Abstract
Background Decision aids help patients make informed treatment decisions. Values clarification (VC) techniques are part of decision aids that help patients assimilate the information with their personal values. There is little evidence that these techniques contribute to enhanced decision making over and above the provision of good quality information.
Objectives To assess whether VC techniques are active ingredients in enhancing informed decision making and explain how and why they work.
Methods Participants were randomly assigned to one of three groups: (i) information only, (ii) information plus implicit task, (iii) information plus explicit task. Thirty healthy women from a UK University participated by making a hypothetical choice between taking part in a clinical trial and having the standard treatment for breast cancer. Verbal protocols were elicited by think‐aloud method and content analysed to assess informed decision making; a questionnaire was completed after the decision assessing decision preference, perceptions of decisional conflict and ambivalence. Data were analysed using multivariate statistics.
Findings No participants changed their decision preference as a result of the VC techniques. Women in the explicit VC group evaluated more information in accord with personal values, expressed lower ambivalence, decisional uncertainty and greater clarity of personal values than those in the implicit VC and control groups. Feelings of ambivalence about both options were related to decisional conflict.
Conclusion Explicit VC techniques are likely to be active ingredients in decision aids. They work by enabling people to deliberate about the decision information in accord with their personal values, which is associated with a better decision experience.
Keywords: cancer, decision aids, informed choice, informed decision making, preferences, trial participation, values clarification
Introduction
Decision aids are interventions to help people make decisions in a better way than they do naturally. 1 In health care this means interventions to help patients make informed decisions about treatment options. 2 , 3 An informed decision requires patients to consider information about all the treatment options and their consequences with reference to their personal values, and make a choice based on a trade‐off of these evaluations. 2 , 4 , 5 , 6 There are many screening and treatment choices for which there is clinical equipoise 7 and the patients’ values are central to the choice of treatment. 8 In the context of (non) participation in clinical trials, enabling patients to make informed decisions is not only desirable but an ethical imperative. 9
Decision aids are complex interventions 10 containing at least two component parts: (i) complete, accurate and non‐biased information about the available options and their consequences and (ii) techniques eliciting patients’ personal values about the decision’s consequences to help them arrive at a choice. 1 , 11 The purpose of the values clarification component is to help people clarify how they feel about the different consequences of options, which consequences are relevant to their personal circumstances, and what trade‐offs they need to make to arrive at a choice. 1 , 12 , 13 The techniques used for values clarification are broadly categorized into implicit or explicit approaches. 14 , 15 , 16 Implicit approaches structure the decision information in a way that clearly illustrates all options of the choice and their consequences, and encourage patients to consider how they feel about the options (e.g. decision boards, attribute tables). Explicit approaches also structure the information in a way that represents clearly the decision problem but include additional, proactive techniques requiring the patient to engage with the information by rating the degree to which the consequences matter to them. 13 , 16 The explicit techniques are varied with some using simple Likert or visual analogue scales (e.g. weigh scale exercise) to rate the personal importance of consequences and some using more complex tasks such as rank ordering the importance of consequences and/or trading‐off options or attributes (e.g. standard gamble). 8 , 12 , 16 , 17 , 18
There is evidence that people using decision aids tend to employ more cognitive and emotional strategies, make more robust evaluations of decision information, have less regret, and express greater satisfaction with the decision. 8 , 19 , 20 However, evidence is lacking on what the component parts of the decision aid add to the intervention in order to enable better decision making. It is unclear if the values clarification techniques contribute to patients making more informed decisions over and above the improved content and structure of the information within decision aids. 10 , 19 If values clarification techniques are an active ingredient in the intervention, there is a paucity of evidence identifying whether different types of clarification techniques are more or less effective in different health contexts with decisions of varying complexity and patients with differing experiences and abilities. 13 , 16
The information processing paradigm provides a framework to help us understand how people make sense of the ‘information out there’ with their cognitions and experiences ‘inside’. 4 From this literature, we can hypothesize how the component parts of decision aids help people make better decisions. 19 , 21 , 22 For example, by structuring the decision problem as a tree and/or attribute table we provide a visual representation of all the options, attributes and consequences of the decision. This representation of the decision problem increases the likelihood of patients evaluating all the decision relevant information without having to rely on partial information provided by either the information provider and/or the patients’ memory. An implicit values clarification statement signposts the need for patients to engage with and evaluate this full information, possibly enabling patients to employ more appropriately their intuitive or usual processes to reach the decision. An explicit values clarification task requires patients to deliberate consciously about their evaluations of the decision information with their beliefs and provides steps which may help patients to integrate more fully the information ‘out there’ with values ‘inside’.
There is debate as to whether encouraging patients to engage deliberatively and systematically with the decision information rather than relying on more intuitive processes to reach a decision helps or hinders decision making. 23 , 24 , 25 Some argue that by making explicit all the patients’ decision processes their evaluation of the decision options and consequences will correspond more closely with their actual experience of decision consequences, 5 i.e. more thorough evaluations lead to more realistic expectations, less conflict with the decision made and a more satisfactory decision experience. 11 , 19 Others argue that consciously exploring the advantages and disadvantages of all the options increases the uncertainty patients feel towards the choice; the ambivalence generated whilst simultaneously holding both positive and negative evaluations of an option results in a worse decision experience. 26 As ambivalence and decisional conflict tend to be investigated independently, it is unclear how these concepts are related. It is likely that values clarification techniques encourage informed decision making but it is unclear how the increased deliberation impacts on perceptions of ambivalence and decisional conflict during decision making.
