Abstract
Amyloid β-peptide (Aβ) in its oligomeric form is often considered as the most toxic species in Alzheimer’s disease (AD), and thus Aβ oligomer is a potentially promising candidate biomarker for AD diagnosis. The development of a sensitive and reliable method for monitoring the Aβ oligomer levels in body fluids is an urgent requirement in order to predict the severity and progression at early or preclinical stages of AD. Here, we show a proof of concept for a sensitive and specific detection of Aβ oligomers by an antibody-aptamer sandwich assay. The antibodies of Aβ oligomers and a nanocomposite of gold nanoparticles with aptamer and thionine (aptamer-Au-Th) were used as the recognition element and the detection probe for specifically binding to Aβ oligomers, respectively. The electrochemical signal of Th reduction could provide measurable electrochemical signals, and a low limit of detection (100 pM) was achieved due to the signal amplification by high loading of Th on the gold nanoparticles. The feasibility of the assay was verified by test of Aβ oligomers in artificial cerebrospinal fluid. The proposed strategy presents valuable information related to early diagnosis of AD process.
Alzheimer’s disease (AD) is the most prevalent progressive dementia marked by memory loss, cognitive decline, behavioral and physical disability, and significant and irreversible brain damage1,2. With a steady increase in the aging population, AD has become a serious social problem. Although some agents have been utilized to alleviate the symptoms of AD patients, there are no powerful therapeutic drugs for radical cure of AD. Thus, early diagnosis of AD is an urgent case for preventative and therapeutic treatment.
Amyloid β-peptide (Aβ) is the major component of the senile plaques that are one of two classical pathological hallmarks of AD3. The amyloid cascade hypothesis enunciates that an increased Aβ aggregation produces firstly to the formation of Aβ oligomers, then fibrils, and ultimately to plaques. It is now widely accepted that the diffusible Aβ oligomers, rather than mature Aβ fibrils and small Aβ monomers, have also been highlighted as the most neurotoxic form4. The neurotoxic increase of the Aβ oligomers could be explained by an increase in the number of toxic β-sheets per total mass of Aβ. The levels of Aβ oligomers in cerebrospinal fluid (CSF) of AD patients are higher than that of normals5. The levels of Aβ oligomers in CSF are efficacious for predicting the severity and progression at early or preclinical stages of AD. Therefore, Aβ oligomers are now considered not only as diagnostic markers but also as therapeutic targets of AD6. However, the detection of Aβ oligomers is a great challenge due to their characteristics of instability and transience to produce heterogeneous mixtures during the analysis process.
Electrochemical sensors7,8,9, surface plasma resonance sensors10,11, and fluorescent sensors12,13,14,15,16,17,18,19,20, and surface-enhanced Raman spectroscopy21 to measure Aβ oligomers have been designed based on the high binding affinity of peptides, proteins, antibodies and small molecules toward Aβ oligomers22. Although a low detection of these assays was obtained, a drawback of above fabricated assays was their low specificity owing to the interference and nonspecific adsorption from body fluids. Detection of Aβ oligomers by large-scale instruments, such as mass spectrometry could achieve the high reproducibility and sensitivity23,24. The inherent shortcomings of expensive instruments and complex operation still existed. Enzyme-linked immunosorbent assay (ELISA) technique is a reliable method for analyzing Aβ oligomers and is easy to realize miniaturization. ELISA, which utilized two approaches based on conformation-specific and sequence-specific antibodies, have been widely reported for detection of Aβ oligomers. The first strategy suffered from the interferences of other oligomeric proteins, such as prion, α-synuclein, polyglutamate, and several heat shock proteins25. The second strategy to measure Aβ oligomers in CSF or human brain ensured the specific and sensitive detection based on using capture and (labeled) detection antibodies to recognize the Aβ oligomers26,27,28,29. However, the problem of time-consuming and requirement of expensive enzyme-lined antibodies should not be ignored. The above problems limit their applications for the routine test of the Aβ oligomers for early diagnosis of AD.
Alternatively, aptamers, which are selected through an in vitro selection process called selective evolution of ligands by exponential enrichment (SELEX), have the comparable binding and specificity with antibodies30,31. Importantly, aptamers are more efficient than antibodies because of the ease in conjugation to various molecules, animal-free synthesis, and improved stability32,33,34. Tsukakoshi et al. isolated Aβ oligomer-specific DNA aptamers by the combination of a gel-shift assay and a competitive screening method35. The selected aptamers could be potentially applied in the biological assay for AD-related research.
