Abstract
This study evaluates stoichiometry patterns in the androdioecious Acer tegmentosum, a species characterized by a rare reproductive system where males and hermaphrodites coexist. Altogether 31 hermaphrodites and 29 male plants were harvested and samples of leaves, current-year shoots, branches and coarse roots were analyzed to explore gender differences in biomass, C, N and P concentrations of these four components. The nitrogen to phosphorus relationship of each component was examined using SMA estimates. Males had significantly greater amounts of leaf and coarse root dry matter content than hermaphrodites. C, N and P stoichiometry differed significantly between genders, especially in the newly emerging vegetative components (leaves and shoots). Males had higher C/N and C/P ratios in current-year shoots and lower C/P ratios in leaves and branches. Hermaphrodites had higher N/P ratios in the leaves and branches. Males had higher rates of increase in leaf P content than hermaphrodites. This study suggests that stoichiometry patterns may be significantly affected by gender.
Androdioecy is a rare sexual dimorphic reproductive system, characterized by the co-existence of hermaphrodites and males in the same reproductive population1. Investigations into evolutionary and maintenance mechanism revealed that most of these species turned out to be functional dioecious species2,3,4. Morphologically, the flowers of the hermaphrodite individuals of an androdioecious species act like female flowers, because they fail to produce fertile pollen.
Some disagreement still exists regarding the role of sexual dimorphism in resource allocation between genders, which was thought to lead to sexual differences in nutrient consumption and in the reproduction functions between males and females5,6,7,8. A number of studies examined gender differences in resource allocation, but some doubt still remains regarding the trade-off patterns in females. In most investigations it is assumed that the higher reproductive allocation is associated with some cost, such as reduced rates of growth7,9,10,11 and higher mortality rates12,13. Hermaphrodites have to allocate their limited energy not only to flowers but also to the production of seeds and fruits, while males invest into flowers only. Contrary to expectations, it was found that females have specific mechanisms which may be compensatory, alleviating the higher reproductive effort7,14,15.
Differences in resource allocation between genders to reproductive and vegetative growth may contribute to differences in nutrient consumption. Comparative tests of sexual differences have come mostly from studies of resource allocation patterns in dioecious plants. Little attention has been paid to stoichiometry patterns, even less when such patterns refer to androdioecious species. Biomass and nitrogen distribution, which reveal the resources allocation pattern of different sexes in dioecious species is particularly important in studies about reproductive costs16. Gender-related differences in the concentrations of the primary elements, C, N and P would advance understanding of resource allocation patterns17. Carbon is the main element constituting the dry matter components of plants18. If males have a higher growth rate than hypothesized, higher carbon content would be found in male components when compared with those in hermaphrodites. The growth metabolism of plants requires proteins and nucleic19,20. Therefore, nitrogen and phosphorus are also important when comparing growth rates between genders. Of particular interest are the differences in the relationship between nitrogen and phosphorus which are essential elements for photosynthetic capacity and growth21. The differences in the gender-related reproductive cost suggest that individual trees vary in their nutrient content and its distribution.
This study explores the C:N:P stoichiometry between the genders of the androdioecious Acer tegmentosum. Gender related differences are expected to be revealed in the nutrient concentrations of leaves, current-year shoots, branches and coarse roots. In particular interest are the relationships between nitrogen and phosphorus concentrations in hermaphrodite and male trees. Accordingly, the objectives of this study were (1): to examine whether the C, N, P concentrations in the four different plant components vary between genders and (2) to evaluate differences in the nitrogen-phosphorus concentration relationships between the genders.
Results
Component amounts of dry matter content of both genders
The amounts of dry matter content of fruits (only for hermaphrodites), leaves, current-year shoots, branches and coarse roots from both male and hermaphrodite trees were first analyzed. The results show that neither the amount of the current-year shoots dry matter content, nor that of branches differed between sexes (Table 1). The amount of dry matter content of the leaves and coarse roots of males were slightly higher than those of the hermaphrodites (p < 0.05).
Table 1. Amounts of dry matter content of various components in both genders.
| Dry matter content (g/kg) | Male | Hermaphrodite | p-value |
|---|---|---|---|
| Fruit dry matter content | — | 280.79 ± 8.71 | — |
| Leaf dry matter content | 254.53 ± 0.72 | 234.65 ± 1.57 | * |
| Current-year shoot dry matter content | 432.02 ± 27.97 | 400.29 ± 3.04 | n.s. |
| Branch dry matter content | 468.28 ± 0.99 | 461.98 ± 0.71 | n.s. |
| Coarse root dry matter content | 466.93 ± 1.92 | 441.96 ± 1.25 | * |
Data show means ± SE; *significant at 0.05 level; n.s., not significant.
