Abstract
Fusarium graminearum contains eight chitin synthase (Chs) genes belonging to seven classes. Previous studies have found that deletion of FgChs3b is lethal to F. graminearum, and deletion of FgChs1, FgChs2, FgChs7 and FgChs5 caused diverse defects in chitin content, mycelial growth, conidiation, virulence or stress responses. However, little is known about the functional relationships among these FgChss. In this study, FgChs2 deletion mutant ΔFgChs2 exhibited reduced mycelial growth and virulence as reported previously. In addition, we found that the mutant produced thickened and “wavy” septa. Quantitative real-time PCR (qRT-PCR) assays showed that the expression levels of FgChs1, FgChs3a, FgChs4, FgChs7, FgChs5 and FgChs6 in ΔFgChs2 were significantly higher than those in the wild type. Therefore, we generated six double deletion mutants of FgChs2 and each of the above six FgChss, and found that FgChs2 shares a function with FgChs1 in regulating mycelial growth, and co-regulates conidiation with FgChs1, FgChs4, FgChs7 and FgChs5. Furthermore, FgChs2 and other six FgChss have overlapped functions in virulence, DON production and septum formation. Taken together, these results indicate that although each chitin synthase of F. graminearum plays certain roles, FgChss may co-regualte various cellular processes in F. graminearum.
Chitin, a β (1, 4)-linked homopolymer of N-acetylglucosamine (GlcNAc), is an essential component of cell walls and septa of all fungi studied to date1,2. The synthesis of chitin is mediated by membrane-bound chitin synthases (Chss), which were divided into seven classes3. There are three Chs genes in budding yeast Saccharomyces cerevisiae, while filamentous fungi generally contain seven or eight Chs genes. Chitin synthases belonging to classes III, V, VI, and VII are only identified in filamentous fungi and some dimorphic yeasts3, which may result in higher chitin content and greater complexity of growth and development of these fungi than the budding yeast. In filamentous fungi, chitin accounts for 10–20% of dry weight content of cell wall in vegetative cells, which is much higher than 1–2% in S. cerevisiae4,5.
In S. cerevisiae, three Chss have been extensively studied and their functions have been well understood. The Class I Chs (ScChs1) repairs the weakened cell wall of daughter cells after separation6. ScChs2 (II) is essential for both septum formation and cell division7. ScChs3 (IV) synthesizes 90% of chitin in the cell walls and is required for chitin ring formation at the base of emerging buds and chitin synthesis in the lateral cell8. However, the functions of individual Chss and their specific involvements and interactions remain poorly understood in filamentous fungi. One of main reasons might be functional overlap among multiple Chss in filamentous fungi.
Fusarium graminearum (teleomorph Gibberella zeae) causes Fusarium head blight (FHB), which is a devastating disease of cereal crops worldwide. Infection of cereal crops with F. graminearum may not only lead to huge yield losses in severe epidemic years, but also pose a serious threat to human and animal health owing to deoxynivalenol (DON) and other mycotoxins in infested grains9,10. Despite the serious damage caused by FHB, efficient strategies for the management of FHB are not available to date11. Previous studies on Magnaporthe oryzae and Botrytis cinerea have showed that chitin synthases play important roles in fungal growth and pathogenicity12,13,14,15,16. Thus, deep understanding the biological functions of Chss in plant pathogenic fungi can provide the basis for the development of chitin synthase-targeted antifungal agents. What’s more, such antifungal agents might be safe to high eukaryotes since chitin and chitin synthases are not present in animals and plants1,3.
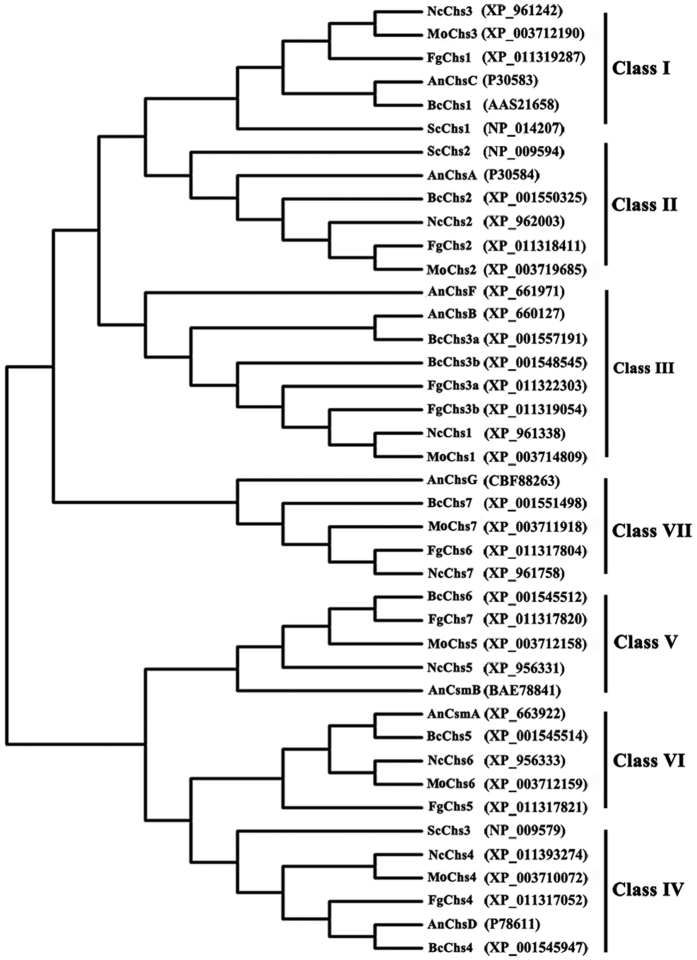
In silico analyses showed that the F. graminearum genome contains eight FgChs genes (Fig. 1). Following the classification proposed by Chigira et al. and Choquer et al.17,18, these genes are referred as FgChs1 (I), FgChs2 (II), FgChs3a (III), FgChs3b (III), FgChs4 (IV), FgChs7 (V), FgChs5 (VI), and FgChs6 (VII) respectively in this study (Fig. 1). Previous reports have indicated that FgChs3b is essential, and the deletion of FgChs1, FgChs2, FgChs7 and FgChs5 led to reduced mycelial growth, virulence or increased sensitivity to various stresses19,20,21. The deletion of FgChs3a, FgChs4 and FgChs6 genes did not cause significant differences from the wild type21. Previous studies were conducted with the different genetic background strains, we therefore constructed various Chs deletion strains in a single progenitor in current study in order to characterize FgChss systemically. Results of this study indicated that FgChs2 and other FgChs genes co-regulate various cellular processes in F. graminearum.
Figure 1. Phylogenetic tree of fungal chitin synthases.
Phylogenetic tree generated using the neighbor-joining method with Mega 5.0 software on the basis of deduced amino acid sequences of chitin synthases from different fungi. FgChs1, FgChs2, FgChs3a, FgChs3b, FgChs4, FgChs5, FgChs6 and FgChs7 from Fusarium graminearum; AnChsA, AnChsB, AnChsC, AnChsD, AnChsF, AnChsG, AnCsmA and AnCsmB from Aspergillus nidulans; BcChs1, BcChs2, BcChs3A, BcChs3B, BcChs4, BcChs5, BcChs6 and BcChs7 from Botrytis cinerea; NcChs1, NcChs2, NcChs3, NcChs4, NcChs5, NcChs6 and NcChs7 from Neurospora crassa; MoChs1, MoChs2, MoChs3, MoChs4, MoChs5, MoChs6 and MoChs7 from Magnaporthe oryzae; ScChs1, ScChs2 and ScChs3 from Saccharomyces cerevisiae. GenBank accession no. of each protein was presented in brackets.
