Abstract
Striae distensae (SD) are common dermal lesions, with significant physical and psychological impact. Many therapeutic modalities are available but none can completely eradicate SD. The most common therapy is the application of topicals used both therapeutically and prophylactically. Even though there are many commercially available topical products, not all have sufficient level of evidence to support their continued use in SD. The aim here was to assess the evidence for the use of topicals in SD and to propose a structured approach in managing SD. A systematic search of published literature and manufacturer website information for topicals in SD was carried out. The results showed that there are few studies (n = 11) which investigate the efficacy of topicals in management of SD. Trofolastin and Alphastria creams demonstrated level‐2 evidence of positive results for their prophylactic use in SD. Additionally, tretinoin used therapeutically showed varying results whilst cocoa butter and olive oil did not demonstrate any effect. Overall, there is a distinct lack of evidence for each topical formulation. The majority of topicals failed to mention their effect on early vs. later stages of SD (striae rubrae compared to striae albae) and their role in both prevention and treatment. In conclusion, there is no topical formulation, which is shown to be most effective in eradicating or improving SD. A structured approach in identification and targeted management of symptoms and signs with the appropriate topical is required. Randomized controlled trials are necessary to assess the efficacy of topical products for treatment and prevention of different stages of SD.
Introduction
Striae distensae (SD) or stretch marks are common dermal lesions which arise due to the stretching of the dermis.1 There are two forms of SD; striae rubrae and striae albae.2 The acute stage (striae rubrae) is characterized by the initial erythematous, red and stretched flat (in some cases appear slightly raised) lesions which are aligned perpendicular to the direction of skin tension and can be symptomatic, whilst the chronic stage (striae albae) is classified when SD have faded and appear atrophic, wrinkled, and hypopigmented (Fig. 1).2, 3, 4
Figure 1.
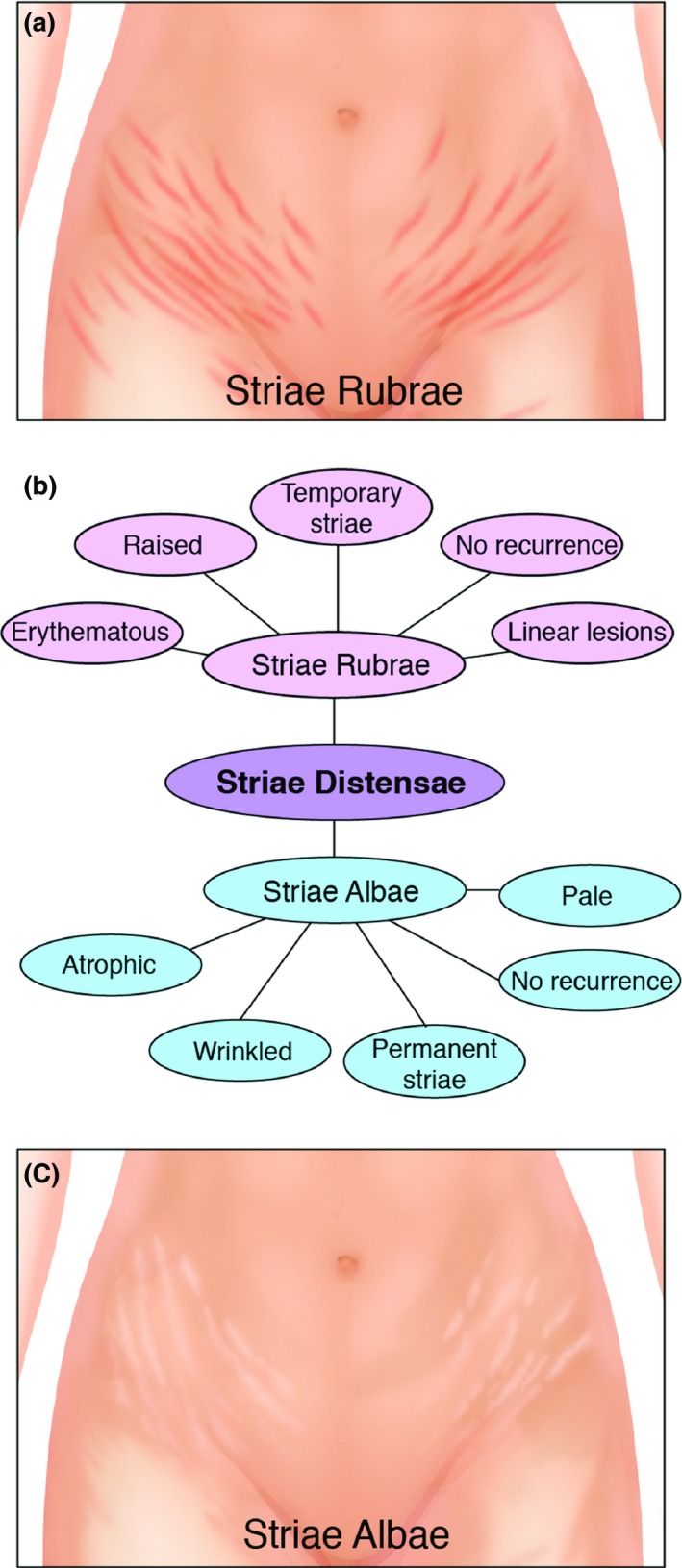
Comparisons between striae albae and striae rubrae. (a) An illustration of striae rubrae on the abdomen. (b) A diagram to demonstrate the difference in characteristics between striae rubrae and striae albae. Striae rubrae are considered as an early form of SD, which are erythematous, red and sometimes slightly raised linear lesions. They do not recur and are classified as temporary striae. Striae albae are atrophic, wrinkled and pale. They also do not recur but are permanent striae. (c) An illustration of striae albae on the abdomen.
There are a number of pathological and histological changes which occur when SD are formed (Fig. 2). Elastolysis of the mid‐dermis is evident due to mast cell degranulation and stimulation of macrophages.5 Other observations include perivascular lymphocytic cuffing, increased glycosaminoglycan, sporadic presence of lymphocytes and oedema in the dermis.6, 7, 8 Gradual atrophy of the epidermis has been noted including loss of rete ridges.7 Vascular changes occur which contribute to the red and erythematous appearance of striae rubrae.9 Additionally, in striae rubrae, collagen fibres become thicker, more densely packed, are arranged in a parallel pattern and show a reduction in elastic fibres.6, 10 Conversely, striae albae have less vascularity and tend to be very pale in colour.11 Striae albae have been described as appearing similar to mature and flattened, or stretched dermal scars.6, 12, 13
Figure 2.