In consideration of the urgency of Aβ oligomer detection and the advantages of aptamers in clinical diagnosis, we introduce an antibody-aptamer sandwich assay as a sensitive, specific, and versatile electrochemical platform for protein detection. The antibodies of Aβ oligomers were used as the recognition element for specifically binding to Aβ oligomers. A nanocomposite of aptamer-Au-Th prepared by in situ modification of gold nanoparticles (AuNPs) with DNA aptamer and thionine (Th) was utilized as the detection probe. The electrochemical signal of Th reduction could provide measurable electrochemical signals and the signal amplification was achieved by high loading of Th on the AuNPs. Finally, the established aptamer-based electrochemical assay was successfully applied for evaluation of Aβ oligomers in artificial CSF samples.
Results and Discussion
Design strategy and characterization of the antibody-aptamer sandwich electrode
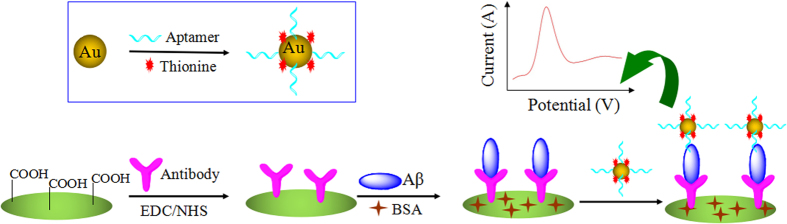
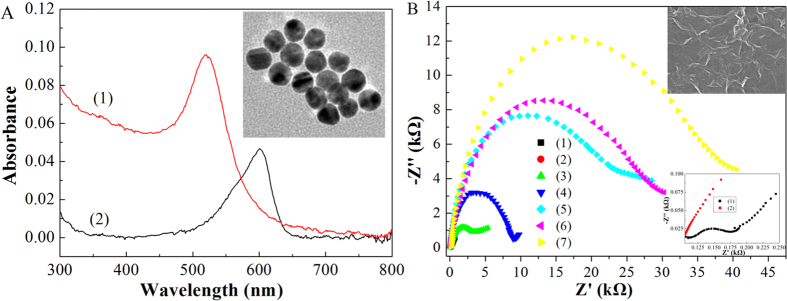
Figure 1 shows the construction procedure of the antibody-aptamer sandwich electrode and its sensing mechanism for Aβ oligomers. AuNPs synthesized as a stabilizer from citrate displayed a maximal absorbance at 520 nm, which was attributed to the surface plasmon resonance of the 20 nm AuNPs verified by characterization of transmission electron microscopy (TEM) (Fig. 2A). The incubation of AuNPs with DNA aptamer for recognition of Aβ oligomers and with Th for electrochemical signal amplification produced the aptamer-Au-Th probe at an appropriate molar ratio. The prepared probe showed an absorbance peak at 600 nm, which was consistent with the reported results36. To further verify the modification of AuNPs, X-ray photoelectron spectroscopy (XPS) was performed to analyze the chemical composition on the surface of AuNPs (Fig. S1). The S 2p and N 1s spectrum peaks of AuNPs after modification of Th or aptamer were observed distinctly at the binding energy of 167 and 399 eV, respectively. After the modification of Th and aptamer together, both of the spectrum peaks increased to some extent. The above results indicated that the aptamer-Au-Th probe was successfully prepared.
Figure 1. A schematic illustration of the electrochemical detection of Aβ oligomers using an antibody-aptamer sandwich assay.
Figure 2.
(A) UV-visible spectra of the aqueous solution of AuNPs (1) and aptamer-Au-Th bioconjugate (2). Inset (a) is a TEM image of AuNPs. (B) Nyquist plots of 10 mM [Fe(CN)6]3−/4− in 0.1 M KCl from 0.1 MHz to 0.1 Hz at ac amplitude of 5 mV under open-circuit potential conditions, obtained at the naked GC electrode (1), and the GC electrodes after the modification of carboxyl graphene (2), the activation of NHS/EDC (3), the immobilization of antibody (4), the recognition of Aβ oligomers (5), the blocking by BSA (6), and the binding of aptamer-Au-Th bioconjugate (7). Inset (b) is a SEM image of (2). Inset (c) is the magnified Nyquist plots for (1) and (2).
The glassy carbon (GC) electrode was fabricated by use of carboxyl graphene as the substrate because of not only its rapid electron transfer process but also its ability to immobilize the antibody onto the electrode surface. The image of scanning electron microscopy (SEM) for the carboxyl graphene-modified GC electrode demonstrated that a wrinkled texture of graphene sheets was formed on the electrode surface (inset b of Fig. 2B). The Aβ oligomers could be specifically recognized by the antibody tethered to the assay, which was followed by the binding of aptamer-Au-Th bioconjugates. The selected aptamers could bind Aβ oligomers specifically because of their high affinity (dissociation constant was estimated as 25 nM)35. The sandwich assay was established and the electrochemical reduction of Th was utilized for the quantitative detection of Aβ oligomers in 0.1 M phosphate buffer solution (PBS, pH 7.4). As we know, this is the first electrochemical assay about determination of the level of AD biomarker based on the binding with DNA aptamer. The formed assay was expected to have high sensitivity and selectivity because of the signal amplification by aptamer-Au-Th probe and the high specificity of antibody and aptamer, respectively.