The purpose of this experimental study was to assess whether values clarification techniques are active ingredients in decision aid interventions, i.e. additions to a complex intervention over and above the provision of good quality information. In particular, the study aimed to (i) explain how and why these values clarification techniques enable people to make informed decisions, (ii) examine the relationship between ambivalence and decisional conflict, and (iii) assess the impact of increased deliberation on perceived decision experience. As this study is a proof of concept study, it was carried out in a sample of healthy women making a hypothetical choice between taking part in a clinical trial and having the standard treatment for their breast cancer.
Methods
Design
A between‐subjects design with participants randomly assigned to one of three groups was employed: (i) routine information only (Control), (ii) routine information plus an implicit values clarification task (Implicit), (iii) routine information plus the explicit values clarification task (Explicit). The study employed qualitative and quantitative methods: a think‐aloud technique employing verbal protocols that were audio tape‐recorded concurrently with decision making, then transcribed and analysed using a thematic content analysis 27 , 28 , 29 , 30 , 31 ; a paper‐based questionnaire was completed immediately after participants had made a decision (not) to participate in the trial. The study had ethical approval from the Leeds Institute of Psychological Sciences Ethics Committee in July 2007.
Sample
All women aged 18 years or older working and/or studying at the University of Leeds, UK, over August to October 2007 were invited to participate via the University’s email distribution list. No women volunteering to participate were excluded. Studies employing think‐aloud techniques often involve small sample sizes as the collection and analysis of verbal protocol data is time consuming and labour intensive. 32 , 33 Ten participants per study group, giving an overall sample size of 30, is comparable to the average sample size used in think‐aloud studies. 34
Materials
Breast cancer treatment clinical trial scenario
Participants were asked to imagine they had been diagnosed with early stage breast cancer, had had the lump removed by surgery and were discussing treatment options with their doctor, who suggested chemotherapy. Participants were told that the clinic was offering participation in a clinical trial, known by the acronym TACT (Taxotere as Adjuvant chemotherapy) and had to decide whether to take part in the trial or have the standard chemotherapy treatment (Appendix S1). TACT was an international phase‐three chemotherapy trial for early stage breast cancer, carried out by the local cancer unit shortly before the time of the study. 35 To enhance the validity of the scenario, participants were asked to consider the impact this diagnosis would have on specific aspects of their life such as work, social life and daily chores 36 , 37 and/or recollect the experiences of any family and friends who had experienced cancer. All participants were provided with details of the University’s counselling service and the hospital’s clinical psychological services in case personal issues were raised as a result of taking part in this research.
Routine decision information
The TACT trial and standard treatment routine information was contained in an A‐4 size paper‐based booklet and included purpose, details of treatments, possible side effects, benefits and risks. The information readability score was 8.0 (equivalent of an eighth grader/age 14 level). 38
Values clarification tasks
The values clarification tasks involved a paper‐based summary of the benefits and risks information for both the options situated on either side of a weigh‐scale as outlined by O’Connor et al. 13 The implicit task asked women to: (i) review this information; (ii) add any other reasons for choosing or not choosing the options in the space provided; (iii) underline the benefits and risks they thought were more likely to happen. In addition to the steps performed in the implicit task, the explicit task asked women to: (iv) indicate the extent to which each benefit and risk mattered to them using stars (zero stars if it did not matter at all and five if it mattered a lot); (v) indicate their leaning towards taking part in the trial or having the standard treatment on a seven‐point scale (Appendix S2).
Measures
Data were elicited by two methods, the think‐aloud method (qualitative) assessing informed decision making and a paper‐based questionnaire (quantitative) assessing socio‐demographic information, decision preference and perceptions of decision making experience.
Informed decision making
A coding frame (Fig. 1) was developed with reference to guidelines on making informed decisions to categorize participants’ utterances resulting from the think‐aloud method. An informed decision requires individuals to consider information about the consequences of all available options, to evaluate the likelihood and desirability of those consequences in accord with his/her personal values, and make a choice based on trade‐offs between these evaluations. 2 , 4 , 5 , 6 , 19 Themes were developed that classified the utterances about the information as attributes of standard and trial options. The resulting themes consisted of eleven trial attributes and eight standard treatment attributes (Fig. 1a). Within the themes, categories classified how the information was processed by participants: attributes referred to (either just repeating or mentioning the attribute); attribute evaluated (judging the attribute as a good or bad); attribute evaluated with reference to personal values (rating the attribute as being good or bad for them) (Fig. 1b). The coding frame was applied systematically to all protocols using the qualitative data management software NVivo 7 (QSR International Pty. Ltd. 2006). The data on the number of times the information processing strategies were used within each theme were analysed quantitatively. In addition, for each attribute classified, women received a score –‘0’ if the information was never evaluated with reference to personal values and ‘1’ if the information was evaluated with reference to personal values at least once. These scores were summed up for all the attributes of the trial and standard treatment to reflect the number of attributes participants evaluated with reference to personal values. Differences in the number of attributes each participant evaluated with reference to personal values were examined using multivariate analysis of variance.
Figure 1.
