Abstract
The identification of early markers that predict the development of specific social trajectories is critical to understand the developmental and neurobiological underpinnings of healthy social development. We investigated, in infant rhesus macaques (Macaca mulatta), whether newborns’ capacity to imitate facial gestures is a valid predictive marker for the emergence of social competencies later in development, at one year of age. Here we first assessed whether infant macaques (N = 126) imitate lipsmacking gestures (a macaque affiliative expression) performed by a human experimenter in their first week of life. We then collected data on infants’ social interactions (aggression, grooming, and play) and self-scratching (a proxy indicator of anxiety) at 11–14 months when infants were transferred into a new enclosure with a large social group. Our results show that neonatal imitators exhibit more dominant behaviours, are less anxious, and, for males only, spend more time in play at one year old. These findings suggest that neonatal imitation may be an early predictor of infant sociality and may help identify infants at risk of neurodevelopmental social deficits.
In humans, there are large inter-individual differences in the ability to establish and maintain social relationships, which have profound consequences on psychological well-being. Individuals with good social skills experience higher levels of happiness, self-esteem, and quality of life1. In contrast, those who lack these skills tend to develop a variety of problems, including social anxiety and phobia, depression, and loneliness2. The identification of early behavioural markers that can anticipate specific developmental trajectories has, therefore, become increasingly important to determine appropriate early intervention strategies for socially impaired individuals3.
Infants’ ability to imitate facial gestures in their first weeks of life is thought to be one of the earliest measures of the presence of inter-individual differences in social skills4. Newborns as young as 42 min are able to imitate open mouth and tongue protrusion gestures5, as well as facial expressions like happiness, sadness, and surprise6. Neonatal imitation has been suggested to enrich infants’ understanding of people7, and strengthen their bonds with caregivers8. Although the presence of this phenomenon at the population level is still debated9, there is clear evidence of ample inter-individual variability in neonatal imitative responses6,10. Heimann and colleagues10 for instance, showed that 44% of infants at 2–3 days of age imitate mouth opening and 61% imitate tongue protrusion, while Field6 found that approximately one-third of human neonates (average age 36 hours) exhibited strong imitation skills, while one-third showed weak imitative responses, and one-third did not imitate the model.
Neonatal imitation is a phenomenon that occurs also in some non-human primates (NHP), namely chimpanzees (Pan troglodytes11), and rhesus macaques (Macaca mulatta12,13). As in human infants, even macaque infants show large inter-individual differences in their imitative abilities14. Although neonatal imitation has been suggested to be an early foundation for infants’ subsequent cognitive and social development15, there are few empirical studies that have investigated this idea. In humans, for instance, compared to non-imitators, infants who are imitators at 2–3 days and 3 weeks old show lower rates of gaze aversion at 3 months old when interacting with their mothers4. Similarly, in rhesus macaques, neonatal imitators look more at faces, especially at the eye region, of conspecifics at 10–28 days old16 and are better at gaze following at 7 months old13 than non-imitators, suggesting that imitators may more readily read social cues16. In the first week of life, imitators, compared to non-imitators, appear to (1) be more attentive during neonatal imitation assessments17, (2) better remember social partners18, and (3) exhibit superior delayed imitation19, suggesting imitators may be more socially advanced than non-imitators. It remains largely unclear, however, whether neonatal imitation is simply the expression of a general behavioural response that is contingent with the context and the stage of development, or whether it represents a basic biological predisposition that could be considered as a trait with clear connections with other traits. In the latter case, it is expected that neonatal imitation is not only linked to other behaviours that are associated with social perception and social interest, but it is expected that it may predict social development at later ages. However, this critical information is missing from the current literature. To fill this gap, we took advantage of the described interindividual variability in neonatal imitative responses and examined whether infant rhesus macaques who imitate facial gestures in their first week of life displayed greater social skills than non-imitators at one year old. We also examined whether imitators exhibit lower anxiety levels in a novel social context since, in both humans and NHP, individuals with poor social skills tend to exhibit increased anxiety and stress20,21.
Rhesus monkeys are an ideal study model in which to investigate this issue. Similar to humans, mother and infant macaques form strong bonds, with mothers engaging in a variety of communicative gestures with infants, such as lipsmacking, exaggerated head movements, and mutual gazing22,23. These exchanges seem to resemble the ‘motherese’ that human mothers display towards their infants22. As in humans, the quality of this early social enviroment can have profound consequences on infant’s cognitive and social development, resulting in large variation in social skills in adolescence and adulthood24,25.