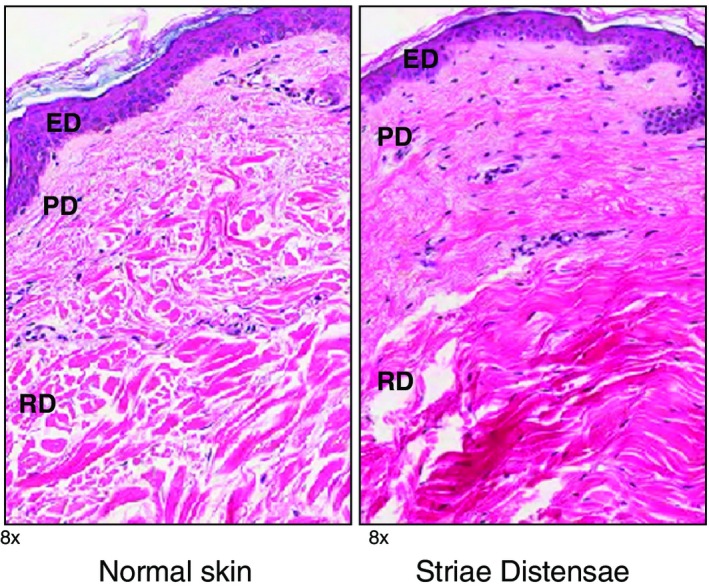
Histological comparison between normal skin and striae distensae (SD) skin H+E stains (magnification ×8.0). The normal epidermis has basket weave appearance and well formed rete ridges. In contrast, SD shows loss of the rete ridge pattern. Additionally, normal dermis demonstrates parallel collagen bundles to the surface, which are evenly spaced, which is in contrast to SD dermis. ED, epidermis; PD, papillary dermis; RD, reticular dermis.
The reported prevalence of SD has been variable in the literature with figures ranging from 11–88%.14, 15, 16 SD severity has been noted to be worse in Black African women compared to Caucasians, within the same geographical region.17, 18 The vast majority of SD has been reported in pregnant women and adolescents. They are associated with Cushings syndrome and chronic steroid use (Fig. 3).17 The causes of SD are proposed to be due to the mechanical effect of tissue stretching;19 from growth in adolescence and rapid increase in the size of certain locations of the body.20, 21 The most common anatomical locations which are affected by SD are the abdomen, breasts, buttocks and thighs (Fig. 4).22 A study by Yamaguchi et al.23 utilized a quality of life questionnaire for female patients with and without SD and demonstrated that those with significant SD had higher scores.23
Figure 3.
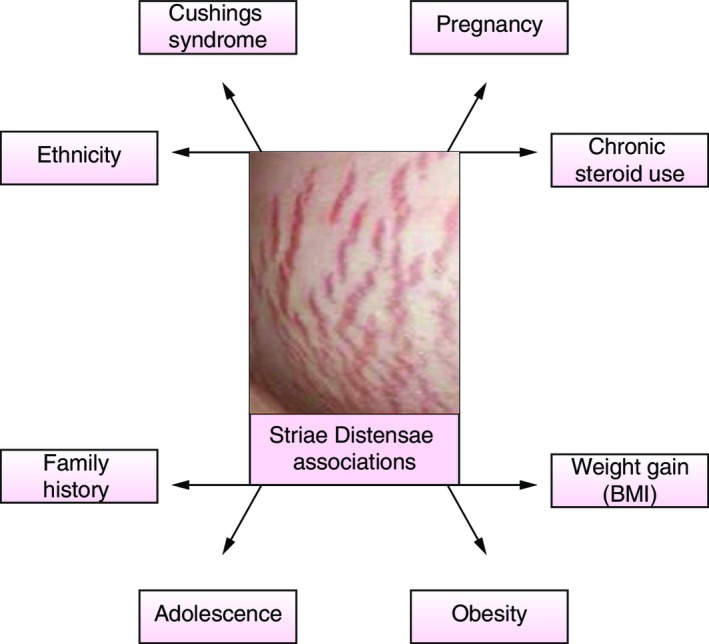
Factors associated with striae distensae. Risk factors and associations with acquiring striae distensae including ethnicity,18 chronic steroid use,17 pregnancy,59 Cushings syndrome,60 weight gain (BMI),61 obesity,17 adolescence,17 family history.62
Figure 4.
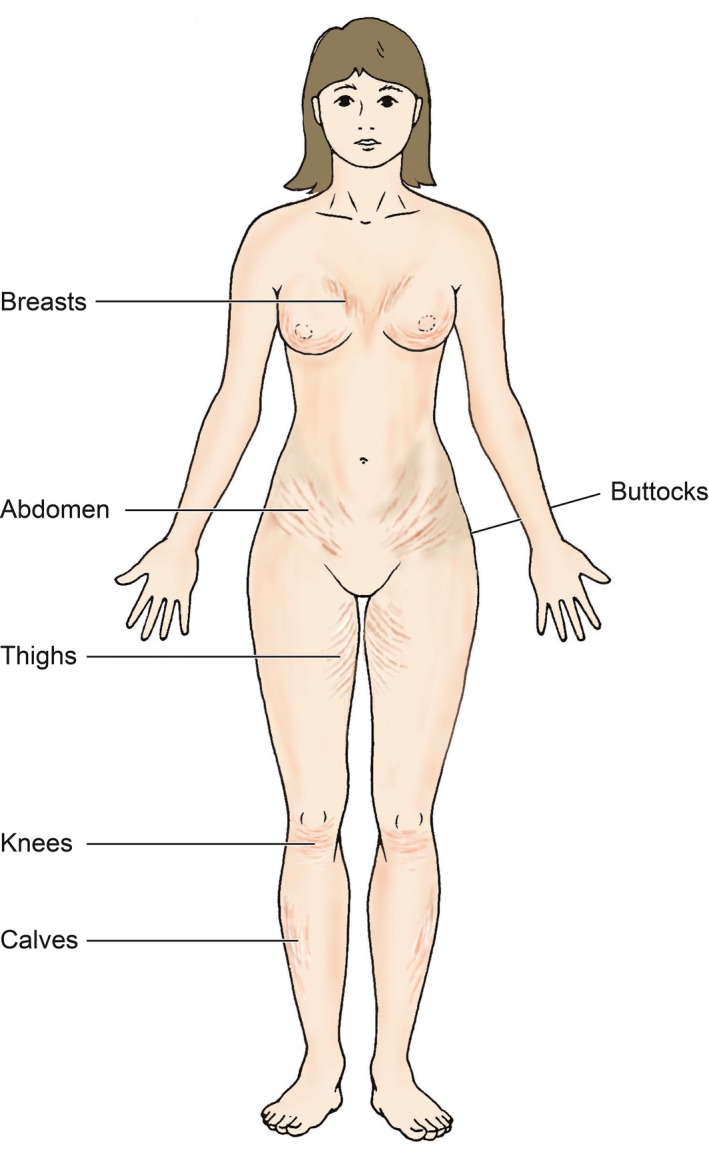
Illustration demonstrating the common anatomical locations affected by striae distensae.
Striae distensae have presented a considerable challenge in terms of both their evaluation and treatment. There are variable responses to therapies, with the main aims being reduction in symptoms and improvement in appearance. Various treatment modalities exist which aim to treat or prevent SD such as laser therapy,24, 25, 26 light therapy,27 acid peel treatments,28 collagen injection,29 laser lipolysis,30 radiofrequency techniques31 and microdermabrasion.32 No single therapy has been advocated to completely eradicate these lesions.
