Abstract
Objective
Enthesitis‐related arthritis (ERA) is a juvenile idiopathic arthritis (JIA) category, primarily affecting entheses and peripheral joints. This study evaluated efficacy, safety, and pharmacokinetics of adalimumab versus placebo in patients with ERA.
Methods
This is a phase III, multicenter, randomized double‐blind study in patients ages ≥6 to <18 years with ERA treated with adalimumab (24 mg/m2, maximum dose 40 mg every other week) or placebo for 12 weeks, followed by up to 192 weeks of open‐label adalimumab. The primary end point was percent change from baseline in number of active joints with arthritis (AJC) at week 12. Samples were collected to determine adalimumab serum concentrations. Adverse events (AEs) were assessed throughout the study.
Results
Forty‐six patients were randomized (31 adalimumab/15 placebo). At baseline, mean age was 12.9 years, mean duration of ERA symptoms was 2.6 years, mean AJC was 7.8, and mean enthesitis count was 8.1. Mean percent change from baseline in AJC at week 12 was greater in the adalimumab group versus placebo (−62.6% versus −11.6%; P = 0.039). Most secondary variables favored adalimumab versus placebo at week 12. Treatment response further increased with continued adalimumab therapy through week 52. Mean steady‐state adalimumab serum concentrations were 7.5–11.8 μg/ml, similar to patients age ≥2 years with polyarticular JIA. AE rates were similar between placebo and adalimumab: any AE (53.3% versus 67.7%), serious AEs (0% versus 3.2%), and infectious AEs (20.0% versus 29.0%).
Conclusion
Adalimumab reduced signs and symptoms of ERA at week 12, with improvement sustained through week 52. The safety profile was consistent with previous adalimumab studies.
INTRODUCTION
Enthesitis‐related arthritis (ERA) is a category of juvenile idiopathic arthritis (JIA), defined by the International League of Associations for Rheumatology (ILAR) classification criteria 1, that primarily affects entheses and peripheral joints, but also can involve the axial skeleton. Most patients with ERA may also be classified as juvenile‐onset spondyloarthritis, but the opposite is not always feasible because of the ERA exclusion criteria. Disease activity and structural change can adversely affect long‐term physical function and quality of life of ERA patients 2, 3, 4, 5, 6, 7.
Box 1. Significance & Innovations.
This is the first randomized, double‐blind, placebo‐controlled study in patients with enthesitis‐related arthritis (ERA) prospectively classified by International League of Associations for Rheumatology criteria.
Results suggest that adalimumab is effective in reducing the signs and symptoms of pediatric ERA at 12 weeks in patients with refractory disease, with improvements sustained through week 52.
The efficacy and safety results from this study suggest that adalimumab may be an appropriate treatment option for patients with active ERA who have failed treatment with nonsteroidal antiinflammatory drugs and disease‐modifying antirheumatic drugs.
There is limited evidence for efficacy of most treatments used in ERA, including pharmacologic and nonpharmacologic measures adapted from treatments used in adult spondyloarthritis (SpA) and other JIA categories 8. In addition to nonsteroidal antiinflammatory drugs (NSAIDs), children with ERA are often treated with sulfasalazine (SSZ) 9, methotrexate (MTX), and glucocorticoids. Several studies suggest that biologic agents, specifically tumor necrosis factor (TNF) inhibitors, improve ERA symptoms 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22.
Adalimumab has been previously demonstrated to be safe and effective in children ages 2–17 years with polyarticular JIA 23, 24, 25, in children ages 12–17 years with refractory juvenile ankylosing spondylitis having axial symptoms 19, and in adults with axial SpA 26, 27.
This study evaluated the efficacy and safety of adalimumab compared to placebo and examined its pharmacokinetics and immunogenicity following subcutaneous administration every other week in children and adolescents with ERA.
PATIENTS and METHODS
Study design and participants
This study (ClinicalTrials.gov: NCT01166282), initiated in September 2010, is an ongoing phase III, multinational, randomized, placebo‐controlled double‐blind study conducted at 16 centers in Canada, France, Germany, Italy, Mexico, Poland, Spain, Sweden, and Switzerland. It was conducted in accordance with the International Conference on Harmonization good clinical practices and the Declaration of Helsinki. Approval of an institutional ethics review board and written informed consent, from either the patient or the parent or legal guardian, were obtained at screening prior to initiating any study procedures. In addition, a verbal or written assent from the patient was obtained if under the legal age of consent.
Eligible patients were ages ≥6 to <18 years at baseline and fulfilled the diagnosis of ERA as defined by the ILAR classification criteria for JIA 1 prior to age 16 years. Inclusion criteria were active disease as defined by fulfillment of the following conditions: at least 3 active joints (AJC; swelling not due to deformity or joints with loss of motion [LOM] plus pain and/or tenderness) and evidence of enthesitis in at least 1 location (either documented in the past or present at baseline), and inadequate response or intolerance to at least 1 NSAID and at least 1 disease‐modifying antirheumatic drug (DMARD), either SSZ or MTX. Patients with a diagnosis of any ILAR JIA category other than ERA, diagnosis of acute inflammatory joint disease not associated with ERA, presence of IgM rheumatoid factor, history of inflammatory bowel disease or psoriasis, and previous biologic therapy, including anti‐TNF therapy, were excluded.