Results
Eight chitin synthase genes of F. graminearum are differentially expressed in both mycelia and conidia
The expression levels of FgChs genes in mycelia cultured in PDB and MM, and in germinating conidia grown in YEPD were determined by quantitative real-time PCR (qRT-PCR) assays. Among the eight genes, the abundance of FgChs6 transcripts was the lowest in both vegetative hyphae and germinating conidia (Fig. 2a). In contrast, the FgChs3b had the highest expression levels (Fig. 2a). The FgChs2, FgChs7 and FgChs5 genes had similar expression profiles with higher expression levels in mycelia grown in MM than in PDB (Fig. 2b). With the exception of FgChs1 and FgChs3b, other FgChs genes exhibited higher expression levels in hyphae than in germinating conidia (Fig. 2b).
Figure 2. Expression profiles of eight FgChs genes assayed by qRT-PCR.
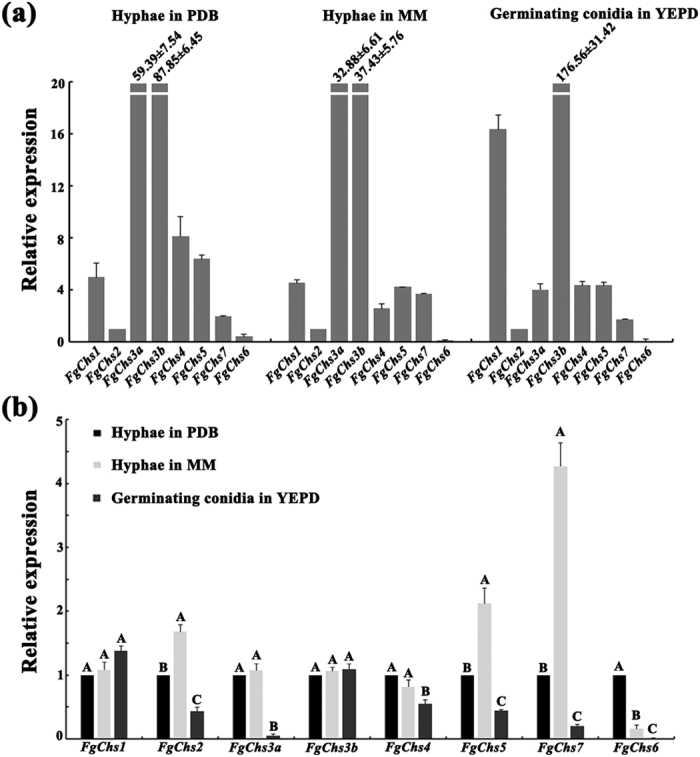
RNA samples of the wild-type PH-1 were isolated from vegetative hyphae grown in PDB or MM, and from germinating conidia cultured in YEPD. The relative expression level of individual FgChs gene was analyzed with the 2−ΔΔCt method with the ACTIN gene as the internal control for normalization. (a) Comparison of the transcript abundance of eight FgChs genes in hyphae grown in PDB and MM, and germinating conidia cultured in YEPD. The expression level of FgChs2 was referred to 1. (b) Comparison of the transcript abundance of individual FgChs genes in hyphae and germinating conidia. The expression level of each FgChs gene in vegetative hyphae grown in PDA was referred to 1. Mean and standard errors were determined with data from three independent replicates. Values on the bars followed by the same letter are not significantly different according to a least significant difference (LSD) test at P = 0.05.
The expression levels of other seven FgChs genes in ΔFgChs2
To explore the relationships of FgChs2 and other FgChss, we determined the expression levels of other seven FgChs genes in the FgChs2 deletion mutant ΔFgChs2 by qRT-PCR assays. As shown in Fig. 3, the expression levels of FgChs1, FgChs3a, FgChs4, FgChs7, FgChs5 and FgChs6 in ΔFgChs2 were significantly higher than those in the wild-type PH-1. Based on the results of qRT-PCR assays, the double mutants of ΔFgChs2/1, ΔFgChs2/3a, ΔFgChs2/4, ΔFgChs2/7, ΔFgChs2/5 and ΔFgChs2/6 were constructed by deletion of FgChs1, FgChs3a, FgChs4, FgChs7, FgChs5 and FgChs6 in ΔFgChs2, respectively (Fig. S1).
Figure 3. Effect of FgChs2 deletion on the transcription of other FgChs genes assayed by qRT-PCR.
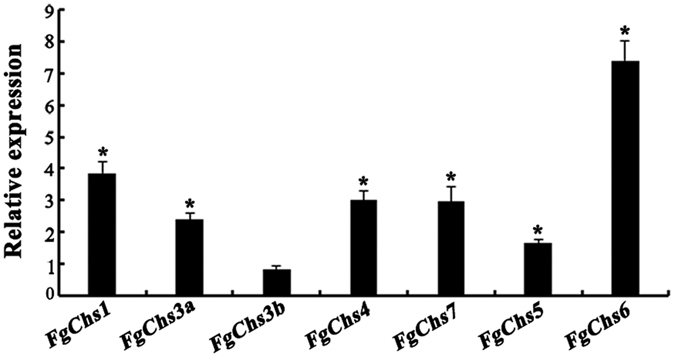
The relative expression level of each FgChs gene in the FgChs2 deletion mutant ΔFgChs2 is the relative amount of mRNA in the wild type. Line bars in each column denote standard errors of three repeated experiments. A t test was performed to determine significant differences, *significant difference for each gene at a 95% coincidence interval.
FgChs2 shares a function with FgChs1 in regulating mycelial growth
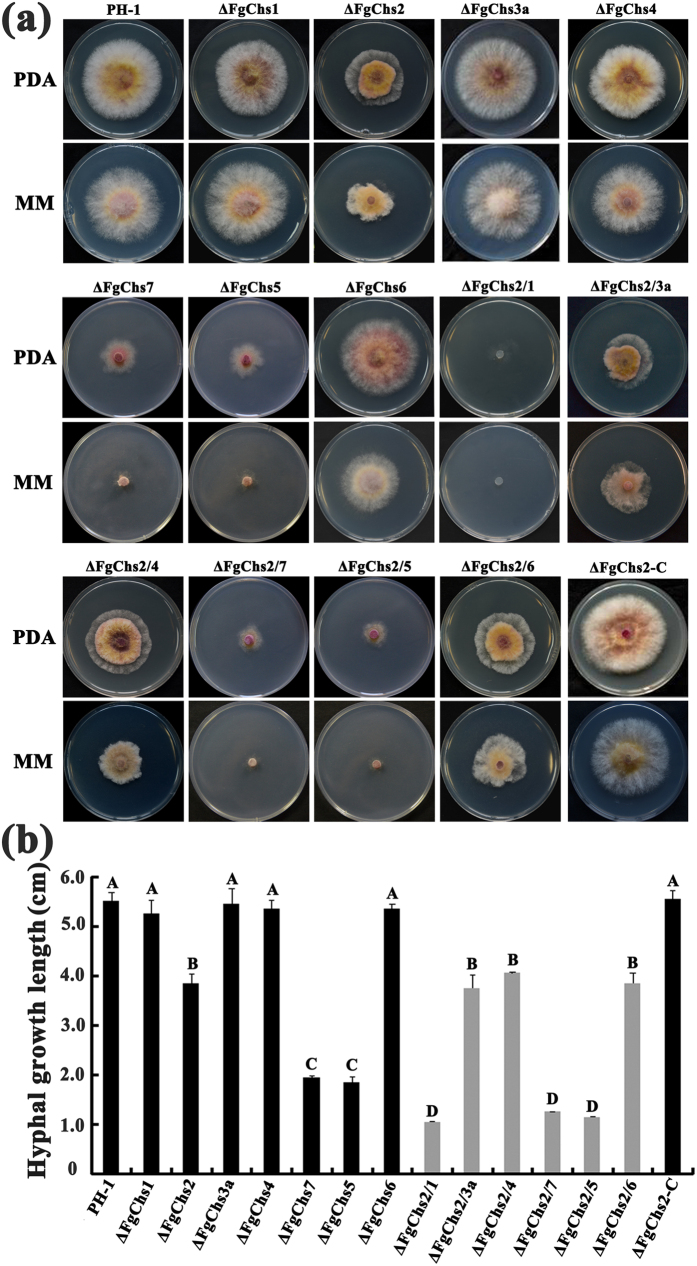
Previous studies have found that deletion of FgChs2, FgChs7 or FgChs5 caused the reduction of mycelial growth in F. graminearum, and the deletion mutants ΔFgChs3a, ΔFgChs1, ΔFgChs4 and ΔFgChs6 had an undistinguishable growth rate compared with that of the wild type19,20,21. In this study, we found that the double mutant ΔFgChs2/1 grew much slower than the single deletion mutants ΔFgChs1 and ΔFgChs2 on both PDA and MM media (Fig. 4a,b). Moreover, aerial hyphae of double mutant ΔFgChs2/1 were developed poorly (Fig. 4a). To a lesser extent, the double mutants ΔFgChs2/7 and ΔFgChs2/5 showed reduced mycelial growth in comparison with the single mutants ΔFgChs2, ΔFgChs7 and ΔFgChs5 (Fig. 4a,b). In contrast, the double mutants ΔFgChs2/3a, ΔFgChs2/4 and ΔFgChs2/6 exhibited similar growth rate with the single mutant ΔFgChs2. These results indicate that FgChs2 has an overlapping function with FgChs1 in regulating mycelial growth in F. graminearum.