Here we predict that at approximately 1 year old, neonatal imitators, compared to non-imitators, will:
spend more time in social play, as play behaviour functions as a way for infants to learn species-specific signals26, form alliances and assess and manipulate social relationships27;
spend more time in social grooming, which, in NHP, is a means by which individuals strengthen social relationships28;
display a greater frequency of dominant behaviours measured as aggression directed to peers as dominant behaviour is commonly linked to an individual’s ability to attain higher ranks29; and
show lower rates of self-scratching, which, in NHP, is considered a proxy indicator of anxiety30.
Results
We assessed neonatal imitation in the first week of life on 126 newborn rhesus macaques, and infants were then classified as either lipsmacking (LPS) imitators or non-imitators (see Methods). Of the 126 infants tested, 48% were classified as LPS imitators. We then measured macaque infants’ social behaviours at approximately one year of age, when placed in a large group with same-aged peers. We ran Linear Mixed Model (LMM) analysis to assess whether infants’ imitation category in the first week of life predicted the time infants spent in play and social grooming as well as infants’ rates of aggression directed at peers and self-scratching at 1 year of age.
Neonatal imitation and social behaviours at 1 year
The amount of time infants spent playing with peers at 1 year of age was significantly predicted by both sex (Estimate ± SE: −0.952 ± 0.204, t = −4.665, p < 0.001, LRT = 20.42, Table 1) and the interaction between sex and neonatal LPS imitation category (Estimate ± SE: −0.777 ± 0.263, t = −2.957, p = 0.004, LRT = 8.41, Table 1): male infants played significantly more (mean ± SD: 13.22 s ± 9.34 s) than females (6.97 s ± 5.60 s). Follow up t-test showed that male imitators played more than male non-imitators (t(80) = 2.265, p = 0.027, d = 0.53, Fig. 1), which is consistent with prediction 1. Contrary to prediction 2, however, no effect of LPS imitation category was found on the amount of grooming exchanged (imitators: 5.05 s ± 6.29 s; non-imitators: 5.66 s ± 5.57 s, Table 1).
Table 1. Results of the LMM model to test the effect of LPS neonatal imitation category, infant sex, rearing condition, and the interaction between LPS neonatal imitation category and sex on social behaviours at one year of age, namely rates of aggression given, play and grooming time.
| Independent | Predictor | Estimate | SE | t-value | P |
|---|---|---|---|---|---|
| Play | Intercept | 3.216 | 0.250 | 12.880 | |
| LPS imitation | 0.302 | 0.201 | 1.499 | 0.127 | |
| Sex | −0.952 | 0.204 | −4.665 | <0.001 | |
| rearing | 0.272 | 0.203 | −1.340 | 0.173 | |
| Sex * LPS imitation | −0.777 | 0.263 | −2.957 | 0.004 | |
| Grooming | Intercept | 1.993 | 0.285 | 6.992 | |
| LPS imitation | −0.217 | 0.201 | −1.079 | 0.278 | |
| Sex | 0.208 | 0.204 | 1.020 | 0.311 | |
| rearing | −0.118 | 0.203 | −0.579 | 0.552 | |
| Sex * LPS imitation | −0.060 | 0.251 | −0.238 | 0.806 | |
| Aggression given | Intercept | 0.351 | 0.089 | 3.927 | |
| LPS imitation | 0.089 | 0.043 | 2.069 | 0.039 | |
| Sex | −0.063 | 0.044 | −1.436 | 0.150 | |
| rearing | 0.044 | 0.044 | 1.005 | 0.307 | |
| Sex * LPS imitation | 0.047 | 0.055 | 0.853 | 0.387 |
Significant predictors (p < 0.05) are shown in bold.
Figure 1. Effect of lipsmacking (LPS) neonatal imitation category on play time at one year old for each sex.
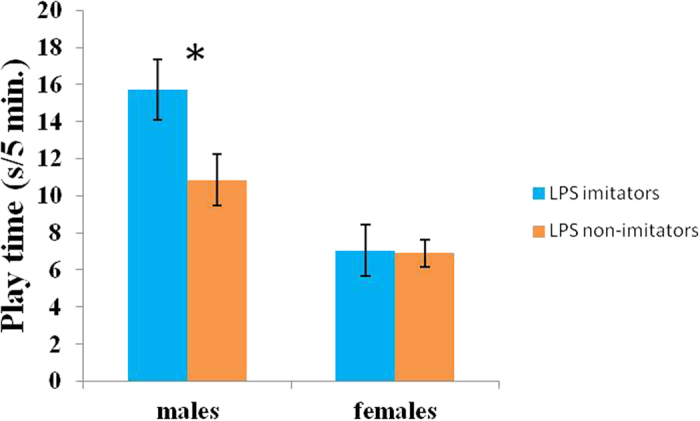
Male imitators spent more time playing with their peers than non-imitators. There was no difference in play time between female imitators and non-imitators. *p < 0.05.
