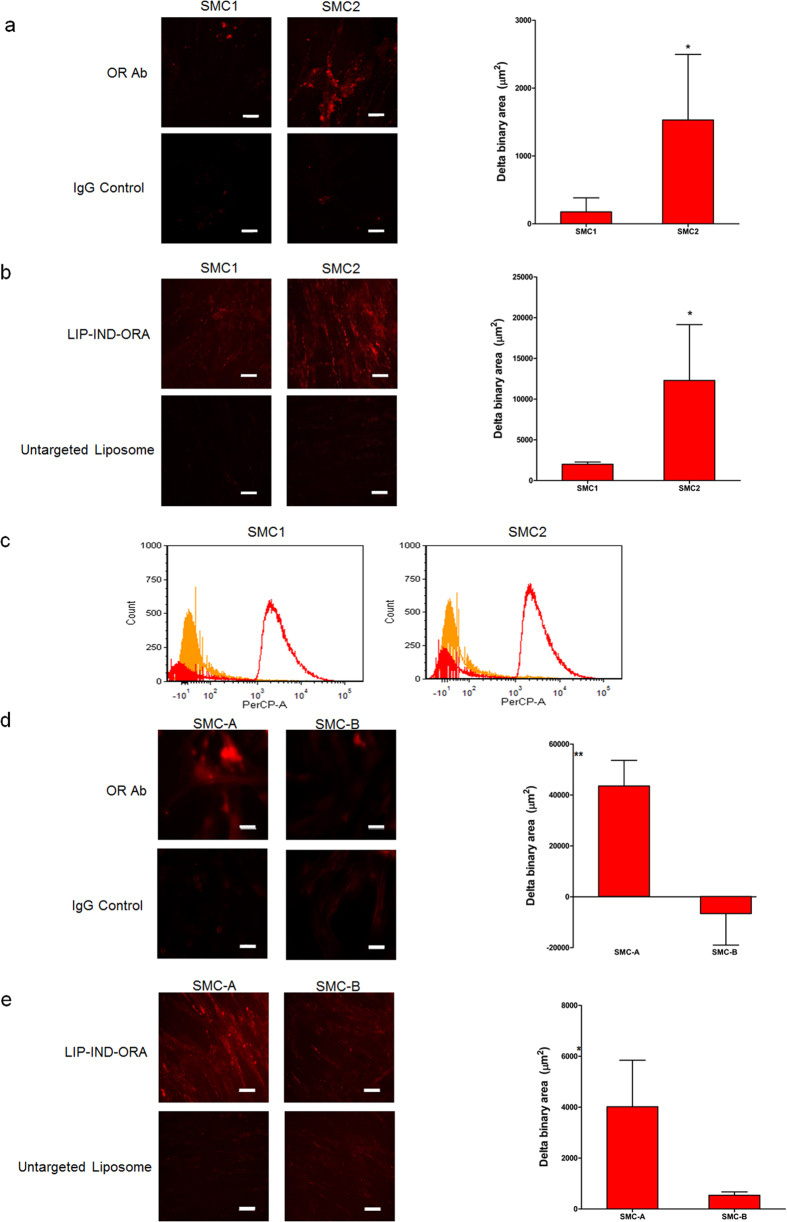
Expression level of oxytocin receptor (OR) was determined using immunofluorescence in smooth muscle cells (SMC) isolated from two pregnant mice (hence SMC1 and SMC2) (Fig. 2a). In both cases, the unspecific binding was tested using an isotypic IgG antibody. Both cells expressed OR, while SMC2 had on average four-times higher expression levels as compared to SMC1. The same trend is seen when the cells are incubated with fluorescently labeled targeted LIP-IND-ORA vs. untargeted liposomes (Fig. 2b). In both primary smooth muscle cells, LIP-IND-ORA enabled better attachment and retention when compared to untargeted liposomes. In terms of the targeting efficiency, LIP-IND-ORA attached on average eight-times more efficiently to SMC2 cells than to SMC1, which is in line with OR expression levels. The results were also confirmed via flow cytometry (Fig. 2c), where we found a significant increase in cells associated with LIP-IND-ORA as compared to untargeted liposome.
Figure 2. In vitro characterization of oxytocin receptor (OR) expression and correlated liposome targeting efficiency.
The in vitro experiments were conducted in triplicates in primary smooth muscle cells SMC1 and SMC2 isolated from pregnant mice (a–c), and in human smooth muscle cell lines SMC-A and SMC-B (d,e). OR expression was verified by immunofluorescence staining with OR antibody (OR-Ab, red) and analyzed via confocal microscopy (a,d), using IgG staining as a negative control. Liposome targeting specificity was analyzed via confocal microscopy (b,e), as well as via flow cytometry (c). All the images were analyzed and quantified using NIS-elements. Mean ± SEM, n = 9 images per sample. Scale bar = 50 μm. *p-value < 0.05, **p-value < 0.01 compared to IgG control (a,d) or to untargeted liposome (b,e).
We have further tested an association of LIP-IND-ORA vs. untargeted liposomes with two human SMC (SMC-A and SMC-B). The results show significant increase of liposome accumulation when ORA targeting is in place by 5-fold and 23-fold, for SMC-A and SMC-B, respectively (Fig. 2d,e). Our data confirm that OR can be successfully targeted on murine and human uterine SMC using the proposed strategy.