Figure 4. Impacts of FgChs single and double deletion on F. graminearum hyphal growth.
(a) Colony morphology of FgChs single and double deletion mutants. The wild-type PH-1, FgChs deletion mutants (ΔFgChs1-7), double deletion mutants of FgChs2 and other FgChss (ΔFgChs2/1-7), and the complemented strain ΔFgChs2-C were grown on PDA or MM at 25 °C for 3 days. (b) Colony diameter of each strain cultured on PDA at 25 °C for 3 days. Line bars in each column denote standard errors of three repeated experiments. Values on the bars followed by the same letter are not significantly different according to a least significant difference (LSD) test at P = 0.05.
Overlapping function in conidiation between FgChs2 and FgChs1, FgChs4, FgChs7 or FgChs5
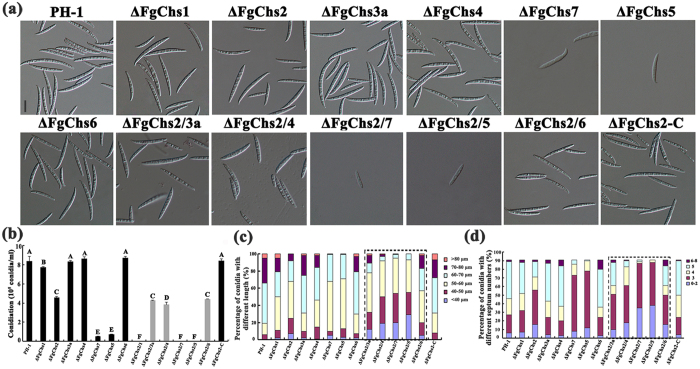
According to the previous studies19,20, conidial production of the mutants ΔFgChs1, ΔFgChs7 and ΔFgChs5 was dramatically reduced. In this study, we determined the phenotypes of asexual development for the single mutants ΔFgChs2, ΔFgChs3a, ΔFgChs4 and ΔFgChs6 and the double mutants ΔFgChs2/1, ΔFgChs2/3a, ΔFgChs2/4, ΔFgChs2/7, ΔFgChs2/5 and ΔFgChs2/6. The mutant ΔFgChs2 showed a decrease of 45.2% in conidiation and produced smaller conidial spores with less septation (Fig. 5a–d). Deletion of FgChs3a, FgChs4 and FgChs6 genes did not cause significant difference in asexual development in comparison with the wild type (Fig. 5a–d). The mutants ΔFgChs2/3a and ΔFgChs2/6 produced similar amount of conidia as ΔFgChs2 (Fig. 5a,b). Whereas, the double mutant ΔFgChs2/1 was unable to produce conidium in CMC even 15 days after inoculation. The mutants ΔFgChs2/4, ΔFgChs2/7 and ΔFgChs2/5 exhibited a significantly reduced conidiation compared with the corresponding single mutants. Moreover, microscopic examination showed that the mutants ΔFgChs2/4, ΔFgChs2/7 and ΔFgChs2/5 had more severe defects in conidium length and septation in comparison with the corresponding single mutants (Fig. 5a,c,d). These results indicated that the functions of FgChs2 in regulating conidiation are partially exchangable with those of FgChs1, FgChs4, FgChs7, and FgChs5.
Figure 5. Involvement of FgChs in regulating conidiation in F. graminearum.
(a) Conidial morphology of the wild type, FgChs deletion mutants (ΔFgChs1-7), double deletion mutants of FgChs2 and other FgChss (ΔFgChs2/1-7), and the complemented strain ΔFgChs2-C. The differential interference contrast (DIC) images of conidia were captured with an electronic microscope. Bar = 20 μm. (b) The quantity of conidia produced by each strain in carboxymethyl cellulose liquid medium (CMC) for 4.5 days in a shaker. Values on the bars followed by the same letter are not significantly different according to a least significant difference (LSD) test at P = 0.05. (c) Comparisons of conidial length among the above strains. A total of 200 conidia were examined for each strain. (d) Comparisons of the percentage of conidia with different septum numbers among the above strains. A total of 200 conidia were examined for each strain.
Although the mutants ΔFgChs1, ΔFgChs2, ΔFgChs7, ΔFgChs5, ΔFgChs2/4, ΔFgChs2/7 and ΔFgChs2/5 produced more shortened conidia with less septa, more than 90% conidia of each mutant as well as the wild type, could germinate after 4 h of incubation in 2% (w/v) sucrose solution (Fig. S2).