Electrochemical impedance spectroscopy was employed to study the interface properties of the electrode surface during the fabrication procedure. The semicircle diameter at higher frequencies of the Nyquist plot is equal to the electron transfer resistance (Ret), which corresponds to the electron-transfer-limited process. Figure 2B illustrated the typical Nyquist plots for the modified electrodes in 0.1 M KCl containing 10 mM [Fe(CN)6]3−/4−. The Ret at the GC electrode could be estimated to be 76 ± 2 Ω. After casting the carboxyl graphene on the GC electrode, the Ret decreased nearly to zero, demonstrating that the graphene layer promoted the electron transfer process between the electrode surface and electrolyte. The Ret increased dramatically to 2.38 ± 0.05 kΩ, 6.24 ± 0.18 kΩ, 15.37 ± 0.36 kΩ, 18.46 ± 0.54 kΩ, and 23.15 ± 0.79 kΩ after the activation of NHS/EDC, the immobilization of antibody, the recognition of Aβ oligomers, blocking by bovine serum albumin (BSA), and binding of aptamer-Au-Th bioconjugate, respectively. The increase in Ret results from the hindered pathway of electron transfer because the most organic and biological molecules are poor electrical conductors and produce hindrance to electron transfer. These results verified the fabrication process of the antibody-aptamer sandwich assay.
Voltammetric determination of Aβ oligomers
Figure 3 shows a comparison of differential pulse voltammetry (DPV) using the antibody-aptamer sandwich assay in the presence and absence of the Aβ oligomers in 0.1 M PBS (pH 7.4). The reduction peak current of Th at −0.20 V for the control experiment showed the non-specific binding of the aptamer-Au-Th bioconjugates over the electrode surface before blocking of BSA. The signal decreased after blocking of BSA due to the less non-specific adsorptions. Obvious increase of reduction peak current of Th in the presence of Aβ oligomers was observed, which resulted from the recognition of Aβ oligomers by both antibody and aptamer. To further verify the antibody recognition, the control experiment without the immobilization of antibody was also carried out and just a very small peak current was obtained because of the bioconjugate adsorption on the electrode surface. The results indicated that the electrochemical assay could be used for the detection of Aβ oligomers.
Figure 3. DPV of the antibody-aptamer sandwich assay in 0.1 M PBS (pH 7.4).
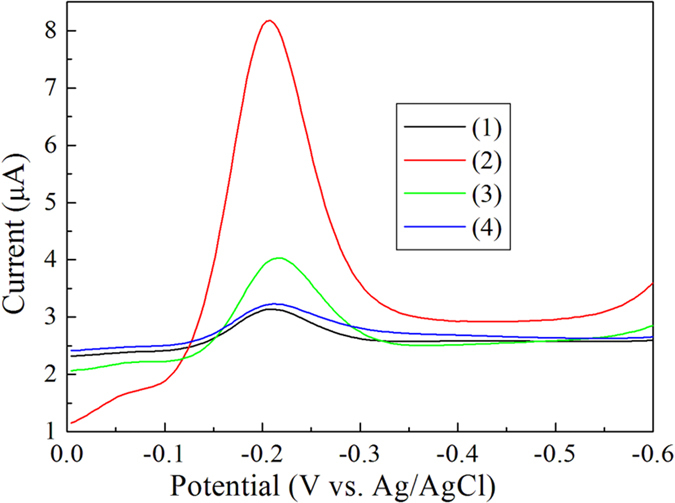
In the absence (1) and presence (2) of 20 nM Aβ oligomers after blocking of BSA. Curve 3 is the absence of 20 nM Aβ oligomers before blocking of BSA. Curve 4 is the presence of 20 nM Aβ oligomers without the immobilization of antibody.
