Abstract
In this clinical trial, we investigated the blood glucose (BG)‐lowering effects of 30, 60 and 90 mg dextromethorphan (DXM) as well as 100 mg sitagliptin alone versus combinations of DXM and sitagliptin during an oral glucose tolerance test (OGTT) in 20 men with T2DM. The combination of 60 mg DXM plus 100 mg sitagliptin was observed to have the strongest effect in the OGTT. It lowered maximum BG concentrations and increased the baseline‐adjusted area under the curve for serum insulin concentrations in the first 30 min of the OGTT (mean ± standard deviation 240 ± 47 mg/dl and 8.1 ± 6.1 mU/l/h, respectively) to a significantly larger extent than did 100 mg sitagliptin alone (254 ± 50 mg/dl and 5.8 ± 2.5 mU/l/h, respectively; p < 0.05) and placebo (272 ± 49 mg/dl and 3.9 ± 3.0 mU/l/h, respectively; p < 0.001). All study drugs were well tolerated, alone and in combination, without serious adverse events or hypoglycaemia. Long‐term clinical trials are now warranted to investigate the potential of the combination of 30 or 60 mg DXM and dipeptidyl peptidase‐4 inhibitors in the treatment of individuals with T2DM, in particular as preclinical studies have identified the β‐cell protective properties of DXM.
Keywords: antidiabetic drug, DPP‐IV inhibitor, insulin secretion, type 2 diabetes, NMDA
Introduction
Recently, we reported that inhibition of N‐Methyl‐D‐Aspartate receptors (NMDARs) in mouse and human pancreatic islets enhanced glucose‐stimulated insulin secretion and islet cell survival 1. Furthermore, we observed that, in vivo, the NMDAR antagonist and over‐the‐counter antitussive agent dextromethorphan (DXM) enhanced glucose tolerance in mice, and that in vitro dextrorphan, the main metabolite of the pro‐drug DXM, amplified the stimulatory effect of exendin‐4, a peptide agonist of the glucagon‐like peptide‐1 receptor, on glucose‐stimulated insulin secretion 1. Moreover, a randomized clinical trial showed that DXM selectively increased postprandial serum insulin concentrations and lowered blood glucose (BG) excursions in individuals with type 2 diabetes mellitus (T2DM) 1.
Like NMDAR antagonists, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, such as sitagliptin, enhance postprandial serum insulin concentrations and improve BG control, but through another mechanism of action 1, 2, 3, 4, 5. The primary objective of the present study was to investigate whether the combination of a low dose of DXM and sitagliptin exerts additive BG‐lowering effects after an oral glucose load compared with sitagliptin alone and DXM alone.
Methods
Eligible subjects were men aged 45–70 years, with a diagnosis of T2DM according to American Diabetes Association criteria at least 4 months before screening, who were on a stable regimen of metformin monotherapy for at least 3 months, and who had a medical history without major pathology, a body mass index of 25–35 kg/m2 and a glycated haemoglobin concentration ≥6.5 and ≤8.0% (Table S1). The study was conducted at Profil, Neuss, Germany. The Ethics committee of the Ärztekammer Nordrhein, Düsseldorf, Germany, approved the trial protocol. The trial was conducted in accordance with the Declaration of Helsinki (2008) and International Conference on Harmonisation Good Clinical Practice (1996), and written informed consent was obtained from all patients. The trial was registered at ClinicalTrials.gov (NCT01936025).