Biodistribution study in vivo
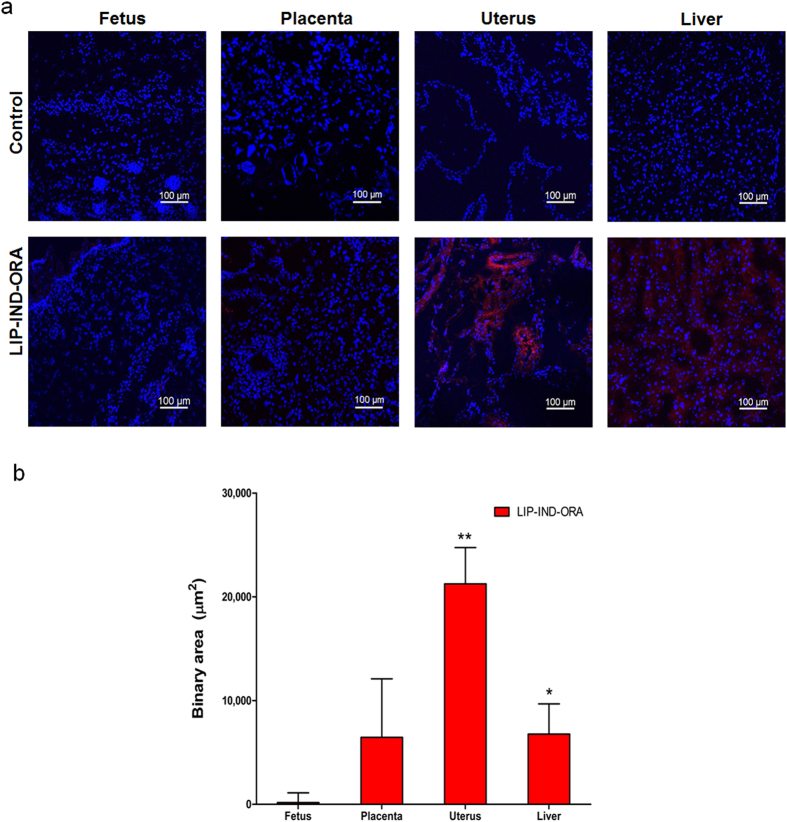
To determine whether the targeted LIP-IND-ORA system would minimize the placental passage and deliver IND to the pregnant uterus, we evaluated the biodistribution of the systems in vivo by fluorescent microscopy of maternal and fetal tissues for tagged LIP-IND-ORA and analysis of IND by HPLC-MS/MS. Fluorescent microscopy was utilized to assess liposome localization to the uterine and fetal tissue. Since, it is known that nanoparticles tend to accumulate in the liver (the major organ of reticulo-endothelial system) the accumulations of the LIP-IND-ORA system and IND in the liver were also measured. Based on the fluorescent microscopy assessment of the tissues, the strongest signal of the system was in the uterus of the pregnant mice. LIP-IND-ORA was primarily confined within the uterus, minimally detected within the liver and placenta, and absent in the fetus as shown in Fig. 3a,b (see also Supplementary Fig. 1). Quantitatively, the binary area of a fluorescent signal associated with LIP-IND-ORA were more than three times higher in the uterus of animals given LIP-IND-ORA compared to liver, placenta and fetus, (uterus: 21,243 ± 3,502; liver: 6,768 ± 2,919; fetus: 168 ± 934 μm2, p < 0.05) [n = 6 per group, mean ± SEM].
Figure 3. Biodistribution of LIP-IND-ORA components in vivo in pregnant mice.
(a) Qualitative analysis of LIP-IND-ORA tissue distribution in the maternal uterus and liver, placenta and in the fetus of pregnant mice. Red: LIP-IND-ORA (lissamin rhodamin labeled phospholipid); Blue- DAPI, nuclear stain. (b) Quantification of the LIP-IND-ORA fluorescent signal in the tissues normalized to tissue auto-fluorescence using NIS elements. Quantitative biodistribution of liposome was obtained from at least 9 randomly selected fields per mouse of each organ. Mean ± SEM, n = 6. *p-value < 0.05, **p-value < 0.01 to fetus.
Likewise, to assess reduction of placental passage of the drug from the LIP-IND-ORA system, the concentrations of IND were measured in the fetal tissue by liquid chromatography-mass spectrometry (LC-MS/MS). The concentration of IND in the uterine tissue was doubled in the pregnant mice receiving LIP-IND-ORA compared to IND alone, (LIP-IND-ORA: 1862.7 ± 503.3 ng/g vs. IND: 965.1 ± 311.7 ng/g, p = 0.006) as shown in Fig. 4. Moreover, there was a 2-fold reduction in levels of IND within the fetus of animals given LIP-IND-ORA compared to IND alone, (LIP-IND-ORA: 121.3 ± 16.8 ng/g vs. IND: 245.3 ± 61.7 ng/g, p = 0.002). Overall, the uterine to fetus IND concentration ratio was four fold higher for LIP-IND-ORA vs. IND. These findings demonstrated the targeting of the LIP-IND-ORA to the pregnant uterus tissue associated with reduction in the placental passage of IND.
Figure 4. IND concentrations in the maternal uterus and fetus.
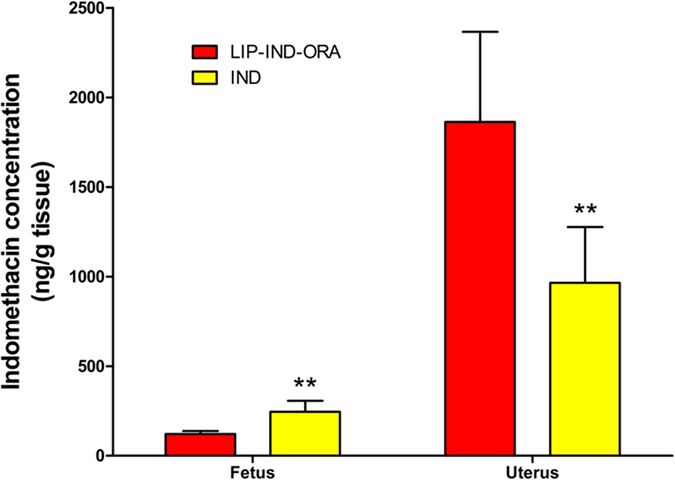
To assess the shift in biodistribution in different formulations, IND was administered either in free form or within LIP-IND-ORA. IND concentrations in the maternal uterus and fetus were determined by LC-MS/MS analysis. Mean ± SEM, n = 6. **p-value < 0.01 to the levels of IND when the drug was administered via LIP-IND-ORA.
Uterine contractility ex vivo
We have further analyzed the efficacy of the proposed LIP-IND-ORA system in inhibiting the pregnant uterine contractility ex vivo. LIP-IND-ORA significantly increased the percent inhibition of uterine contractions compared to control (saline, SAL), (LIP-IND-ORA: 56.0 ± 6.4% vs. SAL: −12.8 ± 18.4%, p = 0.003) [n = 6 per group, mean ± SEM] as shown in Fig. 5a. Moreover, LIP-IND-ORA significantly increased the percent inhibition of uterine contractions compared to LIP, (LIP-IND-ORA, 56.0 ± 6.4, versus LIP −6.0 ± 11.8 p = 0.001). Interestingly, LIP-IND inhibited uterine contractions similarly to IND alone (36.8 ± 5.9% vs. 34.3 ± 6.6%, respectively), demonstrating the tocolytic efficacy of the drug while encapsulated in LIP. Finally, LIP-ORA showed no significant difference in uterine contractility (18.2 ± 20.4) compared to all the other groups. Representation of the myograph experiments for each drug and its effect on uterine contractility are given in Supplementary Fig. 2. Since saline functioned as the absence of a tocolytic agent, its exposure resulted in an increase in uterine contractions as designated by its negative value. The inhibition of uterine activity appeared to be more efficient with LIP-IND-ORA as compared to IND alone (IND: 34.3 ± 5.9). Further, to mimic the oxytocin-induced contractions in the uterus, a dose response curve to oxytocin (OXY) was also performed. In this setting LIP-IND-ORA showed a decreased OXY induced contraction curve compared to both IND and SAL at all doses of oxytocin (Fig. 5b).
