Abstract
Tacrine has been studied in two clinical trials of identical design in patients with probable Alzheimer disease. One trial enrolled patients in the United States, while the other enrolled patients in France. A population pharmacodynamic model has been used to describe the cognitive component of the Alzheimer disease assessment scale (ADASC) using mixed effects nonlinear regression. The model parameters and their population variability and covariance were estimated by using NONMEM. During an observation period of up to 5 months, the rate of disease progression was 6.17 ADASC units/year. The effect of tacrine was described best by a shift in the disease progress curve (-2.99 ADASC units or 177.6 days at a dose of 80 mg/day). The placebo effects associated with tacrine and placebo treatment were similar in magnitude and time course. There was no evidence of tolerance to tacrine but tolerance to the placebo treatment developed during the study. The size of the placebo effect in the French population was 76% larger than in the United States population, and the response to placebo diminished more slowly in the French population.
Full text
PDF

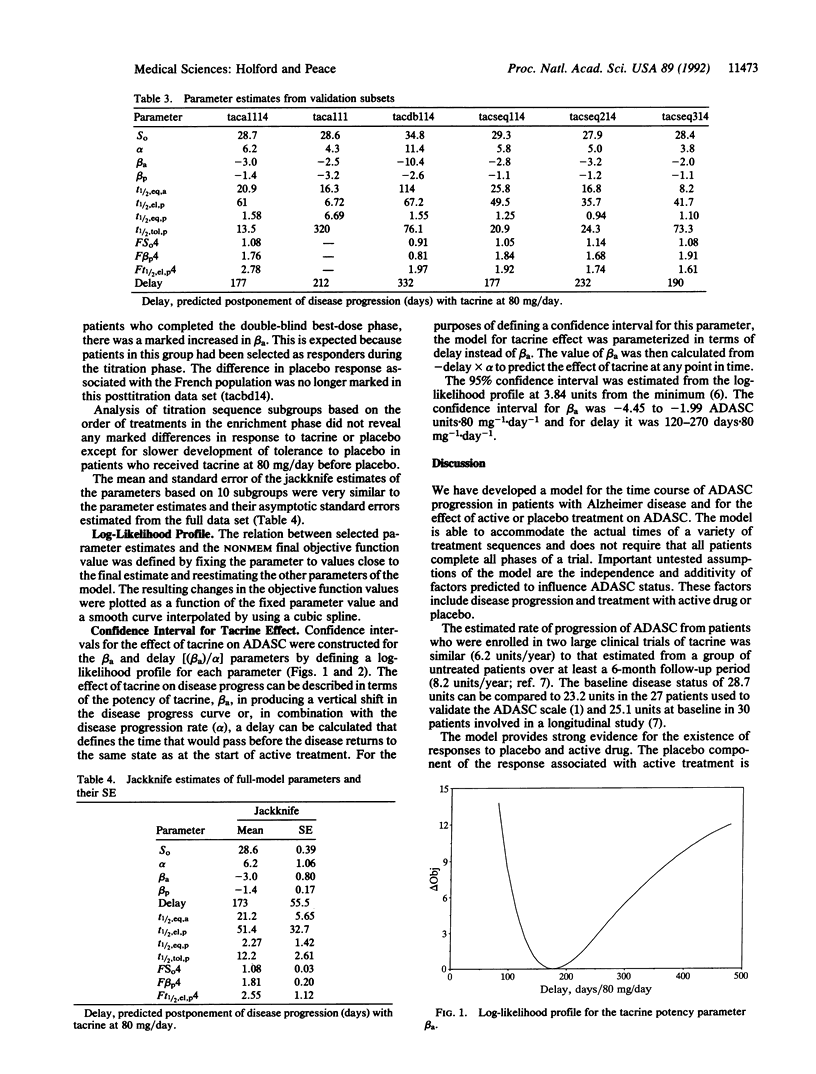
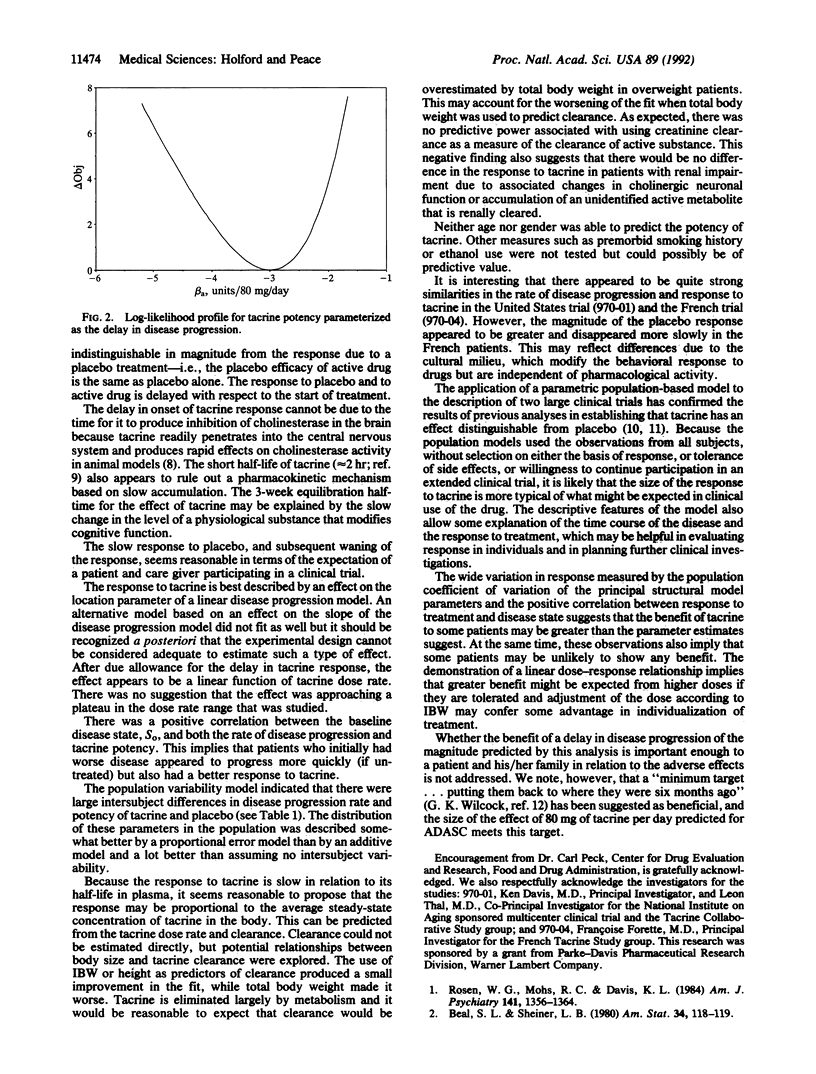