FgChs2 co-regulates virulence and DON biosynthesis with FgChs3a, FgChs1, FgChs4, and FgChs6
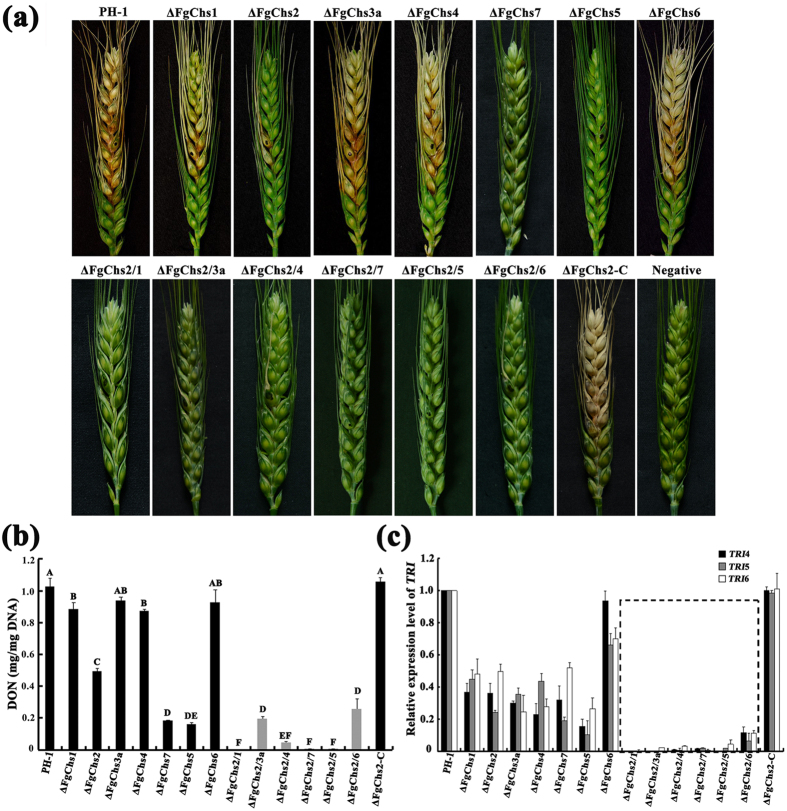
Among the eight FgChss in F. graminearum, FgChs1, FgChs2, FgChs7 and FgChs5 have been found to play important roles in virulence19,20,21. The mutants ΔFgChs7 and ΔFgChs5 almost lost virulence on flowering wheat head19, and the mutants ΔFgChs2 and ΔFgChs1 showed significantly decreased virulence20,21. Here, we determined the virulence of the double mutants on wheat head, and found that the double mutants ΔFgChs2/3a, ΔFgChs2/4 and ΔFgChs2/6 caused the scab symptoms only in the inoculated spikelets and ΔFgChs2/1, ΔFgChs2/7, and ΔFgChs2/5 could not cause any scab symptom in the inoculated spikelets 15 days after inoculation (Fig. 6a). After 25 days of inoculation, the scab symptoms caused by the single mutants ΔFgChs7 and ΔFgChs5 could be observed only in the inoculated spikelets, but no visible scab symptoms were found for the double mutants ΔFgChs2/1, ΔFgChs2/7 and ΔFgChs2/5 (data not shown). These results indicate that there are overlapping functions between FgChs2 and FgChs1, FgChs3a, FgChs4, FgChs7, FgChs5 or FgChs6 in regulating virulence in F. graminearum. Furthermore, cellophane penetration assays were performed for the mutants ΔFgChs7, ΔFgChs5, ΔFgChs2/1, ΔFgChs2/7 and ΔFgChs2/5. As shown in Fig. S3, these mutants could penetrate cellophane sheets, indicating that the dramatically reduced virulence of these mutants might mainly be owing to other factors than host penetration.
Figure 6. Impacts of FgChs deletion on virulence and DON biosynthesis.
(a) Flowering wheat heads were point inoculated with a conidial suspension at 105 conidia/ml of the wild-type PH-1, FgChs deletion mutants (ΔFgChs1-7), double deletion mutants of FgChs2 and other FgChss (ΔFgChs2/1-7), and the complemented strain ΔFgChs2-C. The infected wheat heads were photographed 15 days after inoculation. (b) The amount of DON (per mg fungal DNA) produced by each strain in infected wheat kernels was determined after 30 days of inoculation. Line bars in each column denote standard errors of three replicated experiments. Values on the bars followed by the same letter are not significantly different according to a least significant difference (LSD) test at P = 0.05. (c) Relative expression of DON biosynthetic TRI genes in each strain and bars denote standard errors from three repeated experiments.
DON is an important virulence factor of F. graminearum22,23,24. Therefore, we were interested in analyzing DON biosynthesis in each mutant since studies on functions of FgChss in DON production have not been conducted previously. As shown in Fig. 6b, after culture on sterilized wheat kernels for 30 days, single mutants ΔFgChs1, ΔFgChs2, ΔFgChs4, ΔFgChs7 and ΔFgChs5 showed reduced DON production by 13.7 to 84.5% (Fig. 6b). Double deletion of FgChs2 and any other FgChs gene intensified the decrease of DON production (Fig. 6b).
The trichothecene (TRI) genes are responsible for DON biosynthesis25,26 and the expression of TRI4, TRI5 and TRI6 have a positive correlation with the DON production in F. graminearum27. To confirm the decreased DON in FgChs mutants, we further assayed the expressions of TRI4, TRI5 and TRI6 genes by qRT-PCR assays. The expression levels of three TRI genes in six double mutants were dramatically lower than those in all single mutants (Fig. 6c).
FgChs1, FgChs3a, FgChs4, FgChs7, FgChs5 and FgChs6 have additive effects in septum formation
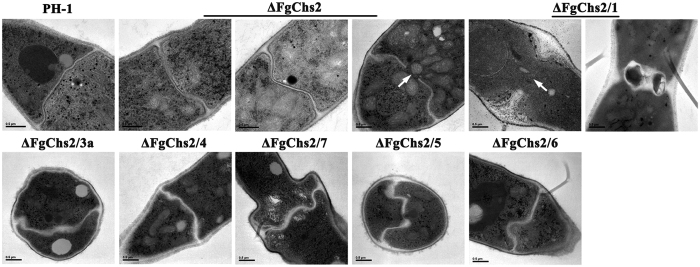
In F. graminearum, only one previous study clearly reported that the septal pores in the mutant ΔGzChs7 (ΔFgChs7) was observed to be plugged by a woronin body-like structure through transmission electron microscopy examination19. Here, we found that deletion of FgChs2 caused thickened (Fig. 7, left panel of ΔFgChs2) and “wavy” septa (Fig. 7, middle panel of ΔFgChs2) occasionally with a larger central pore (Fig. 7, right panel of ΔFgChs2), although deletion of other single FgChs could not result in recognizable changes in septum morphology (data not shown). Interestingly, the double mutants ΔFgChs2/1, ΔFgChs2/3a, ΔFgChs2/4, ΔFgChs2/7, ΔFgChs2/5 and ΔFgChs2/6 produced more thickened septa than ΔFgChs2 (Fig. 7). To a great extent, the double mutant ΔChs2/1 could not form complete septum structure with aberrant thickness and abnormally large pores. These results indicate that although FgChs2 plays an important role in septation, FgChs1, FgChs3a, FgChs4, FgChs7, FgChs5 or FgChs6 have additive effects in septum formation in F. graminearum.
Figure 7. Involvement of FgChs in septum formation in F. graminearum.
Transmission electron microscopic examination of hyphae from the wild-type PH-1, FgChs2 deletion mutant ΔFgChs2, double deletion mutants of FgChs2 and other FgChss, ΔFgChs2/1, ΔFgChs2/3a, ΔFgChs2/4, ΔFgChs2/7, ΔFgChs2/5 and ΔFgChs2/6. Septal pores are indicated by white arrows.