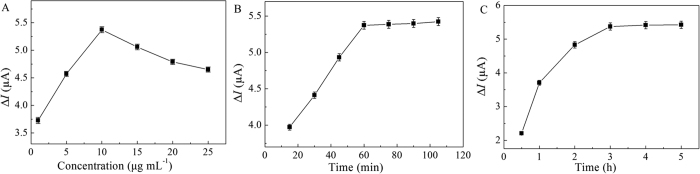
To optimize analytical conditions for voltammetric assay, experimental parameters including antibody concentration, incubation time of Aβ oligomers, and incubation time of aptamer-Au-Th bioconjugate were studied. The increased current ΔI = Ip − I0 was utilized as the binding parameter of Aβ oligomers, where the Ip and I0 was the peak current in the presence and absence of Aβ oligomers, respectively. The effect of antibody concentration on the peak current of Th reduction was investigated from 1 to 25 μg mL−1 as shown in Fig. 4A. The ΔI value gradually increased with increasing concentration from 1 to 10 μg mL−1, and then it began to decrease as the concentration increased over 10 μg mL−1 due to the hindered pathway of electron transfer by the high loading of antibody molecules. Thus, the optimum antibody concentration was used for subsequent experiments. The effect of incubation time of Aβ oligomers (Fig. 4B) and aptamer-Au-Th bioconjugate (Fig. 4C) on the detection of Aβ oligomers was then performed, and the ΔI value reached the steady state over 60 min and 3 h incubation time, respectively. Hence, the incubation time of Aβ oligomers and aptamer-Au-Th bioconjugate was determined to be 60 min and 3 h, respectively.
Figure 4.
Effect of antibody concentration (A), incubation time of Aβ oligomers (B), and incubation time of aptamer-Au-Th bioconjugate (C) for detection of 20 nM Aβ oligomers in 0.1 M PBS (pH 7.4) using the antibody-aptamer sandwich assay.
The antibody-aptamer sandwich assay was examined under the optimum conditions for the determination of Aβ oligomers. In Fig. 5A, the reduction peak current increased upon the increase of the concentration of Aβ oligomers. As shown in Fig. 5B, the ΔI value was linearly related to the concentration of Aβ oligomers within the range of 0.5–30 nM (ΔI = 0.159c + 1.551) with a correlation coefficient of 0.993. The limit of detection (LOD), estimated from 3σ of the baseline signals, was approximately 100 pM for the Aβ oligomers. The obtained LOD of this assay could be comparable to that of other methods such as, ELISA, SERS, MS, SPR, electrochemical and fluorescent sensor7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. The high sensitivity of the proposed assay might be explained by the highly efficient signal amplification by the aptamer-Au-Th bioconjugate. Importantly, this method obviates the utilization of enzyme-linked antibody and the operation of complex and expensive instruments. The analytical properties, advantages, and disadvantages of these techniques are summarized in Table S1. Moreover, the physiological level of Aβ in normal human CSF is about 1–2 nM, and the concentration is lower than that of AD patients3. Thus the proposed method is promising to detect the Aβ oligomers in body fluids.
Figure 5. DPV curves of an increasing concentration of Aβ oligomers in 0.1 M PBS (pH 7.4) using the antibody-aptamer sandwich assay.
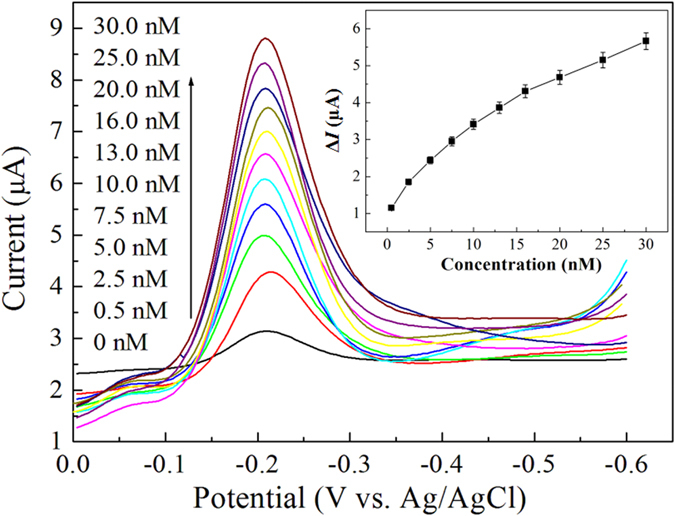
Inset is the linear calibration plot of (Ip − I0) value vs. the concentration of Aβ oligomers.
The performances of the developed electrochemical assay for the detection of Aβ oligomers, including repeatability, reproducibility and stability, were also studied by testing the current response of DPV to 20 nM Aβ oligomers in 0.1 M PBS (pH 7.4). The relative standard deviation (RSD) was 2.9% for 9 successive assays. The fabrication reproducibility was assessed at seven different antibody-aptamer sandwich assay prepared under the same conditions, and the RSD was 6.3%. After being stored in refrigerator (4 °C) for two weeks, 91% of its initial current response was retained. The above results of the modified electrode demonstrated an acceptable stability and reliability, which was comparable with that of the reported assays.