Study participants received either 30, 60 or 90 mg DXM, 100 mg sitagliptin, 30, 60 or 90 mg DXM plus 100 mg sitagliptin, or placebo in the morning after an overnight fast on a total of eight treatment days. One hour after study drug administration an oral glucose tolerance test (OGTT) with 75 g glucose was started. The primary objectives of the present clinical trial were to (i) find the lowest dose of DXM that, compared with placebo, exerted a BG‐lowering effect related to the OGTT, and (ii) investigate whether the combination of DXM and sitagliptin had additive BG‐lowering effects compared with each drug alone (to convert mg/dl to mmol/l, multiply by 0.0555). The primary pharmacodynamic variable was the area under the curve (AUC) of BG concentrations 0–2 h after starting the OGTT: AUCglucose 1–3 h. Further pharmacodynamic variables included AUCglucose 0–1 h, AUCglucose 3–5 h, maximum glucose concentration, AUCinsulin 0–1 h, AUCinsulin 1–1.5 h, AUCinsulin 1–3 h, AUCinsulin 3–5 h, and maximum insulin concentration. Insulin values after starting the OGTT were adjusted for baseline levels to correct for endogenous insulin secretion, meaning that predose concentrations were subtracted from subsequent measurements before calculation.
All statistical analyses were performed using sas software. The primary endpoint AUCglucose 1–3 h was analysed using a mixed model, with treatment as fixed factor and subject as random factor. Normally or log‐normally distributed secondary endpoints were analysed using the same approach as specified for the primary pharmacodynamic analysis using untransformed or log‐transformed endpoints. Time variables and non‐normal or non‐log‐normal distributed endpoints were analysed by non‐parametric technique using Wilcoxon's signed rank test and corresponding Hodges and Lehmann 95% confidence intervals (CIs).
Results
A total of 20 men with T2DM were enrolled and completed the clinical trial (Table S1; Figure S1). To a small, but not significant extent, all doses of DXM alone were found to numerically reduce maximum BG concentrations and AUCglucose 1–3 h, whereas only 60 mg DXM numerically reduced AUCglucose 3–5 h compared with placebo (Table 1). When DXM was used as add‐on to sitagliptin, all doses of DXM plus sitagliptin showed numerically lower values compared with sitagliptin alone for maximum BG concentrations, AUCglucose 1–3 h, and AUCglucose 3–5 h (Table 1); the latter variable showed the least reduction with the lowest dose of DXM (30 mg) plus sitagliptin (Table 1). Notably, 60 mg DXM plus sitagliptin was observed to significantly lower maximum BG concentrations compared with sitagliptin alone (Figure 1A, B; Table 1). For 30, 60 and 90 mg DXM added to sitagliptin, BG reductions within 4 h after starting the OGTT (i.e. % reductions in AUCglucose 1–5 h) of 8.9, 10.5 and 10.7% were observed, respectively, as compared with only 6.5% with sitagliptin alone (Table S2). By contrast, virtually no reductions in pre‐OGTT fasting BG concentrations were observed (Table S3).