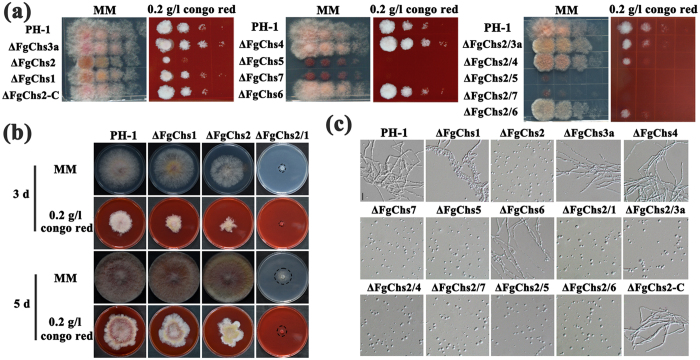
FgChs2, FgChs5 and FgChs7 play an important role in the response to cell wall stress
Among the eight FgChss in F. graminearum, FgChs1, FgChs2, FgChs7 and FgChs5 have been found to be involved in the response to various stresses19,20,21. To explore the function of FgChss in cell wall stress response, serial dilutions of conidial suspension of each strain were spotted on PDA amended with 0.2 g/l of cell wall-damaging agent congo red (CR). After incubation at 25 °C for 3 days, the single gene mutants ΔFgChs2, ΔFgChs5, ΔFgChs7, but not ΔFgChs1, ΔFgChs3a, ΔFgChs4 and ΔFgChs6, showed increased sensitivity to CR dramatically (Fig. 8a). In addition, we determined the sensitivity of ΔFgChs2/1 to CR using mycelial plugs since this mutant was unable to produce conidia. As shown in Fig. 8b, ΔFgChs2/1 and ΔFgChs2, presented similar sensitivity to CR. To further verify this finding, we tested the sensitivity of each strain to cell wall-degrading enzymes. As shown in Fig. 8c, hyphae of the single gene mutants ΔFgChs2, ΔFgChs7, ΔFgChs5, and the double mutants ΔFgChs2/1, ΔFgChs2/3a, ΔFgChs2/4, ΔFgChs2/7, ΔFgChs2/5 and ΔFgChs2/6 all were well digested and released abundant protoplasts after treatment with cellulase, lysozyme and snailase at 30 °C for 30 min. However, the single gene mutants ΔFgChs1, ΔFgChs3a, ΔFgChs4 and ΔFgChs6 could not be digested adequately. These results indicated that ΔFgChs2, ΔFgChs5, ΔFgChs7, but not ΔFgChs1, ΔFgChs3a, ΔFgChs4 and ΔFgChs6, play an important role in response to cell wall stress.
Figure 8. Sensitivity of FgChs single and double deletion mutants to cell wall stress agents.
(a) Serial dilutions of conidial suspension of each strain were spotted on MM (CK) and MM supplemented with 0.2 g/l congo red (CR). All the plates were incubated at 25 °C for 3 days. (b) Mycelial plugs of PH-1, ΔFgChs1, ΔFgChs2 and ΔFgChs2/1 were inoculated on MM (CK) and MM supplemented with 0.2 g/l CR. All the plates were incubated at 25 °C for 3 and 5 days. (c) After treatment with cellulase, lysozyme and snailase at 30 °C for 30 min, mycelia of the mutants ΔFgChs1, ΔFgChs2, ΔFgChs7, ΔFgChs5 and all the double deletion mutants were well digested and released abundant protoplasts. Bar = 10 μm.
Discussion
Chitin synthases from various fungi have been grouped into seven classes3. Neurospora crassa and M. oryzae contain seven chitin synthase genes. However, F. graminearum contains eight predicted FgChs genes (Fig. 1). In this study, we found that eight FgChss exhibited different expression patterns in hypha and conidia. In comparison with other FgChss, FgChs3b exhibited the highest expression levels (Fig. 2a). Consistent with a previous report21, we were unable to knockout FgChs3b, indicating that it might be essential in F. graminearum. Additionally, FgChs2, FgChs7 and FgChs5 exhibited higher expression in hyphae grown in MM than in PDA. Mycelial growth assays found that the growth rates of ΔFgChs2, ΔFgChs7 and ΔFgChs5 on MM plates was much slower than those on PDA plates, in contrast, the growth rate of the wild type was similar on both plates (Fig. 4a). These results indicate that the three FgChss (FgChs2, FgChs7 and FgChs5) might play more important roles in nutrient deficiency conditions.
All Chss responsible for the polymerization of GlcNAc contain conserved chitin synthase and transmenbrane domains. Additionally, classes V and VI Chss have the myosin motor domain (MMD) at their N-terminal end27. Several studies focusing on the functions of Chss in fungi have found that Chss belonging to different classes jointly play roles in hyphal growth, asexual and sexual development, pathogenicity and response to stresses12,27,28,29,30,31, which may indicate functional redundancy in various facets among Chss. In F. graminearum, the individual FgChss have been characterized, the interactions of FgChs2 with other Chss were therefore the main point explored in this study. We found FgChs2 shares functions in mycelial growth with FgChs1, and to a lesser extent, with FgChs7 and FgChs5 in F. graminearum (Fig. 4a,b). The double mutant ΔFgChs2/1 grew dramatically slow and produced fewer aerial hyphae. Although the previous study found that deletion of FgChs1 does not affect biomass and the hyphal growth rate20, our study showed that FgChs1 still plays an important role in hyphal growth. A similar finding has been reported in Aspergillus nidulans and M. oryzae. Fujiwara et al. (2000) reported that the AnchsC (I) AnchsA (II) double mutant of A. nidulans showed fewer aerial hyphae and lower hyphal density29, which is similar to the phenotypes of ΔFgChs2/1 in this study. The hyphae of the AnchsB (III) AnchsD (IV) double mutant showed more disorganized than those of the AnchsB single mutant32. Double deletion of AncsmA (VI) and AncsmB (V) impeded the elongation of germ tubes or hyphae, whereas single deletion of any one rarely caused such defects27. In M. oryzae, MoChs5 (V) and MoChs6 (VI) were also reported to have overlapping functions in maintaining polarized growth in vegetative tissue12. Results of these studies indicated that Chss of different classes may co-regulate vegetative growth in filamentous fungi.
In A. nidulans, a previous study showed that the double disruption of AnchsA and AnchsD caused a severe defect in conidial formation although each single disruptant showed no obvious decrease in conidiation28. The AnchsC and AnchsA double null mutant showed drastically reduced conidiophore population and occasionally produced secondary conidiophores29. These studies indicated that class II Chs shares functions with class I and IV Chss in regulating conidiation in A. nidulans. Similarly, in this study, the double mutant ΔFgChs2/1 was unable to produce conidia and the mutant ΔFgChs2/4 showed significantly less conidiation than those of each single gene deletion mutants ΔFgChs2 and ΔFgChs4 (Fig. 5a,b). Importantly, we found that FgChs2 also has overlapping functions in conidiation with FgChs7 and FgChs5, indicating that the class II FgChs can co-regulate conidiation with multiple classes of Chss in F. graminearum.
A previous study on Wangiella dermatitidis found that the double disruption of WdCHS2 (I) and WdCHS3 (III) caused marked virulence defects although the single gene mutants showed no loss of virulence33. Zheng et al. (2006) reported that disruption mutant of both WdCHS2 and WdCHS1 (II) grew abnormally and almost lost virulence at 25 °C, while single gene disruption strains remained virulence as the wild type, indicating overlapping functions in virulence between these Chs genes34. Functional overlap in virulence of Chss has also been found in our study. FgChs2 shares functions in pathogenicity not only with FgChs1 and FgChs3a, but also with FgChs4, FgChs7, FgChs5 and FgChs6. In addition, our study found that all chitin synthases except FgChs3a and FgChs6 are involved in regulating the production of DON. The reduced DON production in the FgChs deletion mutants further verify the involvement of FgChss in virulence since DON plays an important role in the extension of F. graminearum in plant tissue23. In M. oryzae, Mochs6 mutant was non-pathogenic, and Mochs1 (III) and Mochs7 (VII) single gene mutants were reported to cause only rare lesions on rice seedlings12. In B. cinerea, BcChs1 (I), BcChs3a (III), BcChs6 (V) or BcChs7 (VII) deletion mutant exhibited reduced virulence13,14,15,16. Muszkieta et al. (2014) found that AfcsmA (VI) and AfcsmB (V) mutants of Aspergillus fumigatus were responsible for the virulence to Galleria mellonella and mouse35. These studies indicate that Chss play an important role in virulence in pathogenic fungi.