Eligible patients were centrally randomized 2:1, via an Interactive Voice and Web Response service, to receive subcutaneous injections of adalimumab (24 mg/m2 body surface area [BSA] up to 40 mg every other week) or matching placebo for 12 weeks. All patients and investigators were blinded to treatment allocation. Patients who completed the double‐blind period were eligible to receive open‐label adalimumab up to an additional 192 weeks. An option for early escape to the open‐label adalimumab treatment period was available at week 4 if worsening occurred, defined as ≥30% increase in AJC with a minimum of at least 2 additional active joints compared with baseline, or at week 8, for failure to improve, defined as <30% improvement in AJC compared with baseline.
Patients could enter the study on stable doses of concomitant NSAIDs, DMARDs (SSZ [≤50 mg/kg body weight per day with a maximum of 3 gm per day] and MTX [≤15 mg/m2 BSA with a maximum of 25 mg per week]), and glucocorticoids (prednisone equivalent ≤10 mg per day or 0.2 mg/kg body weight per day, whichever was less); doses remained stable during the first 12 weeks, except as medically required due to an adverse event (AE). Dose adjustment or induction of treatment with these agents was permitted after week 12. Nondrug therapy (e.g., physiotherapy, hydrotherapy) was allowed during the study.
Procedures
Primary efficacy assessment
The primary efficacy end point was percent change from baseline to week 12 in the AJC (0–68).
Secondary efficacy end points
Secondary variables analyzed at week 12 and through week 52 included total enthesitis count (0–35), the Maastricht Ankylosing Spondylitis Enthesitis Score (0–13) 28, the Spondyloarthritis Canadian Research Consortium Enthesitis Index (0–16) 29, tender joint count (TJC; 0–72), swollen joint count (SJC; 0–68), number of joints with LOM (0–66), presence of dactylitis (0–20), patient's assessment of total back pain (0–100 mm visual analog scale [VAS]), parent's assessment of patient's pain (0–100 mm VAS), physician's global assessment of disease activity (PGA, 0–100 mm VAS), 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index 30, and American College of Rheumatology (ACR) Pediatric 30 (Pedi 30), 50, 70, and 90 responses 31. The percent change in AJC from baseline through week 52 was also analyzed. AEs were collected throughout the study and for 70 days after the last dose of study medication.
Pharmacokinetics and immunogenicity
Blood samples for adalimumab trough serum concentration measurements were obtained just prior to dosing at baseline and at weeks 2, 4, 8, 12, 24, 36, and 52. Blood samples for anti–adalimumab antibodies (AAA) measurements were collected just prior to dosing at baseline and weeks 12, 24, 36, and 52.
Statistical analysis
The sample size was calculated using nQuery Advisor 6.0. With a total sample size of 45 patients (2:1 randomization; n = 30 for adalimumab and n = 15 for placebo) and an expected percentage change of 70% for adalimumab versus 35% for placebo, assuming a common SD of 33%, the study provided 90% power to detect the treatment difference using a one‐way analysis of variance (ANOVA) at a 2‐sided alpha level of 0.05.
The intent‐to‐treat (ITT) analysis set consisted of all patients who were randomized and received at least 1 dose of the study drug. The primary end point was analyzed using an analysis of covariance model, adjusting for the number of active joints at baseline. For patients who did not have an AJC at week 12 or who had escaped early to open‐label adalimumab treatment, their last available joint count from the double‐blind period was used (last observation carried forward). Two sensitivity analyses were performed on the ITT set: one excluding patients who early escaped prior to week 12, the other applying a nonparametric exact Wilcoxon's test to account for the non‐normally distributed data and small sample size. Eight patients in the ITT analysis set (3 in the placebo group and 5 in the adalimumab group) did not fully fulfill the study inclusion/exclusion criteria. Of the 3 patients in the placebo group, 1 patient had insufficient disease activity, 1 patient used a prohibited medication (tramadol at stable dose) during the screening and blinded periods, and 1 patient entered the study on a higher dose of MTX than was allowed. Of 5 patients in the adalimumab group, 2 patients did not have a prior trial or MTX or SSZ and did not have a contraindication to these medications, 1 patient was not on a stable dose of MTX prior to baseline and decreased the dose during the double‐blind period, 1 patient was already age 18 years at enrollment, and 1 patient entered the study on a higher dose of prednisone than allowed.
For the comparison of secondary variables between the 2 treatment groups, Fisher's exact test was used for discrete variables, and one‐way ANOVA was used for continuous variables. Kaplan Meier analysis was used to determine time to achieve SJC = 0, TJC = 0, and enthesitis count = 0 for all patients from time of first adalimumab injection.
The safety population consisted of all patients who were randomized and received at least 1 dose of study medication. Safety results represent data collected through week 52, or up to 70 days following the last study dose of adalimumab for patients who discontinued prior to week 52. AEs were summarized as the number and percentage of patients experiencing AEs, using Medical Dictionary for Regulatory Activities (version 15.1).
Adalimumab serum concentrations were summarized by treatment groups at each time point using descriptive statistics. A patient was considered to be AAA+ if the patient had at least 1 AAA concentration >20 ng/ml and the sample was collected within 30 days after an adalimumab dose. The number and percentage of patients who became AAA+ were determined. Data collected through week 52 were included in this analysis.