Selectivity of the assay
In order to evaluate the selectivity of the system for the detection of Aβ oligomers, the fabricated assay was used to test the Aβ molecules including Aβ1–42 monomers, Aβ1–40 monomers, Aβ1–42 oligomers, Aβ1–40 oligomers, Aβ1–42 fibrils, and Aβ1–40 fibrils, under the identical conditions. Figure 6 shows the comparison of the electrochemical response of the above Aβ molecules. It was clear that Aβ monomers and Aβ fibrils had minor influences for the detection of Aβ oligomers owing to high recognition ability of antibodies to Aβ oligomers. In addition, the similar current response for both of Aβ1–42 oligomers and Aβ1–40 oligomers indicated that the total Aβ oligomers were detected by the electrochemical assay. Therefore, the outstanding advantages of the assay with high selectivity and stability could be potentially applicable in real samples of body fluids.
Figure 6. Selectivity of the antibody-aptamer sandwich assay in 0.1 M PBS (pH 7.4) containing 20 nM Aβ.
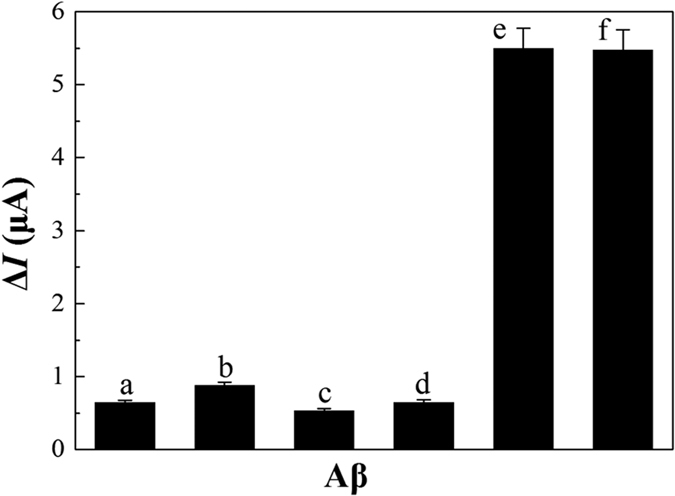
(a) Aβ1–42 monomers, (b) Aβ1–40 monomers, (c) Aβ1–42 fibrils, (d) Aβ1–40 fibrils, (e) Aβ1–42 oligomers, (f) Aβ1–40 oligomers.
Application in CSF samples
To demonstrate the viability of this technique, the total Aβ oligomers were analyzed in real samples of artificial CSF, as illustrated in Table 1. The standard addition method analyzed by calibration curve and the recovery during the experiments for spiked samples were utilized to test the accuracy of the assay. The data in Table 1 showed the acceptable recovery, which indicated the validity of the electrochemical analysis for the Aβ oligomers in CSF samples using the fabricated assay. Thus, the simplicity, high sensitivity and selectivity of the antibody-aptamer sandwich assay make it as a new potential platform for the detection of Aβ oligomers for real samples in body fluids of AD patients.
Table 1. Results of the detection of Aβ oligomers in artificial CSF using the antibody-aptamer sandwich assay.
| Sample no. | Sample | Added (nM) | Found (nM) | Recovery(%) |
|---|---|---|---|---|
| 1 | Aβ oligomer | 1.0 | 0.97 | 97.0 |
| 2 | Aβ oligomer | 7.0 | 7.4 | 105.7 |
| 3 | Aβ oligomer | 12.0 | 12.3 | 102.5 |
| 4 | Aβ oligomer | 18.0 | 17.6 | 97.8 |
| 5 | Aβ oligomer | 23.0 | 23.7 | 103.0 |
Conclusions
In summary, this study demonstrates the effective determination of Aβ oligomers based on the antibodies of Aβ oligomers and a nanocomposite of aptamer-Au-Th as the recognition element and the detection probe, respectively. Compared with the known method for detection of Aβ oligomers, the fabricated electrochemical assay offers some advantages: (i) high sensitivity due to the signal amplification by high loading of Th on the AuNPs, (ii) high specificity owing to the high specific recognition of antibodies and DNA aptamers with Aβ oligomers, and (iii) obviation of the utilization of enzyme-linked antibody and the operation of complex and expensive instruments. All of these factors make the antibody-aptamer sandwich assay as an ideal platform for Aβ oligomers. We believe that our work represents a significant step forward to the routine detection of Aβ oligomers and would be valuable for the early diagnosis of AD.