We found support for prediction 3: our LMM model revealed that LPS imitation significantly predicted rates of aggression directed at peers (Estimate ± SE: 0.089 ± 0.043, t = 2.069, p = 0.039, LRT = 4.31, Table 1). LPS imitators displayed significantly higher rates of aggression compared to non-imitators (Fig. 2).
Figure 2. Effect of lipsmacking (LPS) neonatal imitation category on aggression rates.
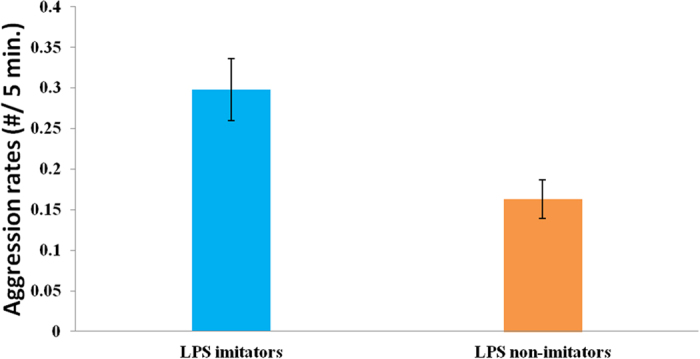
LPS imitators directed more aggression to peers compared to non-imitators.
Neonatal imitation and rates of self-scratching at 1 year
We found that LPS imitation significantly predicted rates of self-scratching (Estimate ± SE: 0.087 ± 0.044, t = −1.981, p = 0.048, LRT = 3.93, Table 2), with LPS imitators showing significantly lower rates of self-scratching than LPS non-imitators (Fig. 3), suggesting that imitators exhibited lower anxiety levels than non-imitators.
Table 2. Results of the LMM model to test the effect of LPS neonatal imitation category, infant sex, rearing condition, and the interaction between LPS neonatal imitation category and sex on rates of self-scratching at one year of age.
| Independent | Predictor | Estimate | SE | t-value | P |
|---|---|---|---|---|---|
| Self-scratching | Intercept | 1.031 | 0.077 | 13.497 | |
| LPS imitation | −0.087 | 0.044 | −1.981 | 0.048 | |
| Sex | −0.046 | 0.044 | −1.045 | 0.292 | |
| rearing | 0.016 | 0.044 | 0.370 | 0.706 | |
| Sex * LPS imitation | −0.068 | 0.055 | −1.246 | 0.212 |
Significant predictor (p < 0.05) is shown in bold.
Figure 3. Effect of lipsmacking (LPS) neonatal imitation category on self-scratching rates.
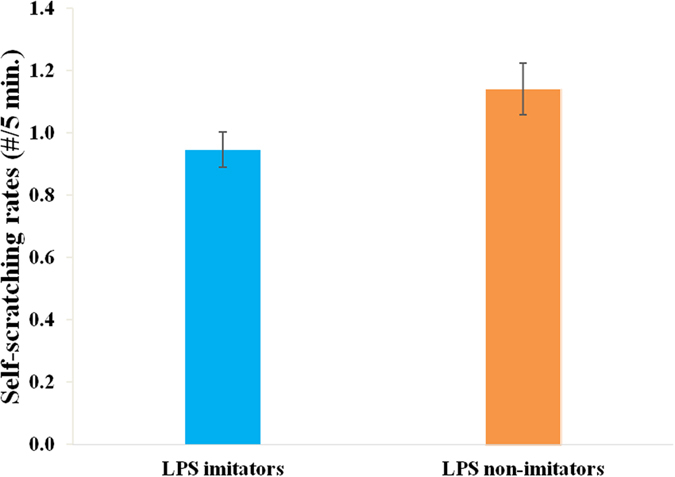
Imitators self-scratched less frequently than non-imitators.
Discussion
Our results build up on previous reports that infant rhesus monkeys display large inter-individual variability in their neonatal imitative abilities, with nearly half of the infants showing the capacity to imitate LPS gestures13,14,16,17,18,19. However, by using a large cohort of infants, our work provides the first evidence that infants’ ability to match a caregiver’s gestures predicts infant social behaviour and temperament at one year old, as LPS imitators exhibited greater dominance behaviour (expressed through increased aggression rates) and lower anxiety levels, and male imitators tended to play more than non-imitators at 1 year of age. These results indicate that neonatal imitative abilities not only reflect infants’ social skills or interest in the first weeks or months of life, as previous work in human and macaque has shown10,13,14,16,19, but also reflect a longer developmental trajectory that encompasses the first year of life.