The most common method for treating SD is the use of topicals.33 Only a limited number of topical agents have been evaluated in formal studies for the treatment of SD (such as tretinoin,34 Trofolastin,35 Alphastria,36 cocoa butter,37 olive oil38 and silicone gel39), although, there are many topical products, available commercially on the market, which claim benefits for prevention and/or treatment of SD, which have not been formally evaluated in any clinical study.40
The use of topical therapies aims to provide lasting improvements in pigmentation and texture of both striae rubrae and albae, with minimal side‐effects, in patients of all skin types.41 However, in order to improve the appearance and reduce the symptoms associated with SD, the following processes should occur; increased collagen production and fibroblastic activity, increase in elasticity and blood perfusion, improvement in cell proliferation, increased skin hydration and anti‐inflammatory properties.35, 37, 42
It is important to assess the evidence available for the use of these topical formulations. Overall, there has been insufficient clinical evaluation of these products and many studies have failed to demonstrate efficacy. Indeed, no single treatment or management plan has been advocated for SD and the literature provides no gold standard therapy; all hampered by a paucity of level 1 evidence. The aim of this paper is to evaluate the available evidence for the use of topical treatments for the management of SD and to propose a structured management approach.
Materials and method
A thorough search of information on topical products for SD on the manufacturers websites was carried out. Additionally, a systematic search of published literature was carried out, using PubMed, Scopus and Embase, on commonly available topical products for management of SD. All relevant articles published in the English language, from 1980 onwards and in humans were utilized. Keywords, used in the search are outlined in Fig. 5. All retrieved articles were reviewed for their relevance and 11 were considered suitable for inclusion in this review. Each study was assigned a level of evidence (LOE) (outlined in the summary Table 1): LOE 1 = randomized control trial; LOE‐2 = cohort study; LOE‐3 = case–control study; LOE‐4 = Case series study; LOE‐5 = expert opinion or case report.
Figure 5.
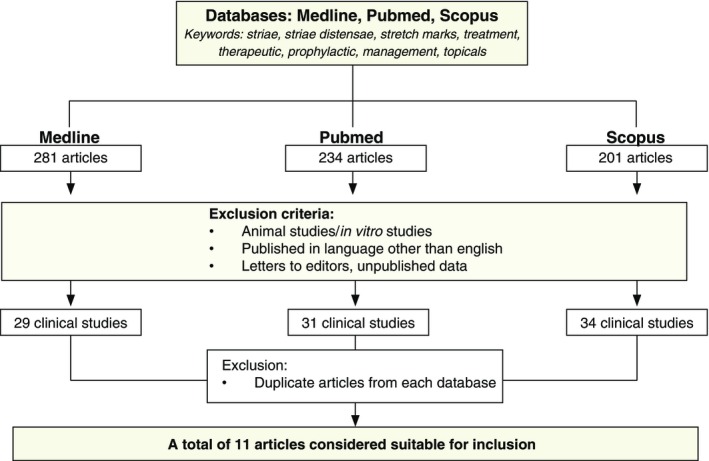
A flow chart demonstrating the methodology and process of selecting relevant articles for review.
Table 1.
A table summarizing the studies available for topical treatment for striae distensae and the levels of evidence for each study
| Author | Treatment | Type of treatment | Type of striae | Dosage | No. of subjects | Outcome | Adverse effects | Study design | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|
| Kang45 | Tretinoin vs. placebo | Therapeutic | Rubrae | 0.1%, Daily, 6 months | 22 | Reduced length and width of SD with tretinoin. No significant difference in dermal collagen and elastic fibres in treatment compared to placebo. | Erythema or scaling first 2 months (55%) | RCT | 2 |
| Rangel34 | Tretinoin | Therapeutic | Not stated | 0.1%, Daily, 3 months | 20 | 20% reduction in length of SD. Significant improvement from baseline. No control and norandomization. | Erythema or scaling in first month (55%) | Case series | 4 |
| Mallol35 | Trofolastin (Centella Asiatica) vs. placebo | Prophylactic | Not stated | Daily, 12th week pregnancy until labour | 80 | In women with a history of SD during puberty, the treatment cream gave a significant absolute prevention of SD during pregnancy compared with the placebo (89%). | None stated | RCT | 2 |
| Ud‐Din39 | Silicone vs. placebo | Therapeutic | Not stated | Daily for 6 weeks | 20 | Increased melanin, decreased haemoglobin, collagen and pliability with both gels. Collagen levels were significantly higher and melanin lower with silicone gel compared to controls. | None stated | RCT | 2 |
| de Buman36 | Alphastria vs. placebo | Prophylactic | Not stated | Unknown | 90 | 10% in prophylactically treated compared to 70% in placebo treatment developed SD. | None stated | RCT | 2 |
| Osman37 | Cocoa butter vs. placebo | Prophylactic | Not stated | Daily, 12–18 weeks gestation until delivery | 175 | No significant difference in development of SD between groups. | None stated | RCT | 2 |
| Buchanan52 | Cocoa butter vs. placebo | Prophylactic | Not stated | Daily, 12–15 weeks gestation until delivery | 300 | No significant difference in development of SD. | None stated | RCT | 2 |
| Taavoni38 | Olive oil | Prophylactic | Not stated | Twice daily, 18–20 weeks gestation, for 8 weeks | 70 | No significant difference in development of SD between treatment and control groups. | None stated | RCT | 2 |
| Timur53 | Almond oil with massage vs. almond oil alone vs. control | Prophylactic | Not stated | Cream very other day, 19–32nd week gestation | 141 | Significant difference in frequency of SD between groups: almond oil and massage 20%, almond oil alone 38.8%, control 41.2%. | None stated | Non‐randomized comparative study | 3 |
| Pribanich46 | Tretinoin | Therapeutic | Not stated | 0.025% daily for 7 months | 11 | No difference between treatment and controls. | None stated | Double‐blind controlled study | 2 |
| Hexsel47 | Tretinoin vs. dermabrasion | Therapeutic | Rubrae | 0.05% daily for 16 weeks | 32 | Both treatments effective but no difference between treatments. | Pruritus, erythema, scaling | Prospective randomized open‐label | 2 |
Results
There are a number of topical products available for the treatment of SD. These products are now discussed, categorized by their mechanisms of action, including stimulation of collagen production, increasing elasticity, improving cell proliferation, anti‐inflammatory properties and rehydration (Tables 1 and 2).
Table 2.