RESULTS
Patient disposition and baseline characteristics
Forty‐six patients were randomized, 15 to placebo and 31 to adalimumab (Figure 1). All patients received the study drug. No patients discontinued the study during the double‐blind period; however, 7 patients early escaped and entered open‐label adalimumab treatment (3 placebo, 4 adalimumab). Three patients (2 adalimumab, 1 placebo) started open‐label adalimumab at week 4, and 4 patients (2 adalimumab, 2 placebo) started at week 8 (Figure 1). Through week 52, 3 of 46 patients (6.5%) discontinued the study during the open‐label period, all due to AEs, 1 patient randomized to placebo treatment and 2 randomized to adalimumab (one of whom reported lack of efficacy as the primary reason for discontinuation).
Figure 1.
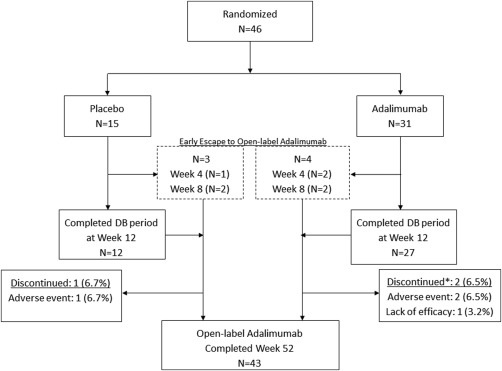
Patient disposition through week 52. DB = double‐blind; * = a patient may have had more than 1 reason for study discontinuation.
Demographics and disease characteristics were comparable between treatment groups (Table 1), with no significant differences noted between adalimumab and placebo patients. The majority of patients were male and white, and the mean ± SD age was 12.9 ± 2.9 years with an average duration of symptoms of 2.6 ± 2.3 years. Patients had a moderate to high level of disease activity as demonstrated by a mean ± SD AJC of 7.8 ± 6.6, mean ± SD enthesitis count of 8.1 ± 8.4, and mean parent's assessment of patient's pain and PGA both greater than 50 (0–100 mm). HLA–B27 was positive in 67.4% of patients. All patients had prior treatment with NSAIDs; 93% and 90% of the placebo and adalimumab groups, respectively, had previously used DMARDs. The majority of patients in both groups received a concomitant NSAID or DMARD at baseline.
Table 1.
Baseline demographics and disease characteristicsa
| Placebo (n = 15) | Adalimumab (n = 31) | P b | |
|---|---|---|---|
| Demographics | |||
| Male, no. (%) | 9 (60.0) | 22 (71.0) | 0.514 |
| White, no. (%) | 10 (66.7) | 25 (80.6) | 0.462 |
| Age, years | 11.9 ± 2.9 | 13.4 ± 2.9 | 0.091 |
| HLA–B27 positive, no. (%) | 11 (73.3) | 18 (64.3) | 0.869 |
| Concomitant NSAIDs, no. (%)c | 14 (93.3) | 27 (87.1) | 1.000 |
| Concomitant DMARDs, no. (%)c | 11 (73.3) | 21 (67.7) | 1.000 |
| Sulfasalazine | 3 (20.0) | 6 (19.4) | |
| Methotrexate | 8 (53.3) | 16 (51.6) | |
| Disease characteristics | |||
| Symptom duration, years | 2.7 ± 2.5 | 2.6 ± 2.3 | 0.868 |
| Duration since diagnosis, years | 2.2 ± 2.4 | 1.7 ± 1.9 | 0.389 |
| Active joints with arthritis (0–68) | 6.7 ± 5.3 | 8.4 ± 7.1 | 0.411 |
| Tender joint count (0–72) | 11.9 ± 9.3 | 13.4 ± 10.5 | 0.658 |
| Swollen joint count (0–68) | 5.2 ± 3.7 | 6.7 ± 7.3 | 0.446 |
| Joints with loss of motion (0–66) | 4.5 ± 4.1 | 5.1 ± 3.2 | 0.550 |
| Total enthesitis count (0–35) | 7.8 ± 7.5 | 8.3 ± 8.9 | 0.855 |
| MASES (0–13) | 3.0 ± 3.4 | 3.5 ± 4.2 | 0.659 |
| SPARCC enthesitis index (0–16) | 4.3 ± 3.5 | 4.5 ± 3.8 | 0.854 |
| Patient's assessment of total back pain (0–100) | 34.9 ± 30.5 | 35.4 ± 30.0 | 0.962 |
| Physician's global assessment of disease activity (0–100) | 52.6 ± 20.5 | 53.3 ± 22.5 | 0.917 |
| Parent's global assessment of patient's well‐being (0–100) | 49.0 ± 20.8 | 52.6 ± 25.2 | 0.633 |
| Parent's assessment of patient's pain (0–100) | 52.7 ± 27.2 | 57.3 ± 21.0 | 0.529 |
| C‐HAQ (0–3) | 0.8 ± 0.5 | 0.8 ± 0.7 | 0.996 |
| hsCRP, mg/liter | 14.4 ± 23.7 | 6.3 ± 10.1 | 0.109 |
| Dactylitis count (0–20) | 0.1 ± 0.3 | 0.4 ± 1.5 | 0.367 |
| BASDAI (0–10) | 4.7 ± 2.5 | 4.7 ± 2.5 | 0.947 |
Values are the mean ± SD unless indicated otherwise. NSAIDs = nonsteroidal antiinflammatory drugs; DMARDs = disease‐modifying antirheumatic drugs; MASES = Maastricht Ankylosing Spondylitis Enthesitis Score; SPARCC = Spondyloarthritis Canadian Research Consortium; C‐HAQ = Childhood Health Assessment Questionnaire; hsCRP = high‐sensitivity C‐reactive protein; BASDAI = Bath Ankylosing Spondylitis Disease Activity Index.