Biomass components as a proportion and C, N and P content
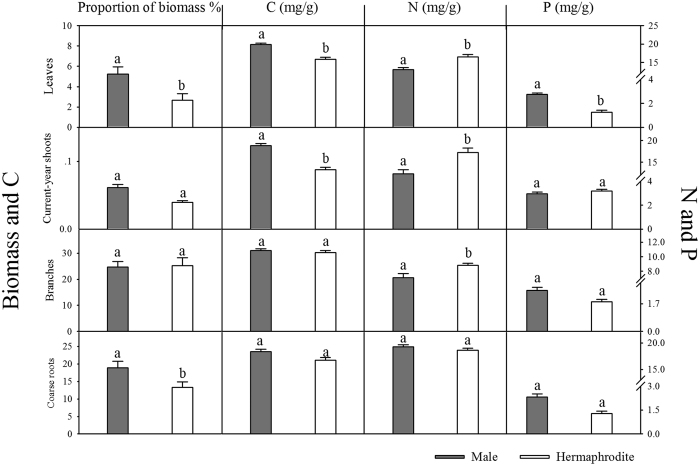
The four biomass components of hermaphrodites and males were determined as a proportion of the total biomass. Compared with males, hermaphrodites had a lower ratio of leaf and coarse root biomass. This result suggests that hermaphrodites have a different biomass allocation strategy than males. In terms of element content, males had higher amounts of C in the newly emerging vegetative components (leaves and shoots) than hermaphrodites and a higher P content in the leaves. However, hermaphrodites contain higher amounts of N than males, in the leaves, in current-year shoots and in branches. The element amounts in the coarse roots were not significantly different between the sexes. The results are shown in Fig. 1.
Figure 1. Comparison of biomass proportion and C, N and P concentrations of the four components in hermaphrodite and male trees.
Different letters within each plot indicate significant differences (p < 0.01) between genders, in an ANCOVA model with DBH and gender as covariates.
Differences of stoichiometric ratios in both genders
As shown in Table 2, mean C/N, C/P and N/P ratios in all components are gender-specific (based on the results of t-tests), save for coarse roots and the N/P ratio in current-year shoots. A closer look at the data shows that males have higher C/N ratios in leaves, current-year shoots and branches as well as a higher C/P ratio in current-year shoots, but lower C/P ratios in leaves and branches. The N/P ratios differed significantly between the sexes, with higher concentrations in leaves and branches of hermaphrodites than in males.
Table 2. C/N, C/P and N/P ratios of the various components on a mass basis.
| Items | Components | Genders | p-value | |
|---|---|---|---|---|
| Males | Hermaphrodites | |||
| C/N | Fruits | — | 33.41 ± 2.13 | — |
| Leaves | 47.17 ± 0.21 | 27.83 ± 0.30 | *** | |
| Current-year Shoot | 44.51 ± 0.45 | 22.70 ± 0.48 | *** | |
| Branches | 82.83 ± 0.82 | 65.73 ± 0.64 | ** | |
| Coarse roots | 30.83 ± 0.39 | 28.58 ± 0.33 | n.s. | |
| C/P | Fruits | — | 245.20 ± 14.44 | — |
| Leaves | 603.83 ± 17.03 | 875.33 ± 25.90 | *** | |
| Current-year Shoot | 223.55 ± 4.38 | 141.18 ± 4.39 | *** | |
| Branches | 335.27 ± 7.60 | 496.00 ± 10.75 | ** | |
| Coarse roots | 776.25 ± 32.89 | 882.29 ± 38.78 | n.s. | |
| N/P | Fruits | — | 10.78 ± 0.83 | – |
| Leaves | 12.96 ± 0.36 | 32.01 ± 0.90 | *** | |
| Current-year Shoot | 5.15 ± 0.08 | 6.87 ± 0.29 | n.s. | |
| Branches | 4.29 ± 0.11 | 8.01 ± 0.20 | ** | |
| Coarse roots | 22.57 ± 0.81 | 29.98 ± 1.22 | n.s. | |
Results show means ± SE; **significant at 0.01 level; ***significant at 0.001 level; n.s., not significant.