A list of some of the known active ingredients contained in the topical products used for striae distensae and the evidence associated with these ingredients either directly associated with striae distensae or indirectly associated with another condition. References can be found in Supplementary Material
| Name | Description | Direct association – Proposed action on striae distensae | Indirect association | Indirect association ‐Proposed action on conditions other than striae distensae | Evidence (Reference no.) |
|---|---|---|---|---|---|
| Almond oil | An oil expressed from bitter almonds, rich in vitamin D and E | Maintain skin elasticity and hydration | N/A | N/A | 26 |
| Centella Asiatica extract | A small herbaceous plant of the Mackinlayaceae family native to the wetlands in Asia | Prevents progression and appearance of SD, stimulates cell production and fibroblasts in SD. | Human fibroblast cells, human dermal fibroblasts | Stimulatory effect on extracellular matrix synthesis of collagen and fibronectin | 3, 4, 5, 6 |
| Chamomile | A European plant of the daisy family | N/A | Human skin | Reduction in skin itch and irritation | 43 |
| Cocoa butter | A fatty substance obtained from cocoa beans | Reduce hyperpigmentation and moisturizing effects although no significant effects on SD noted | N/A | N/A | 28, 29 |
| Coconut oil | A fatty oil obtained from the flesh of the coconut | N/A | Atopic dermatitis | Anti‐inflammatory properties, increase in skin capacitance in human, rats | 13, 14 |
| Collagen‐elastin hydrolysates | A combination of collagen and elastin by breaking down the molecular bonds | N/A | Biomaterials for tissue regeneration, rabbit skin | Promotes cell adhesion | 8, 9 |
| Crowberry | A fruit which contains mostly water and is a species of empetrum (an evergreen plant) | N/A | Anthocyanins in crowberry | Strong antioxidant properties | 10 |
| Darutoside | A botanical ingredient from the plant siegesbeckia orientalis | Proposed to improve skin elasticity and more regular alignment of collagen matrix | N/A | N/A | No studies found |
| Glycine soja | A plant in the legume family which contains significant amounts of amino acids and proteins | N/A | Porcine pancreatic elastase and human leucocyte elastase and human study comparing anti‐wrinkle cosmetics | Stimulates collagen production | 23, 24 |
| Hepapeptide‐7 | A synthetic peptide consisting of arginine, asparagine, glycine, isoleucine and methionine | N/A | Menopausal skin | Promotes keratinocyte proliferation | 31 |
| Hyaluronic acid | A viscous fluid carbohydrate present in connective tissue and synovial fluid | N/A | Facial dermatitis | Hydration and anti‐inflammatory effects | 35, 36 |
| Licorice extract | Extracted from the root of the licorice plant | N/A | Human skin | Helps to reduce pigmentation although can cause leucoderma effects | 32 |
| Lupin seed extract | A genus of flowering plant in the legume family | N/A | Rats | No beneficial effects | 2 |
| Marine collagen | This is a fibrous protein extracted from the scales of saltwater fish | N/A | Human skin | Stimulates skin cells and improves extra‐cellular matrixand photo ageing | 37, 38 |
| Marine elastin | This is obtained by hydrolysed enzymatic from connective tissue of some fish species | N/A | Human skin | Improves elasticity and photo ageing effects | 37, 38 |
| Meadowfoam seed oil | An edible seed oil, extracted from the seeds of meadowfoam (Seeds contain 20–30% oil and contains over 98% long chain fatty acids) | N/A | Activated meadowfoam seed oil | Potential uses as a bioherbicide | 30 |
| NIA‐114™ molecule | A molecule which delivers Niacin (Vitamin B3) (a water‐soluble micronutrient) to skin cells | N/A | Actinic keratosis | Stimulates DNA repair, promotes release of leptin, stimulates a receptor to decrease hyperpigmentation | 1 |
| Olive oil | An oil obtained from olives | Rich in vitamin E and softens the skin, although clinical trials demonstrate no effect. | N/A | N/A | 11, 12 |
| Palm oil | An oil from the fruit of the West African oil palm | N/A | Human malignant melanoma | Suppresses melanoma cell proliferation | 15 |
| Registril | A combination of Phaseolus lunatus (green bean extract) and rutin (from fruits and berries) | Proposed to stimulate production of fibroblasts and collagen | N/A | N/A | No studies found |
| Resveratrol | A polyphenol compound found in certain plants and has antioxidant properties | N/A | Human keratinocytes and guinea pig skin | Inhibits keratinocyte proliferation | 33, 34 |
| Retinyl palmitate | An ester of retinol and palmitic acid and is considered to be a milder form of vitamin A | N/A | Skin ageing | Increases cell production and enhances skin metabolic rate | 22 |
| Shea butter | A fatty substance obtained from the nuts of a shea tree | N/A | Keloid fibroblasts | Reduces actively proliferating keloid fibroblasts | 27 |
| Siegesbeckia | A species of plant native to eastern Asia used in traditional medicine | N/A | Siegesbeckia pubescens and wound healing properties in mice | Increases rate of wound contraction and anti‐inflammatory agent | 16, 17 |
| Silicon dioxide | A chemical compound which is a dioxide of silicone (a hard, unreactive, colourless compound) | Hydration and oxygen transmission | Scars | Hydration and oxygen transmission | 25 |
| Tamanu oil | An oil which is pressed from nuts which yield 70–75% of inedible oil | N/A | Human skin – contact dermatitis | Anti‐inflammatory and antioxidant properties | 39 |
| Tocopherol acetate | An ester of acetic acid and tocopherol (vitamin E) | N/A | Human cadaveric skin | Contains antioxidant activity and non‐irritant to skin | 7 |
| Tretinoin | A preparation of retinoic acid | Increases collagen production | N/A | N/A | 18, 19, 20, 21 |
| Vitamin B5 (Panthenol) | Pantothenic acid is a water‐soluble vitamin from the B group of vitamins | N/A | Healthy human volunteers | Promotes cell growth and differentiation | 41 |
| Vitamin E | A group of lipid‐soluble 3 compounds that include tocopherols and tocotrienols | N/A | Nanofibrous mats | Antioxidant and skin barrier stabilizer | 42 |
| Wheat germ oil | This is extracted from the germ of the wheat kernel and is high in octacosanol | N/A | Human hyperkeratotic skin conditions | Anti‐inflammatory properties | 40 |
Stimulation of collagen production
The majority of topical products claim to improve the appearance of SD by the stimulation of collagen production in order to increase skin elasticity. StriVectin‐SD® (StriVectin®, New York, USA) is a skin repair cream, which is indicated for use on SD, wrinkles and ageing skin.41 The active ingredient is the molecule NIA‐114™. It is thought to work by increasing collagen type I and IV synthesis. However, there is no published evidence for its efficacy on SD.
Another topical on the market is Cussons® Mum and Me Bump Stretch Marks Cream (PZ Cussons Ltd, Manchester, UK) which is recommended by the manufacturers for use in preventing SD. It contains Lupin Seed extract, which is used to increase collagen in the skin. It is claimed that this product is clinically proven.43 Their topical formulation was tested against 5 ml of skin treatment oil. Twenty‐eight women applied the two formulations twice daily for 12 weeks. The results showed that there was a 16% increase in elasticity of the skin using the cream, within 12 weeks. This study was briefly reported on the company website, but there are no published peer‐reviewed clinical trials.
A number of products contain the active ingredient Centella Asiatica which is suggested to stimulate cell production.35 Liforma® Stretch Mark Day Gel and Night Cream (Liforma®, Reigate, UK) is a moisturizing cream which contains Centella Asiatica and claims to prevent SD. A product comparative study was undertaken with 25 women, who completed a questionnaire.44 They reported that Liforma® scored the highest in all outcomes. However, these results were based on subjective measures.
Trofolastin has been used in a study for the prevention of SD and also contains the active ingredients; Centella asiatica, tocopherol and collagen‐elastin hydrolysates.35, 42 It is suggested that Centella asiatica stimulates fibroblasts and has an antagonistic effect against glucocorticoids.35 A randomized controlled trial compared the treatment cream to a placebo in 80 subjects.35 Their results demonstrated that 56% of subjects in the placebo group compared to 34% in the treatment group developed SD. Additionally, in women who had a history of SD during puberty, the treatment cream gave 100% prevention of SD, compared to 89% in the placebo group.
Clarins® Stretch Marks Cream (Clarins® Ltd, London, UK) is indicated for reducing the appearance of SD. The active ingredients in this product are, Crowberry and Centella Asiatica extracts, olive, coconut and palm oils and siegesbeckia. There are no clinical trials associated with this product.