Table 1.
Glucose, insulin and glucagon concentrations after starting the oral glucose tolerance test.
| Maximum glucose, mg/dl | Maximum insulin, mU/l | Maximum glucagon, pmol/l | AUCinsulin 1–1.5 h, mU/l/h | AUCglucose 1–3 h, mg/dl/h | AUCinsulin 1–3 h, mU/l/h | AUCglucagon 1–3 h, pmol/l/h | AUCglucose 3–5 h, mg/dl/h | AUCinsulin 3–5 h, mU/l/h | |
|---|---|---|---|---|---|---|---|---|---|
| Study drug | After starting the OGTT | 0–30 min after OGTT | 0–2 h after starting the OGTT | 2–4 h after starting the OGTT | |||||
| Placebo | 271.8 ± 49.3 | 56.2 ± 35.9 | 56.6 ± 11.4 | 3.9 ± 3.0 | 444.7 ± 69.3 | 63.9 ± 38.6 | 102.3 ± 19.6 | 388.4 ± 99.4 | 49.6 ± 36.5 |
| Sitagliptin | 254.4 ± 49.7* | 71.8 ± 43.5* | 55.7 ± 11.7 | 5.8 ± 2.5 | 412.4 ± 74.3* | 77.1 ± 39.4* | 100.7 ± 19.6 | 366.5 ± 101.5 | 74.1 ± 42.2* |
| DXM 30 | 264.5 ± 43.6 | 57.6 ± 30.6‡ | Not analysed | 5.6 ± 4.2 | 430.2 ± 64.2 | 68.7 ± 39.3 | Not analysed | 390.3 ± 89.7‡ | 56.4 ± 39.0 |
| DXM 30 + sitagliptin | 243.1 ± 45.0**† | 71.9 ± 42.5*† | 8.4 ± 6.4**†‡ | 393.2 ± 77.9**† | 81.4 ± 47.8*† | 365.6 ± 91.9† | 73.2 ± 40.8*† | ||
| DXM 60 | 262.4 ± 45.0 | 69.1 ± 46.8 | 56.1 ± 10.1 | 3.9 ± 2.2 | 425.2 ± 78.0 | 74.2 ± 46.0 | 100.1 ± 15.2 | 374.6 ± 104.6 | 63.5 ± 49.8 |
| DXM 60 + sitagliptin | 240.1 ± 47.0**†‡ | 78.6 ± 52.6**† | 55.7 ± 12.8 | 8.1 ± 6.1**††‡ | 393.3 ± 73.1**† | 89.0 ± 53.5**† | 101.2 ± 20.7 | 352.8 ± 100.4* | 70.8 ± 42.7* |
| DXM 90 | 270.2 ± 50.4‡ | 60.1 ± 42.3‡ | Not analysed | 4.4 ± 5.2 | 433.4 ± 76.2 | 67.0 ± 49.3‡ | Not analysed | 390.2 ± 103.0 | 53.4 ± 40.2‡ |
| DXM 90 + sitagliptin | 243.4 ± 52.6**†† | 87.0 ± 57.5**††‡ | 7.6 ± 4.7**† | 391.0 ± 92.2**†† | 88.3 ± 51.2**†† | 353.1 ± 103.7*† | 74.1 ± 47.9*† | ||
Data are presented as means ± standard deviation. Maximum insulin concentrations and AUC insulin data were baseline adjusted. Statistical analyses for BG concentrations and serum insulin concentrations were performed using a linear mixed model, with treatment as fixed factors and subject as a random factor using untransformed and, when needed, log‐transformed endpoints. Statistical analyses of plasma glucagon concentrations were performed using the Wilcoxon signed rank test based on a two‐sided α of 5% and calculation of Hodges and Lehman estimates and corresponding non‐parametric confidence intervals. AUC, area under the curve; DXM, dextromethorphan; OGTT, oral glucose tolerance test.
*p < 0.05 and **p < 0.001 sitagliptin alone, DXM alone and sitagliptin + DXM versus placebo; †p < 0.05 and ††p < 0.001 sitagliptin + DXM versus DXM alone at same dose; ‡p < 0.05 DXM alone and sitagliptin + DXM versus sitagliptin alone.
Figure 1.
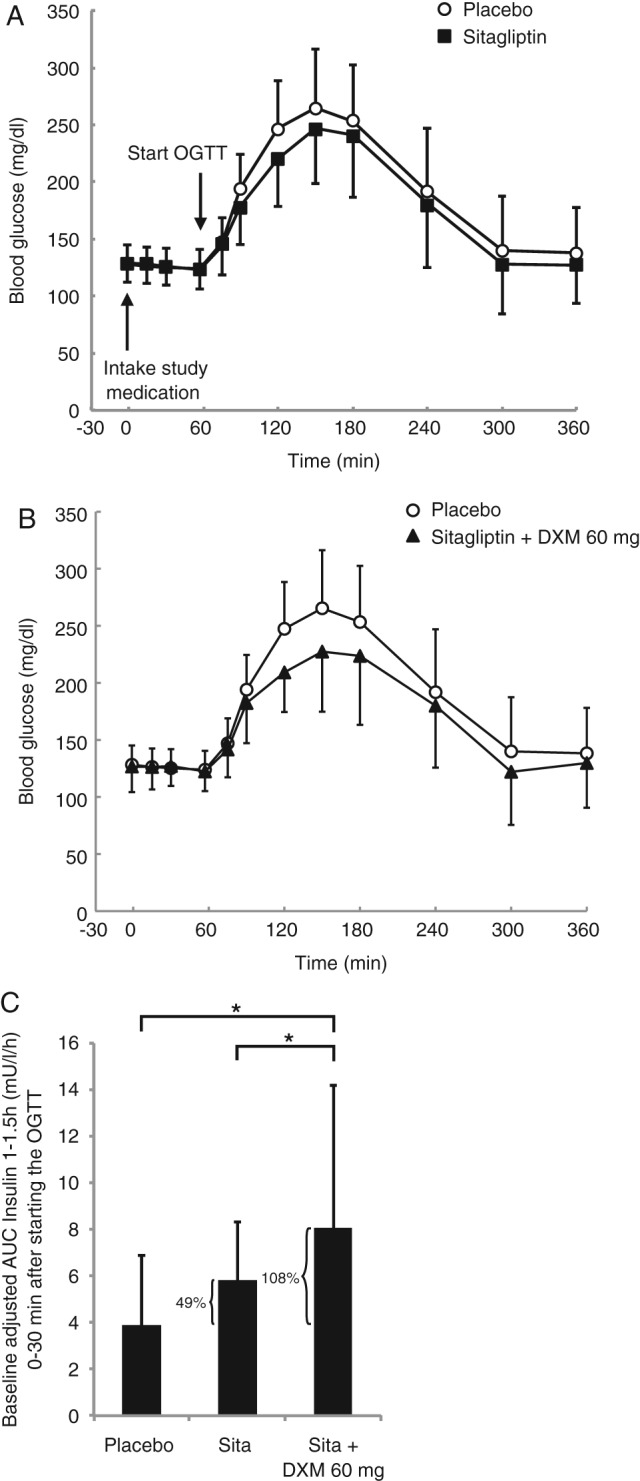