Methods
Chemicals and materials
Aβ(1–40) and Aβ(1–42) were purchased from DgPeptides Co., Ltd (Shanghai, China) with purity of >95%. The aptamer sequence of Aβ oligomers (5′-HS-GCCTGTGTTGGGGCGGGTGCG) was screened out by Tsukakoshi et al.35, and were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). Anti-Aβ oligomer antibody was provided by Abcam (Cambridge, England). Chloroauric acid trihydrate (HAuCl4·3H2O), Th, NHS (N-hydroxysuccinimide), EDC (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride), and BSA were purchased from Sigma-Aldrich. Carboxyl graphene dispersion was provided by the XFNANO Co., Ltd (Nanjing, China). PBS were prepared by varying the volume ratios of the solution containing Na2HPO4 and NaH2PO4. All other chemicals were purchased from commercial suppliers and used as received. Deionized water (18 MΩ cm, Milli-Q gradient system, Millipore) was used throughout the experiments.
Artificial CSF used in determination of the samples was prepared by 150 mM NaCl, 3.0 mM KCl, 1.4 mM CaCl2·2H2O, 1 mM phosphate, and 0.8 mM MgCl2·6H2O 37,38.
Instruments
All electrochemical measurements were carried out on a CHI660D electrochemical workstation (Shanghai CH Instruments, China) using a conventional three electrode system, with a modified GC disk electrode (3.0-mm diameter) as working electrode, a platinum foil as counter electrode, and a saturated Ag/AgCl as reference electrode. UV-vis absorption characterization was performed on a UV-vis 2300 spectrometer (Shimadzu, Japan). TEM samples, which were prepared by dropping 20 μL of gold colloidal solution onto a copper grid (3 mm, 300 mesh) coated with carbon film, were examined with Tecnai G2 20 S-TWIN (FEI, America) transmission electron microscope. The surface morphology of the modified electrodes was observed by SEM (Quonxe-2000, FEI). XPS samples of AuNPs were obtained by purification at high speed centrifugation (10,000 rpm) for three times. 20 μL of gold colloidal solution was dropped onto a silicon chip, and XPS spectra were carried out on an X-ray photoelectron spectrometer (PHI-5400).
Treatment of Aβ solution
To obtain Aβ monomers, lyophilized peptides were dissolved in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) and then the above Aβ solution (2 mg mL−1) was incubated overnight at room temperature39. The solvent of HFIP was evaporated off by treatment of N2 gas and the Aβ was redissolved in dimethylsulfoxide. The prepared Aβ monomer solution (11.5 μM) was stored at −20 °C as stock solution. The Aβ oligomers and fibrils were obtained by incubation of the Aβ monomer solution (11.5 μM) at 37 °C at dark for 24 h and 72 h, respectively.
Preparation of aptamer-Au-Th bioconjugate
The citrate stabilized36 20 nm AuNPs were synthesized as follows: 3.75 mL trisodium citrate solution (1%) was added to a boiling HAuCl4 solution (0.01%, 250 mL) with rapid stirring, and then the mixture color changed from pale yellow to deep purple within 2 min. The solution was kept boiling, stirred for 15 min, after which, the AuNPs solution was cooled to room temperature. 7.0 mL of saturated Th was added into 35 mL of AuNPs solution, and then the resulting solution was mixed for 24 h. The above solution was concentrated to 5.0 mL by centrifugation (8, 000 rpm) for 15 min, followed by washing and resuspension with 0.1 M PBS (pH 7.4). 45 μL of aptamer aqueous solution (100 μM) was then added into the 5.0 mL of Au-Th solution and incubated for 4 h (140 rpm) at room temperature. The above mixed solution was treated by centrifugation (8, 000 rpm, 15 min), washing and resuspension into 460 μL of 0.1 M PBS (pH 7.4), and then the bioconjugate of aptamer-Au-Th was obtained.
Fabrication of the antibody-aptamer sandwich assay
Before modification, the GC electrode was polished with α-alumina slurry (1.0, 0.3, and 0.05 μm) on a polishing cloth. Then, the electrode was sonicated in acetone and deionized water each for 10 min, and then dried under N2 gas. Next, 10 μL of carboxyl graphene dispersion (2 mg mL−1) was dropped onto the GC electrode surface. After drying by infrared lamp and washing with distilled water, the electrode was treated by activation with EDC/NHS (30 mM/2 mM) for 2 h and washed with distilled water. Subsequently, the electrode was immersed into 10 μg mL−1 antibody dissolved in 0.1 M PBS (pH 7.4) for 4 h. Followed by washing with 0.1 M PBS (pH 7.4), the above electrode was blocked with 10 μL of 1% BSA solution to eliminate the non-specific binding effects. After washing with PBS, the antibody-modified sensor was fabricated.