Gender-related nitrogen-phosphorus relationships
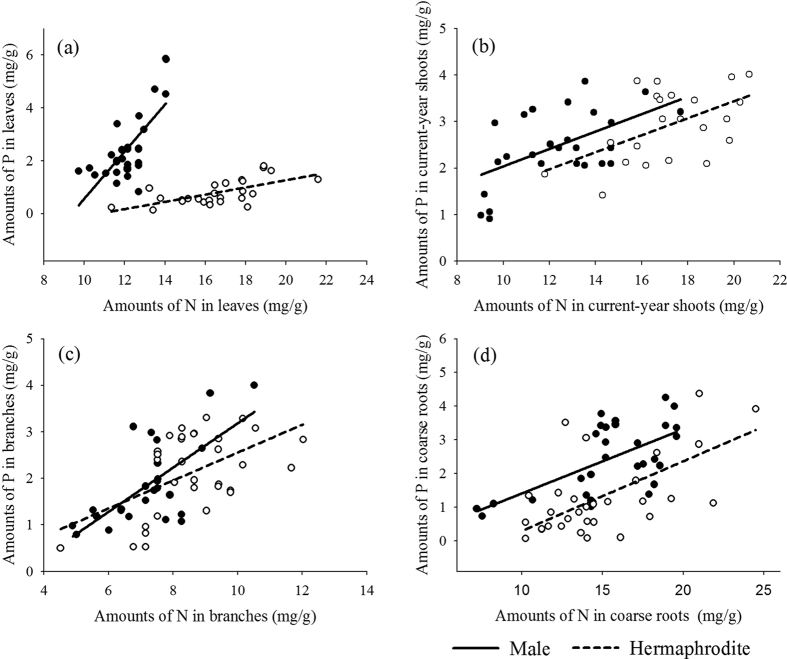
The nitrogen-phosphorus relationships were also analyzed for the genders and among their SMA component estimates. Figure 2 and Table 3 show for all the observed components that the P concentration increased with an increase in N concentration. However, both the regression intercepts and the slopes vary between male and hermaphrodite trees. The data for current-year shoots, branches and coarse roots show that the scales of phosphorus concentrations with respect to nitrogen concentrations differ between the two gender groups although in both the slope < 1.0.
Figure 2. Phosphorus concentrations as a function of the amount of nitrogen in four components of hermaphrodite and male trees.
Details of these relationships, calculated from standardized major axis (SM) regressions, are presented in Table 3.
Table 3. Summary of standardized major axis (SMA) regression results for the components examined in both genders.
| Components | n | Intercept | 95% CI | Slope | 95% CI | R2 | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| Male trees | |||||||||
| Leaves | 29 | −12.69 | −16.90 | −8.47 | 1.25** | 0.95 | 1.64 | 0.51 | p < 0.001 |
| Current-year shoots | 29 | −1.81 | −3.26 | −0.35 | 0.35*** | 0.25 | 0.48 | 0.29 | p < 0.01 |
| Branches | 29 | −3.19 | −4.81 | −1.56 | 0.70** | 0.51 | 0.95 | 0.46 | p < 0.001 |
| Coarse roots | 29 | −2.22 | −3.69 | −0.75 | 0.30*** | 0.22 | 0.41 | 0.40 | p < 0.001 |
| Hermaphrodite trees | |||||||||
| Fruits | 31 | −2.43 | −4.25 | −0.61 | 0.31*** | 0.21 | 0.45 | 0.22 | p < 0.05 |
| Leaves | 31 | −2.76 | −3.93 | −1.60 | 0.21*** | 0.16 | 0.30 | 0.41 | p < 0.001 |
| Current-year shoots | 31 | −3.05 | −5.45 | −0.65 | 0.35*** | 0.24 | 0.51 | 0.28 | p < 0.05 |
| Branches | 31 | −2.83 | −4.44 | −1.21 | 0.58** | 0.42 | 0.79 | 0.27 | p < 0.01 |
| Coarse roots | 31 | −3.72 | −5.26 | −2.18 | 0.34*** | 0.25 | 0.45 | 0.37 | p < 0.001 |
*Significant deviation of slope from 1, **p < 0.01, ***p < 0.001.
This result shows that increases in the amount of P fail to keep pace with increasing N concentrations (Fig. 2b–d and Table 3). The SMA regression slope of leaves in males exceeds 1, which is statistically significant, while the slope in hermaphrodites is less than 1. The OLS regression shows the same result of non-overlapping slopes (Table 4). The results of these two tests suggests that the amount of P in the leaves of males accelerates faster than a unit increase in the amount of N in leaves, i.e., the rate of increase in the amount of P in male trees is greater than that of hermaphrodites (Tables 3 and 4).
Table 4. Data range of slopes using OLS regression (test both N against P concentration and 1/[P against N concentration]) in various components in both genders.
| Components | Males | Hermaphrodites | ||
|---|---|---|---|---|
| Range | p-value | Range | p-value | |
| Fruits | — | — | 0.15–0.65 | p < 0.001 |
| Leaves | 0.89–1.75 | p < 0.01 | 0.14–0.34 | p < 0.001 |
| Current-year shoots | 0.19–0.65 | p < 0.001 | 0.18–0.66 | p < 0.001 |
| Branches | 0.48–1.03 | p < 0.001 | 0.30–1.11 | p < 0.001 |
| Coarse roots | 0.19–0.48 | p < 0.001 | 0.21–0.55 | p < 0.001 |
p-value shows the significant deviation of slope from one.