Both aggressive and play behaviours serve important roles for infants’ development of social skills and establishment in a new social group26,31. Aggression is commonly used in primates as a way to achieve and maintain high ranks29, which ultimately increases individuals’ inclusive fitness. Furthermore, third-party agonistic interventions in aggressive interactions play a fundamental role in stabilizing social groups32, as well as in establishing and maintaining dominance relationships and cementing alliances, contributing thereby to the formation of social bonds33. Finally, aggression has the fundamental function of obtaining and securing resources for both sexes, such as mates for males and feeding sources for females, which can explain why we did not find sex differences in the rates of aggression infants directed to peers. This finding is consistent with previous studies on rhesus macaques showing that while adult males and females display differences in aggression rates34, no sex difference in aggression is apparent in the first three years of life35. In free-ranging populations of rhesus monkeys, mother’s rank, and the presence of close relatives have a strong influence on infant’s ability to achieve high-ranks, as dominant females and family members are more likely to support infants than low-ranking females or unrelated group members36. Our work suggests that, when infants are raised in the absence of mothers or close relatives, and under homogeneous environmental conditions (except for the difference in rearing condition, which, in our study, did not predict any of the examined behaviours), infants’ biological predisposition to exhibit advanced social skills can be fundamental in the acquisition of dominant positions at least at one year of age (but see Bastian et al.37). Our work therefore indicates that these greater social skills can already be detected in the first week of life.
By including behavioural patterns typical of ‘serious’ functional contexts, such as mating, agonistic, and anti-predatory behaviours38, play behaviour offers a fundamental contribution for infant cognitive and social development across a broad range of animal species26. Through play, infants learn context-specific signals39, develop coordinate and cooperative skills40, and help infants assess the strength of their future competitors41. Accordingly, among yellow-bellied marmots (Marmota flaviventris), the outcome of play interactions predicts adult dominance rank42, while in brown bears (Ursus arctos) play behaviour increases infant chances to survive to independence43. In humans, peer play among children has been shown to have a strong impact on social development, through the acquisition, for instance, of conflict-resolution and cooperative-learning skills44: children who experience peer-rejection, for instance, suffer psychosocial and conduct problems45, which can also have negative consequences on children’s learning skills, leading to poor school performance46.
One of the core aspects of play, especially in the first years of life, is the ability to imitate peers: the performance of same acts is the predominant mode of social interactions among toddlers, and these imitative exchanges promote continued social interactions by communicating a common understanding of ongoing activities47 and are fundamental for the development of more advanced play skills at later ages48. Accordingly, children between 4 and 6 years of age tend to be attentive to peers’ activity, and this ability to observe peer behaviour has been found to have positive effects on children’s own performance of these activities48. The importance of imitation during social play is also supported by work conducted on NHP: in geladas (Theropithecus gelada), and Tonkean macaques (M. tonkeana), for instance, play bouts in which participants engage in rapid mimicry of facial expressions tend to last longer than bouts where this form of facial imitation is absent49,50. This capacity to imitate peers’ behaviours likely originates from newborn’s ability to match caregiver’s behaviours. For instance, infant rhesus macaques who engage in more face-to-face interactions with their caregiver in the first month of life display enhanced social development at later ages25.
Notably, children with neurodevelopmental disorders, such as autism spectrum disorder (ASD), exhibit impaired play activity with peers51 as well as deficit in imitative abilities52. This difficulty of children with ASD to imitate a model’s actions has been shown to be specific to non-meaningful (i.e. non goal-directed) gestures and appears to be due to an impaired selection mechanism (i.e. ‘what’ to imitate) which is related to these children’s impaired capacity to attend social stimuli and recognize intentional actions52.
Although our results need to be replicated in humans, our work suggests that these imitation skills can already be detected in newborns, and can be used as reliable indicators of specific developmental outcomes, at least until one year of age in macaques. In other words, in humans, classifying newborns based on their neonatal imitative behaviours may potentially lead to the identification of infants who might exhibit impaired social development. This classification, in turn, may facilitate testing of intervention programs aimed at improving the social development in infants and children who lack social skills. To date, a variety of methods have been developed to treat infants with neurodevelopmental disorders (e.g. ASD), either through pharmacological treatments53, or by providing a richer social environment54. However, these treatments are generally provided at an advanced developmental stage, when the toddler or child has already been diagnosed with ASD. Therefore, it is crucial to be able to identify early markers that can help detect neurodevelopmental disorders at an earlier developmental stage, when the brain is more plastic, and the course of early behavioural and brain development can be altered3. Our work, thus, should encourage more longitudinal studies in humans to assess to what extent children who imitate facial gestures when neonates are also more social in the first years of life (the developmental stage of a one-year old rhesus monkey corresponds approximately to the developmental stage of a four-year old human child55).