Oral glucose tolerance test and serum insulin concentrations in individuals with type 2 diabetes (T2DM). Blood glucose concentrations in 20 men with T2DM before and during an oral glucose tolerance test (OGTT) shown for (A) placebo versus 100 mg sitagliptin and (B) placebo versus 100 mg sitagliptin plus 60 mg dextromethorphan (DXM). (C) Serum insulin concentrations during the first 30 min after starting the OGTT after administration of placebo, 100 mg sitaglipin and 100 mg sitagliptin plus 60 mg DXM. Significance (*p < 0.05) was determined by a linear mixed model, with treatment as a fixed factor and subject as a random factor using untransformed endpoints. All values are means ± standard deviation.
With regard to postprandial serum insulin concentrations, all doses of DXM alone compared with placebo were found to numerically increase maximum insulin concentrations to a small, but not significant extent (Table 1). Sitagliptin alone and all sitagliptin plus DXM combinations led to significant increases in maximum insulin concentrations, but the combination of 90 mg DXM plus sitagliptin resulted in significantly higher increases in the maximum insulin concentrations versus sitagliptin alone (Table 1). All doses of DXM plus sitagliptin numerically increased the baseline‐adjusted AUCinsulin 1–1.5 h; that is, the amount of serum insulin during the first 30 min of the OGTT, compared with sitagliptin alone (Table 1). This increase was significant for the combination of 30 mg DXM plus sitagliptin and 60 mg DXM plus sitagliptin (95% CI 0.46, 4.73; p = 0.017, and 95% CI 0.11, 4.38; p = 0.039, respectively) when compared with sitagliptin alone (Figure 1C, Table 1). The combination of 30 mg DXM plus sitagliptin and 60 mg DXM plus sitagliptin compared with placebo increased the baseline‐adjusted AUCinsulin 1–1.5 h by ∼110% versus an increase of only ∼50% for sitagliptin alone compared with placebo (Figure 1C, Table 1). Notably, within the setting of the present trial, 100 mg sitagliptin alone failed to significantly increase the baseline‐adjusted AUCinsulin 1–1.5 h (95% CI −0.22, 4.05; p = 0.078). Sitagliptin versus placebo, and sitagliptin plus all DXM combinations versus placebo, however, led to significant increases in the baseline‐adjusted AUCinsulin 1–3 h after starting the OGTT, with higher significance noted for the combinations of sitagliptin plus DXM (Table 1). By contrast, the addition of DXM to sitagliptin did not increase the baseline‐adjusted AUCinsulin 3–5 h compared with sitagliptin alone (Table 1). All DXM dosages plus sitagliptin versus placebo were observed to result in small increases in fasting serum insulin concentrations before starting the OGTT, whereas no significant changes in fasting BG concentrations were observed (Table S3). As compared with placebo, plasma glucagon concentrations remained virtually unchanged after administration of the study medications, both before and after starting the OGTT (Tables 1 and S3).