In this study, we found that the mutant ΔFgChs2 showed thickened and “wavy” septa occasionally with a larger central pore (Fig. 7). In S. cerevisiae, C. albicans and W. dermatitidis, class II Chss are also found to be responsible for septum formation7,34,36, which is consistent with our finding. Unexpectedly, all the double mutants of FgChs2 and other FgChss, especially ΔFgChs2/1, showed more serious defects on septal morphology than the single mutants, indicating that all classes of FgChss are involved in septation. The FgChs1 FgChs2 double mutant produced aberrantly thick septa with an abnormally large pore, which is very similar to those of the double mutant AnchsC and AnchsA of A. nidulans30. However, ΔAnchsA and ΔAnchsC single mutants did not show different appearances in comparison with the wild type30. In ΔAncsmB and ΔAncsmA, the generation of intrahyphal hyphae was associated with the closing of septal pores, indicating that class V and VI chss in A. nidulans might be involved in the formation of septal pores37. But in our study, the single deletion mutants ΔFgChs7 and ΔFgChs5 did not show obvious defects on septal morphology, moreover, the double mutants ΔFgChs2/7 and ΔFgChs2/5 did not exhibit additional defects on septal pores in comparison with ΔFgchs2, which might indicate the functions in septum formation of classes V and VI Chss in F. graminearum may different from those in A. nidulans.
Similar to our case, localization analysis of seven chitin synthases in N. crassa showed that all of them localize at septa indicating all Chss might involved in septum formation38,39,40. However, only the class VI heterokaryotic KA6 strain produced aberrant and particularly “wavy” septa in B. cinerea16. Characterization of all Chss of M. oryzae showed that only class III Chs deletion mutant displayed more than 90% of abnormal conidia without any septum12. In Fusarium oxysporum, the FochsVb (V) single and FochsV (VI) FochsVb double mutants exhibited morphological abnormalities in septum formation and distribution31, whereas other FoChs1 (I), FoChs2 (II) and FoChs7 (IV) single mutants showed similar septation with the wild type41. These studies strongly indicated that different classes of Chss may be responsible for septum formation in different filamentous fungi.
In M. oryzae, the Mochs1 Mochs3 (I) double mutant exhibited increased susceptibility to high osmotic and oxidative stresses12. In A. nidulans, the double disruptant of AnchsC and AnchsA showed high sensitivity to SDS, chitin-binding dyes and chitin synthase inhibitors, although the single AnChsC and AnChsA mutants did not show defects in responses to these stresses29, indicating that AnChsC and AnChsA may have compensatory functions in responses to stresses. In contrast to what is seen in A. nidulans and M. oryzae, our study found that FgChss do not co-regulate the response to cell wall-damaging stress in F. graminearum (Fig. 8). These results indicate functions of Chss in stress responses are species-specific.
Experimental Procedures
Strains and culture conditions
F. graminearum strain PH-1 was used as the wild-type progenitor for the construction of FgChs deletion mutants. The wild type, resultant mutants and complemented strains generated in this study were grown on potato dextrose agar (PDA) (200 g potato, 20 g glucose, 20 g agar, and 1 l water) or minimal medium (MM) (10 mM K2HPO4, 10 mM KH2PO4, 4 mM (NH4)2SO4, 2.5 mM NaCl, 2 mM MgSO4, 0.45 mM CaCl2, 9 mM FeSO4, 10 mM glucose, and 1 l water, pH 6.9) for mycelial growth tests, carboxymethyl cellulose liquid medium (CMC; 15 g carboxylmethyl cellulose, 1 g yeast extract, 0.5 g MgSO4, 1 g NH4NO3, 1 g KH2PO4 and 1 l water) for conidiation tests, and 2% sugar water and yeast extract peptone dextrose liquid medium (YEPD; 1% yeast extract, 2% peptone, 2% dextrose, and 1 l water, pH 6.7) for conidial germination tests.
Generation of gene deletion and complementation mutants
The double-joint PCR approach42 was used to generate the gene replacement construct for each target gene (Fig. S1a,d). In briefly, the 5′ and 3′ flanking regions of each gene were amplified with the primer pairs listed in Table S1, and the amplified sequences were then fused with the appropriate resistance gene cassette. The resulting PCR products for each gene were transformed into protoplasts of the wild-type progenitor PH-1 respectively, as described previously23,43. Hygromycin B (Calbiochem, La Jolla, CA) was added to a final concentration of 100 mg/l for transformant selection. When other FgChs gene deletion mutants were constructed in the FgChs2 deletion background, the geneticin resistance gene cassette (NEO) was used as a second marker. In order to complement the FgChs2 deletion mutant with the entire wild-type FgChs2, the entire FgChs2 was inserted into pYF11 vector which contained NEO by the yeast homologous recombination approach44.
Putative gene deletion mutants were identified by PCR assays with the relevant primers (Table S1), and were further analyzed by the Southern blotting assays (Fig. S1). DNA of each strain was extracted and then digested by the appropriate restriction endonucleases, as indicated in Fig. S1a,d. The probes used for each strain (Fig. S1a,d) were labeled with digoxigenin (DIG) using a High Prime DNA Labeling and Detection Starter kit II according to the manufacturer’s instructions (Roche Diagnostics; Mannheim, Germany).
In this study, we totally obtained seven single mutants ΔFgChs2, ΔFgChs1, ΔFgChs3a, ΔFgChs4, ΔFgChs7, ΔFgChs5 and ΔFgChs6, six double mutants of FgChs2 and each of other FgChss, ΔFgChs2/1, ΔFgChs2/3a, ΔFgChs2/4, ΔFgChs2/7, ΔFgChs2/5 and ΔFgChs2/6, and one complemented strain ΔFgChs2-C (Fig. S1b,c,e,f). We failed to obtain FgChs3b mutant although we had obtained more than 100 ectopic transformants from 4 transformation experiments independently, which indicates that the deletion of this gene may be lethal. All of the mutants generated in this study were preserved in 15% glycerol at −80 °C.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA of the wild type was extracted from mycelia grown in potato dextrose broth (PDB) and MM at 25 °C for 2 days in the dark, and from germinating conidia in YEPD at 25 °C for 6 hours, by using the TaKaRa RNAiso Reagent (TaKaRa Biotechnology Co., Dalian, China). Ten mg of each RNA sample was used for reverse transcription with a RevertAid H Minus First Strand cDNA Synthesis Kit employing the oligo(dT)18 primer (Fermentas Life Sciences, Burlington, ON, Canada). The expression levels of FgChs genes were determined by qRT-PCR with the primers listed in Table S1. For each sample, PCR amplification with the primer pair Actin-F + Actin-R (Table S1) for quantification of transcription of ACTIN gene was performed as a reference. The expression level of each gene in each strain was calculated using the 2−ΔΔCt method45. The experiment was repeated three times.
To assay the expression levels of in FgChs genes in the FgChs2 deletion mutant ΔFgChs2, the wild type and ΔFgChs2 were grown in PDB at 25 °C for 2 days in the dark. To determine the expression levels of TRI genes, the wild type and deletion mutants were inoculated into mycotoxin synthetic (MS) medium46 (0.5 g KH2PO4, 0.6 g K2HPO4, 0.017 g MgSO4, 1 g (NH4)2SO4, 20 g glucose, 0.1 ml Vogel’s trace elements stock solution and 1 l water) and cultured for 4 days at 25 °C in the dark. RNA extraction and qRT-PCR were performed as described above. The experiment was repeated three times.