Tretinoin is a topical used for SD and has been studied in four clinical trials.34, 45, 46, 47 These studies have shown that it has been used successfully in the treatment of striae rubrae and is of limited value in striae albae.34, 46 A randomized controlled trial was conducted with 22 subjects who had striae rubrae.45 It was administered at a dose of 0.1% daily for 6 months. The results showed a reduction in the length and width of SD. Adverse effects were reported, including erythema or scaling in the first 2 months. Another randomized controlled study used a dose of 0.025% of tretinoin daily for 7 months, in 11 women with SD. There was no difference compared to placebo and the topical was ineffective in improving SD. However, the small sample size could have affected this.
A case series also evaluated the therapeutic effect of tretinoin, in 20 subjects with SD (type not stated).34 Tretinoin was applied at a dose of 0.1% daily for 3 months. The findings demonstrated a reduction of 20% in the length of SD. However, there was no control in this study and no randomization. A prospective randomized, open‐label study compared the effect of topical 0.05% tretinoin cream vs. dermabrasion in the treatment of striae rubrae. They showed that both treatments were efficacious on SD but there were no differences between treatments.47
Bio‐Oil® is recommended by the manufacturers for use in helping improve the appearance of scars, SD and uneven skin tone. There have been two randomized controlled studies performed to assess the efficacy of Bio‐Oil® in improving the appearance of SD.48, 49 However, these studies were only reported on the company website and the full details and results of the studies are not available. The first, a single‐blinded, randomized controlled trial, demonstrated that 50% of subjects had improvement in appearance of their SD at 8 weeks.48 As this study was only single blinded, this could have led to a positive bias. Additionally, the magnitude of the change recorded was small and there was a small sample size.
The second double‐blinded, randomized and placebo controlled trial involved 38 subjects of various ethnicities, different causes of SD and different locations of SD.49 The results demonstrated improvement in the appearance of their SD up to 8 weeks.
Kelo‐Cote® (Sinclair Pharma plc, London, UK) is a silicone gel, which has been recommended for use on atrophic scars resulting from acne, chickenpox and SD. The website states that in clinical trials, 75% of subjects advocated the use of this topical for improving the appearance of existing SD. However, the full information regarding these trials is not available. In a double‐blinded, controlled trial, the effects of this topical formulation were compared to a placebo on SD, using non‐invasive objective measures and immunohistochemical analysis of sequential tissue biopsies.39 Both histological and clinical data demonstrated that melanin increased, whilst collagen, elastin, vascularity and pliability decreased over the 6‐week period with both gels. Furthermore, collagen levels were significantly higher with the treatment gel and melanin levels were significantly lower with the treatment gel compared to the placebo. The benefit of this topical formulation remains unknown, as the changes in the skin could have been due to the effect of massage.
Another product by the same company, named Kelo‐Stretch™ (Sinclair is Pharma plc), contains titrated Centella Asiatica extract (0.5%), sweet almond oil and shea butter. It is claimed that the scientific evidence for this product was demonstrated in a study of women using this topical twice daily. They noted a significant improvement in skin elasticity, a 10% reduction in the width of SD and 60% of subjects noted a reduction in existing SD.50 This study has not been published.
Apothederm™ Stretch Marks Cream (Apothederm™, Bothwell, WA, USA) claims to strengthen the skin and repair existing SD, by increasing collagen levels and reducing the appearance of striae rubrae and striae albae. The active ingredients include natural emollients: shea butter, cocoa butter, olive oil and meadowfoam seed oil. A dermatologist, used this product on subjects with SD and demonstrated positive results.51 This study has not been published.
Skinception™ Intensive Stretch Marks Therapy Cream (Skinception™, Johnson City, Tennessee, USA) contains active ingredients (Regestril, Darutoside, Pro‐Col‐One and Pro‐Sveltyl) to promote the production of collagen. It is claimed that this product is clinically proven, although no information is published.
Alphastria has been used prophylactically for the prevention of SD.36 It contains the active ingredient hyaluronic acid, which is suggested to stimulate fibroblast activity and the production of collagen.36 One randomized controlled study demonstrated that 10% of women in the treated group compared with 70% in the placebo group developed SD.36
Improve cell proliferation
Two topical products suggest their mechanism of action on SD is by improving cell proliferation. Thalgo® Stretch Marks Cream (Thalgo UK Ltd, London, UK) aims to prevent SD formation and diminish existing SD. The active ingredients are; Marine Elastin and Marine Collagen, Centella Asiatica Plant Extract, Tamanu Oil, Wheat Germ Oil. There is no clinical evidence for the effectiveness of this topical formulation for the treatment and prevention of SD. However, this product contains the active ingredient, Centella Asiatica, which has been evaluated in a study that was discussed previously. Furthermore, one of the active ingredients in Liforma® Stretch Marks Cream is Vitamin B5 (Panthenol) which is suggested to improve cell production in order to diminish the appearance of SD.
Anti‐inflammatory properties
Two topical formulations use active ingredients, which they claim can reduce redness in SD, thereby displaying anti‐inflammatory properties. SilDerm™ Stretch Mark Repair Cream (SilDerm™ Ltd, Cheshire, UK) contains active ingredients Registril and Darutoside and is recommended by the manufacturer in treating existing SD. However, there is no description of how this product was formally tested and evaluated. Additionally, there is no published peer‐reviewed research on this topical product. Liforma® Stretch Marks Cream also contains Vitamin E and chamomile which act as anti‐inflammatories.
Rehydration
Palmers® Cocoa Butter cream is used for prevention and to reduce the appearance of SD by maintaining epidermis elasticity and rehydration of the skin. This topical is made from cocoa butter and vitamin E, elastin and collagen. There have been two randomized controlled trials, which have investigated the effects of cocoa butter vs. a placebo, for the prophylactic treatment of SD.37, 52 Both studies found that there were no significant differences between the creams.37, 52
Other topical products
Various oils have been used in the prevention of SD, some of which have been evaluated in the literature. Taavoni et al.38 performed a randomized controlled trial, using olive oil (type not specified).38 There were no significant differences noted overall between the groups. However, a non‐randomized, comparative study investigated the effect of almond oil in the prevention of SD.53 They noted significant differences in the frequency of SD between the groups (almond oil and massage 20%, almond oil alone 38.8%, control 41.2%).
TriLASTIN‐SR® (TriLASTIN‐SR®, Chadds Ford, Pennsylvania, USA) is indicated for use on red, white and silver SD and for the prevention of SD. There is no available published research for this product. RegimA® Scar Repair and Anti‐Stretch Complex (RegimA®, Gauteng, South Africa) is a topical product, which is used for both skin scars and SD in particular striae rubrae. There is no published research evidence found to support the use of this product for SD.
Discussion
There are a number of topical products used either therapeutically or prophylactically for the management of SD. There are several mechanisms of action reported, (none with level 1 evidence), to be associated with these topical formulations, which include the ability to stimulate collagen production, increase elasticity, improve cell proliferation, anti‐inflammatory properties as well as rehydration simultaneously.
There is a paucity of a high level of evidence reported in the literature, to support the use of topical products in the prevention of SD. Furthermore, most of the evidence suggests that there is limited proof of the efficacy of the commonly available topical preventative agents (Figs 6 and 7).54 The majority state that the product was clinically proven, however, there were few published clinical trials. The available peer‐reviewed literature demonstrated positive results (LOE‐2) for some topical treatments, such as Alphastria and Trofolastin, through randomized controlled trials with large sample sizes, although, the type of striae was not stated. Additionally, tretinoin showed varying results across three studies and demonstrated positive results on striae rubrae. One study using Kelo‐Cote® silicone gel found some equivocal differences between the placebo and treatment group, however, it was determined that the benefit of this product for SD remains unknown. Two studies using cocoa butter and one study using olive oil demonstrated that there were no significant effects to SD. The majority of topical products did not have any peer‐reviewed evidence to support their use on SD. Overall, there is limited evidence for the efficacy of topical therapy for the treatment of SD.