P values for categorical variables are from Fisher's exact test; P values for continuous variables from one‐way analysis of variance with treatment in the model.
At any time during treatment through week 52.
Efficacy
Mean percent decrease in AJC from baseline to week 12 was greater in the adalimumab group (−62.6%) compared to placebo (−11.6%) (P = 0.039). Sensitivity analyses excluding patients who started early escape treatment (adalimumab −83.3 versus placebo −32.1; P = 0.018), and applying nonparametric testing (adalimumab median −88.9 versus placebo median −50.0, P = 0.025), produced similar results. Treatment response was maintained with adalimumab therapy in the open‐label period (Figure 2A) with 88.7% mean reduction in AJC from baseline to week 52.
Figure 2.
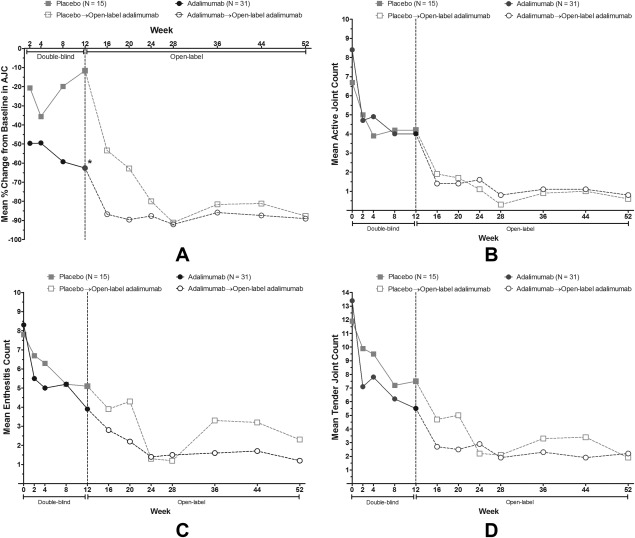
Clinical response during the 12‐week, double‐blind period and 40‐week, open‐label adalimumab period. A, mean percent change from baseline in active joints with arthritis (AJC) by treatment over time through week 52; * indicates P = 0.039, adalimumab versus placebo. B, mean active joint count by treatment over time through week 52. C, mean enthesitis count by treatment over time through week 52. D, mean tender joint count by treatment over time through week 52. Analyses are last observation carried forward.
Decreases in AJC and enthesitis count continued through week 52 with a mean ± SD AJC of only 0.7 ± 2.0 (Figure 2B) and mean ± SD enthesitis count of 1.5 ± 4.9 at week 52 (Figure 2C), compared to 7.8 ± 6.6 and 8.1 ± 8.4 at baseline, respectively. At week 52 patients who received placebo for the first 12 weeks followed by 40 weeks of open‐label adalimumab achieved approximately the same improvement in joint counts as patients who received adalimumab for the entire 52 weeks (Figures 2B and 2D). Most secondary variables showed numerically greater, but not statistically significant, improvement at week 12 in favor of adalimumab compared to placebo (Table 2). The majority demonstrated trends in favor of adalimumab, with results being sustained or improving further during the open‐label period through week 52.
Table 2.