Growth and conidiation tests
To measure hyphal growth of each strain, mycelial plugs were taken from the edge of 3-day-old colony and placed on the center of PDA and MM plates at 25 °C in the dark. After incubation for 3 days, colony diameter in each plate was measured in two perpendicular directions with the original mycelial plug diameter (5 mm) subtracted from each measurement. The experiment was repeated three times independently.
For conidiation assays, fresh mycelia (50 mg) of each strain were inoculated in a 50-ml flask containing 20 ml CMC liquid media. The flasks were incubated at 25 °C for 4.5 and 15 days in a shaker (180 rpm). Then the conidial number in each flask was determined using a hemacytometer. Conidial morphology was observed with a Nikon ECLIPSE E100 microscope (Nikon Co., Tokyo, Japan). Furthermore, conidia (approximately 10 conidia/μl) of each strain were incubated in 2% sugar water at 25 °C for 4 hours, and conidium germination was examined under a Nikon ECLIPSE E100 microscope (Nikon Co., Tokyo, Japan). The experiment was repeated three times independently.
Pathogenicity assays
Pathogenicity of each strain on flowering wheat heads was performed as described previously47. Briefly, a 10-μl aliquot of fresh conidial suspension was injected into a floret in the central section spikelet of single flowering wheat heads of susceptible cultivar Jimai 22 and the control heads were inoculated with 10-μl of sterilized water. Fifteen replicates were experimented for each strain. After inoculation, the plants were kept at 22 ± 2 °C under 95–100% humidity with 12 h of daylight. After inoculating for 15 and 25 days, the infected spikelets of each inoculated wheat head were recorded. The experiment was repeated four times.
To further analyze the virulence defects of the mutants in details, penetration behavior of each strain was examined on cellophane membranes as described previously48. Briefly, each strain was grown on minimal medium covered with a cellophane membrane. After 2 days of incubation, the cellophane membrane with the colony was removed from each plate. After the plates were incubated for two additional days, mycelial growth on each plate was examined. The presence of mycelial growth on the plate indicates penetration of the cellophane membrane. The experiment was repeated three times.
Determination of DON production
To determine DON biosynthesis, a 50-g aliquot of healthy wheat kernels was sterilized and inoculated with five mycelial plugs of each strain. After incubation at 25 °C for 30 days, DON was extracted using a previously described protocol49. The DON extracts were purified with PuriToxSR DON column TC-T200 (Trilogy analytical laboratory), and the amount of DON (per mg fungal DNA) in each sample was determined by using a HPLC system Waters 1525 (Waters Co., America)50. Additionally, the amount of F. graminearum DNA of each sample was determined using qRT-PCR assays47. The experiment was repeated three times, and data were analyzed using analysis of variance (SAS version 8.0; SAS Institute, Cary, NC).
Determination of sensitivity to cell wall stress agents
Serial dilutions of conidial suspension of each strain were spotted on MM amended with the cell wall-damaging agent CR (0.2 g/l). After the plates were incubated at 25 °C for 3 days, the growth of each strain in each plate was examined. Give that the mutant ΔFgChs2/1 could not produce conidium, we determined the sensitivity to CR using mycelial plugs. Mycelial plugs (5-mm in diameter) of each strain taken from the periphery of a 3- or 5-day-old colony were incubated on MM amended 0.2 g/l CR. After the plates were incubated at 25 °C for 3 and 5 days, the growth of each strain in each plate was examined. Each experiment was repeated three times independently.
For each strain, fresh hyphae of each strain were harvested, and treated with cellulase, lysozyme and snailase (2% w/v, each) (Kaiyang Co., Shanghai, China) for 30 min in 0.7 M NaCl at 30 °C. The resulting protoplast of each strain was examined under a Nikon ECLIPSE E100 microscope (Nikon Co., Tokyo, Japan). Each experiment was repeated three times independently.
Transmission electron microscopy (TEM) assays
For the transmission electron microscopy assay, the 1.5-day-old mycelia cultured in PDB were fixed with 2.5% (v/v) glutaraldehyde. The specimens were dehydrated in a graded series of ethanol and embedded in Epon812. Ultrathin sections were cut with an ultramicrotome (LKB-V, Sweden), stained with uranyl acetate and lead citrate, and observed with an H-7650 transmission electron microscope (Hitachi, Japan).
Additional Information
How to cite this article: Liu, Z. et al. The chitin synthase FgChs2 and other FgChss co-regulate vegetative development and virulence in F. graminearum. Sci. Rep. 6, 34975; doi: 10.1038/srep34975 (2016).
Supplementary Material
Acknowledgments
This research was supported by the 973 Project (2013CB127802), National Science Foundation (31571945 and 31300128), and China Agriculture Research System (CARS-3-1-15).
Footnotes
Author Contributions Z.L., X.Z., X.L., C.F. and X.H. carried out the experiments. Y.Y. and Z.M. proposed the hypothesis, figured out strategy, designed experiments, supervised the project, and wrote the manuscript. All authors contributed to the data collection and analysis and the manuscript preparation.
References
- Latgé J. P. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66, 279–290 (2007). [DOI] [PubMed] [Google Scholar]
- Munro C. & Gow N. Chitin synthesis in human pathogenic fungi. Med. Mycol. 39, 41–53 (2001). [PubMed] [Google Scholar]
- Lenardon M. D., Munro C. A. & Gow N. A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 13, 416–423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid V. J. et al. Molecular basis of cell integrity and morphogenesis In Saccharomyces cerevisiae. Microbiol. Rev. 59, 345–386 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F. M. Review: cell wall assembly in yeast. Yeast 10, 851–869 (1994). [DOI] [PubMed] [Google Scholar]
- Ford R. A., Shaw J. A. & Cabib E. Yeast chitin synthases 1 and 2 consist of a non-homologous and dispensable N-terminal region and of a homologous moiety essential for function. Mol. Gen. Genet. 252, 420–428 (1996). [DOI] [PubMed] [Google Scholar]
- Roncero C. & Sánchez Y. Cell separation and the maintenance of cell integrity during cytokinesis in yeast: the assembly of a septum. Yeast 27, 521–530 (2010). [DOI] [PubMed] [Google Scholar]
- Schmidt M., Bowers B., Varma A., Roh D. H. & Cabib E. In budding yeast, contraction of the actomyosin ring and formation of the primary septum at cytokinesis depend on each other. J. Cell Sci. 115, 293–302 (2002). [DOI] [PubMed] [Google Scholar]
- Goswami R. S. & Kistler H. C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5, 515–525 (2004). [DOI] [PubMed] [Google Scholar]
- Pestka J. J. & Smolinski A. T. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Heal. Part B. 8, 39–69 (2005). [DOI] [PubMed] [Google Scholar]
- Steiner B., Kurz H., Lemmens M. & Buerstmayr H. Differential gene expression of related wheat lines with contrasting levels of head blight resistance after Fusarium graminearum inoculation. Theor. Appl. Genet. 118, 753–764 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L. A. et al. Different chitin synthase genes are required for various developmental and plant infection processes in the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 8, e1002526 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulié M. C., Piffeteau A., Choquer M., Boccara M. & Vidal-Cros A. Disruption of Botrytis cinerea class I chitin synthase gene Bcchs1 results in cell wall weakening and reduced virulence. Fungal Genet. Biol. 40, 38–46 (2003). [DOI] [PubMed] [Google Scholar]