All dosages of study medication were well tolerated, and few adverse events were observed after administration of DXM alone or none after the combination of 30 mg DXM plus sitagliptin. With regard to the combinations of 60 mg or 90 mg DXM plus sitagliptin, only one mild adverse event (fatigue) was observed each.
Discussion
In this single‐dose, double‐blinded clinical trial, we provided a proof of concept that addition of low doses of DXM, including 30 mg (a dose used as over‐the‐counter medication for cough suppression), to sitagliptin substantially increased early postprandial serum insulin concentrations and reduced BG excursions during an OGTT without inducing hypoglycaemia. More specifically, the postprandial serum insulin concentrations during the first 30 min of the OGTT were strongly increased by the addition of DXM to sitagliptin, whereas postprandial serum insulin concentrations during the last 2 h of the OGTT were not increased at all. This points to the possibility that the combination of DXM and sitagliptin preferentially restores the early phase of insulin release in individuals with T2DM, with BG‐lowering effects lasting throughout the entire OGTT; however, the question of whether a normal β‐cell physiology could be restored in individuals with T2DM by this combination requires further clinical tests, more directly addressing insulin secretion in these patients 6. The findings of the present trial highlight an additive action of DXM and sitagliptin (Figure 1), most likely because they target pancreatic β‐cell NMDARs and incretin receptors, respectively, which enhance glucose‐stimulated insulin release from human pancreatic islets via different glutamate‐dependent molecular mechanisms 1, 2, 7, 8.
The present study shows for the first time that addition of DXM increases the BG‐lowering effects of the DPP‐4 inhibitor sitagliptin, which is a popular oral antidiabetic medication, but only reduces BG levels in patients with T2DM to a moderate extent compared with incretin‐based peptides requiring regular injections 9. Consistently, sitagliptin only lowered AUCglucose 1–3 h by ∼7% in the present study, whereas the combinations of a low dose of DXM with sitagliptin reduced this primary endpoint by ∼12%. Our findings now warrant further and long‐term clinical trials on the combined action of DXM and incretin‐based diabetes therapies so as to draw conclusions on the clinical relevance of this combination, in particular concerning the preservation of β‐cell function and mass that has been described for DXM in preclinical studies 1, 10.
Conflict of Interest
J. M., T. M. and E. L. declare the following competing financial interest: these authors are pursuing a patent application (WO 2013/029762 A1) entitled ‘Morphinan‐derivatives for treating diabetes and related disorders’.
J. M. and E. L. designed the structure of manuscript and figures and wrote the manuscript with input from the other authors. J. M. generated figures and tables with input from E. L., A. S., A. W. and S. O. E. L. suggested to T. H. to perform a clinical trial on a combination of a low dose of DXM with a DPP‐4 inhibitor, while T. H. suggested the use of sitagliptin. A.S., F. Sievers, F. Schliess, and T. H. guided the clinical trial whose protocol was written by A. S. with input from E. L., J. M., T. H., F. Schliess, T. M., A. W. and S. O. A. F. performed the statistical analyses. S. W. determined the insulin and glucagon concentrations. All authors were involved in drafting or revising the content of the paper and approved the final version of the manuscript.
Supporting information
Figure S1. Consort flow diagram.
Table S1. Baseline demographic and clinical characteristics of the study sample.