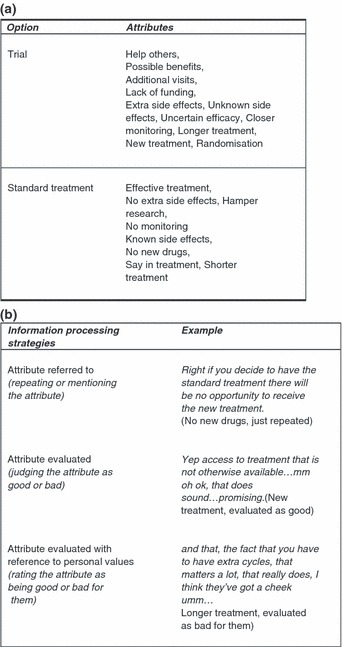
Think‐aloud protocol analysis coding frame: (a) attributes of trial and standard treatment options, (b) categories classifying how the attribute information was processed.
Socio‐demographic information
Age, ethnic origin, occupation, educational level, marital status, personal history of cancer diagnosis and treatment, and people known with cancer in the social network.
Decision preference
Initial decision preference was assessed after the decision scenario but before receipt of the full trial information using a categorical response (take part in the trial: have the standard treatment; undecided). Final decision preference was assessed after receipt of full trial information (control group) or after the values clarification task (values clarification groups) using a categorical response (take part in the trial: have the standard treatment).
Perceptions of decision making experience during decision making
Perceptions of decision making experience during decision making were assessed after the final decision had been made by asking participants to reflect on the entire decision making process.
Attitudinal ambivalence (felt)
For each option, three items rated the reactions participants felt towards that option 39 (1 = completely one sided reactions/no conflict at all/no indecision at all to 7 = completely mixed reactions/maximum conflict/maximum indecision). Scores on the three scales were summed up for each option; higher scores indicated higher levels of felt ambivalence.
Attitudinal ambivalence (potential)
For each option, two items rated participants’ judgements about the positive and negative attributes ascribed to that option (scored 1–7). 40 The two judgements were combined using the ‘Griffin’ formula to give the potential ambivalence score for each option 41 : [(P + N)/2] – |P–N|; higher scores reflect equally strong positive and negative judgements and hence greater ambivalence.
Decisional conflict scale 42
Perceived decisional conflict was assessed after the final decision for two time points: perceptions during decision making were assessed by asking participants to reflect on the entire decision making process (uncertainty, values clarity, and informed subscales); and perceptions after the decision were assessed by asking participants how they had felt after they had made the decision (uncertainty, values clarity, informed and effective decision making subscales). The scores were converted to the equivalent 0–100 scale; higher scores indicate higher decisional conflict.
Procedure
The study sessions took place in a quiet room. Participants were allocated to the study groups using random permuted blocks of three to ensure equal numbers of participants in each group as the study progressed. At the beginning of the session, participants received written instructions about how the session would proceed followed by instructions to think‐aloud. Participants read the decision scenario and were asked to indicate their initial preference. Following the scenario, they received detailed information about the trial and standard treatment options and were asked for their final decision preference. The explicit and implicit groups received the values clarification tasks after they had read the detailed information but before they were asked for their final decision. Participants were asked to say aloud all thoughts that came to their mind from the time they received the decision scenario until they had indicated their final decision, without needing to explain or justify their approach to the task. The researcher reminded them by saying ‘please keep talking’ if they remained silent for more than a few minutes. The think‐aloud protocols were audio‐recorded. Participants completed the paper‐based questionnaire on completion of the study task.
Data analysis
Logistic regression analyses compared between group differences in demographic characteristics and decision preferences; one set compared the explicit and implicit groups with the control, and the other the implicit and explicit groups.
Multivariate analyses of variance with two sets of orthogonal planned comparisons were carried out to test whether the values clarification techniques were more effective in enhancing informed decision making and improving decision making experience than routine information alone and whether the explicit technique is more effective than the implicit one; the first set compared the control group with the mean of implicit and explicit groups and the second the implicit and explicit groups. Where clarification of relationships was needed, pairwise comparisons with Bonferroni correction were performed to explore if either or both were significantly different from the control. 43 Regression analysis was carried out to assess the relationship between decisional conflict and ambivalence.
Results
Thirty women, aged between 19 and 60 years (mean = 36 years, SD = 13.8) took part in the study. The sample was predominantly Caucasian (n = 28); 17 were students; 14 were married or living as married. The majority (n = 28) knew someone who had, or had previously suffered from, cancer in their social network: 10 had close relatives, 18 distant relatives or friends. The differences by study group with respect to demographic characteristics (Table 1) were not statistically significant. However, as this may be due to the small sample size, the demographic variables were controlled for in subsequent analyses comparing groups.
Table 1.
Participant characteristics, initial and final decision preference by group
| Explicit (n = 10) | Implicit (n = 11) | Control (n = 9) | Total (n = 30) | |
|---|---|---|---|---|
| Participant characteristics | ||||
| Mean age (years) | 38.3 (SD = 13.4) | 39.8 (SD = 13.5) | 28.4 (SD = 12.9) | 36 (SD = 13.8) |
| Occupation: Staff (n) | 6 | 5 | 2 | 13 |
| Occupation: Students (n) | 4 | 6 | 7 | 17 |
| Marital status: Married/living as married (n) | 3 | 8 | 3 | 14 |
| Women with close relatives with cancer (n) | 4 | 6 | 0 | 10 |
| Initial decision preference (n) | ||||
| Trial | 4 | 4 | 5 | 13 |
| Standard treatment | 3 | 0 | 1 | 4 |
| Uncertain | 3 | 7 | 3 | 13 |
| Final decision preference (n) | ||||
| Trial | 6 | 9 | 6 | 21 |
| Standard treatment | 4 | 2 | 3 | 9 |
Decision preference
No participants changed their decision from their initial preference but of the 13 that were undecided, eight chose to take part in the trial and five to have the standard treatment. The differences in the initial and final trial decision preferences by study group were not significant (Table 1).