Figure 5. Inhibition of uterine contractility ex vivo.
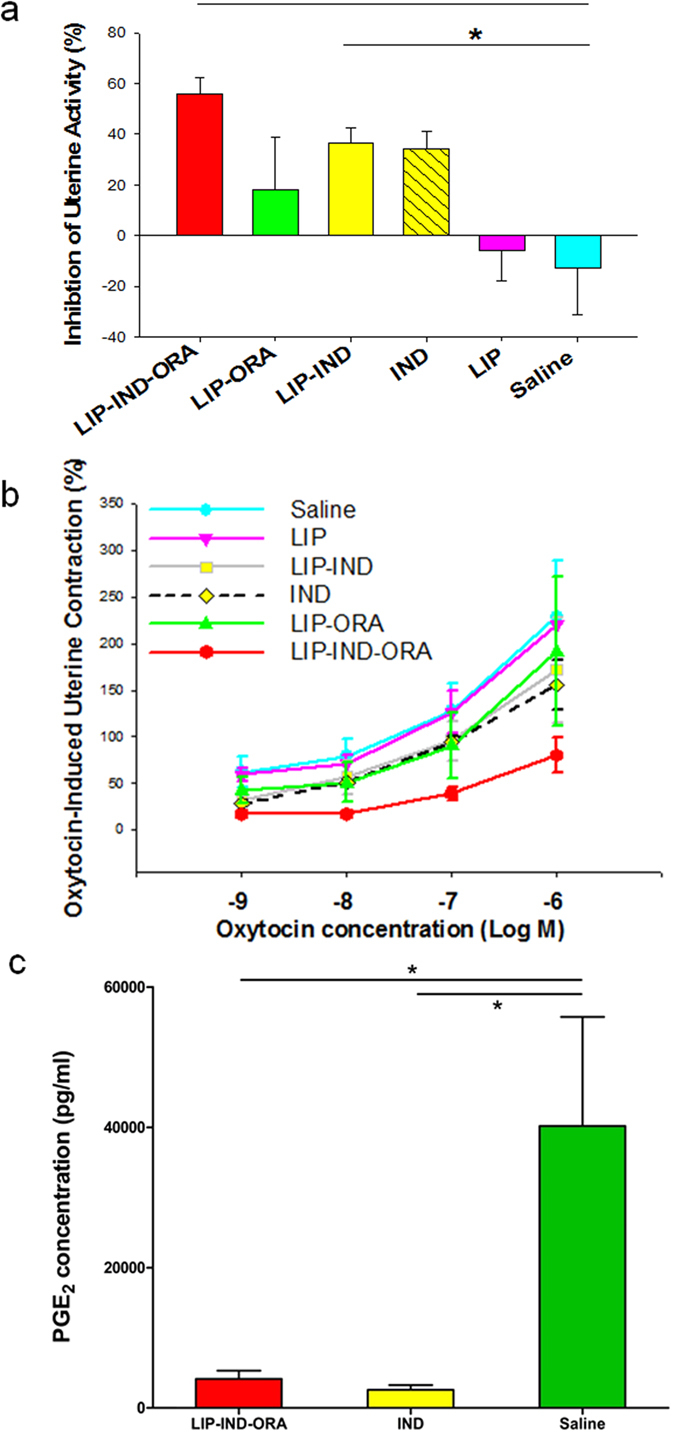
The efficacy of LIP-IND-ORA to inhibit contractility of uterus isolated from pregnant mice (GD 19) was examined. (a) Inhibition (%) of uterine contractions between LIP-IND-ORA, as compared to LIP-ORA, LIP-IND, IND, LIP and untreated control (saline, SAL) were determined in the absence or in the presence of various doses of oxytocin (an inducer of the uterine contractility) (b). (c) Prostaglandin E2 (PGE2) concentrations as determined by ELISA. Mean ± SEM. n = 6. *p-value < 0.05, **p-value < 0.01 vs. untreated control.
To evaluate the pharmacological activity of IND encapsulated in the LIP-IND-ORA system, the levels of prostaglandin E2 (PGE2) were determined in the uterus. PGE2 levels were significantly reduced in the uterus exposed to LIP-IND-ORA and IND compared to SAL, (4,127.9 ± 1,178.6, 2,587.4 ± 676.5 and 40,188.7 ± 15,555.6 pg/mL, respectively, p = 0.019) [n = 6 per group, mean ± SEM] as described in Fig. 5c. This illustrates that the encapsulation of IND within the targeted LIP, LIP-IND-ORA, does not alter the pharmacological activity of IND.
Preterm birth in vivo
LIP-IND-ORA significantly reduced the rate of lipopolysaccharide (LPS) induced preterm birth compared to SAL, (LIP-IND-ORA, n = 13: 46.2% vs. SAL, n = 8: 87.5%, p = 0.029) as shown in Fig. 6. There was a trend towards reduced rate of preterm birth by 15% with LIP-IND-ORA compared to IND alone (IND, n = 11: 54.5%), though this was not statistically significant. Additionally, although the length of pregnancy in hours was prolonged by 31% in mice treated with LIP-IND-ORA compared to IND and SAL, this was not statistically significant, (LIP-IND-ORA: 44.0 ± 4.5 h vs. IND: 30.0 ± 4.9 h vs. SAL: 17.5 ± 3.2 h, p = 0.076) [mean ± SEM].
Figure 6. In vivo therapeutic efficacy in prevention of preterm birth.
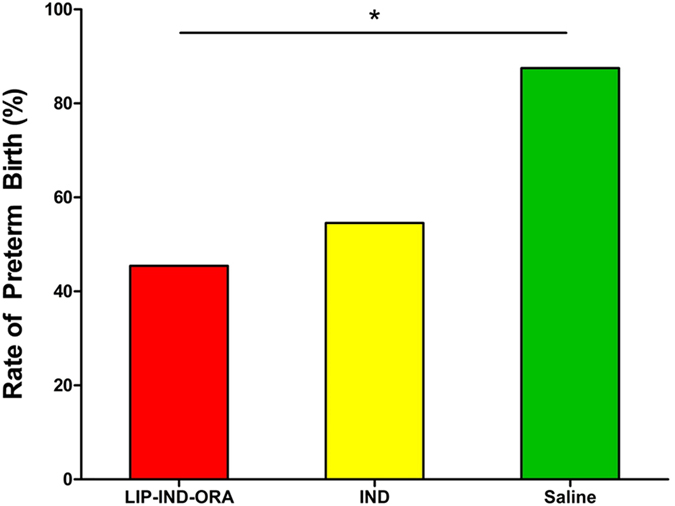
The efficacy of LIP-IND-ORA to prevent preterm birth was tested in an LPS-induced preterm pregnant mouse model. On GD 15, timed-pregnant CD1 mice were administered LPS (25 μg/kg) via intraperitoneal injection. Animals were randomly divided into 3 groups and given daily treatments (GD 15–17) via tail vein injection: IND (N = 11), LIP-IND-ORA (N = 13), and SAL (N = 8). IND concentrations were 1 mg/kg. Preterm birth rates (%) were compared between groups. *p-value = 0.029 compared to saline control.