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornsson T. D. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet. 1979 May-Jun;4(3):200–222. doi: 10.2165/00003088-197904030-00003. [DOI] [PubMed] [Google Scholar]
- Davis K. L., Thal L. J., Gamzu E. R., Davis C. S., Woolson R. F., Gracon S. I., Drachman D. A., Schneider L. S., Whitehouse P. J., Hoover T. M. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer's disease. The Tacrine Collaborative Study Group. N Engl J Med. 1992 Oct 29;327(18):1253–1259. doi: 10.1056/NEJM199210293271801. [DOI] [PubMed] [Google Scholar]
- Forsyth D. R., Wilcock G. K., Morgan R. A., Truman C. A., Ford J. M., Roberts C. J. Pharmacokinetics of tacrine hydrochloride in Alzheimer's disease. Clin Pharmacol Ther. 1989 Dec;46(6):634–641. doi: 10.1038/clpt.1989.199. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Peace K. E. Methodologic aspects of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11466–11470. doi: 10.1073/pnas.89.23.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen W. G., Mohs R. C., Davis K. L. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984 Nov;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Yesavage J. A., Poulsen S. L., Sheikh J., Tanke E. Rates of change of common measures of impairment in senile dementia of the Alzheimer's type. Psychopharmacol Bull. 1988;24(4):531–534. [PubMed] [Google Scholar]