The detection of Aβ oligomers based on the antibody-aptamer sandwich strategy were performed by incubation of the prepared assay with a 10 μL of Aβ oligomers aqueous solutions with different concentrations at 37 °C. After washing with 0.1 M PBS (pH 7.4) for three times, the resultant sensor was then incubated with 10 μL of the aptamer-Au-Th bioconjugate at 37 °C. Followed by rinsing throughly with 0.1 M PBS (pH 7.4) to remove the unbound conjugate, the voltammetric responses of the sandwich assay were recorded by DPV from 0 to −0.5 V with a pulse amplitude of 0.005 V and a pulse width of 0.1 s for the detection of Aβ oligomers. The buffer solutions were purged with high purity N2 gas for at least 15 min prior to each electrochemical measurement.
Additional Information
How to cite this article: Zhou, Y. et al. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci. Rep. 6, 35186; doi: 10.1038/srep35186 (2016).
Supplementary Material
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (Grant Nos 21675109, 21475084, 21405102, 21305085), China Postdoctoral Science Foundation (2016M590684), Program for Science & Technology Innovation Talents in Universities of Henan Province (16HASTIT005, 14HASTIT016), and Innovation Scientists and Technicians Troop Construction Projects of Henan Province (No. 41).
Footnotes
Author Contributions Y.Z. and L.L. contributed to design the study and write the manuscript. H.Z. and C.L. prepared the electrochemical sensor and performed the electrochemcial measurements. Z.C. and X.Z. performed UV and TEM measurements. M.X. and B.Y. analyzed the data. All authors contributed to discuss of the results and review the manuscript.
References
- Rauk A. The chemistry of Alzheimer’s disease. Chem. Soc. Rev. 38, 2698–2715 (2009). [DOI] [PubMed] [Google Scholar]
- Kepp K. P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 112, 5193–5239 (2012). [DOI] [PubMed] [Google Scholar]
- Hamley I. W. The Amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and Fibrillization. Chem. Rev. 112, 5147–5192 (2012). [DOI] [PubMed] [Google Scholar]
- Viles J. H. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and Prion disease. Coord. Chem. Rev. 256, 2271–2284 (2012). [Google Scholar]
- Hayne D. J., Lim S. & Donnelly P. S. Metal complexes designed to bind to amyloid-β for the diagnosis and treatment of Alzheimer’s disease. Chem. Soc. Rev. 43, 6701–6715 (2014). [DOI] [PubMed] [Google Scholar]
- Murakami K. et al. Monoclonal antibody with conformational specificity for a toxic conformer of amyloid β42 and its application toward the Alzheimer’s disease diagnosis. Sci. Rep. 6, 29038/1–29038/12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. et al. A general way to assay protein by coupling peptide with signal reporter via supermolecule formation. Anal. Chem. 85, 1047–1052 (2013). [DOI] [PubMed] [Google Scholar]
- Rushworth J. V. et al. A label-free electrical impedimetric biosensor for the specific detection of Alzheimer’s amyloid-beta oligomers. Biosens. Bioelectron. 56, 83–90 (2014). [DOI] [PubMed] [Google Scholar]
- Veloso A. J. et al. Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillar aggregation processes. Anal. Chem. 86, 4901–4909 (2014). [DOI] [PubMed] [Google Scholar]
- Haes A. J., Chang L., Klein W. L. & Duyne R. P. V. Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc. 127, 2264–2271 (2005). [DOI] [PubMed] [Google Scholar]
- Yi X. Y., Feng C. T., Hu S. Q., Li H. F. & Wang J. X. Surface plasmon resonance biosensors for simultaneous monitoring of amyloid-beta oligomers and fibrils and screening of select modulators. Analyst 141, 331–336 (2015). [DOI] [PubMed] [Google Scholar]
- Matveeva E. G., Rudolph A., Moll J. R. & Thompson R. B. Structure-selective anisotropy assay for amyloid beta oligomers. ACS Chem. Neurosci. 3, 982–987 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T. & Mihara H. FRET detection of amyloid β-peptide oligomerization using a fluorescent protein probe presenting a pseudo-amyloid structure. Chem. Commun. 48, 1568–1570 (2012). [DOI] [PubMed] [Google Scholar]
- Teoh C. L. et al. Chemical fluorescent probe for detection of Aβ oligomers. J. Am. Chem. Soc. 137, 13503–13509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennmalm S., Chmyrov V., Widengren J. & Tjernberg L. O. Highly sensitive FRET-FCS detects amyloid β-peptide oligomers in solution at physiological concentrations. Anal. Chem. 87, 11700–11705 (2015). [DOI] [PubMed] [Google Scholar]