Informed decision making
When the study groups were compared on the frequency with which the trial and standard treatment attributes were referred to, evaluated and evaluated with reference to personal values, multivariate effects were significant for both the trial attributes (F[6,42] = 2.8, P < 0.05) and the standard treatment attributes (F[6,42] = 4.3, P < 0.01) (Table 2). For trial attributes, the values clarification groups evaluated the attributes more often and evaluated more with reference to personal values than the control group. The explicit group evaluated more with reference to personal values than the implicit group (P < 0.10). For standard treatment attributes there was a similar pattern. The values clarification groups evaluated attributes more often than the control group, and the explicit group evaluated more than the implicit group. The explicit group evaluated with reference to personal values more than the implicit group, but the difference between the values clarification and control groups was not significant. Pairwise comparisons (Bonferroni adjusted) confirmed that the implicit group was not significantly different from the control group, leading to this result.
Table 2.
Use of information processing strategies by groupa
| Explicit (n = 10) | Implicit (n = 11) | Control (n = 9) | F | Planned comparisons | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| Frequency with which information processing strategies were used | |||||||||
| Trial attributes Multivariate F[6,42] = 2.8, P < 0.05 | Referred to only | 7.2 | 3.3–11.1 | 7.5 | 3.9–11.1 | 4.1 | 0–8.2 | 0.84 | |
| Evaluated at all | 18.1 | 14.4–21.8 | 18.8 | 15.3–22.2 | 11.8 | 7.9–15.7 | 4.0* | C v E&I, P < 0.01; | |
| Evaluated with reference to personal values | 9.3 | 6.7–11.8 | 6.3 | 4.0–8.7 | 3.2 | 0.5–5.9 | 5.5* | C v E&I, P < 0.05; E v I, P < 0.10; | |
| Standard treatment attributes Multivariate F[6,42] = 4.3, P < 0.01 | Referred to only | 2.2 | 0.2–4.2 | 4.6 | 2.8–6.5 | 1.0 | −1.1 to 3.2 | 3.3* | |
| Evaluated at all | 6.0 | 4.6–7.5 | 2.5 | 1.1–3.9 | 1.7 | 0.1–3.3 | 10.2*** | C v E&I, P < 0.05; E v I, P < 0.01 | |
| Evaluated with reference to personal values | 2.9 | 1.7–4.1 | 0.5 | −0.6 to 1.7 | 0.9 | −0.4 to 2.2 | 4.9* | E v I, P < 0.01 | |
| Number of attributes evaluated with reference to personal values, Multivariate F [4,44] = 4.1, P < 0.01 | |||||||||
| Trial attributes (0–11) | 6.7 | 5.2–8.2 | 4.1 | 2.7–5.5 | 2.6 | 1.0–4.2 | 7.7** | C v E&I, P < 0.01; E v I, P < 0.05 | |
| Standard treatment attributes (0–8) | 2.4 | 1.6–3.3 | 0.5 | −0.2 to 1.3 | 0.5 | −0.4 to 1.4 | 7.6** | C v E&I, P < 0.10; E v I, P < 0.01 | |
*P < 0.05, **P < 0.01, ***P < 0.001.
aValues are adjusted for demographic covariates.
The study groups were compared on the number of attributes each participant evaluated with reference to personal values. A significant main effect was found for both trial (F[2,28] = 7.7, P < 0.01) and standard treatment (F[2,28] = 7.6, P < 0.01) attributes. Planned comparisons show that for both trial and standard treatment attributes, the values clarification groups evaluated more attributes with personal values than the control group and the explicit group evaluated more attributes with personal values than the implicit group (Table 2). Pairwise comparisons (Bonferroni adjusted) revealed no significant differences between the control and the implicit group, however, suggesting that the results in the planned comparisons are driven by the explicit group.
Felt and potential ambivalence
The multivariate effects for felt (F[4,44] = 1.8, n.s.) or potential (F[4,44] = 0.41, n.s.) ambivalence were not significant by study group, neither were the main effects for trial or standard treatment. However, the planned comparisons suggested women in the explicit group felt significantly less ambivalent about both the trial and standard treatment than those in the implicit group (Table 3). There were no differences between the values clarification and control groups, and pairwise comparisons indicated that the implicit and control groups were not significantly different, indicating a difference between the explicit group and the others.
Table 3.