Discussion
Preterm birth continues to be the leading cause of perinatal mortality in the United States (US)4. The most recent National Vital Statistics Reports in 2015 report that death from prematurity (2.81 per 1000 births in 2013) is 2.3-fold higher than death from cancer (1.71 per 1000 adults from 2008–2012)41,42. Of the children that survive, 25–50% suffer long-term neurological impairment4. Based on a 2007 Institute of Medicine report, the annual financial burden from prematurity is estimated to be $26.2 billion or more than $51,000 per premature infant. Despite current use of prophylactic progesterone, pessaries, and cerclages4, there continues to be negligible improvements in the rate of preterm birth less than 34 weeks, 3.7% in 2006 to 3.4% in 201241. Tocolytics remain the primary treatment for preterm labor to delay delivery4. The fundamental problems with tocolytic therapies are the inability to improve neonatal outcomes and potential adverse effects to the fetus22,23,24,25,26,27,28,29,30,31,32. Unfortunately, although highly demanded, there has been no significant improvement in tocolytic therapies for the past three decades, which can be ascribed to scant innovation in the field of drug therapies for preterm labor. In this manuscript, we pioneer the nanomedicine approach of targeted tocolytic therapy to address these fundamental problems unique to pregnancy by making therapeutics function better and safer for both mom and baby.
We were able to demonstrate successful delivery of indomethacin directly to the pregnant uterus with our customized liposome consistently across three approaches, in vitro, in vivo and ex vivo. A few months ago, King et al. have shown using fluorescent qualitative analysis that RGD-targeted nanoparticles can increase accumulate more in the mouse placenta than untargeted particles43. Our greatest achievement was reduction in the drug levels detected in the fetus, while maintaining indomethacin’s tocolytic efficacy. The targeted liposome significantly decreased prostaglandin levels in the uterus thereby inhibiting uterine contractions. This resulted in prolonging pregnancy by 31% and reducing the rate of preterm birth by 15% as compared to the free drug. To enable faster clinical translation of the proposed approach, we used a clinically used40 oxytocin receptor antagonist (ORA, or Atosiban) as our targeting element. In therapeutic concentration, ORA was shown to induce minimal adverse effects40. In the current study, the total administered dose of ORA was several times below the minimal therapeutic dose (see calculations in Supplementary information) and, consequently, ORA levels were not detectable in the maternal uterus and fetal tissue by LC-MS/MS. In this work we report for the first time in the field of obstetrics the use of targeted nanoparticles to improve high-risk pregnancy complicated with preterm labor. This promising approach opens new horizons for drug development in obstetrics that could greatly impact preterm birth, which currently has no successful treatments36.
Methods
Liposome design and fabrication
LIP, LIP-IND, LIP-ORA and LIP-IND-ORA were prepared by lipid hydration-extrusion method. First, the lipids were dissolved in 3 mL ethanol at the following concentrations: 9.6–12.2 mg soy bean phosphatidylcholine (Lipoid S100, Lipoid, Germany), 0–0.77 mg cholesterol (Sigma) and 1–3 mg DSPE-PEG(2000) Carboxylic Acid (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000] (ammonium salt)) (Avanti, Alabama, USA). To fluorescently label LIP, fluorescent phospholipid Lissamine rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (rhodamine-DHPE, Invitrogen), 2% of the total lipid was incorporated to all liposome formulations. The incorporated Lissamine rhodamine B has excitation/emission maxima of 568/583 nm and was utilized for fluorescence microscopy and flow cytometry analysis. 0.45 mg of IND (Sigma), which corresponds to 10% of phospholipids molar content, was added to the above ethanolic mixture for LIP-IND and LIP-IND-ORA formulations. A thin film was formed by evaporating the solvent for 30 minutes (min), 41 °C at 150 rpm using rotary evaporator (Rotavapor, Buchi, Switzerland). The film was rehydrated with 1 mL PBS pH 7.2. Liposomes were extruded 10 times using each of the following 800-, 400-, and 200-nm Nuclepore Track-Etch Membrane (Whatman) filters with Lipex Biomembrane extruder. The resulting liposomes were ultracentrifuged (60,000 × g, 2 hours [h]) using Solvall WX ultra series ultracentrifuge (Thermo Scientific). Supernatant was removed and the LIP and LIP-IND were resuspended with 1 mL PBS.
ORA-NHS was prepared for conjugation with liposome by adding 1.9 mg EDC (1-ethyl-3-[3-dimethylaminopropyl]carbodiimide) (Life technologies) and 2.9 mg NHS (N-hydroxysuccinimide) to each mg ORA in MES buffer and incubated in rotator at room temperature (RT) for at least 15 min. For LIP-ORA and LIP-IND-ORA preparation, the systems were resuspended with 1 mL MES (2-[morpholino]ethanesulfonic acid) buffer containing ORA-NHS equivalent to 0.35 mg ORA weight. Conjugation was conducted at RT overnight, and unbound ORA were washed from liposome by ultracentrifugation (60,000 × g, 2 h).
The size and zeta potential of the liposomes were assessed by dynamic light scattering using Zetasizer (Malvern, Worcestershire, UK). Five separately prepared batches of each formulation were analyzed in triplicates each. The morphology and structure of LIP were observed by scanning electron microscopy as previously described39.
The levels of IND in the LIP were assessed using high-performance liquid chromatography (HPLC). Supernatant from ultracentrifugation after conjugation was used for measurement of unbound ORA concentration to determine the ORA conjugation efficiency indirectly. An aliquot of LIP-ORA and LIP-IND-ORA were dissolved in ethanol and used for direct measurement of conjugated ORA.
IND was analyzed by isocratic detection using ultraviolet diode-array HPLC system Column Hitachi Elite LaChrom, Column oven L-2300, Autosampler L-2200, Diode Array Detector L-2455, Pump L-2130 Hitachi D-2000 Elite v3.0 software. Kinetex 2.6 m XB-C18 100 Ǻ (100 × 4.6 mm; Phenomenex) column was used for the separation at 237 nm. Chromatography was performed using an isocratic elution with mobile phase composed of 0.2% phosphoric acid in acetonitrile at a flow rate of 0.6 mL/min with average retention time of 7.2 min.