Mean change from baseline in clinical end points and ACR Pediatric responder statusa
| Double‐blind period | Open‐label period | |||
|---|---|---|---|---|
| Placebo (n = 15), week 12 | Adalimumab (n = 31), week 12 | P b | Adalimumab (n = 46), week 52 | |
| Tender joint count (0–72) | −4.5 ± 9.0 | −7.9 ± 8.3 | 0.209 | −10.8 ± 9.5 |
| Swollen joint count (0–68) | −2.4 ± 4.7 | −3.5 ± 5.6 | 0.509 | −5.7 ± 6.3 |
| Joints with loss of motion (0–66) | −1.1 ± 3.8 | −3.3 ± 3.9 | 0.077 | −3.4 ± 3.8 |
| Total enthesitis count (0–35) | −2.7 ± 5.0 | −4.4 ± 6.2 | 0.382 | −6.6 ± 7.6 |
| MASES (0–13) | −0.7 ± 2.3 | −1.7 ± 2.6 | 0.208 | −2.6 ± 3.3 |
| SPARCC enthesitis index (0–16) | −2.4 ± 2.7 | −2.6 ± 3.3 | 0.804 | −3.7 ± 3.2 |
| Dactylitis count (0–20) | 0.0 | −0.4 ± 1.5 | 0.380 | −0.1 ± 1.7 |
| Patient assessment of total back pain (0–100) | −9.5 ± 23.9 | −14.6 ± 24.2 | 0.518 | −21.5 ± 33.0 |
| Physician's global assessment of disease activity (0–100) | −22.1 ± 23.3 | −31.4 ± 24.8 | 0.231 | −45.7 ± 23.9 |
| Parent's global assessment of patient's well‐being (0–100) | −16.5 ± 10.5 | −29.2 ± 29.8 | 0.117 | −35.5 ± 28.2 |
| Parent's assessment of patient's pain (0–100) | −19.9 ± 21.7 | −32.5 ± 29.0 | 0.142 | −40.2 ± 30.5 |
| C‐HAQ (0–3) | −0.1 ± 0.4 | −0.2 ± 0.6 | −0.4 ± 0.6 | |
| hsCRP, mg/liter | 4.8 ± 23.1 | 0.4 ± 16.4 | 0.378 | −7.1 ± 16.0 |
| Response status, no. (%)c | ||||
| ACR Pedi 30 response | 9 (60.0) | 22 (71.0) | 0.514 | 39 (84.8) |
| ACR Pedi 50 response | 6 (40.0) | 21 (67.7) | 0.111 | 39 (84.8) |
| ACR Pedi 70 response | 3 (20.0) | 17 (54.8) | 0.031 | 35 (76.1) |
| ACR Pedi 90 response | 2 (13.3) | 13 (41.9) | 0.092 | 28 (60.9) |
| BASDAI 50 response | 4 (26.7) | 19 (61.3) | 0.057 | 33 (71.7) |
Values are the mean ± SD unless indicated otherwise. ACR = American College of Rheumatology; MASES = Maastricht Ankylosing Spondylitis Enthesitis Score; SPARCC = Spondyloarthritis Canadian Research Consortium; C‐HAQ = Childhood Health Assessment Questionnaire; hsCRP = high‐sensitivity C‐reactive protein; Pedi 30 = ACR Pediatric criteria for 30% improvement; BASDAI 50 = Bath Ankylosing Spondylitis Disease Activity Index criteria for 50% improvement.
P values for categorical variables are from Fisher's exact test; P values for continuous variables from one‐way analysis of variance with treatment in the model.
Nonresponder imputation analysis.
Statistically significant improvement in ACR Pedi 70 and numerically superior ACR Pedi 30/50/90 response rates were observed in favor of adalimumab at week 12 (Table 2). During the open‐label period, more than 80% of patients achieved ACR Pedi 30 and ACR Pedi 50, more than 75% achieved ACR Pedi 70, and more than 60% achieved ACR Pedi 90 at week 52.
During treatment with adalimumab through week 52, 93.5% of patients achieved complete resolution of their swollen joints (SJC = 0) (Figure 3A), with a median of 41 days of adalimumab treatment to achieve SJC = 0. Similar percentages achieved TJC = 0 and enthesitis count = 0 (82.6% and 87.0%, respectively) (Figures 3B and 3C). Median treatment duration to achieve TJC = 0 was 108 days, and to achieve enthesitis count = 0 was 56 days. During the open‐label adalimumab treatment period through week 52, 16 patients (35%) were able to discontinue concomitant NSAIDs, 5 patients (11%) discontinued concomitant DMARDs (2 SSZ, 3 MTX), and 7 patients (15%) stopped corticosteroids without needing to restart these therapies.
Figure 3.
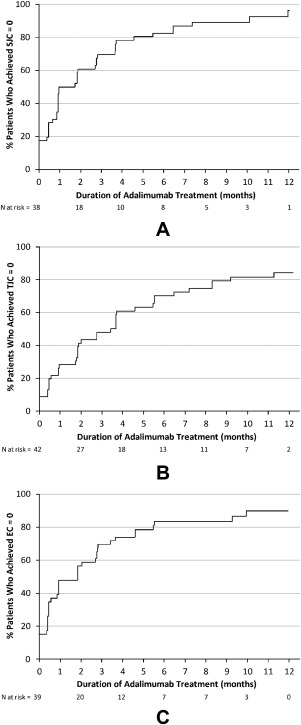
Kaplan Meier curves for time to achieve joint or enthesitis counts = 0, from first adalimumab injection through week 52. A, time to achieve swollen joint count (SJC) = 0. B, time to achieve tender joint count (TJC) = 0. C, time to achieve total enthesitis count (EC) = 0. N = number of patients.
Safety
Mean duration of treatment was 338.2 days for patients who received adalimumab at any time through week 52 (blinded or open‐label). During the double‐blind period, similar proportions of patients in the 2 treatment groups experienced any AE (Table 3). The most common events (reported by ≥2 patients in a group) in the placebo group were upper respiratory tract infections (13.3%) and headache (12.9%). Within the adalimumab group, common AEs included upper respiratory tract infection, injection site pain, and increased alanine aminotransferase (ALT) in 9.7%, and gastroenteritis, upper abdominal pain, nausea, and syncope in 6.5% of patients. One patient on adalimumab reported serious AEs of upper abdominal pain and headache during the double‐blind period. This patient also later reported serious AEs of worsening of underlying ERA and pain in the open‐label period, which caused the patient to prematurely discontinue from the study. Three other patients reported serious AEs during the open‐label period that included appendicitis, concussion, and musculoskeletal chest pain (in a patient who had early‐escaped from placebo to open‐label adalimumab at week 8). One patient reported a serious AE of worsening of underlying ERA 32 days after discontinuing treatment. Other than infections, which were those commonly observed in the general population, none of the other risks associated with anti‐TNFs were reported. During the double‐blind period, 1 patient in the adalimumab group experienced hepatic cytolysis. This patient had transient elevations in ALT beginning at screening and at most study visits, with a maximum value 3.7 times the upper limit of normal (ULN) occurring on day 30, at which time aspartate aminotransferase was 1.87 times the ULN. Total bilirubin and alkaline phosphatase were not elevated during the study. During the open‐label period through week 52, 1 patient each reported events of cutaneous vasculitis and new‐onset psoriasis. There were no malignancies, opportunistic infections, tuberculosis, lupus‐like syndrome, demyelinating disease, or deaths reported through week 52.