Attitudinal ambivalence and decisional conflict by groupa
| Explicit (N = 10) | Implicit (N = 11) | Control (N = 9) | F | Planned comparisons | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95%CI | Mean | 95%CI | Mean | 95%CI | |||
| Felt ambivalence during decision making, Multivariate F(4,44) = 1.8, n.s. | ||||||||
| Felt ambivalence, Trial (low‐high: 3–21) | 3.0 | 1.8–4.2 | 4.9 | 3.8–6.0 | 3.7 | 2.4–5.0 | 2.9* | E v I, P < 0.05 |
| Felt ambivalence, ST (low–high: 3–21) | 2.6 | 1.4–3.8 | 4.2 | 3.0–5.3 | 4.0 | 2.7–5.3 | 2.2 | E v I, P < 0.10 |
| Potential ambivalence during decision making, Multivariate F(4,44) = 0.42, n.s. | ||||||||
| Potential ambivalence, Trial (low–high: −2 to 7) | 1.6 | 0.1–3.2 | 2.2 | 0.7–3.6 | 3.1 | 1.5–4.8 | 0.86 | |
| Potential ambivalence, ST (low–high: −2 to 7) | 2.1 | 0.7–3.4 | 2.0 | 0.8–3.3 | 2.0 | 0.6–3.4 | 0 | |
| Perceptions of decisional conflict during decision making (low–high: 0–100), Multivariate F[6,42] = 2.5, P < 0.05 | ||||||||
| Uncertainty | 31.6 | 12.3–50.9 | 68.6 | 50.5–86.7 | 57.7 | 37.1–78.3 | 4.3** | E v I, P < 0.01 |
| Uninformed | 30.6 | 17.7–43.6 | 25.7 | 13.6–37.9 | 19.4 | 5.5–33.2 | 0.72 | |
| Unclear values | 11.3 | −4.4 27.1 | 48.0 | 33.2–62.7 | 33.8 | 16.9–50.6 | 6.1*** | E v I, P < 0.01 |
| Perceptions of decisional conflict after the decision (low–high: 0–100), Multivariate F[8,40] = 1.6, n.s. | ||||||||
| Uncertainty | 21.4 | 5.8–36.9 | 49.7 | 35.1–64.2 | 34.6 | 18.0–51.2 | 3.7** | E v I, P < 0.05 |
| Uninformed | 17.9 | 5.9–30.0 | 28.0 | 16.7–39.4 | 14.4 | 1.6–27.3 | 1.4 | |
| Unclear values | 15.9 | 5.3–26.5 | 30.7 | 20.8–40.7 | 24.3 | 13.0–35.7 | 2.2 | E v I, P < 0.05 |
| Ineffective decision | 20.2 | 7.4–33.1 | 24.0 | 11.9–36.0 | 20.9 | 7.1–34.6 | 0.1 | |
*P < 0.10, **P < 0.05, ***P < 0.01.
aValues are adjusted for demographic covariates.
Perceptions of decisional conflict
The explicit group women were less uncertain about their decision and less unclear about their values both during decision making and after the decision than those in the implicit group. Differences between the average scores for values clarification groups and control were not significant, and again pairwise comparisons (Bonferroni adjusted) indicated that the implicit and control groups were not significantly different, indicating a difference between the explicit group and the others (Table 3). Overall, women felt more certain about their decision and clearer about their values after the decision than during decision making; feelings of being informed were unchanged (Table 4).
Table 4.
Paired t‐tests for decisional conflict at the time of decision making and after decision
| During decision | After decision | Paired t‐tests | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | Sig. | |
| Uncertainty (good–poor:0–100) | 52.5 | 30.4 | 35.3 | 22.7 | 4.5 | 0.000 |
| Uninformed (good–poor:0–100) | 25.5 | 18.5 | 20.8 | 16.8 | 1.6 | 0.111 |
| Unclear values (good–poor:0–100) | 31.1 | 25.6 | 23.3 | 15.5 | 1.9 | 0.065 |
| Total decisional conflict (3 scales) (good–poor:0–100) | 36.3 | 20.4 | 26.4 | 16.0 | 3.5 | 0.001 |
Relationship between ambivalence and decisional conflict
Felt ambivalence about the trial and standard treatment were positively associated with decisional conflict (r = 0.51, P < 0.01); higher decisional conflict during decision making was associated with higher felt ambivalence about both trial (r = 0.74, P < 0.01) and the standard treatment (r = 0.75, P < 0.01). There was no correlation with potential ambivalence measures (r = 0.31 and r = 0.14, n.s.). Felt ambivalence about both the options explained a total of 73% of the variance in decisional conflict; the unique variance explained by ambivalence about the standard treatment was 18% and the unique variance explained by felt ambivalence about the trial was 17%, the shared variance was explained by both variables was 38% (Table 5).
Table 5.
Regression of decisional conflict at the time of decision making onto ambivalence about trial and standard treatment (n = 30)
| β | SE | t | Unique variance explained | R 2 | |
|---|---|---|---|---|---|
| Model | 0.73*** | ||||
| Felt ambivalence, ST | 2.041 | 0.48 | 4.3*** | 0.18 | |
| Felt ambivalence, Trial | 1.914 | 0.45 | 4.2*** | 0.17 |
***P < 0.001.
Discussion
The study provides evidence that values clarification techniques are likely to be active ingredients in patient decision aid interventions. All participants perceived themselves to be equally informed suggesting the information component of the decision aid provided knowledge about the decision problem but not how to evaluate this knowledge in accord with their beliefs. 8 However, participants’ engagement with the decision information was differentially associated with their study group; those in the explicit group evaluating more attributes more often in accord with their personal values than the implicit or information only groups, and those in the implicit group evaluating the attributes more often than the information only group. Participants in the explicit study group had lower felt ambivalence and decisional conflict scores than the other groups suggesting that deliberating about the seemingly incompatible attributes of both options in accord with your own beliefs was associated with a better decision experience than relying on more intuitive strategies. 19 The study found decisional conflict and attitudinal ambivalence to be conceptually similar measures of perceived decisional experience. 41 , 44 Felt, but not potential ambivalence, was associated with decisional conflict suggesting that decisional conflict occurs only when individuals are aware of the simultaneous positive and negative evaluations of options, not when these evaluations are dormant. This is the first time a link has been demonstrated between a construct used in the applied field of decision aids and that used in the theoretical frameworks of attitude research.