Figure 6.
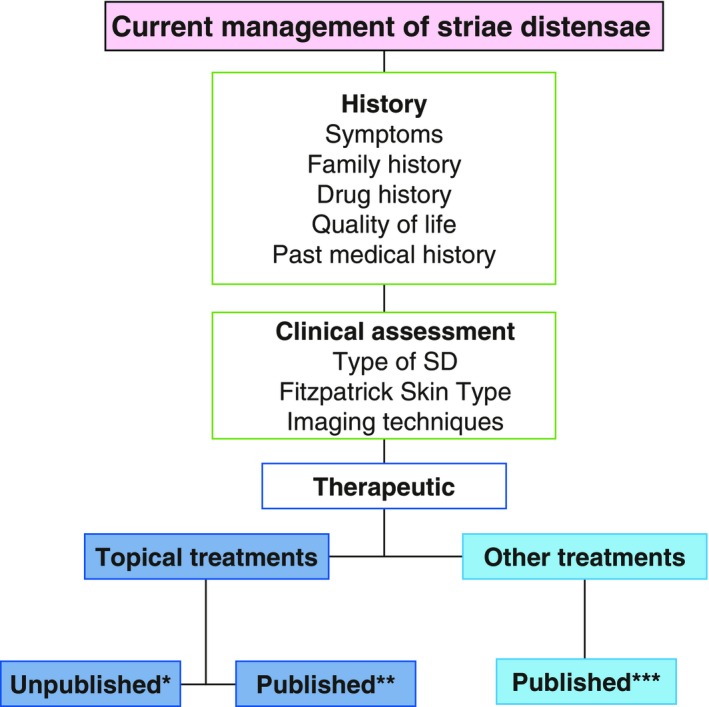
Management of striae distensae. A flow chart to summarize the management of patients with striae distensae with particular emphasis on the topical formulations, which are used therapeutically and which have published evidence to support their use. *Kelo‐Stretch™, Apothederm™, Bio‐Oil®, StriVectin‐SR ®, Clarins®, RegimA®, Thalgo®, SilDerm™, Skinception™; **Kelo‐Cote®39, Tretinoin34,43,44; ***Laser therapy24‐26, light therapy27, acid peels28, collagen injections29, radiofrequency devices31, microdermabrasion32.
Figure 7.
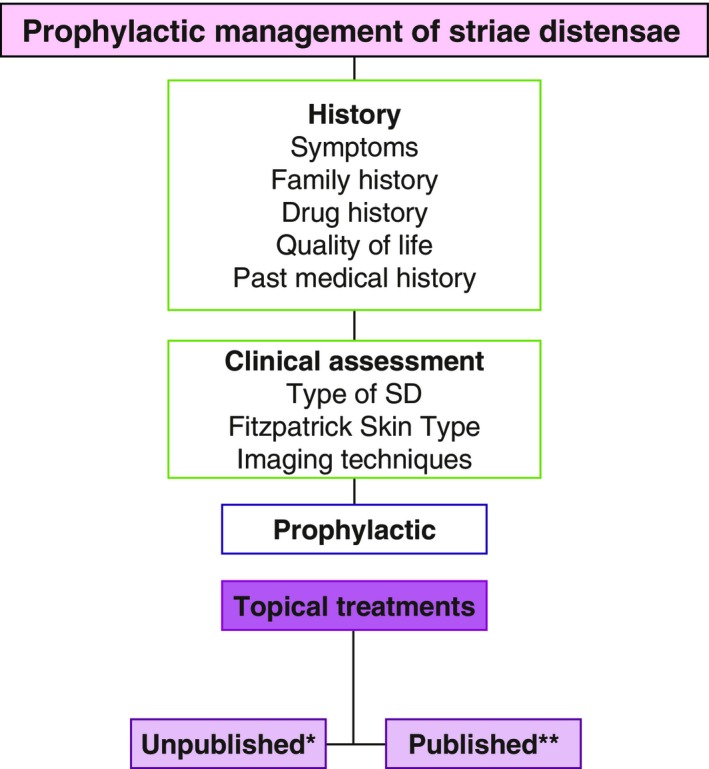
A flow chart to summarize the management of patients with striae distensae with particular emphasis on the topical formulations, which are used prophylactically and which have published evidence to support their use. *Cussons®, Liforma®, Kelo‐Cote®, Thalgo®, TriLASTIN‐SR ®, Kelo‐Stretch™; **Alphastria36, Cocoa butter37,45, Olive Oil38, Almond oil46, Trofolastin35.
Although a number of studies showed that some topical products for SD had beneficial outcomes, it was difficult to determine if the results were due to the act of massage. There is limited evidence that shows how massage affects the skin, although it is recommended that patients with scars, should massage their scars daily to improve the scar quality.55, 56
It is evident that different types of SD demonstrate variable degrees of improvement when treated by topical formulations. Some topical treatments are advocated for use on either striae rubrae or striae albae.57 However, not all topicals are beneficial for use on both types of SD. It is important to identify the pathological effects vs. the symptom relief by these topical products. Patients with striae rubrae require a reduction in pigmentation and erythema, whilst reducing the symptom of itch, which is common in early striae. Conversely, those with striae albae require an increase in pigmentation and may not have symptomatic striae. Therapeutic interventions at the initial stages may minimize structural alterations in the epidermis that occur later. It is important to identify which topical formulation targets which signs and symptoms based on the type of SD and the patient's Fitzpatrick skin type.20 A strategic approach could be useful to fully assess a patient in order to create a patient‐specific management/treatment plan (Fig. 8).58 A thorough focused, clinical assessment can allow the clinician to tailor the appropriate management strategy to an individual with SD.
Figure 8.
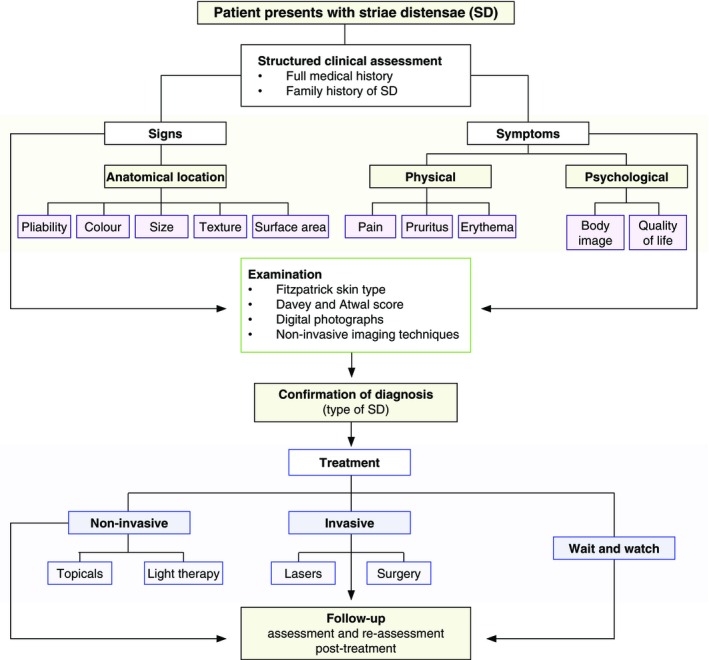
A proposed strategic approach to create a specific management/treatment plan for patients with striae distensae.