Discussion
Sex-differential biomass allocation and dry matter content
Earlier studies on sexual dimorphism revealed different resource allocation patterns between female and male trees11,12,13. It was found that females usually had a greater energy demand during reproduction. Higher reproductive costs affected the vegetative growth of female plants, resulting in different growth rates and biomass allocation patterns7,22,23,24. However, in many cases no differences were found between the genders in resource allocation8,25,26.
Our analysis of the contribution to total biomass of the four vegetative structures revealed significant differences between the genders, hermaphrodites having lower ratios of leaf to coarse root biomass than males (Fig. 1). This suggests that A. tegmentosum has a gender-specific biomass allocation strategy, with males allocating more resources to leaves and coarse roots.
The reproductive investment can be measured by the dry matter content of its reproductive and vegetative parts27. The fact that some potentially limiting resource, such as carbon, is not evenly distributed between the different plant organs is often ignored28. Biomass as well as energy can pass between vegetative and reproductive organs29. The amounts of dry matter content can reflect the nutrient-holding and resource-retention capacity of plants30,31. The amount of dry matter content of leaves is a leaf trait, easily measured and often reported. Díaz-Barradas suggested that female plants of the dioecious Empetrum rubrum species have greater amounts of leaf dry matter content and may exhibit compensatory mechanisms to face their higher reproductive costs32. It has also been reported that males have lower amounts of dry matter content but higher levels of protein33. In this study, higher amounts of leaf and coarse root dry matter content were found in males than in hermaphrodites in A. tegmentosum (Table 1), suggesting that males have a greater ability of acquiring and retaining carbon in leaves and coarse roots.
Differential C, N and P concentration between genders
For sexually dimorphic species, the growth rate of female and male plants were found to be significantly different, because of the differences in reproductive investment between them15. The indispensable elements C, N and P have a bearing on the regulation of organismal growth rates34. But only a few studies deal with the nutrient concentrations in relation to the growth rates, and most of these studies were carried out under controlled conditions. Gehring (1994), analysed the sexual differences of growing individual male and female plants of the dioecious species Silene latifolia under specific conditions and found that higher female reproductive costs did not significantly affect their ability to assimilate carbon35. Che-Castaldo (2015) demonstrated that female plants allocate more resources (in terms of carbon, nitrogen and phosphorus) to reproduction than males36. Similarly, Wallace (1979) reported that male plants allocate a greater proportion of their resources (including biomass, nitrogen and phosphorus) to reproductive tissues relative to female plants37. Zhang et al.11 suggested that the strategies to cope with N and P deficiencies is different between the sexes and that deficiencies in the amounts of N and P would have greater negative effects on females than on males38. The amount of nitrogen is one of the most important factors affecting photosynthetic efficiency, especially nitrogen in the leaves which is mostly allocated to the photosynthetic organs39,40. Photosynthetic efficiency differs among different dioecious species. Simmondsia chinensis, Silene latifolia and Populus tremuloides are reported to have a higher photosynthetic capacity in males than in females, but contrary results were found in Acer negundo and Simmondsia chinensis35,37,41,42,43,44 Tolvanen (2001) studied the dioecious dwarf shrub Salix arctica and reported that males appeared to have lower amounts of N in current shoots and leaves in the early season, and that females were able to allocate more C and N to plant growth45.
Figure 1 shows that males have higher levels of C in their new vegetative components (leaves and current-year shoots) than hermaphrodites. Males also have higher P concentrations in their leaves than hermaphrodites. P content is thought to be related to the P concentration in the rRNA, which influences the growth rate. Our results show that, compared with hermaphrodites, males allocated more C and P to vegetative growth. Regarding N concentrations, our results are similar with those of Tolvanen (2001). We also found that hermaphrodites have higher N concentrations in their leaves, current-year shoots and branches (Fig. 1) than males.
During the reproductive season, females tend to adjust their resource allocation patterns by increasing photosynthesis to meet the demands of flowers or fruits14,42. In this study, the samples were collected in the fruiting season, which may explain the higher N content in hermaphrodites than in males, owing to the C demands in fruits. Our results show a clear, gender-differential pattern of nutrient content. This supports the hypothesis that sexual dimorphic traits have evolved to meet the different resource and nutrient requirements for males and hermaphrodites.