Table 3.
Number and percentage of patients with adverse events through week 12 and week 52a
| Double‐blind period | Any adalimumab (n = 46)b | ||
|---|---|---|---|
| Placebo (n = 15) | Adalimumab (n = 31) | ||
| Any adverse event | 8 (53.3) | 21 (67.7) | 43 (93.5) |
| Serious adverse event | 0 | 1 (3.2) | 5 (10.9) |
| Adverse event leading to discontinuation of study drug | 0 | 0 | 3 (6.5) |
| Infectious adverse event | 3 (20.0) | 9 (29.0) | 37 (80.4) |
| Serious infection | 0 | 0 | 1 (2.2) |
| Hepatic‐related adverse event | 0 | 1 (3.2) | 1 (2.2) |
Values are the number (percentage).
Adverse events from first dose of adalimumab through week 52.
Immunogenicity and pharmacokinetics
Mean serum adalimumab trough concentrations at steady‐state for pediatric patients with ERA were 7.5–11.8 μg/ml between weeks 12 and 52. Serum adalimumab concentrations appeared to be slightly higher in the adalimumab treatment group taking concomitant MTX (9.7–11.8 μg/ml, n = 16) compared to the non‐MTX group (7.5–9.4 μg/ml, n = 15).
During the 52‐week period, an overall AAA+ rate of 10.9% (5 of 46) was observed. Two of the AAA+ patients received placebo for the first 12 weeks, followed by adalimumab (1 with concomitant MTX and 1 without) and 3 of the AAA+ patients received adalimumab for the 52‐week period (1 with concomitant MTX and 2 without). Week 52 mean AJC reduction in this small sample of AAA+ patients was not affected by the presence of AAA.
DISCUSSION
While the prevalence of chronic, inflammatory arthritis in children is difficult to estimate because of differences in nomenclature between studies, it is estimated that approximately 0.07 to 4.01 per 1,000 children have JIA 32 and that between 3% and 22% of these have ERA 33, 34, although estimates vary widely 35, 36, 37. Long‐term effects of ERA, such as pain, fatigue, and poor physical function, negatively impact quality of life 2, with few effective drugs available to reduce disease activity and improve physical functioning and the quality of life of these children. Evidence only shows a slight positive effect of SSZ in ERA, specifically the PGA 9, and published data are based on treatment response in other JIA categories and SpA 38. Therefore, biologic therapy has become an increasingly important treatment option for refractory ERA.
This is the first randomized controlled study in the ERA patient population prospectively identified by ILAR criteria. Results demonstrate that adalimumab improved the signs and symptoms of disease in children and adolescents with ERA. In these NSAID‐ and DMARD‐refractory or intolerant patients, adalimumab was significantly better than placebo in reducing AJC at 12 weeks, with improvement sustained with continued adalimumab therapy through week 52.
Adalimumab also resulted in improvements in other signs and symptoms of ERA at week 12 with improvements continuing through week 52 of the study, most of which did not achieve statistical significance. The more common design of JIA studies is open‐label with treatment withdrawal and time to flare. As results from randomized, controlled, double‐blind studies have the highest evidence level and can be interpreted as true treatment effects, the results of this study are more robust than those observed in withdrawal studies. Due to ethical concerns with enrolling more pediatric patients in a placebo‐controlled trial than are needed to address the primary hypothesis, the study was not statistically powered to detect differences in the secondary variables. However, the positive trends observed support the efficacy of adalimumab in the ERA patient population and are important in this population with severe disease and limited treatment options. While numerically superior response rates in ACR Pedi 30/50/90 were observed, adalimumab performed significantly better than placebo in the more stringent measure of the ACR Pedi 70. Change from baseline in SJC, TJC, and enthesitis count did not reach statistical significance due to low baseline values in some patients, making change more difficult to detect; however, more than 80% of patients treated with adalimumab had complete resolution of swollen joints, tender joints, or enthesitis (achieved SJC, TJC, or enthesitis count of 0) by week 52, with SJC improving faster than TJC or enthesitis count. Clinical improvement during the first 52 weeks of the study resulted in 35% of patients being able to discontinue NSAIDs.
Safety results were similar to those obtained in adalimumab trials of polyarticular JIA 23, 24, 25 and adults with rheumatoid arthritis and SpA 26, 27, 39. No AEs were identified that were unique to patients with ERA. Infections commonly observed in the general population were the most frequently reported AEs. Few events identified as risks with anti‐TNF therapy were observed, and no tuberculosis, malignancies, or deaths were reported.