Currently the evidence explaining why the component parts of decision aid interventions facilitate patient decision making is limited. 8 , 16 , 19 , 45 , 46 The paucity of evidence is partly attributable to the methods needed to unpack the active ingredients of a decision aid rather than assess its effectiveness. We employed a think‐aloud technique to encourage participants to verbalize their thinking about the decision they were making. This is an established technique amongst decision scientists 27 , 28 , 47 and is believed to capture accurately the reasoning people usually employ when engaged in decision making. The main difficulty of using a think‐aloud technique to evaluate interventions that encourage deliberation is a possible confound between the think‐aloud method and the active components of the decision aid. More specifically, it is feasible that the think‐aloud method acted as an intervention to encourage conscious deliberation of the decision information. However, as there was a differential effect of the groups on the informed decision making measures, it seems more likely that conscious deliberation was a result of the values clarification techniques than the research method.
One criticism of the think‐aloud technique, and all self‐report measures as methods to ascertain people’s cognitions, is that they describe only those processes available to conscious attention; processes that are sub‐conscious and/or difficult to verbalize are not measured. 48 , 49 The implication from this would be that people in the implicit and information only groups could have processed the information in the same way as those in the explicit group but did not verbalize these processes either because they were not accessible to conscious report or were not the focus of attention. 50 This criticism is difficult to refute because reasoning is a noumenon 51 – a thing‐in‐itself whose existence can only be postulated and not directly observed via our senses. We have to use observable manifestations like verbal or written responses to infer about these hidden processes. Our finding that the groups differed on their perceived decision experience provides indirect evidence that the processing of information could not have been the same across the three groups. Decision and behaviour scientists elicit observable responses to classify aspects of people’s reasoning (e.g. stated evaluations of decision attributes, and attitudes and risk perceptions) rather than perceptions of how they reason (e.g. preferences for involvement in decision making and decision making style) and use theoretical frameworks to make predictions about how these phenomena are related. A theoretically informed interpretation of our findings overall is that values clarification techniques increased people’s awareness of the justifications/reasons for their choice by bringing the evaluative processes to their conscious attention and it was this increased awareness that led to a better rated decision making experience. The value of these techniques, therefore, lies in managing individuals’ attentional resources more effectively by prompting evaluation and assimilation of information. 52
A particular strength of this study was using measures to describe both the information strategies people employed to make the decision and their perception of the decision experience. By having these measures, we ascertained that, as hypothesized, information provides knowledge about the decision problem only, implicit techniques enable evaluation of attributes only, and explicit techniques enable evaluation of attributes and assimilation with existing beliefs. In addition, we found that deliberation and assimilation of information during decision making was associated with lower decisional conflict and felt ambivalence. In other words consciously attending to and evaluating the positive and negative attributes of the decision options during decision making reduces feelings of conflict and ambivalence about the decision post‐choice, i.e. facilitating informed decision making is associated with a better perceived decision experience.
This method meant our sample size was small and lacked the power to detect possible group differences such as those in initial preference and knowledge of people with cancer. In addition, participants were women making a hypothetical rather than a real‐world choice. However, there are some reasons to believe that our findings may be relevant to patients making these decisions. First, the decision scenario we used was a choice patients with cancer were making between standard treatment and trial participation in our local NHS Trust. Second, the decision aid component parts were developed with reference to real‐world patient decision aids. 8 , 11 , 19 Third, most women who participated had had or knew someone with cancer suggesting an understanding of the health area. Fourth, although people’s values and preferences are labile, 53 the strategies they employ to process information and make decisions are the same as everyone else. 54
This study provides evidence for a proof of concept for the effectiveness of values clarification techniques in decision aids on people’s decision making. Like the evidence from a phase 2 trial of a biomedical intervention, the next level of evidence could be elicited by carrying out this study in a sample of patients who have had cancer and/or are making a decision about trial participation. We hypothesize that although trial information will lead to patients making informed choices, the use of an explicit values clarification technique within a decision aid will help patients make more informed decisions and improve their decision experience. Despite the findings from this study, a body of evidence is still needed explaining the role of the component parts of decision aids in facilitating patient decision making. For example, in this decision scenario, choosing between standard treatment and taking part in a trial, an explicit values clarification technique was necessary to assimilate the decision attributes with existing values. It may be that for simpler decisions, such as choosing between different treatment options and/or less serious consequences, an implicit values clarification technique is sufficient. 55
Source of funding
UK Overseas Research Scholarship.
Conflict of interest
None.
Supporting information
Appendix S1. Breast cancer treatment clinical trial scenario.
Appendix S2. The explicit and implicit values clarification tasks.
Supporting info item
Paper presented at the 5th International Shared Decision Making Conference, Boston, June 2009.