Gender-related stoichiometric ratios and the nitrogen-phosphorus relationship
C, N and P make important contributions to the formation of carbohydrates, lignin, cellulose, proteins and other substances associated with the regulation of growth rates, which are especially affected by N and P20. High phosphorous levels may result in lower C/P and N/P ratios46. Usually, the N/P ratio in leaves accurately reflects the dynamic characteristic of the nutrient status47. This ratio has therefore been used as a diagnostic indicator of the limitation of vegetative growth by these nutrients47,48. The C:N:P ratios of organisms are mainly determined by their P content49. The growth rate hypothesis (GRH) states that plants themselves will change their specific organ C:N:P ratios to adapt to the change of growth rates. Therefore, the organisms with higher growth rates exhibit relatively lower C:P and N:P ratios20.
For sexually dimorphic species, the higher reproductive investment may affect the growth rate of females such that male plants usually have higher vegetative growth rates12,15,50. According to that growth rate hypothesis, the sexually differential growth rates may be reflected in the C:N:P ratios of particular plant components between males and hermaphrodites. A limitation of specific nutritional elements may transform the chemical constitution of individual plants, contributing to changes in the stoichiometric ratios. As a result, the C/N, C/P and N/P ratios are decreasing with increasing growth rates51. When the P concentration in plant cells increases erratically, the growth rate increases but the N/P ratio drops20,52.
As shown in Table 2, hermaphrodites have a lower C/N ratio in leaves, current-year shoots and branches, as well as a lower C/P ratio in current-year shoots, but a higher C/P ratio in leaves and branches. In terms of N/P ratios, males show lower ratios in leaves and branches, suggesting that males may have higher rates of growth in leaves and branches than hermaphrodites.
We also analyzed the nitrogen-phosphorus relationships of the four components. The results of slopes based on SMA and OLS regressions were consistent, showing different intercepts and slopes between genders (Tables 3 and 4). The P concentration increased with increasing N in both sexes, but the slope of the regression of male leaves exceeds 1, while it is less than 1 in hermaphrodites. The scaling slope of leaves was greater in males than in hermaphrodites, suggesting that leaf P increased faster relative to leaf N in males and that males have a greater ability to absorb phosphorus.
These new findings on the androdioecious A. tegmentosum may hopefully lead to comparative studies on stoichiometry patterns of other sexually dimorphic species, especially regarding the different reproductive and vegetative components.
Methods
Study region
The original experiments were conducted at the Jiaohe Experimental Forest in Jilin Province (43°57.5′N, 127°44.1′–127°44.7′E) with a monsoon climate, at an average elevation of 459 m. The region has a mean annual temperature of 3.8 oC, precipitation is low in winter but at higher elevations very high in the summer, with a mean annual precipitation as high as 695.9 mm. Located in northeastern China, the study site is a multi-species near-mature forest dominated by Pinus koraiensis, Fraxinus mandshurica, Tilia amurendsis and Acer mono. The soil is a dark brown forest soil.
The androdioecious species A. tegmentosum in this study is a small tree widely distributed in the conifer and broad-leaved mixed forests in northeastern China, which blossoms in early spring with racemes of green flowers. The fruits of the hermaphrodite plants emerge with a dipterous appearance and change from green to yellowish-brown when ripe in late August.
Sampling in the field
We randomly identified the sex of 60 flowering A. tegmentosum (29 males and 31 hermaphrodites) in the spring of 2014 and marked their flowering branches. All the sample trees were cut at ground level in order to harvest their leaves and shoots, as well as the fruits from the hermaphrodites. The flowering branches were dissected into leaves, current-year shoots and branches. The coarse roots (diameter ≥ 5 mm) of each tree were dug out and cleaned. The fresh mass of each component was determined separately in the field using a platform balance to the nearest 0.1 kg. Representative samples of each of the four components, weighing between 500 and 1000 g were taken to the laboratory for analysis.
Measurements of biomass components and element analysis
After measuring the fresh weights, the samples collected in the field were dried in an oven to a constant mass and weighed. To calculate the total biomass (expressed as dry matter) of each section, we multiplied the ratio of samples (dry to fresh matter) by total fresh weight as measured in the field53. Proportion of biomass was defined as the ratio of biomass of the corresponding components to total biomass. The sample dry matter content was measured by the ratio of dry mass to fresh mass (g/kg)54.
Before the element analysis, the dried samples were ground into a powder. The C concentrations were determined from approximately 100 mg of homogenously ground material of each sample, using a potassium dichromate volumetric method55. The total amount of N of each sample was determined by the Kjeldahl Method and P by the Mo-Sb colorimetric method55.