In conclusion, there are a limited number of research studies available which investigate the efficacy of different types of topical products used for this common condition. There is no topical formulation, which is most effective in eradicating or improving SD. Furthermore, there is a need for a structure to identify which topical treatments can address the patient's signs and symptoms and type of SD. Further high‐quality randomized controlled trials are necessary to assess the efficacy of topical products for the prevention and treatment of SD.
Supporting information
Table S1. Table 2 References.
Conflict of interest
The authors have no conflict of interest or financial disclosures.
Funding sources
None.
The copyright line for this article was changed on 12 October 2015 after original online publication.
References
- 1. de Angelis F, Kolesnikova L, Renato F, Liguori G. Fractional nonablative 1540‐nm laser treatment of striae distensae in Fitzpatrick skin types II to IV: clinical and histological results. Aesthet Surg J 2011; 31: 411–419. [DOI] [PubMed] [Google Scholar]
- 2. Elson ML. Topical tretinoin in the treatment of striae distensae and in the promotion of wound healing: a review. J Dermatol Treat 1994; 5: 163–165. [Google Scholar]
- 3. Kang S. Topical tretinoin therapy for management of early striae. J Am Acad Dermatol 1998; 39: S90–S92. [DOI] [PubMed] [Google Scholar]
- 4. Kim BJ, Lee DH, Kim MN et al Fractional photothermolysis for the treatment of striae distensae in Asian skin. Am J Clin Dermatol 2008; 9: 33–37. [DOI] [PubMed] [Google Scholar]
- 5. Sheu HM, Yu HS, Chang CH. Mast cell degranulation and elastolysis in the early stage of striae distensae. J Cutan Pathol 1991; 18: 410–416. [DOI] [PubMed] [Google Scholar]
- 6. Watson RE, Parry EJ, Humphries JD et al Fibrillin microfibrils are reduced in skin exhibiting striae distensae. Br J Dermatol 1998; 138: 931–937. [DOI] [PubMed] [Google Scholar]
- 7. Singh G, Kumar LP. Striae distensae. Indian J Dermatol Venereol Leprol 2005; 71: 370–372. [DOI] [PubMed] [Google Scholar]
- 8. Goldman A, Rossato F, Prati C. Stretch marks: treatment using the 1,064‐nm Nd:YAG laser. Dermatol Surg 2008; 34: 686–692. [DOI] [PubMed] [Google Scholar]
- 9. Devillers C, Pierard‐Franchimont C, Schreder A et al High resolution skin colorimetry, strain mapping and mechanobiology. Int J Cosmet Sci 2010; 32: 241–245. [DOI] [PubMed] [Google Scholar]
- 10. Gilmore SJ, Vaughan BL Jr, Madzvamuse A, Maini PK. A mechano‐chemical model for striae distensae. Math Biosci 2012; 240: 141–147. [DOI] [PubMed] [Google Scholar]
- 11. Hermanns JF, Piérard GE. High‐resolution epiluminescence colorimetry of striae distensae. J Eur Acad Dermatol Venereol 2006; 20: 282–287. [DOI] [PubMed] [Google Scholar]
- 12. Zheng P, Lavker RM, Kligman AM. Anatomy of striae. Br J Dermatol 1985; 112: 185–193. [DOI] [PubMed] [Google Scholar]
- 13. Piérard GE, Nizet JL, Adant JP et al Tensile properties of relaxed excised skin exhibiting striae distensae. J Med Eng Technol 1999; 23: 69–72. [DOI] [PubMed] [Google Scholar]
- 14. Bleve M, Capra P, Pavanetto F, Perugini P. Ultrasound and 3D skin imaging: methods to evaluate efficacy of striae distensae treatment. Dermatol Res Pract 2012; 673706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang ALS, Agredano YZ, Kimball AB. Risk factors associated with striae gravidarum. J Am Acad Dermatol 2004; 51: 881–885. [DOI] [PubMed] [Google Scholar]
- 16. Ghasemi A, Gorouhi F, Rashighi‐Firoozabadi M et al Striae gravidarum: associated factors. J Eur Acad Dermatol Venereol 2007; 21: 743–746. [DOI] [PubMed] [Google Scholar]
- 17. Cho S, Park ES, Lee DH et al Clinical features and risk factors for striae distensae in Korean adolescents. J Eur Acad Dermatol Venereol 2006; 20: 1108–1113. [DOI] [PubMed] [Google Scholar]
- 18. Elbuluk N, Kang S, Hamilton T. Differences in clinical features and risk factors for striae distensae in African American and white women. J Am Acad Dermatol 2009; 1: AB56. [Google Scholar]
- 19. Atwal GSS, Manku LK, Griffiths CEM, Polson DW. Striae gravidarum in primiparae. Br J Dermatol 2006; 155: 965–969. [DOI] [PubMed] [Google Scholar]
- 20. García Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol 2002; 3: 497–506. [DOI] [PubMed] [Google Scholar]
- 21. Weber FP. Idipathic stria atrophicae of puberty. Lancet 1935; 229: 1347. [Google Scholar]
- 22. Sisson WR. Colored striae in adolescent children. J Pediatr 1954; 45: 520–530. [DOI] [PubMed] [Google Scholar]
- 23. Yamaguchi K, Suganuma N, Ohashi K. Quality of life evaluation in Japanese pregnant women with striae gravidarum: a cross‐sectional study. BMC Res Notes 2012; 21: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho SB, Lee SJ, Lee JE et al Treatment of striae alba using the 10,600 nm carbon dioxide fractional laser. J Cosmet Laser Ther 2010; 12: 118–119. [DOI] [PubMed] [Google Scholar]
- 25. Belo V, Arceo‐Cruz MC, Buse E et al The efficacy of fractional resurfacing using an erbium glass laser in the treatment of stretch marks on Asian skin. Laser Surg Med 2009; 41: 92. [Google Scholar]
- 26. Alexiades‐Armenakas M, Sarnoff D, Gotkin R, Sadick N. Multi‐center clinical study and review of fractional ablative CO2 laser resurfacing for the treatment of rhytides, photoaging, scars and striae. J Drugs Dermatol 2011; 10: 352–362. [PubMed] [Google Scholar]
- 27. Sadick NS, Magro C, Hoenig A. Prospective clinical and histological study to evaluate the efficacy and safety of a targeted high‐intensity narrow band UVB/UVA1 therapy for striae alba. J Cosmet Laser Ther 2007; 9: 79–83. [DOI] [PubMed] [Google Scholar]
- 28. Mazzarello V, Farace F, Ena P et al A superficial texture analysis of 70% glycolic acid topical therapy and striae distensae. Plast Reconstr Surg 2012; 129: 589–590. [DOI] [PubMed] [Google Scholar]
- 29. Aust MC, Knobloch K, Vogt PM. Percutaneous collagen induction therapy as a novel therapeutic option for Striae distensae. Plast Reconstr Surg 2010; 126: 219–220. [DOI] [PubMed] [Google Scholar]
- 30. Freedman B. Striae reduction following laser lipolysis. Laser Surg Med 2010; 42: 96–97. [Google Scholar]