Animals
Pregnant female (strain CD1, stock N 022) were purchased form Charles River. For the targeting efficiency study and the biodistribution study, mice were used at term gestation, gestation day 18 (GD 18). For the ex-vitro uterine contractility study pregnant mice were used at gestational day 19 (GD 19), just before mice are about to deliver and uterine contractility is maximal. For the preterm study, mice were obtained at mid gestation (GD 14). The animal care and experiments were in accordance with the protocol #HSC-AWC-13-154 approved in January 2014 by the University of Texas Health Science Center at Houston (UTHSC-H) Animal Welfare Committee (AMC). The mice were housed separately in temperature and humidity-controlled quarters with constant 12:12-hours light-dark cycles in the animal care facility at the (UTHSC-H).
Targeting efficiency in vitro
To confirm the localization of the oxytocin receptor on uterine cells, pregnant mouse uterine cell lines were created from two timed-pregnant CD1 mice on gestational day (GD) 1844,45. These pregnant mice were not involved in any prior study. After CO2 inhalation euthanasia, laparotomy was performed and the pregnant uterus was retrieved and placed in Hank’s balanced salt solution. The uterine tissue was cut into 1–2 mm fragments with a razor then digested in 0.1% trypsin (Sigma, USA) and 0.1% deoxyribonuclease (Sigma, USA) for 30 min at 37 °C in shaker incubator, followed by 0.1% collagenase (Sigma, USA) for another 30 min. After filtering the tissue through gauze, the cells were washed then plated on collagen I-coated 75 mm flasks (BD Biosciences, USA) with RPMI 1640 media (Sigma, USA), 10% fetal bovine serum (FBS, Sigma, USA) and Penstrept (Sigma, USA). The media was changed daily until Day 4. In addition, biopsies were obtained from two women undergoing cesarean section to create a cell culture of uterine cells. Both were obtained from singleton, non-laboring pregnancies at 39 weeks of gestation (Human A is Hispanic, BMI 29 kg/m2 and Human B is Caucasian, BMI 33 kg/m2). Institutional review board was approved August 2014 at UTHSCH, #HSC-MS-14-0370.
The study of liposome attachment was conducted in triplicates, where the cells were seeded with a density of 2 × 105 cells/mL and 0.5 mL/well in 8-well chamber slide. The cells were incubated at 37 °C overnight for cell attachment. 10 μL (12.2 mg/mL) of either targeted or non-targeted liposomes that were tagged with lissamine rhodamine were added to each well and gently shaken for a homogenous distribution in the well. The slides were incubated at 37 °C for 4 h to allow for interaction between cells and liposomes. After the incubation, the medium containing liposomes were discarded and the cells were washed twice with PBS. Cells were fixed afterwards with 4% paraformaldehyde in PBS for 30 min. Slide chambers were removed with the provided slide separator and the slides were mounted using Prolong Gold Antifade reagent (Life Technologies) and sealed with Cytoseal XYL (Thermo Scientific). Fluorescence signal from liposomes was detected using Nikon Eclipse Ti fluorescence microscope and analyzed using NIS Elements software. Nine randomly selected microscopy area were taken for quantification of OR and accumulating liposomes.
For flow cytometry analysis, the cells were detached from the flasks by using the cell dissociation buffer (Life Technologies). Medium was removed from the flask and the cell layer was washed with calcium-/magnesium-free PBS. After PBS removal, 2 mL of the cell dissociation buffer was added to the cells and the flask was incubated at 37 °C for 10 min. Cells that are still attached after the time were detached by firmly tapping the flask. The cells were gathered by addition of medium and counted. At least 2 × 105 cells were incubated with targeted and non-targeted liposomes in concentration of 0.244 mg/well for 4 h at 37 °C. After incubation time, the medium containing liposomes were removed by centrifugation at 400 × g for 5 min. The cells were analyzed using BD FACS Fortessa (Becton Dickinson, San Jose, CA) detected in the PerCP channel, using untreated cells as control. The data was post-processed using FCS Express Flow 5 software.
Biodistribution study in vivo
The concentration of IND delivered to the pregnant uterus from LIP-IND-ORA was compared to free IND using our established in vivo pregnant mouse model39. On GD 18, timed-pregnant CD1 mice (N = 6/group) were randomly allocated to receive either LIP-IND-ORA, IND or saline (SAL) via tail vein injection at a volume of 0.1 mL. When the drug was used (LIP-IND-ORA or IND), the dose of IND was 1 mg/kg (range 50–60 mg per animal based on maternal weight)31,37. The in vivo doses were maintained across the study. After 4 h, pregnant mice were sacrificed by CO2 inhalation, followed by laparotomy to retrieve maternal liver, uteri, placentas and fetuses. The onset of action of LIP-IND-ORA is unknown when administered intravenously. However, indomethacin’s onset of action is 2–3 h when given orally to humans and rodents22, and is 4–5 h when encapsulated with liposomes and administered via intraperitoneal38. Moreover, our prior study showed an increase of IND in the uterus and reduction in the fetus when encapsulated within LIP after 4 h following administration39. Based on these prior studies, 4 h was chosen as the period of exposure.
Liposome distribution was determined by immunofluorescence as previously described39. Briefly, tissue localization of LIP was qualitatively assessed using fluorescent microscopy identifying the absence or presence of LIP (tagged with fluorescent dye as previously described) within the liver, uterus, placenta and fetus. For this analysis, the excised tissues were placed in cryo-molds, embedded in the cryo-preserving media (OCT) and immediately frozen using liquid nitrogen. The blocks were stored in −80 °C until sectioning using cryo-microtome. During mounting on the slides, the tissue slices were stained with DAPI (4′,6-diamidino-2-phenylindole) fluorescent stain to identify nuclear structures of cells. Images were taken with the BX51 fluorescent microscope (Nikon, USA) using filters for DAPI and Cy3 at 100× magnification. Six animals per group were utilized in this study and their organs were sectioned and analyzed. Quantification was conducted in randomly selected area of at least 9 areas per animal to ensure the objectivity, while an image was chosen to represent the fluorescence signal46. Quantitative biodistribution of liposome was determined using an NIS elements image processing software (Nikon, USA)46. The binary area of a fluorescent signal reported in μm2 as mean ± sem.
The concentrations of indomethacin in IND and LIP-IND-ORA samples were determined by LC-MS/MS using multiple reactions monitoring assay with Phenacetin-ethoxy-D5 (Sigma, USA) as internal standard. Uterine and fetal tissues were homogenized in 1 mL of ice–cold methanol/water (7:3 v/v). Phenacetin (final concentration 20 ng/mL) was added to each sample before centrifugation at 15,000 rpm for 10 min. Supernatant was dried under nitrogen and reconstituted in 0.1% formic acid aqueous solution followed by protein precipitation with acetonitrile (1:2). Samples were centrifuged again, supernatant was transferred to vial and 10 μL was injected to Shimadzu triple quad 8040 MS connected to LC system. Indomethacin concentrations were determined in ng/mL against calibrators. Calibration curve was prepared by spiking in calibrator levels (15.6; 31.2; 62.4; 125; 250 and 500 ng/mL) in control tissue (no indomethacin) followed by extraction procedure identical to sample preparation. Method parameters were: LOD = 3.9 ng/mL (accuracy 76%, S/N > 3); LOQ = 15.6 ng/mL (accuracy 85%; CV < 10%; S/N > 10) with correlation coefficient for linear regression R2 = 0.992. Indomethacin concentrations were further normalized per tissue weight (ng/g) and reported as mean ± SEM.