Data analysis
Analyses of covariance (ANCOVA) with diameter at breast height (DBH) and gender as covariates were applied to examine sexual differences in biomass proportion and the C, N and P concentrations of the various components. Standardized major axes estimation (SMA) is a procedure for assessing the heterogeneity of regression slopes, to identify the best fit bivariate line between two variables56,57. The most common use of the null hypothesis is that the slopes are equal to one, which implies isometry, with y and x increasing at a constant rate58. This method was used to fit a regression line for the N concentrations against the P concentrations, and then the slopes of these lines were compared between genders. If the slopes significantly differed from 1, the relationship between N against P concentrations would not be described as isometric. Measurement error (ME) is the difference between the measured values and actual values, which can cause a biased estimate of the slopes in linear regression58. McArdle (2003) pointed out that one efficient way to deal with ME is to test the slopes of both y on x and x on y59. In this study, the ordinary least squares (OLS) regression was also used to analyze the nitrogen-phosphorus relationship in the four components between genders. We regressed both N against P concentration and P against N concentration to deal with the ME in the variables.
All analyses were performed by R, version 3.1.3; the R library “smatr” was used for SMA analyses.
Additional Information
How to cite this article: Zhang, X. et al. Stoichiometry patterns in the androdioecious Acer tegmentosum. Sci. Rep. 6, 35022; doi: 10.1038/srep35022 (2016).
Acknowledgments
This research is supported by the Program of the National Natural Science Foundation of China (31600480; 31670643), the Fundamental Research Funds for the Central Universities (Nos BLYJ201608) and the 12th five year National Science and Technology plan of China (2012BAC01B03).
Footnotes
Author Contributions All authors have materially participated in the research and/or the article preparation. X. Zhang major contribution to field works and interpretation of results; J.Y. research concept, and interpretation of results; C.F. and L.T. element analysis; C.Z. and K.v.G. data analysis and text; J.W. research concept, data; X. Zhao project coordination, data. All authors have approved the final article.
References
- Yampolsky C. & Yampolsky H. Distribution of sex forms in the phanerogamic flora. Bibliotheca genetica (Leipzig, 1922). [Google Scholar]
- Charlesworth D. Androdioecy and the evolution of dioecy. Biol J Linn Soc. 22(4), 333–348 (1984). [Google Scholar]
- Anderson G. J. & Symon D. E. Functional dioecy and andromonoecy in Solanum. Evolution. 43, 204–219 (1989). [DOI] [PubMed] [Google Scholar]
- Vassiliadis C., Valero M. & Saumitou L. P. A model for the evolution of high frequencies of males in an androdioecious plant based on a cross-compatibility advantage of males. Heredity. 85, 413–422 (2000). [DOI] [PubMed] [Google Scholar]
- Delph L. F. Sexual dimorphism resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 71, 1342–1351 (1990). [Google Scholar]
- Roff D. A. The evolution of life histories: theory and analysis, 1st edn. Chapman and Hall (New York, 1992). [Google Scholar]
- Obeso J. R. The costs of reproduction in plants. New Phytol. 155, 321–348 (2002). [DOI] [PubMed] [Google Scholar]
- Petzold A., Pfeiffer T., Jansen F., Eusemann P. & Schnittler M. Sex ratios and clonal growth in dioecious Populus euphratica Oliv., Xinjiang Prov., Western China. Trees. 27, 729–744 (2013). [Google Scholar]
- Antos J. A. & Allen G. A. A comparison of reproductive effort in the dioecious shrub Oemleria cerasiformis using nitrogen, energy and biomass, as currencies. Am Midl Nat. 124, 254–262 (1990). [Google Scholar]
- Cornelissen T. & Stiling P. Sex-biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos. 111, 488–500 (2005). [Google Scholar]
- Zhang X. N., Zhang C. Y. & Zhao X. H. Effect of sex ratio, habitat factors and neighborhood competition on stem growth in the dioecious tree Fraxinus mandshurica. Ecol Res. 29, 309–317 (2014). [Google Scholar]
- Allen G. A. & Antos J. A. Sex ratio variation in the dioecious shrub Oemleria cerasiformis. Am Nat. 141, 537–553 (1993). [DOI] [PubMed] [Google Scholar]