In the present study, we found the association between neonatal imitative response and play behaviour at 1 year old exclusively in males. In rhesus macaques, while females remain in their natal group, males transfer to neighbouring groups at approximately 3 years old. This process of migration is risky, as males face high predation risks and need to successfully integrate and be accepted into a new group. Accordingly, males suffer higher mortality than females, especially when they transfer to other groups56. These conditions might have selected males to have evolved a specific set of social skills from the earliest weeks of life than females. While we found no sex differences in neonatal imitation rates, a previous study reported female infant macaques appear more socially interested than males between 2–5 weeks of age, looking more to faces, especially the eyes, and exhibiting more affiliative behaviours towards human caretakers57. Together, with the present findings, these data suggest there may be sex differences in both early and later social trajectories. Future work in humans should explore the presence of early sex-specific behavioural markers that can help detect the development of neurodevelopmental disorders, especially for those disorders that are biased towards a specific sex, such as ASD58.
Finally, we found that neonatal imitators displayed lower anxiety levels, assessed through the measurement of self-scratching rates, compared to non-imitators. Dettmer et al.59 showed that relocation to a new enclosure with a larger social group (which includes also mother-reared infants) is a major source of stress for nursery-reared infants within the first year after the relocation. Our results suggest that imitators might be better able to cope and adapt with the new environment and better integrate into the new social group. It is plausible that the mechanism behind imitators’ greater social skills and lower anxiety levels coincide, as a large body of research has consistently found a positive link between peer acceptance and sociality, and a negative association between sociality and anxiety in both human and NHP21.
Overall, our study shows that neonatal imitation in rhesus macaques may be employed as an early marker that can help anticipate specific developmental trajectories, as neonatal imitators exhibit more dominant behaviours, are less anxious, and male imitators play for longer than non-imitators. More importantly, our results contribute to a body of research that aims to identify early markers of impaired social and cognitive competencies. While replications in human infants are necessary, we believe this study represents a large step forward, highlighting the utility of considering neonatal imitation as a meaningful, predictive early marker of sociality.
Materials and Methods
Subjects and housing conditions
The study was conducted on 126 healthy nursery-reared infants, raised at the Laboratory of Comparative Ethology at the National Institutes of Health in eight cohorts between 2007 and 2014. All procedures described below adhered to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the NICHD Animal Care and Use Committee.
Infants were separated from their mothers on the day of birth, and raised in a nursery following the protocol reported by Shannon et al.60. In brief, for the first 14 days of life, infants were housed in plastic incubators (51 × 38 × 43 cm), before being moved to cages (65 × 73 × 83 cm) from the third week of life until approximately 8 months old. In both housing conditions, infants were provided with an inanimate cloth-covered surrogate, along with pieces of fabric fleece and various toys. For unrelated studies, infants were randomly assigned to one of two rearing conditions when the youngest infant of the group turned 36 days: some (N = 57) were reared with three to four peers (peer-rearing), while others (N = 69) were reared with their surrogate and were given 2-hr play sessions with three to four peers (surrogate-peer-rearing) each weekday. At about 8 months of age, infants were relocated into a single social group, which included peer-reared, surrogate-peer-reared, and mother-reared infants. The size of this social group ranged between 14 and 59 infants, with an average group size of 40.6 (median: 42) infants. The enclosure consisted of an indoor and outdoor area: the indoor enclosure measured 7.3 × 3.4 × 3.7 m and was equipped with perches, barrels, swings, and wood shavings, while the outdoor area was a circular corn-crib enclosure measuring 5.03 m in diameter by 5.49 m high. All infants had free access to both areas, except when they were partitioned to either enclosure for routine cleaning, or to the inside during inclement weather59.
Neonatal imitation task
Following previous studies16,19, we tested infants on a neonatal imitation task in their first week of life, two times a day, every other day (days 1–2, 3–4, 5–6, and 7–8), with at least an hour break between each test session. In each test session, infants were held by one experimenter, while a second experimenter presented the stimuli, and a third experimenter was the time-keeper who signaled the start and end time of each phase of the test. We presented infants with two different stimuli, one during each session, at a distance of approximately 30 cm at eye-level with the infant: 1) a lipsmacking gesture (LPS) which is a common affiliative gesture in macaque and consists of the rapid opening and closing of the mouth; and 2) a nonsocial control condition (a white plastic disk with black/red or green/yellow orthogonal stripes slowly rotated clockwise and counter-clockwise). The order in which stimuli were presented remained the same for each infant across days, but was randomized across infants.
The test lasted 3 minutes total: in the first 40 seconds (BASELINE 1) the demonstrator either displayed a calm, neutral facial expression in the LPS condition or held the disk still in the control condition. In the following STIMULUS period, the demonstrator either displayed a lipsmacking gesture, or rotated the disk in the control condition, for 20 seconds. Then, for 20 sec the demonstrator showed again either a neutral facial expression or a still disk. This movement-still face sequence was repeated three times (STIMULUS), with the final still face expression period lasting 40 sec (BASELINE 2). We videotaped all sessions using a Sony Digital Video camcorder (either a ZR600 or HDR-CX560V).