Uterine contractility ex vivo
Our established ex vivo pregnant mouse model of uterine contractility was used to measure the ability of LIP-IND-ORA to inhibit uterine contractions47,48,49. On GD19, timed-pregnant mice (N = 6) underwent CO2 inhalation euthanasia, the maternal uteri were excised and placed into Krebs physiological solution. Uterine ring segments, 4 mm in width, were cut, and the fetuses and placentas were gently removed. The uterine rings were positioned between tungsten-wire (250 μm in diameter) stirrups and placed in an organ chamber containing 10 mL Krebs buffer, bubbled with 5% carbon dioxide in 95% oxygen maintained at constant temperature and pH (37 °C, pH 7.4). Passive tension was gradually applied to the optimal level of 1 g during an equilibration period of 60 min. Once the uterine tissue contracted spontaneously, uterine rings were then incubated with either: LIP-IND-ORA (10−5 mol/L), LIP-ORA, LIP-IND (10−5 mol/L), IND (10−5 mol/L), LIP and saline (SAL) as control for 40 min. After incubation of the study drugs, dose response curves to oxytocin (OXY) were obtained (10−9mol/L to 10−6mol/L; 20 min between OXY doses) to produce increased stable uterine contractions. The final concentration of IND administered into the organ chamber is equal to the concentration of IND (1 mg/kg) in LIP-IND and LIP-IND-ORA. To confirm tissue viability, potassium chloride (KCL 60 mmol/L) was added in each chamber at the end of the experiment. IND and OXY dose concentrations were based from previous uterine contractility studies50,51,52. OXY and IND (Sigma, USA) were dissolved in water and ethanol respectively. The final concentration of ethanol in the organ chamber solution (1.3 × 10−4mol/L) was 130 times inferior to a plasma concentration that could possibly account for a tocolytic effect53.
Changes in isometric tension were recorded with isometric force transducers (Harvard Apparatus, South Natik, MA) connected and stored to an online computer with data acquisition software (WINDAQ-200; DATAQ). The data was acquired and analyzed using Windaq data acquisition system (Dataq Instruments Inc, Akron, OH). Spontaneous contractile activity for each uterine ring was analyzed as an integral activity over 40 min before (basal activity) and after application of each study drug. Baseline activity was defined as the integral activity over the 40 min following stabilization of uterine contractions. The effect of the IND alone and LIP-IND-ORA were determined by calculating the integral activity expressed as a percent change from the baseline integral activity. OXY induced contractile response was expressed as an integral activity over 20 min of each OXY dose and the baseline uterine contractility54,55,56. The percent inhibition of uterine contractions and the dose response curve to oxytocin were calculated using software (Sigma Plot and GraphPad Prism, version 3.00 for Windows; GraphPad Software, San Diego, CA). Data was expressed as mean ± SEM.
Since the pharmacological action of IND involves the inhibition of prostaglandin production by cyclooxygenase, prostaglandin E2 (PGE2) levels were measured in uterine tissue using ELISA (ADI-901-001, Enzo Life Science, New York, USA). Another set of pregnant CD1 mice at GD 19 (n = 5) was used to evaluate uterus PGE2 levels. The uterus from the pregnant mice was obtained as described previously and incubated with LIP-IND-ORA, IND or saline as control for 40 min. The 40 min exposure time was chosen since this was the same length of time the uterine tissue was exposed to LIP-IND-ORA or IND in the uterine contractility experiments. PGE2 levels in each sample were then determined based on the instructions in the ELISA kit (ADI-901-001, Enzo Life Science, New York, USA). The concentration of PGE2 were expressed as pg/mL and reported as the mean ± SEM.
Preterm birth in vivo
Our established in vivo preterm pregnant mouse model was used to test the ability of LIP-IND-ORA to prevent preterm birth. LPS is a commonly used model to induce labor in murine models31,37. Prior studies have demonstrated that when pregnant rodents are exposed to 50 μg/kg of LPS, preterm labor is induced in 90% of animals31. IND reduces the preterm birth rate in pregnant mice exposed to LPS by as much as 60–70%31,37. On GD 15, timed-pregnant CD1 mice received LPS (L2880, from Escherichia coli 055:B5, Sigma, USA) (25 μg/kg) via intraperitoneal injection. Animals were randomly divided into 3 groups and given daily treatments (GD15-17) via tail vein injection (100 μl volume) according to group randomization: IND (N = 11), LIP-IND-ORA (N = 13), and SAL (N = 8). IND concentrations for both IND and LIP-IND-ORA were again 1 mg/kg (range 50–60 mg per animal based on maternal weight) based on prior studies31,37. Since the LIP-IND-ORA solution color was visibly pink and due to limited resources to create a placebo, the randomization was not blinded. Each day after the LPS administration, the preterm birth rate was determined as the number of pregnant mice spontaneously delivering prior to GD18. Animals were single housed and were monitored continuously by direct observation and by video camera for the duration of the treatment period to confirm timing of delivery. The rate of preterm birth was expressed as percent of preterm delivery and length of pregnancy was expressed in hours after LPS administration. Results were reported as median ± sem.
A sample size calculation was performed based on prior experiments involving IND in the prevention of preterm birth in pregnant rodents. Mice treated with IND had a 30% rate of preterm birth compared to 90% in control31,37. Based on an effect of 33% with an α of 0.05 (2-tailed) and β of 0.80, we determined that 7 maternal mice were needed in each group.
Statistical Analysis
Differences in isometric tensions of myometrium, IND concentrations in the uterus and fetus, PGE2 concentrations in the uterus, preterm birth rates and length of pregnancy between groups were analyzed using one-way analysis of variance with Tukey post hoc test. STATA software (version 12.1) were used and a P value < 0.05 was considered significant.
Additional Information
How to cite this article: Refuerzo, J. S. et al. Uterus-targeted liposomes for preterm labor management: studies in pregnant mice. Sci. Rep. 6, 34710; doi: 10.1038/srep34710 (2016).
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD08947. We would also like to thank Francesca Ferrari, M.D., Esther Tamayo, and Lan Yongsheng for assisting in the conduction of the in vivo, ex vivo and in vitro experiments.