- Nanami S., Kawaguchi H. & Yamakura T. Sex ratio and gender dependent neighboring effects in Podocarpus nagi, a dioecious tree. Plant Ecol. 177, 209–222 (2005). [Google Scholar]
- Tozawa M., Ueno N. & Seiwa K. Compensatory mechanisms for reproductive costs in the dioecious tree Salix integra. Botany. 87, 315–323 (2009). [Google Scholar]
- Àlvarez-Cansino L., Zunzunegui M., Diaz Barradas M. C. & Paz Esquivias M. Gender-specific costs of reproduction on vegetative growth and physiological performance in the dioecious shrub Corema album. Ann Bot. 106, 989–998 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenborough S. A., Burslem D. F., Garwood N. C. & Valencia R. Determinants of biased sex ratios and inter-sex costs of reproduction in dioecious tropical forest trees. Am J Bot. 94(1), 67–78 (2007). [DOI] [PubMed] [Google Scholar]
- Sterner R. W. Elemental stoichiometry of species in ecosystems. In Linking Species & Ecosystems. Springer: US, 240–252 (1995). [Google Scholar]
- Etherington J. R. Plant physiological ecology. Edward Arnold Ltd, 11–16 (1978). [Google Scholar]
- Aerts R. & Chapin F. S. The mineral nutriation of wild Plants revised:a re-evaluation of processes and patterns. Ecol Res. 30, l–67 (2000). [Google Scholar]
- Elser J. J., Acharya K. & Kyle M. Growth rate stoichiometry couplings in diverse biota. Ecol Lett. 6, 936–943 (2003). [Google Scholar]
- Reich P. B., Oleksyn J., Wright I. J., Niklas K. J., Hedin L. & Elser J. J. Evidence of a general 2/3-power law of scaling leaf nitrogen to phosphorus among major plant groups and biomes. P Roy Soc Lond B Bio. 277(1683), 877–883 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J. R., Alvarez S. M. & Retuerto R. Sex ratios, size distributions, and sexual dimorphism in the dioecious tree Ilex aquifolium (Aquifoliaceae). Am J Bot. 85, 1602–1608 (1998). [PubMed] [Google Scholar]
- Leigh A., Cosgrove M. J. & Nicotra A. B. Reproductive allocation in a gender dimorphic shrub: anomalous female investment in Gynatrix pulchella? J Ecology. 94(6), 1261–1271 (2006). [Google Scholar]
- Zhang C. Y., Wang J., Zhao X. H., Xia F. C. & Gadow K. V. Sexual dimorphism in reproductive and vegetative allometry for two dioecious Rhamnus plants in north-eastern China. Eur J For Res. 131, 1287–1296 (2012). [Google Scholar]
- Nicotra A. B. Sexually dimorphic growth in the dioecious tropical shrub Siparuna grandiflora. Funct Ecol. 13, 322–331 (1999). [Google Scholar]
- Massei G., Watkins R. & Hartley S. E. Sex-related growth and secondary compounds in Juniperus oxycedrus macrocarpa. Acta Oeco. 29(2), 135–140 (2006). [Google Scholar]
- Bloom A. J., Chapin F. S. & Mooney H. A. Resource limitation in plants: an economic strategy. Ann Rev Ecol Syst. 16, 363–392 (1985). [Google Scholar]
- Laure G. P., Salager J. L., Martine H. M. & Jacques R. Carbon allocation to volatiles and other reproductive components in male Ficus Carica (Moraceae). Am J Bot. 88, 2214–2220 (2001). [PubMed] [Google Scholar]
- Harper J. L. The value of a leaf. Oecologia. 80, 53–58 (1989). [DOI] [PubMed] [Google Scholar]
- Wilson P. J., Thompson K. & Hodgson J. G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 143(1), 155–162 (1999). [Google Scholar]
- Arredondo J. T. & Sehnyder H. Components of leaf elongation rate and their relationship to specific leaf area in contrasting grasses. New Phytol. 158, 305–314 (2003). [Google Scholar]
- Díaz-Barradas M. C., Zunzunegui M., Collantes M., Álvarez-Cansino L. & Novo F. G. Gender-related traits in the dioecious shrub Empetrum rubrum in two plant communities in the Magellanic steppe. Acta Oecol. 60, 40–48 (2014). [Google Scholar]
- Makhlouf-Gafsi I., Mokni-Ghribi A., Bchir B., Attia H., Blecker C. & Besbes S. Physico-chemical properties and amino acid profiles of sap from Tunisian date palm. Sci Agr. 73(1), 85–90 (2016). [Google Scholar]
- Sterner R. W. & Elser J. J. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press (2002). [Google Scholar]
- Gehring J. L. & Monson R. K. Sexual differences in gas exchange and response to environmental stress in dioecious Silene latifolia (Caryophyllaceae). Am J Bot. 166–174 (1994). [Google Scholar]
- Che-Castaldo C., Crisafulli C. M., Bishop J. G. & Fagan W. F. What causes female bias in the secondary sex ratios of the dioecious woody shrub Salix sitchensis colonizing a primary successional landscape? Am J Bot. 102(8), 1309–1322 (2015). [DOI] [PubMed] [Google Scholar]