References
- 1. Edwards W, Fasolo B. Decision technology. Annual Review of Psychology, 2001; 52: 581–606. [DOI] [PubMed] [Google Scholar]
- 2. Bekker H, Thornton JG, Airey CM et al. Informed decision making: an annotated bibliography and systematic review. Health Technology Assessment, 1999; 3: 1–156. [PubMed] [Google Scholar]
- 3. O’Connor AM, Stacey D, Entwistle V et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 2005; 2: CD001431. [DOI] [PubMed] [Google Scholar]
- 4. Baron J. Thinking and Deciding, 3rd edn Cambridge: Cambridge University Press, 2000. [Google Scholar]
- 5. Frisch D, Clemen R. Beyond expected utility theory: rethinking behavioural decision research. Psychological Bulletin, 1994; 116: 46–54. [DOI] [PubMed] [Google Scholar]
- 6. Janis IL, Mann L. Decision Making: A Psychological Analysis of Conflict, Choice, and Commitment. London: Free Press; Collier Macmillan, 1977. [Google Scholar]
- 7. Elwyn G, Edwards A, Kinnersley P, Grol R. Shared decision making and the concept of equipoise: the competences of involving patients in healthcare choices. British Journal of General Practice, 2000; 50: 892–899. [PMC free article] [PubMed] [Google Scholar]
- 8. Bekker HL, Hewison J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial. Prenatal Diagnosis, 2004; 24: 265–275. [DOI] [PubMed] [Google Scholar]
- 9. Declaration of Helsinki . Declaration of Helsinki: ethical principles for medical research involving human subjects. 1964.
- 10. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. British Medical Journal, 2008; 337: 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Connor A, Edwards A. The role of decision aids in promoting evidence‐based patient choice In: Edwards A, Elwyn G. (eds) Shared Decision Making In Health Care: Achieving Evidence‐Based Patient Choice. Oxford: Oxford University Press, 2009: 2. [Google Scholar]
- 12. Feldman‐Stewart D, Brennenstuhl S, Brundage MD, Roques T. An explicit values clarification task: development and validation. Patient Education and Counseling, 2006; 63: 350–356. [DOI] [PubMed] [Google Scholar]
- 13. O’Connor A, Wells G, Tugwell P, Laupacis A, Elmslie T, Drake E. The effects of an ‘explicit’ values clarification exercise in a woman’s decision aid regarding postmenopausal hormone therapy. Health Expectations, 1999; 2: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan M, Scott D, Reeves C et al. Eliciting public preferences for healthcare: a systematic review of techniques. Health Technology Assessment, 2001; 5: 1–186. [DOI] [PubMed] [Google Scholar]
- 15. Stiggelbout AM, de Haes JCJM. Patient preference for cancer therapy: an overview of measurement approaches. Journal of Clinical Oncology, 2001; 19: 220–230. [DOI] [PubMed] [Google Scholar]
- 16. Llewellyn‐Thomas H. Values clarification In: Edwards A, Elwyn G. (eds) Shared Decision Making In Health Care: Achieving Evidence‐Based Patient Choice. Oxford: Oxford University Press, 2009: 123–134. [Google Scholar]
- 17. Elit LM, Levine MN, Gafni A et al. Patients’ preferences for therapy in advanced epithelial ovarian cancer: development, testing, and application of a bedside decision instrument. Gynecologic Oncology, 1996; 62: 329–335. [DOI] [PubMed] [Google Scholar]
- 18. Llewellyn‐Thomas H, Williams I, Levy L, Naylor C. Using a trade‐off technique to assess patients’ treatment preferences for benign prostatic hyperplasia. Medical Decision Making, 1996; 16: 262–272. [DOI] [PubMed] [Google Scholar]
- 19. Bekker H, Hewison J, Thornton J. Understanding why decision aids work: linking process with outcome. Patient Education and Counseling, 2003; 50: 323–329. [DOI] [PubMed] [Google Scholar]
- 20. Feldman‐Stewart D, Brundage M, Van Manen L, Svenson O. Patient‐focussed decision‐making in early‐stage prostate cancer: insights from a cognitively based decision aid. Health Expectations, 2004; 7: 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bekker H. Using decision making theory to inform clinical practice In: Edwards A, Elwyn G. (eds) Evidence‐Based Patient Choice: Inevitable or Impossible? 2nd edn Oxford: Oxford University Press, 2009: 45–52. [Google Scholar]
- 22. Feldman‐Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Education and Counseling, 2004; 54: 265–273. [DOI] [PubMed] [Google Scholar]
- 23. Charles C, Gafni A, Whelan T, O’Brien M. Treatment decision aids: conceptual issues and future directions. Health Expectations, 2005; 8: 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frisch D, Jones S. Assessing the accuracy of decisions. Theory & Psychology, 1993; 3: 115–135. [Google Scholar]
- 25. Acker F. New findings on unconscious versus conscious thought in decision making: additional empirical data and meta‐analysis. Judgement and Decision Making, 2008; 3: 292–303. [Google Scholar]
- 26. Gigerenzer G. Gut Feelings: Short Cuts to Better Decision Making. London: Penguin, 2008. [Google Scholar]
- 27. Ericsson A. Valid and non‐reactive verbalization of thoughts during performance of tasks: towards a solution to the central problems of introspection as a source of scientific data. Journal of Consciousness Studies, 2003; 10: 1–18. [Google Scholar]
- 28. Ericsson K, Simon HA. Verbal reports as data. Psychological Review, 1980; 87: 215–251. [Google Scholar]