Footnotes
Author Contributions J.S.R. developed the concept of targeted liposome for treatment of preterm labor, performed the in vivo experiments, analyzed the data and wrote the manuscript. F.L. designed and created the targeted liposomes, tested and analyzed their targeting efficiency in vitro and in vivo and assisted in editing the manuscript. N.B. and D.G. performed the measurement of indomethacin level by LC-MS/MS. G.C. conducted the statistical analysis for the ex vivo experiments. A.O. assisted in performing the in vitro and in vivo experiments and data collection/organization. M.L. contributed to the concept of targeted liposome for treatment of preterm labor, performed the in vitro and in vivo experiments, analyzed the data and assisted in writing the manuscript. B.G. developed the concept of targeted liposome for treatment of preterm labor and the overall study, designed, formulated and characterized the nanoscale delivery system, tested its targeting efficiency, assisted in performing the in vivo experiments, analyzed the data and wrote the manuscript. The authors report no conflict of interest. First authorship is to be shared by J.S.R. and F.L. who have contributed equally. Last authorship is to be shared by M.L. and B.G. who have contributed equally.
References
- March of Dimes. 2015 Premature birth report card. National Center for Health Statistics, final natality data. Date of access 07/08/2016, from www.marchofdimes.org/Peristats/pdflib/998/premature-birth-report-card-United-States.pdf.
- World Health Organization. Preterm birth. Fact sheet No 363. Updated November 2015. Date of access 07/08/2016, from www.who.int/mediacentre/factsheets/fs363/en/.
- Premature rupture of membranes. Practice Bulletin No. 160. American College of Obstetricians and Gynecologists. Obstet Gynecol127, e39–51 (2016). [DOI] [PubMed]
- Management of preterm labor. Practice Bulletin No. 159. American College of Obstetricians and Gynecologists. Obstet Gynecol 127, e29–38 (2016). [DOI] [PubMed] [Google Scholar]
- Glover D. D., Amonkar M., Rybeck B. F. & Tracy T. S. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol 188, 1039–1045 (2003). [DOI] [PubMed] [Google Scholar]
- Refuerzo J. S. et al. Use of over-the-counter medications and herbal remedies in pregnancy. Am J Perinatol 22, 321–324, 10.1055/s-2005-873235 (2005). [DOI] [PubMed] [Google Scholar]
- van der Aa E. M., Peereboom-Stegeman J. H., Noordhoek J., Gribnau F. W. & Russel F. G. Mechanisms of drug transfer across the human placenta. Pharm World Sci 20, 139–148 (1998). [DOI] [PubMed] [Google Scholar]
- Garland M. Pharmacology of drug transfer across the placenta. Obstet Gynecol Clin North Am 25, 21–42 (1998). [DOI] [PubMed] [Google Scholar]
- Syme M. R., Paxton J. W. & Keelan J. A. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 43, 487–514, 10.2165/00003088-200443080-00001 (2004). [DOI] [PubMed] [Google Scholar]
- Keelan J. A., Leong J. W., Ho D. & Iyer K. S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 10, 2229–2247, 10.2217/nnm.15.48 (2015). [DOI] [PubMed] [Google Scholar]
- Peer D., Karp J. M., Hong S., Farokhzad O. C., Margalit R. & Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology 2, 751–760 (2007). [DOI] [PubMed] [Google Scholar]
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nature Reviews Cancer 5, 161–171, 10.1038/nrc1566 (March 2005). [DOI] [PubMed] [Google Scholar]
- Riehemann K., Schneider S. W., Godin B., Ferrari M. & Fuchs H. Nanomedicine–challenge and perspectives. Angew Chem Int Ed Engl 48, 872–897, 10.1002/anie.200802585 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz Y., Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release 160(2), 117–34. doi: 10.1016/j.jconrel.2012.03.020 (2012). [DOI] [PubMed] [Google Scholar]
- Touitou E. & Godin B. Vesicles for enhanced delivery into and through the skin. In: Touitou, E., Barry, B. W. (Eds). Enhancement in drug delivery, CRC Press, Taylor & Francis Group, Boca Raton-London-New York, pp. 255–278 (2006).
- Hallak M. et al. The effect of tocolytic agents (indomethacin and terbutaline) on fetal breathing and body movements: a prospective, randomized, double-blind, placebo-controlled clinical trial. Am J Obstet Gynecol 167, 1059–1063 (1992). [DOI] [PubMed] [Google Scholar]
- Hallak M., Moise K. J. Jr., Smith E. O. & Cotton D. B. The effects of indomethacin and terbutaline on human fetal umbilical artery velocimetry: a randomized, double-blind study. Am J Obstet Gynecol 168, 865–868 (1993). [DOI] [PubMed] [Google Scholar]
- Klauser C. K. et al. A comparison of three tocolytics for preterm labor: a randomized clinical trial. J Matern Fetal Nonatal Med 27, 801–806, 10.3109/14767058.2013.847416 (2014). [DOI] [PubMed] [Google Scholar]
- Besinger R. E., Niebyl J. R., Keyes W. G. & Johnson T. R. Randomized comparative trial of indomethacin and ritodrine for the long-term treatment of preterm labor. Am J Obstet Gynecol 164, 981–986; discussion 986-988 (1991). [DOI] [PubMed] [Google Scholar]
- Kurki T., Eronen M., Lumme R. & Ylikorkala O. A randomized double-dummy comparison between indomethacin and nylidrin in threatened preterm labor. Obstet Gynecol 78, 1093–1097 (1991). [PubMed] [Google Scholar]
- Morales W. J. & Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: a randomized study. Am J Obstet Gynecol 169, 97–102 (1993). [DOI] [PubMed] [Google Scholar]
- Moise K. J. Jr. et al. Placental transfer of indomethacin in the human pregnancy. Am J Obstet Gynecol 162, 549–554 (1990). [DOI] [PubMed] [Google Scholar]
- Vermillion S. T., Scardo J. A., Lashus A. G. & Wiles H. B. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol 177, 256–259; discussion 259-261 (1997). [DOI] [PubMed] [Google Scholar]
- Suarez V. R. et al. The effect of in utero exposure to indomethacin on the need for surgical closure of a patent ductus arteriosus in the neonate. Am J Obstet Gynecol 187, 886–888 (2002). [DOI] [PubMed] [Google Scholar]
- Moise K. J. Jr. Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol 168, 1350–1353 (1993). [DOI] [PubMed] [Google Scholar]
- Hendricks S. K., Smith J. R., Moore D. E. & Brown Z. A. Oligohydramnios associated with prostaglandin synthetase inhibitors in preterm labour. Br J Obstet Gynaecol 97, 312–316 (1990). [DOI] [PubMed] [Google Scholar]