- 31. Suh DH, Chang KY, Son HC et al Radiofrequency and 585‐nm pulsed dye laser treatment of striae distensae: a report of 37 Asian patients. Dermatol Surg 2007; 33: 29–34. [DOI] [PubMed] [Google Scholar]
- 32. Abdel‐Latif AM, Albendary AS. Treatment of striae distensae with microdermabrasion: a clinical and molecular study. JEWDS 2008; 5: 24–30. [Google Scholar]
- 33. Kelekci KH, Kelekci S, Destegul E et al Prematurity: is it a risk factor for striae distensae? Int J Dermatol 2011; 50: 1240–1245. [DOI] [PubMed] [Google Scholar]
- 34. Rangel O, Arias I, Garcia E, Lopez‐Padilla S. Topical tretinoin 0.1% for pregnancy‐related abdominal striae: an open‐label, multicenter, prospective study. Adv Ther 2001; 18: 181–186. [DOI] [PubMed] [Google Scholar]
- 35. Mallol J, Belda MA, Costa D et al Prophylaxis of Striae gravidarum with a topical formulation. A double blind trial. Int J Cosmet Sci 1991; 13: 51–57. [DOI] [PubMed] [Google Scholar]
- 36. de Buman M, Walther M, de Weck R. [Effectiveness of Alphastria cream in the prevention of pregnancy stretch marks (striae distensae). Results of a double‐blind study]. Gynakol Rundsch 1987; 27: 79–84. [PubMed] [Google Scholar]
- 37. Osman H, Usta IM, Rubeiz N et al Cocoa butter lotion for prevention of striae gravidarum: a double‐blind, randomised and placebo‐controlled trial. BJOG 2008; 115: 1138–1142. [DOI] [PubMed] [Google Scholar]
- 38. Taavoni S, Soltanipour F, Haghani H et al Effects of olive oil on striae gravidarum in the second trimester of pregnancy. Complement Ther Clin Pract 2011; 17: 167–169. [DOI] [PubMed] [Google Scholar]
- 39. Ud‐Din S, McAnelly SL, Bowring A et al A double‐blind controlled clinical trial assessing the effect of topical gels on striae distensae (stretch marks): a non‐invasive imaging, morphological and immunohistochemical study. Arch Dermatol Res 2013; 305: 603–617. [DOI] [PubMed] [Google Scholar]
- 40. Thomas RGR, Liston WA. Clinical associations of striae gravidarum. J Obstet Gynaecol 2004; 24: 270–271. [DOI] [PubMed] [Google Scholar]
- 41. Beer KR. Comparative evaluation of the safety and efficacy of botulinum toxin type A and topical creams for treating moderate‐to‐severe glabellar rhytids. Dermatol Surg 2006; 32: 184–197. [DOI] [PubMed] [Google Scholar]
- 42. Brinkhaus B, Lindner M, Schuppan D, Hahn EG. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica . Phytomedicine 2000; 7: 427–448. [DOI] [PubMed] [Google Scholar]
- 43. Cussons . Cussons Mum & Me UK. [WWW document] 2014. URL http://www.mumandme.com/en-gb/bump/stretch-mark-cream (last accessed: 7 October 2014).
- 44. Liforma . Liforma – the stretch marks specialists. [WWW document] 2014. URL http://liforma.tumblr.com (last accessed: 7 October 2014).
- 45. Kang S, Kim KJ, Griffiths CE et al Topical tretinoin (retinoic acid) improves early stretch marks. Arch Dermatol 1996; 132: 519–526. [PubMed] [Google Scholar]
- 46. Pribanich S, Simpson FG, Held B et al Low‐dose tretinoin does not improve striae distensae: a double‐blind, placebo‐controlled study. Cutis 1994; 54: 121–124. [PubMed] [Google Scholar]
- 47. Hexsel D, Soirefmann M, Porto MD et al Superficial dermabrasion versus topical tretinoin on early striae distensae: a randomized, pilot study. Dermatol Surg 2014; 40: 537–544. [DOI] [PubMed] [Google Scholar]
- 48. Bio‐Oil . Photobiology Laboratory of the Medical University of South Africa. [WWW document] 2005. URL http://www.bio-oilprofessional.co.uk/clinical-research/ (last accessed: 7 October 2014).
- 49. Bio‐Oil . proDERM institute for applied dermatological research, Hamburg, Germany. [WWW document] 2010. URL http://www.bio-oilprofessional.co.uk/clinical-research/ (last accessed: 7 October 2014).
- 50. Sinclair . Kelo‐stretch™ . [WWW document] 2014. URL https://www.sinclairisonline.com/ProductRange.aspx?id=26 (last accessed: 7 October 2014).
- 51. Apothederm . Stretch Mark Cream. [WWW document] 2014. URL http://apothederm.com/shop-products/body/stretch-mark-cream/ (last accessed: 7 October 2014).
- 52. Buchanan K, Fletcher HM, Reid M. Prevention of striae gravidarum with cocoa butter cream. Int J Gynaecol Obstet 2010; 108: 65–68. [DOI] [PubMed] [Google Scholar]
- 53. Timur S, Kafkasli A. The effect of bitter almond oil and massaging on striae gravidarum in primiparaous women. J Clin Nurs 2012; 21: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 54. Al‐Himdani S, Ud‐Din S, Gilmore S, Bayat A. Striae distensae: a comprehensive review and evidence‐based evaluation of prophylaxis and treatment. Br J Dermatol 2014; 170: 527–547. [DOI] [PubMed] [Google Scholar]
- 55. Rapaport MH, Schettler P, Bresee C. A preliminary study of the effects of a single session of Swedish massage on the hypothalamic‐ pituitary‐adrenal and immune function in normal individuals. J Altern Complement Med 2010; 16: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shin TM, Bordeaux JS. The role of scar management: a literature review. Dermatol Surg 2012; 38: 414–423. [DOI] [PubMed] [Google Scholar]
- 57. Brennan M, Young G, Devane D. Topical preparations for preventing stretch marks in pregnancy. Cochrane Database Syst Rev 2012; 11: CD000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ud‐Din S, Bayat A. New insights on keloids, hypertrophic scars, and striae. Dermatol Clin 2014; 32: 193–209. [DOI] [PubMed] [Google Scholar]
- 59. Murphy KW, Dunphy B, O'Herlihy C. Increased maternal age protects against striae gravidarum. J Obstet Gynaecol 1992; 12: 297–300. [Google Scholar]
- 60. Kharb S, Gundgurthi A, Dutta MK et al Striae atrophicans: a mimic to Cushing's cutaneous striae. Indian J Endocrinol Metab 2012; 16(Suppl 1): S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. García‐Hidalgo L, Orozco‐Topete R, Gonzalez‐Barranco J et al Dermatoses in 156 obese adults. Obes Res 1999; 7: 299–302. [DOI] [PubMed] [Google Scholar]
- 62. Jaramillo‐Garcia CM, Lopera‐Calderon MC, Zuluaga De Cadena A. Related factors with atrophic stretch marks in adolescent female students from two private educational establishments from the city of Medellin, 1997–1999. CES Med 2009; 23: s69–s79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Table 2 References.