- Wallace C. S. & Rundel P. W. Sexual dimorphism and resource allocation in male and female shrubs of Simmondsia chinensis. Oecologia. 44(1), 34–39 (1979). [DOI] [PubMed] [Google Scholar]
- Zhang S., Jiang H., Zhao H., Korpelainen H. & Li C. Sexually different physiological responses of Populus cathayana to nitrogen and phosphorus deficiencies. Tree Physiol. tpu025 (2014). [DOI] [PubMed] [Google Scholar]
- Makino A. & Osmond B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplast and mitoehondria in pea and wheat. Plant Physiol. 96, 355–362 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcroft A. S., Whitehead D., Silvester W. B. & Kelliher F. M. The response of photosynthetic model parameters to temperature and nitrogen concentration in Pinus radiata D. Don. Plant Cell Environ. 20(11), 1338–1348 (1997). [Google Scholar]
- Bourdeau P. F. Photosynthetic and respiratory rates in leaves of male and female quaking aspents. Forest Sci. 4, 331–334 (1958). [Google Scholar]
- Laporte M. M. & Delph L. F. Sex-specific physiology and source-sink relations in the dioecious plant Silene latifolia. Oecologia. 106, 63–72 (1996). [DOI] [PubMed] [Google Scholar]
- Marshall J. D., Dawson T. E. & Ehleringer J. R. Gender-related differences in gas exchange are not related to host quality in the xylem-tapping mistletoe, Phoradendron juniperinum (Viscaeae). Am J Bot. 35, 557–567 (1993). [Google Scholar]
- Wang X. Z. & Gurtis P. S. Gender-specific response of Populus tremuloides to atmospheric CO2 enrichment. New Phytol. 150, 657–684 ( 2001). [Google Scholar]
- Tolvanen A. & Henry G. H. Responses of carbon and nitrogen concentrations in high arctic plants to experimental warming. Can J Bot. 79(6), 711–718 (2001). [Google Scholar]
- Demars B. O. & Edwards A. C. Tissue nutrient concentrations in freshwater aquatic macrophytes: high inter‐taxon differences and low phenotypic response to nutrient supply. Freshwater Biol. 52(11), 2073–2086 (2007). [Google Scholar]
- Cornelissen J. H. C., Lavorel S., Gamier E. et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide[J], Aust J Bot. 51(4), 335–380 (2003). [Google Scholar]
- Tessier J. T. & Raynal D. J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J Appl Eco. 40(3), 523–534 (2003). [Google Scholar]
- Vanni M. J., Flecker A. S., Hood J. M. & Headworth J. L. Stoichiometry of nutrient recycling by vertebrates in a tropical stream: linking species identity and ecosystem processes. Ecol Lett. 5(2), 285–293 (2002). [Google Scholar]
- Zhang C., Zhao X., Gao L. & Gadow K. V. Gender, neighboring competition and habitat effects on the stem growth in dioecious Fraxinus mandshurica trees in a northern temperate forest. Ann Forest Sci. 66, 8–812 (2009). [Google Scholar]
- Ågren G. I. The C: N: P stoichiometry of autotrophs–theory and observations. Ecol Lett. 7(3), 185–191 (2004). [Google Scholar]
- Gernusak L. A., Winter K. & Turner B. L. Leaf nitrogen to phosphotus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytol. 185, 770–779 (2009). [DOI] [PubMed] [Google Scholar]
- Mate R., Johansson T. & Sitoe A. Biomass equations for tropical forest tree species in Mozambique. Forests. 5(3), 535–556 (2014). [Google Scholar]
- Garnier E., Shipley B., Roumet C. & Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct Ecol. 15(5), 688–695 (2001). [Google Scholar]
- Bao S. D. Analysis method of soil agricultural chemistry. Third edition. Chinese Agricultural Press. 135–155 (Beijing, 2000). [Google Scholar]
- Warton D. J., Wright I. J., Falster D. S. & Westoby M. Bivariate line-fitting methods for allometry. Biol Rev. 81, 259–291 (2006). [DOI] [PubMed] [Google Scholar]
- Fanin N., Fromin N., Buatois B. & Hättenschwiler S. An experimental test of the hypothesis of non‐homeostatic consumer stoichiometry in a plant litter–microbe system. Ecol lett. 16(6), 764–772 (2013). [DOI] [PubMed] [Google Scholar]
- Sterner R. W., Andersen T., Elser J. J., Hessen D. O., Hood J. M., McCauley E. & Urabe J. Scale-dependent carbon: nitrogen: phosphorus seston stoichiometry in marine and freshwaters. Limnol Oceanogr. 53(3), 1169 (2008). [Google Scholar]
- McArdle Brian, H. Lines, models, and errors: regression in the field. Limnol Oceanogr. 48(3), 1363–1366 (2003). [Google Scholar]