Social interactions with peers at 1 year
Four observers who were blind to the research questions scored infants’ behaviours in their social group between ~11 months (mean ± SD = 339 ± 41 days) and ~14 months (mean ± SD = 405 ± 58 days) of age, using the J-watcher software. To facilitate data collection, during behavioural observations infants were partitioned in the indoor enclosure. Social interactions and self-directed behaviours were recorded through 5 minutes focal animal sampling, twice a week, once in the morning and once in the afternoon. The following data were collected:
Aggression: frequency of bites, hair pulls, aggressive chases, threats, hitting, slapping, or displacements.
Play: duration of play behaviours that included play face, non-aggressive chasing, tagging, swatting, bobbing, biting, pulling, lunging, mouthing, and wrestling (rough and tumble).
Social grooming: duration of cleaning or manipulating the fur of another individual.
Self-scratching: frequency count, common usage.
Data analysis
For the neonatal imitation assessment, we coded infants’ mouth movements off-line, frame-by-frame (30 frames per second). Coders had at least 6 months of experience with macaque infants, were familiar with their gestures, and were blind to the stimulus presented (disk or lipsmacking gesture). We defined lipsmacking as a high frequency opening and closing of the mouth within 2 seconds. Infants were classified as LPS imitators if they displayed on average across the four testing days higher rates of LPS gestures during the Stimulus period than the Baseline period in the LPS condition, and this increase was larger in the LPS condition than in the disk control condition16.
We ran linear mixed model analysis (LMM), using the lmer function in the lme4 package implemented in R v3.1.2, in which we included mean duration of play and social grooming and mean rates of aggression given and self-scratching across focal observations as dependent variables in separate models, while LPS imitation category, infant sex, and rearing condition prior to weaning, as well as the interaction between imitation category and sex, were set as fixed factors in all models. All models included the cohort identity as random factor, to control for a potential cohort-effect. All dependent variables were square root transformed to normalize their distribution. We used the drop1 function to calculate the likelihood ratio test (LRT) to compare the full models (the model with all the fixed effects) to a reduced model (the model without the variable of interest).
Additional Information
How to cite this article: Kaburu, S. S. K. et al. Neonatal imitation predicts infant rhesus macaque (Macaca mulatta) social and anxiety-related behaviours at one year. Sci. Rep. 6, 34997; doi: 10.1038/srep34997 (2016).
Acknowledgments
This research was supported by the Division of Intramural Research, NICHD, and NICHD P01HD064653. We thank Valentina Sclafani, Neal Marquez, Amanda Dettmer, Michelle Miller, Kristen Byers and the animal care staff and volunteers in the Laboratory of Comparative Ethology for help with data collection.
Footnotes
Author Contributions S.S.K.K., A.P. and P.F.F. designed the study. E.A.S., S.S.K.K. and A.P. collected the neonatal imitation data. A.P. and S.S.K.K. collected the data at 1-year. A.P. and E.A.S. coded the neonatal imitation videos. S.J.S. provided the laboratory facility and resources. S.S.K.K. analyzed the data and wrote the manuscript. All authors edited the manuscript and provided critical feedback.