Serum adalimumab concentrations were comparable to those observed in patients ages 2–17 years with polyarticular JIA 24, 25. Although sustained efficacy in AAA+ patients was observed, the number of AAA+ patients was too small to provide meaningful assessment of the impact of immunogenicity on adalimumab trough concentrations, efficacy, and safety in the ERA patient population.
This study has several limitations. A small number of patients were available for study enrollment due to the rarity of the disease. ERA patients tend to have few joints involved, although in our refractory population, the mean AJC at baseline was high, indicating more severe disease 2, 40 and reflecting longer disease duration 41. While there were no statistical differences in baseline disease activity between the placebo and adalimumab treatment groups, it is acknowledged that there is potential for numerical differences to affect outcome, given the small sample size of the study. With the exception of high‐sensitivity C‐reactive protein (hsCRP), we believe these small imbalances are unlikely to have biased the study, as most differences are small, not clinically meaningful, and are not likely to affect the probability to detect a difference between treatment groups. Not all patients had elevated inflammatory markers at baseline, even in the context of active disease. This may have limited the ability to observe statistically significant ACR Pedi responses and decreases in hsCRP. Additionally, 40% of the patients had oligoarthritis at baseline and, as ACR pediatric response criteria were designed to evaluate disease in patients with polyarthritis, observable change would have been more likely if all patients had higher baseline AJCs. While an important proportion had an oligoarticular disease course, results should be extrapolated with caution to patients with fewer than 3 active joints (requirement for study inclusion), as many ERA patients present with oligoarticular disease. Importantly, patients with fewer than 3 active joints were excluded from the study as they would be likely to respond to limited intraarticular corticosteroid injections and not require systemic therapy. Furthermore, patients in this study did not have imaging performed to document development of axial involvement over time. Future studies should be done in ERA to evaluate the effect of biologic treatment on the occurrence and progression of axial involvement.
In summary, 12 weeks of adalimumab therapy reduced the signs and symptoms of ERA, with improvement sustained with continued adalimumab therapy through week 52. The safety profile of adalimumab observed in pediatric patients with ERA was consistent with that observed in patients age ≥2 years treated for polyarticular JIA. The efficacy and safety results from this study suggest that adalimumab may be an appropriate treatment option for patients with active ERA who have failed conventional treatments.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Burgos‐Vargas had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Burgos‐Vargas, Tse, Pangan, Unnebrink, Anderson.
Acquisition of data
Burgos‐Vargas, Tse, Horneff, Pangan, Kalabic, Unnebrink, Anderson.
Analysis and interpretation of data
Burgos‐Vargas, Tse, Horneff, Pangan, Kalabic, Goss, Unnebrink, Anderson.
ROLE OF THE STUDY SPONSOR
AbbVie funded the study, contributed to its design, and was involved in the collection, analysis, and interpretation of the data, and in the writing, review, and approval of the manuscript. Medical writing support was provided by Kathleen V. Kastenholz, PharmD, MS, and Gaurav Patki, PhD, of AbbVie. Statistical programming support was provided by Angelika Freitag, a former employee of AbbVie, and Carina Zimmermann, of AbbVie.
ClinicalTrials.gov identifier: NCT01166282.
REFERENCES
- 1. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 2. Flato B, Hoffmann‐Vold AM, Reiff A, Forre O, Lien G, Vinje O. Long‐term outcome and prognostic factors in enthesitis‐related arthritis: a case‐control study. Arthritis Rheum 2006;54:3573–82. [DOI] [PubMed] [Google Scholar]
- 3. Sarma PK, Misra R, Aggarwal A. Outcome in patients with enthesitis related arthritis (ERA): juvenile arthritis damage index (JADI) and functional status. Pediatr Rheumatol Online J 2008;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone M, Warren RW, Bruckel J, Cooper D, Cortinovis D, Inman RD. Juvenile‐onset ankylosing spondylitis is associated with worse functional outcomes than adult‐onset ankylosing spondylitis. Arthritis Rheum 2005;53:445–51. [DOI] [PubMed] [Google Scholar]
- 5. Tupper SM, Rosenberg AM, Pahwa P, Stinson JN. Pain intensity variability and its relationship with quality of life in youths with juvenile idiopathic arthritis. Arthritis Care Res 2013;65:563–70. [DOI] [PubMed] [Google Scholar]
- 6. Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schontube M, et al. Long‐term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum 2002;46:2392–401. [DOI] [PubMed] [Google Scholar]