- Soulié M. C. et al. Botrytis cinerea virulence is drastically reduced after disruption of chitin synthase class III gene (Bcchs3a). Cell. Microbiol. 8, 1310–1321 (2006). [DOI] [PubMed] [Google Scholar]
- Cui Z., Wang Y., Lei N., Wang K. & Zhu T. Botrytis cinerea chitin synthase BcChsVI is required for normal growth and pathogenicity. Curr. Genet. 59, 119–128 (2013). [DOI] [PubMed] [Google Scholar]
- Morcx S. et al. Disruption of Bcchs4, Bcchs6 or Bcchs7 chitin synthase genes in Botrytis cinerea and the essential role of class VI chitin synthase (Bcchs6). Fungal Genet. Biol. 52, 1–8 (2013). [DOI] [PubMed] [Google Scholar]
- Chigira Y., Abe K., Gomi K. & Nakajima T. ChsZ, a gene for a novel class of chitin synthase from Aspergillus oryzae. Curr. Genet. 41, 261–267 (2002). [DOI] [PubMed] [Google Scholar]
- Choquer M., Boccara M., Gonçalves I. R., Soulié M. C. & Vidal-Cros A. Survey of the Botrytis cinerea chitin synthase multigenic family through the analysis of six euascomycetes genomes. Eur. J. Biochem. 271, 2153–2164 (2004). [DOI] [PubMed] [Google Scholar]
- Kim J. E. et al. Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr. Genet. 55, 449–459 (2009). [DOI] [PubMed] [Google Scholar]
- Xu Y. B. et al. Disruption of the chitin synthase gene CHS1 from Fusarium asiaticum results in an altered structure of cell walls and reduced virulence. Fungal Genet. Biol. 47, 205–215 (2010). [DOI] [PubMed] [Google Scholar]
- Cheng W. et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 13, 1335–1345 (2015). [DOI] [PubMed] [Google Scholar]
- Desjardins A. E. et al. Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant-Microbe Interact. 9, 775–781 (1996). [Google Scholar]
- Proctor R. H., Hohn T. M. & McCormick S. P. Reduced virulence of Gibberella zeae caused by disruption of a trichthecine toxin biosynthetic gene. Mol. Plant-Microbe Interact. 8, 593–601 (1995). [DOI] [PubMed] [Google Scholar]
- Seong K. Y. et al. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 72, 354–367 (2009). [DOI] [PubMed] [Google Scholar]
- Alexander N. J., Proctor R. H. & McCormick S. P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxins Rev. 28, 198–215 (2009). [Google Scholar]
- Schmidt-Heydt M., Parra R. & Geisen R. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. R. Soc. Interface 8, 117–126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita N., Yamashita S., Ohta A. & Horiuchi H. Aspergillus nidulans class V and VI chitin synthases CsmA and CsmB, each with a myosin motor-like domain, perform compensatory functions that are essential for hyphal tip growth. Mol. Microbiol. 59, 1380–1394 (2006). [DOI] [PubMed] [Google Scholar]
- Motoyama T. et al. The Aspergillus nidulans genes chsA and chsD encode chitin synthases which have redundant functions in conidia formation. Mol. Gen. Genet. 251, 442–450 (1996). [DOI] [PubMed] [Google Scholar]
- Fujiwara M. et al. Evidence that the Aspergillus nidulans class I and class II chitin synthase genes, chsC and chsA, share critical roles in hyphal wall integrity and conidiophore development. J. Biochem. 127, 359–366 (2000). [DOI] [PubMed] [Google Scholar]
- Ichinomiya M., Yamada E., Yamashita S., Ohta A. & Horiuchi H. Class I and class II chitin synthases are involved in septum formation in the filamentous fungus Aspergillus nidulans. Eukaryot. Cell 4, 1125–1136 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Urdíroz M., Roncero M. I. G., González-Reyes J. A. & Ruiz-Roldán C. ChsVb, a class VII chitin synthase involved in septation, is critical for pathogenicity in Fusarium oxysporum. Eukaryot. Cell 7, 112–121 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinomiya M. et al. Repression of chsB expression reveals the functional importance of class IV chitin synthase gene chsD in hyphal growth and conidiation of Aspergillus nidulans. Microbiology 148, 1335–1347 (2002). [DOI] [PubMed] [Google Scholar]
- Wang Z. et al. WdChs2p, a class I chitin synthase, together with WdChs3p (class III) contributes to virulence in Wangiella (Exophiala) dermatitidis. Infect. Immun. 69, 7517–7526 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L. et al. WdChs1p, a class II chitin synthase, is more responsible than WdChs2p (Class I) for normal yeast reproductive growth in the polymorphic, pathogenic fungus Wangiella (Exophiala) dermatitidis. Arch. Microbiol. 185, 316–329 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszkieta L. et al. Deciphering the role of the chitin synthase families 1 and 2 in the in vivo and in vitro growth of Aspergillus fumigatus by multiple gene targeting deletion. Cell. Microbiol. 16, 1784–1805 (2014). [DOI] [PubMed] [Google Scholar]
- Munro C. A. et al. Chs1 of Candida albicans is an essential chitin synthase required for synthesis of the septum and for cell integrity. Mol. Microbiol. 39, 1414–1426 (2001). [DOI] [PubMed] [Google Scholar]
- Takeshita N., Ohta A. & Horiuchi H. CsmA, a class V chitin synthase with a myosin motor-like domain, is localized through direct interaction with the actin cytoskeleton in Aspergillus nidulans. Mol. Biol. Cell 16, 1961–1970 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M. et al. Spitzenkörper localization and intracellular traffic of green fluorescent protein-labeled CHS-3 and CHS-6 chitin synthases in living hyphae of Neurospora crassa. Eukaryot. Cell 6, 1853–1864 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-León E. et al. Traffic of chitin synthase 1 (CHS-1) to the Spitzenkörper and developing septa in hyphae of Neurospora crassa: actin dependence and evidence of distinct microvesicle populations. Eukaryot. Cell 10, 683–695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo-Somera R. A. et al. Dissecting the function of the different chitin synthases in vegetative growth and sexual development in Neurospora crassa. Fungal Genet. Biol. 75, 30–45 (2015). [DOI] [PubMed] [Google Scholar]
- Martín-Udíroz M., Madrid M. P. & Roncero M. I. G. Role of chitin synthase genes in Fusarium oxysporum. Microbiology 150, 3175–3187 (2004). [DOI] [PubMed] [Google Scholar]
- Yu J. H. et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41, 973–981 (2004). [DOI] [PubMed] [Google Scholar]
- Hou Z. et al. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 15, 1119–1127 (2002). [DOI] [PubMed] [Google Scholar]
- Ma H., Kunes S., Schatz P. J. & Botstein D. Plasmid construction by homologous recombination in yeast. Gene 58, 201–216 (1987). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Merhej J., Boutigny A. L., Pinson-Gadais L., Richard-Forget F. & Barreau C. Acidic pH as a determinant of TRI gene expression and trichothecene B biosynthesis in Fusarium graminearum. Food Addit. and Contam. 27, 710–717 (2010). [DOI] [PubMed] [Google Scholar]
- Jiang J. et al. A type 2C protein phosphatase FgPtc3 is involved in cell wall integrity, lipid metabolism, and virulence in Fusarium graminearum. PloS one 6, e25311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Berges M. S., Rispail N., Prados-Rosales R. C. & Di Pietro A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. The Plant Cell 22, 2459–2475 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Kolaczkowski E., Xie W., Yu H. & Jelen H. Analysis of deoxynivalenol and its derivatives (batch and single kernel) using gas chromatography/mass spectrometry. J. Agr. Food Chem. 46, 1414–1418 (1998). [Google Scholar]
- Liu X. et al. Paralogous cyp51 genes in Fusarium graminearum mediate differential sensitivity to sterol demethylation inhibitors. Fungal Genet. Biol. 48, 113–123 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.