References
- Demir M., Jaafar J., Bilyk N. & Mohd Ariff M. R. Social skills, friendship and happiness: A cross-cultural investigation. J. Soc. Psychol. 152, 379–385 (2012). [DOI] [PubMed] [Google Scholar]
- Segrin C. Social skills deficits associated with depression. Clin. Psychol. Rev. 20, 379–403 (2000). [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev. Psychopathol. 20, 775–803 (2008). [DOI] [PubMed] [Google Scholar]
- Heimann M. Neonatal imitation, gaze aversion, and mother-infant interaction. Infant Behav. Dev. 12, 495–505 (1989). [Google Scholar]
- Meltzoff A. N. & Moore M. K. Imitation of facial and manual gestures by human neonates. Science 198, 75–78 (1977). [DOI] [PubMed] [Google Scholar]
- Field T. Individual differences in the expressivity of neonates and young infants in Development of nonverbal behavior in children (ed. Feldman R. W.) 279–298 (Springer, 1982). [Google Scholar]
- Meltzoff A. N. & Moore M. K. Early imitation within a functional framework: The importance of person identity, movement, and development. Infant Behav. Dev. 15, 479–505 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund D. F. A note on neonatal imitation. Dev. Rev. 7, 86–92 (1987). [Google Scholar]
- Oostenbroek J. et al. Comprehensive longitudinal study challenges the existence of neonatal imitation in humans. Curr. Biol. 26, 1334–1338 (2016). [DOI] [PubMed] [Google Scholar]
- Heimann M., Nelson K. E. & Schaller J. Neonatal imitation of tongue protrusion and mouth opening: Methodological aspects and evidence of early individual differences. Scand. J. Psychol. 30, 90–101 (1989). [DOI] [PubMed] [Google Scholar]
- Myowa‐Yamakoshi M., Tomonaga M., Tanaka M. & Matsuzawa T. Imitation in neonatal chimpanzees (Pan troglodytes). Dev. Sci. 7, 437–442 (2004). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F. et al. Neonatal imitation in rhesus macaques. PLoS Biol. 4, 1501 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. A., Miller G. M., Ferrari P. F., Suomi S. J. & Paukner A. Neonatal imitation and early social experience predict gaze following abilities in infant monkeys. Sci. Rep. 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P. F. et al. Interindividual differences in neonatal imitation and the development of action chains in rhesus macaques. Child Dev. 80, 1057–1068 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N. The roots of social and cognitive development: Models of man’s original nature in Social perception in infants (eds Field T. M. & Fox N. A.) 1–30 (Ablex, 1985). [Google Scholar]
- Paukner A., Simpson E. A., Ferrari P. F., Mrozek T. & Suomi S. J. Neonatal imitation predicts how infants engage with faces. Dev. Sci. 17, 833–840 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. A., Paukner A., Suomi S. J. & Ferrari P. F. Visual attention during neonatal imitation in newborn macaque monkeys. Dev. Psychobiol. 56, 864–870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. A., Paukner A., Sclafani V., Suomi S. J. & Ferrari P. F. Lipsmacking imitation skill in newborn macaques is predictive of social partner discrimination. PloS one 8, e82921 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukner A., Ferrari P. F. & Suomi S. J. Delayed imitation of lipsmacking gestures by infant rhesus macaques (Macaca mulatta). PloS one 6, e28848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary M. R. & Kowalski R. M. Social anxiety (Guilford Press, 1997). [Google Scholar]
- Sapolsky R. M. The influence of social hierarchy on primate health. Science 308, 648–652 (2005). [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Paukner A., Ionica C. & Suomi S. J. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr. Biol. 19, 1768–1772 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer A. M. et al. First‐time rhesus monkey mothers, and mothers of sons, preferentially engage in face‐to‐face interactions with their infants. Am. J. Primatol. 78, 238–246 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer A. M. et al. Associations between early life experience, chronic HPA axis activity, and adult social rank in rhesus monkeys. Soc. Neurosci. 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer A. M. et al. Neonatal face-to-face interactions promote later social behavior in infant rhesus monkeys. Nat. Commun. 7, 11940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power T. G. Play and exploration in children and animals (Psychology Press, 1999). [Google Scholar]
- Pellis S. M. Keeping in touch: Play fighting and social knowledge in The cognitive animal: Empirical and theoretical perspectives on animal cognition (ed. Bekoff M., Allen C. & Burghardt G. M.) 421–427 (MIT Press, 2002). [Google Scholar]
- Dunbar R. I. M. Primate social systems (Springer Science & Business Media, 1988). [Google Scholar]
- Deag J. M. Aggression and submission in monkey societies. Anim. Behav. 25, 465–474 (1977). [Google Scholar]
- Maestripieri D., Schino G., Aureli F. & Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Anim. Behav. 44, 967–979 (1992). [Google Scholar]
- Bernstein I. S. & Gordon T. P. The function of aggression in primate societies: Uncontrolled aggression may threaten human survival, but aggression may be vital to the establishment and regulation of primate societies and sociality. Am. Sci. 62, 304–311 (1974). [PubMed] [Google Scholar]
- Flack J. C., Girvan M., De Waal F. B. & Krakauer D. C. Policing stabilizes construction of social niches in primates. Nature 439, 426–429 (2006). [DOI] [PubMed] [Google Scholar]
- Kaplan J. R. Fight interference and altruism in rhesus monkeys. Am. J. Phys. Anthropol. 49, 241–249 (1978). [Google Scholar]
- Bernstein I. S. & Ehardt C. L. Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. J. Comp. Psychol. 99, 115 (1985). [PubMed] [Google Scholar]
- Kulik L., Amici F., Langos D. & Widdig A. Sex Differences in the development of aggressive behavior in Rhesus macaques (Macaca mulatta). Int. J. Primatol. 36, 764–789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman C. M. Early agonistic experience and rank acquisition among free-ranging infant rhesus monkeys. Int. J. Primatol. 1, 153–170 (1980). [Google Scholar]
- Bastian M. L., Sponberg A. C., Suomi S. J. & Higley J. D. Long‐term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta). Dev. Psychobiol. 42, 44–51 (2003). [DOI] [PubMed] [Google Scholar]
- Pellis S. M. & Pellis V. C. On knowing it’s only play: the role of play signals in play fighting. Aggress. Violent Behav. 1, 249–268 (1996). [Google Scholar]
- Palagi E. et al. Rough‐and‐tumble play as a window on animal communication. Biol. Rev. Camb. Philos. Soc. 91, 311–327 (2015). [DOI] [PubMed] [Google Scholar]
- Palagi E., Cordoni G., Demuru E. & Bekoff M. Fair play and its connection with social tolerance, reciprocity and the ethology of peace. Behaviour (in press).