Table S2. Glucose, insulin and glucagon concentrations during the entire oral glucose tolerance test.
Table S3. Glucose, insulin and glucagon concentrations after study drug administration, but before starting the OGTT. Data are presented as means ± SD. Statistical analyses for BG concentrations and serum insulin concentrations were performed using a linear mixed model with treatment as fixed factors and subject as a random factor using untransformed and, when needed, log‐transformed endpoints. Statistical analyses of plasma glucagon concentrations were performed using the Wilcoxon Signed Rank test based on a two‐sided alpha of 5% and calculation of Hodges and Lehman estimates and corresponding nonparametric confidence intervals. Data are presented as means ± SD. *p < 0.05 versus placebo; †p < 0.05 versus DXM alone at same dose; ‡p < 0.05 versus Sita alone.
Acknowledgements
The study was supported by the Heinrich Heine University Düsseldorf, Profil Neuss, the German Centre for Diabetes Research (DZD e.V.), the German Diabetes Centre (DDZ), the Federal Ministry of Health, and the Ministry for Innovation, Science and Research of North‐Rhine‐Westphalia.
We thank E. Mayatepek for actively supporting the exchange between the Department of Pediatrics and the Institute of Metabolic Physiology at the Heinrich Heine University Düsseldorf and Birgit Kronshage for carefully checking the statistics of the clinical study. We would also like to thank all patients for participating in this clinical trial.
References
- 1. Marquard J, Otter S, Welters A et al. Characterization of pancreatic NMDA receptors as possible drug targets for diabetes treatment. Nat Med 2015; 21: 363–372. [DOI] [PubMed] [Google Scholar]
- 2. Wollheim CB, Maechler P. Beta cell glutamate receptor antagonists: novel oral antidiabetic drugs? Nat Med 2015; 21: 310–311. [DOI] [PubMed] [Google Scholar]
- 3. Ahrén B. Use of DPP‐4 inhibitors in type 2 diabetes: focus on sitagliptin. Diabetes Metab Syndr Obes 2010; 3: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenstock J, Zinman B. Dipeptidyl peptidase‐4 inhibitors and the management of type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2007; 14: 98–107. [DOI] [PubMed] [Google Scholar]
- 5. Cefalu WT, Buse JB, Del Prato S et al. Beyond metformin: safety considerations in the decision‐making process for selecting a second medication for type 2 diabetes management: reflections from a diabetes care editors' expert forum. Diabetes Care 2014; 37: 2647–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kahn SE, Carr DB, Faulenbach MV, Utzschneider KM. An examination of beta‐cell function measures and their potential use for estimating beta‐cell mass. Diabetes Obes Metab 2008; S4: 63–76. [DOI] [PubMed] [Google Scholar]
- 7. Gheni G, Ogura M, Iwasaki M et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep 2014; 9: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maechler P, Wollheim CB. Mitochondrial glutamate as a messenger in glucose‐induced insulin exocytosis. Nature 1999; 402: 685–689. [DOI] [PubMed] [Google Scholar]
- 9. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farilla L, Bulotta A, Hirshberg B et al. Glucagon‐like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144: 5149–5158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Consort flow diagram.
Table S1. Baseline demographic and clinical characteristics of the study sample.
Table S2. Glucose, insulin and glucagon concentrations during the entire oral glucose tolerance test.
Table S3. Glucose, insulin and glucagon concentrations after study drug administration, but before starting the OGTT. Data are presented as means ± SD. Statistical analyses for BG concentrations and serum insulin concentrations were performed using a linear mixed model with treatment as fixed factors and subject as a random factor using untransformed and, when needed, log‐transformed endpoints. Statistical analyses of plasma glucagon concentrations were performed using the Wilcoxon Signed Rank test based on a two‐sided alpha of 5% and calculation of Hodges and Lehman estimates and corresponding nonparametric confidence intervals. Data are presented as means ± SD. *p < 0.05 versus placebo; †p < 0.05 versus DXM alone at same dose; ‡p < 0.05 versus Sita alone.