- 29. Payne JW, Bettman JR, Johnson EJ. The Adaptive Decision Maker. Cambridge: Cambridge University Press, 1993. [Google Scholar]
- 30. Smead R, Wilcox J, Wilkes R. How valid are product descriptions and protocols in choice experiments? Journal of Consumer Research, 1981; 8: 37–42. [Google Scholar]
- 31. Svenson O. Process descriptions of decision making. Organizational Behavior and Human Performance, 1979; 23: 86–112. [Google Scholar]
- 32. Gilhooly K, Green C. Protocol analysis: theoretical background In: Richardson T. (ed) Handbook of Qualitative Research Methods for Psychology and the Social Sciences. Leicester: British Psychological Society, 1996: 43–54. [Google Scholar]
- 33. Kuusela H, Paul P. A comparison of concurrent and retrospective verbal protocol analysis. The American Journal of Psychology, 2000; 113: 387–404. [PubMed] [Google Scholar]
- 34. Ford JK, Schmitt N, Schechtman SL, Hults BM, Doherty ML. Process tracing methods: contributions, problems, and neglected research questions. Organizational Behaviour and Human Decision Processes, 1989; 43: 75–117. [Google Scholar]
- 35. Ellis P, Barrett‐Lee P, Johnson L et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open‐label, phase III, randomised controlled trial. The Lancet, 2009; 373: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hughes R. Considering the vignette technique and its application to a study of drug injecting and HIV risk and safer behaviour. Sociology of Health and Illness, 1998; 20: 381–400. [Google Scholar]
- 37. Hughes R, Huby M. The application of vignettes in social and nursing research. Journal of Advanced Nursing, 2002; 37: 382–386. [DOI] [PubMed] [Google Scholar]
- 38. Flesch R. A new readability yardstick. Journal of Applied Psychology, 1948; 32: 221–233. [DOI] [PubMed] [Google Scholar]
- 39. Priester JR, Petty RE. The gradual threshold model of ambivalence: relating the positive and negative bases of attitudes to subjective ambivalence. Journal of Personality & Social Psychology, 1996; 71: 431–449. [DOI] [PubMed] [Google Scholar]
- 40. Kaplan KJ. On the ambivalence‐indifference problem in attitude theory and measurement: a suggested modification of the semantic differential technique. Psychological Bulletin, 1972; 77: 361–372. [Google Scholar]
- 41. Thompson M, Zanna M, Griffin D. Let’s not be indifferent about (attitudinal) ambivalence In: Petty KE, Krosnick JA. (eds) Attitude Strength: Antecedents and Consequences. Hillsdale, NJ: Lawrence Erlbaum, 1995: 361–386. [Google Scholar]
- 42. O’Connor AM. Validation of a decisional conflict scale. Medical Decision Making, 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 43. Field A. Discovering Statistics Using SPSS, 2nd edn London: Sage, 2005. [Google Scholar]
- 44. Newby‐Clark I, McGregor I, Zanna M. Thinking and caring about cognitive inconsistency: when and for whom does attitudinal ambivalence feel uncomfortable? Journal of Personality & Social Psychology, 2002; 82: 157–166. [PubMed] [Google Scholar]
- 45. Fagerlin A. Getting down to details in the design and use of decision aids. Medical Decision Making, 2009; 29: 409–411. [DOI] [PubMed] [Google Scholar]
- 46. O’Connor A, Rostom A, Fiset V et al. Decision aids for patients facing health treatment or screening decisions: systematic review. British Medical Journal, 1999; 319: 731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Payne JW, Braunstein ML, Carroll JS. Exploring predecisional behavior: an alternative approach to decision research. Organizational Behaviour and Human Performance, 1978; 22: 17–44. [Google Scholar]
- 48. Crutcher R. Telling what we know: the use of verbal report methodologies in psychological research. Psychological Science, 1994; 5: 241–244. [Google Scholar]
- 49. De Neys W, Glumicic T. Conflict monitoring in dual process theories of thinking. Cognition, 2008; 106: 1248–1299. [DOI] [PubMed] [Google Scholar]
- 50. White P. Limitations on verbal reports of internal events: a refutation of Nisbett and Wilson and of Bem. Psychological Review, 1980; 87: 105–112. [Google Scholar]
- 51. Kant I. Critique of pure reason. 1781.
- 52. Payne J, Bettman J. Walking with the scarecrow: the information‐processing approach to decision research In: Koehler D, Harvey N. (eds) Blackwell Handbook of Judgment and Decision Making. Oxford: Blackwell Publication, 2004: 110–132. [Google Scholar]
- 53. Jansen SJ, Stiggelbout AM, Wakker P, Nooji M, Evert M, Kievit J. Unstable preferences: a shift in valuation or an effect of the elicitation procedure? Medical Decision Making, 2000; 20: 62–71. [DOI] [PubMed] [Google Scholar]
- 54. Kuhberger A, Schulte‐Mecklenbeck M, Perner J. Framing decisions: hypothetical and real. Organizational Behavior and Human Decision Processes, 2002; 89: 1162–1175. [DOI] [PubMed] [Google Scholar]
- 55. Wong S, Thornton JG, Gbolade B, Bekker HL. A randomised controlled trial of a decision‐aid leaflet to facilitate women’s choice between pregnancy termination methods. BJOG: An International Journal of Obstetrics and Gynaecology, 2006; 113: 688–694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Breast cancer treatment clinical trial scenario.
Appendix S2. The explicit and implicit values clarification tasks.
Supporting info item