- Lv G. L. et al. A spiropyran-based fluorescent probe for specific detection of β-amyloid peptide oligomers in Alzheimer’s disease. Chem. Commun. 52, 8865–8868 (2016). [DOI] [PubMed] [Google Scholar]
- Xia N. et al. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 85, 625–632 (2016). [DOI] [PubMed] [Google Scholar]
- Zhu L. L. et al. Selective amyloid β oligomer assay based on a basic site-containing molecular beacon and enzyme-free amplification. Biosens. Bioelectron. 78, 206–212 (2016). [DOI] [PubMed] [Google Scholar]
- Park M. C. et al. Droplet-based magnetic bead immunoassay using microchannel connected multiwell plates (μCHAMPs) for the detection of amyloid beta oligomers. Lap on a Chip 16, 2245–2253 (2016). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. A graphene oxide-based fluorescent platform for selective detection of amyloid-β oligomers. Anal. Methods 7, 8727–8732 (2015). [Google Scholar]
- Chou I.-H. et al. Nanofluidic biosensing for β-Amyloid detection using surface enhanced Raman spectroscopy. Nano Lett. 8, 1729– 1735 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. L., Liu L. T., Hao Y. Q. & Xu M. T. Detection of Aβ monomers and oligomers: early diagnosis of Alzheimer’s disease. Chem. Asian J. 11, 805–817 (2016). [DOI] [PubMed] [Google Scholar]
- Cernescu M. et al. Laser-induced liquid bead ion desorption mass spectrometry: an approach to precisely monitor the oligomerization of the β-amyloid peptide. Anal. Chem. 84, 5276–5284 (2012). [DOI] [PubMed] [Google Scholar]
- Tay W. M. et al. A mass spectrometric approach for characterization of amyloid-β aggregates and identification of their post-translational modifications. Biochemistry 51, 3759–3766 (2012). [DOI] [PubMed] [Google Scholar]
- Morgado I. et al. Molecular basis of β-amyloid oligomer recognition with a conformational antibody fragment. Proc. Natl. Acad. Sci. USA 109, 12503–12508 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H. et al. High-molecular-weight β-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 24, 2716–2726 (2010). [DOI] [PubMed] [Google Scholar]
- Bruggink K. A. et al. Amyloid-β oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal. Biochem. 433, 112–120 (2013). [DOI] [PubMed] [Google Scholar]
- Herskovits A. Z., Locascio J. J., Peskind E. R., Li G. & Hyman B. T. A luminex assay detects amyloid β oligomers in Alzheimer’s disease cerebrospinal fluid. Plos one 8, e67898/1–e67898/11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. A. et al. Magnetic bead droplet immunoassay of oligomer amyloid β for the diagnosis of Alzheimer′s disease using micro-pillars to enhance the stability of the oil–water interface. Biosens. Bioelectron. 67, 724–732 (2015). [DOI] [PubMed] [Google Scholar]
- Ellington A. D. & Szostak J. W. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990). [DOI] [PubMed] [Google Scholar]
- Tuerk C. & Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Aptamer/Graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 132, 9274–9276 (2010). [DOI] [PubMed] [Google Scholar]
- Tang L. H. et al. Colorimetric and ultrasensitive bioassay based on a dual-amplification system using aptamer and DNAzyme. Anal. Chem. 84, 4711–4717 (2012). [DOI] [PubMed] [Google Scholar]
- Wang M. J. et al. Electrochemical detection of DNA immobilized on gold colloid particles modified self-assembled monolayer electrode with silver nanoparticle label. J. Pharm. Biomed. Anal. 33, 1117–1125 (2003). [DOI] [PubMed] [Google Scholar]
- Tsukakoshi K., Abe K., Sode K. & Ikebukuro K. Selection of DNA aptamers that recognize α-synuclein oligomers using a competitive screening method. Anal. Chem. 84, 5542–5547 (2012). [DOI] [PubMed] [Google Scholar]
- Yu Y. Y. et al. A method for evaluating the level of soluble β-amyloid(1–40/1–42) in Alzheimer’s disease based on the binding of gelsolin to β-amyloid peptides. Angew. Chem. Int. Ed. 53, 12832–12835 (2014). [DOI] [PubMed] [Google Scholar]
- Hegnerová K. et al. Surface plasmon resonance biosensors for detection of Alzheimer disease biomarker. Sensor. Actuat. B 139, 69–73 (2009). [Google Scholar]
- Liu L. et al. Competitive electrochemical immunoassay for detection of β-amyloid (1–42) and total β-amyloid peptides using p-aminophenol redox cycling. Biosens. Bioelectron. 51, 208–212 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou Y. L., Dong H., Liu L. T. & Xu M. T. Simple colorimetric detection of amyloid β-peptide (1–40) based on aggregation of gold nanoparticles in the presence of copper ions. Small 11, 2144–2149 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.