- Pellis S. M., Burghardt G. M., Palagi E. & Mangel M. Modeling play: Distinguishing between origins and current functions. Adapt. Behavi. 1059712315596053 (2015). [Google Scholar]
- Blumstein D. T., Chung L. K. & Smith J. E. Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris). Proc. R. Soc. Lond. [Biol] 280, 20130485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen R. & Fagen J. Juvenile survival and benefits of play behaviour in brown bears, Ursus arctos. Evol. Ecol. Res. 6, 89–102 (2004). [Google Scholar]
- Fisher E. P. The impact of play on development: A meta-analysis. Play Cult. 5, 159–181 (1992). [Google Scholar]
- Kupersmidt J. B., Burchinal M. & Patterson C. J. Developmental patterns of childhood peer relations as predictors of externalizing behavior problems. Dev. Psychopathol. 7, 825–843 (1995). [Google Scholar]
- Coolahan K., Fantuzzo J., Mendez J. & McDermott P. Preschool peer interactions and readiness to learn: Relationships between classroom peer play and learning behaviors and conduct. J. Educ. Psychol. 92, 458–465 (2000). [Google Scholar]
- Ingersoll B. The social role of imitation in autism: Implications for the treatment of imitation deficits. Infants Young Child. 21, 107–119 (2008). [Google Scholar]
- Morrison H. & Kuhn D. Cognitive aspects of preschoolers’ peer imitation in a play situation. Child Dev. 1054–1063 (1983). [Google Scholar]
- Mancini G., Ferrari P. F. & Palagi E. In play we trust. Rapid facial mimicry predicts the duration of playful interactions in geladas. PloS one 8, e66481 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopa C. & Palagi E. Mimic me while playing! Social tolerance and rapid facial mimicry in macaques (Macaca tonkeana and Macaca fuscata). J. Comp. Psychol. 130, 153–161 (2016). [DOI] [PubMed] [Google Scholar]
- Dominguez A., Ziviani J. & Rodger S. Play behaviours and play object preferences of young children with autistic disorder in a clinical play environment. Autism 10, 53–69 (2006). [DOI] [PubMed] [Google Scholar]
- Vanvuchelen M., Van Schuerbeeck L., Roeyers H. & De Weerdt W. Understanding the mechanisms behind deficits in imitation: Do individuals with autism know ‘what’ to imitate and do they know ‘how’to imitate? Res. Dev. Disabil. 34, 538–545 (2013). [DOI] [PubMed] [Google Scholar]
- Cook E. H., Rowlett R., Jaselskis C. & Leventhal B. L. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am. Acad. Child Adolesc. Psychiatry 31, 739–745 (1992). [DOI] [PubMed] [Google Scholar]
- Sallows G. O., Graupner T. D. & MacLean W. E. Jr. Intensive behavioral treatment for children with autism: Four-year outcome and predictors. Am. J. Ment. Retard. 110, 417–438 (2005). [DOI] [PubMed] [Google Scholar]
- Harlow H. F. & Mears C. E. Emotional sequences and consequeces in Emotion: Theory, research, experience. Vol. 2. Emotions in early development (ed. Plutchik R. & Kellerman H.) 171–198 (Academic Press, 1983). [Google Scholar]
- Fedigan L. M. & Zohar S. Sex differences in mortality of Japanese macaques: Twenty-one years of data from the Arashiyama West population. Am. J. Phys. Anthropol. 102, 161–175 (1997). [DOI] [PubMed] [Google Scholar]
- Simpson E. A. et al. Experience-independent sex differences in newborn macaques: Females are more social than males. Sci. Rep. 6, 19669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav. Brain Res. 176, 170–186 (2007). [DOI] [PubMed] [Google Scholar]
- Dettmer A. M., Novak M. A., Suomi S. J. & Meyer J. S. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology 37, 191–199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C., Champoux M. & Suomi S. J. Rearing condition and plasma cortisol in rhesus monkey infants. Am. J. Primatol. 46, 311–321 (1998). [DOI] [PubMed] [Google Scholar]