- Kirshon B., Moise K. J. Jr., Mari G. & Willis R. Long-term indomethacin therapy decreases fetal urine output and results in oligohydramnios. Am J Perinatol 8, 86–88 (1991). [DOI] [PubMed] [Google Scholar]
- Major C. A., Lewis D. F., Harding J. A., Porto M. A. & Garite T. J. Tocolysis with indomethacin increases the incidence of necrotizing enterocolitis in the low-birth-weight neonate. Am J Obstet Gynecol 170, 102–106 (1994). [DOI] [PubMed] [Google Scholar]
- Suarez R. D., Grobman W. A. & Parilla B. V. Indomethacin tocolysis and intraventricular hemorrhage. Obstet Gynecol 97, 921–925 (2001). [DOI] [PubMed] [Google Scholar]
- Sakai M. et al. Evaluation of the tocolytic effect of a selective cyclooxygenase-2 inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Mol Hum Reprod 7, 595–602 (2001). [DOI] [PubMed] [Google Scholar]
- Karadas B. et al. Comparison of effects of cyclooxygenase inhibitors on myometrial contraction and constriction of ductus arteriosus in rats. Eur J Pharmacol 485, 289–298, doi:S0014299903027134 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- Momma K. & Takao A. In vivo constriction of the ductus arteriosus by nonsteroidal antiinflammatory drugs in near-term and preterm fetal rats. Pediatr Res 22, 567–572, 10.1203/00006450-198711000-00018 (1987). [DOI] [PubMed] [Google Scholar]
- Panter K. R. et al. The effect of indomethacin tocolysis in preterm labour on perinatal outcome: a randomised placebo-controlled trial. Br J Obstet Gynaecol 106, 467–473 (1999). [DOI] [PubMed] [Google Scholar]
- Niebyl J. R. et al. The inhibition of premature labor with indomethacin. Am J Obstet Gynecol 136, 1014–1019 (1980). [DOI] [PubMed] [Google Scholar]
- Reinebrant H. E. et al. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev 6, CD001992, doi: 10.1002/14651858.CD001992.pub3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovici A., Cantu J. & Jenkins S. M. Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin North Am 39, 77–87, doi: 10.1016/j.ogc.2011.12.003 (2012). [DOI] [PubMed] [Google Scholar]
- Lee P. R. et al. Therapeutic effect of cyclo-oxygenase inhibitors with different isoform selectivity in lipopolysaccharide-induced preterm birth in mice. Am J Obstet Gynecol 189, 261–266, doi: S000293780300471X [pii] (2003). [DOI] [PubMed] [Google Scholar]
- Srinath P., Vyas S. P. & Diwan P. V. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev Ind Pharm 26, 313–321, doi: 10.1081/DDC-100100359 (2000). [DOI] [PubMed] [Google Scholar]
- Refuerzo J. S. et al. Liposomes: a nanoscale drug carrying system to prevent indomethacin passage to the fetus in a pregnant mouse model. Am J Obstet Gynecol 212, 508 e501–507, doi: 10.1016/j.ajog.2015.02.006 (2015). [DOI] [PubMed] [Google Scholar]
- Flenady V., Reinebrant H. E., Liley H. G., Tambimuttu E. G. & Papatsonis D. N. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst Rev, 3, CD004452, doi: 10.1002/14651858.CD004452.pub3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. A., Hamilton B. E., Osterman M. J., Curtin S. C. & Matthews T. J. Births: final data for 2013. Natl Vital Stat Rep 64(1), 1–65 (2015). [PubMed] [Google Scholar]
- Matthews T. J., MacDorman M. F. & Thoma M. E. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep 64(9), 1–30 (2015). [PubMed] [Google Scholar]
- King, A. N. C. et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci Adv 2 e1600349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga M., Ku C. Y., Dodge K. & Sanborn B. M. Oxytocin-stimulated responses in a pregnant human immortalized myometrial cell line. Biol Reprod 55, 427–432 (1996). [DOI] [PubMed] [Google Scholar]
- Ohmichi M. et al. Role of mitogen-activated protein kinase pathway in prostaglandin F2alpha-induced rat puerperal uterine contraction. Endocrinology 138, 3103–3111, 10.1210/endo.138.8.5305 (1997). [DOI] [PubMed] [Google Scholar]
- Tasciotti E. et al. Near-infrared imaging method for the in vivo assessment of the biodistribution of nanoporous silicon particles. Mol Imaging 10, 56–68 (2011). [PMC free article] [PubMed] [Google Scholar]
- Longo M. et al. Effects of L-type Ca(2+)-channel blockade, K(+)(ATP)-channel opening and nitric oxide on human uterine contractility in relation to gestational age and labour. Mul Hum Reprod 9, 159–164 (2003). [DOI] [PubMed] [Google Scholar]
- Chiossi, G. et al. In vitro myometrial contractility profiles of different pharmacological agents used for induction of labor. Am J Perinatol 29, 699–704, 10.1055/s-0032-1314891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M. M., Friel A. M., Healy D. G. & Morrison J. J. Uterine relaxant effects of cyclooxygenase-2 inhibitors in vitro. Obstet Gynecol 98, 563–569 (2001). [DOI] [PubMed] [Google Scholar]
- Sawdy R. J., Sullivan M. H. & Bennett P. R. The effects of non-steroidal anti-inflammatory compounds on human myometrial contractility. Eur J Obstet Gynecol Reprod Biol 109, 33–40, doi:S0301211502004815 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- Sawdy R., Knock G. A., Bennett P. R., Poston L. & Aaronson P. I. Effect of nimesulide and indomethacin on contractility and the Ca2+ channel current in myometrial smooth muscle from pregnant women. Br J Pharmacol 125, 1212–1217, 10.1038/sj.bjp.0702211 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikland M., Lindblom B., Wilhelmsson L. & Wiqvist N. Oxytocin, prostaglandins, and contractility of the human uterus at term pregnancy. Acta Obstet Gyneco Scand 61, 467–472 (1982). [DOI] [PubMed] [Google Scholar]
- Schrock A., Fidi C., Low M. & Baumgarten K. Low-dose ethanol for inhibition of preterm uterine activity. Am J Perinatol 6, 191–195, 10.1055/s-2007-999574 (1989). [DOI] [PubMed] [Google Scholar]
- McCallum L. A., Pierce S. L., England S. K., Greenwood I. A. & Tribe R. M. The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med 15, 577–586, 10.1111/j.1582-4934.2010.01021.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey C. et al. The inwardly rectifying K+ channel KIR7.1 controls uterine excitability throughout pregnancy. EMBO Mol Med 6, 1161–1174, 10.15252/emmm.201403944 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod J. S., Rada C. C., Pierce S. L., England S. K. & Lamping K. G. Altered contribution of RhoA/Rho kinase signaling in contractile activity of myometrium in leptin receptor-deficient mice. Am J Physiol Endocrinol Metab 301, E362–E369, 10.1152/ajpendo.00696.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.