- 7. Weiss PF, Beukelman T, Schanberg LE, Kimura Y, Colbert RA. Enthesitis‐related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: the Childhood Arthritis and Rheumatology Research Alliance Registry. J Rheumatol 2012;39:2341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tse SM, Burgos‐Vargas R, Colbert RA. Juvenile spondyloarthritis treatment recommendations. Am J Med Sci 2012;343:367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burgos‐Vargas R, Vazquez‐Mellado J, Pacheco‐Tena C, Hernandez‐Garduno A, Goycochea‐Robles MV. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies [abstract]. Ann Rheum Dis 2002;61:941–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burgos‐Vargas R, Casasola‐Vargas JC, Gutierrez‐Suarez R, Vazquez‐Mellado J. An open, observational, extension study of a three‐month, randomized, placebo‐controlled trial to assess the long‐term efficacy and safety of infliximab in juvenile‐onset spondyloarthritis (Jo‐Spa) [abstract]. Arthritis Rheum 2008;58 Suppl:S578. [Google Scholar]
- 11. Henrickson M, Reiff A. Prolonged efficacy of etanercept in refractory enthesitis‐related arthritis. J Rheumatol 2004;31:2055–61. [PubMed] [Google Scholar]
- 12. Tse SM, Burgos‐Vargas R, Laxer RM. Anti–tumor necrosis factor α blockade in the treatment of juvenile spondylarthropathy. Arthritis Rheum 2005;52:2103–8. [DOI] [PubMed] [Google Scholar]
- 13. Hugle B, Burgos‐Vargas R, Inman RD, O'Shea F, Laxer RM, Stimec J, et al. Long‐term outcome of anti‐tumor necrosis factor alpha blockade in the treatment of juvenile spondyloarthritis. Clin Exp Rheumatol 2014;32:424–31. [PubMed] [Google Scholar]
- 14. Tse SM, Laxer RM, Babyn PS, Doria AS. Radiologic improvement of juvenile idiopathic arthritis enthesitis–related arthritis following anti–tumor necrosis factor‐α blockade with etanercept. J Rheumatol 2006;33:1186–8. [PubMed] [Google Scholar]
- 15. Sulpice M, Deslandre CJ, Quartier P. Efficacy and safety of TNFα antagonist therapy in patients with juvenile spondyloarthropathies. Joint Bone Spine 2009;76:24–7. [DOI] [PubMed] [Google Scholar]
- 16. Schmeling H, Horneff G. Infliximab in two patients with juvenile ankylosing spondylitis. Rheumatol Int 2004;24:173–6. [DOI] [PubMed] [Google Scholar]
- 17. Otten MH, Prince FH, Twilt M, Ten Cate R, Armbrust W, Hoppebnreijs EP, et al. Tumor necrosis factor–blocking agents for children with enthesitis‐related arthritis: data from the Dutch Arthritis and Biologicals in Children Register, 1999–2010. J Rheumatol 2011;38:2258–63. [DOI] [PubMed] [Google Scholar]
- 18. Burgos‐Vargas R, Casasola‐Vargas J, Gutierrez‐Suarez R, Vazquez‐Mellado J. Efficacy, safety, and tolerability of infliximab in juvenile‐onset spondyloarthropathies (JO‐SpA): results of the three‐month, randomized, double‐blind, placebo‐controlled trial phase [abstract]. Arthritis Rheum 2007;56 Suppl:S319. [Google Scholar]
- 19. Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle‐Deschner J, Tzaribacev N, et al. Double‐blind, placebo‐controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther 2012;14:R230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horneff G, Burgos‐Vargas R, Constantin T, Foeldvari I, Vojinovic J, Chasnyk VG, et al. Efficacy and safety of open‐label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis‐related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Ann Rheum Dis 2014;73:1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Papsdorf V, Horneff G. Complete control of disease activity and remission induced by treatment with etanercept in juvenile idiopathic arthritis. Rheumatology (Oxford) 2011;50:214–21. [DOI] [PubMed] [Google Scholar]
- 22. Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF‐α inhibitor therapy for juvenile idiopathic arthritis: enthesitis‐related arthritis predicts persistent active disease. J Rheumatol 2011;38:2675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med 2008;359:810–20. [DOI] [PubMed] [Google Scholar]
- 24. Imagawa T, Takei S, Umebayashi H, Yamaguchi K, Itoh Y, Kawai T, et al. Efficacy, pharmacokinetics, and safety of adalimumab in pediatric patients with juvenile idiopathic arthritis in Japan. Clin Rheumatol 2012;31:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kingsbury DJ, Bader‐Meunier B, Patel G, Aroro V, Kalabic J, Kupper H. Safety, effectiveness, and pharmacokinetics of adalimumab in children with polyarticular juvenile idiopathic arthritis aged 2 to 4 years. Clin Rheumatol 2014;33:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2006;54:2136–46. [DOI] [PubMed] [Google Scholar]
- 27. Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non‐radiographic axial spondyloarthritis: results of a randomised placebo‐controlled trial (ABILITY‐1). Ann Rheum Dis 2013;72:815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heuft‐Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van der Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 30. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 31. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 32. Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis: why does it vary so much? J Rheumatol 2002;29:1520–30. [PubMed] [Google Scholar]
- 33. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 34. Boiu S, Marniga E, Bader‐Meunier B, Mouy R, Compeyrot‐Lacassagne S, Quartier P, et al. Functional status in severe juvenile idiopathic arthritis in the biologic treatment era: an assessment in a French paediatric rheumatology referral centre. Rheumatology (Oxford) 2012;51:1285–92. [DOI] [PubMed] [Google Scholar]
- 35. Foeldvari I, Bidde M. Validation of the proposed ILAR classification criteria for juvenile idiopathic arthritis: International League of Associations for Rheumatology. J Rheumatol 2000;27:1069–72. [PubMed] [Google Scholar]
- 36. Danner S, Sordet C, Terzic J, Donato L, Velten M, Fischbach M, et al. Epidemiology of juvenile idiopathic arthritis in Alsace, France. J Rheumatol 2006;33:1377–81. [PubMed] [Google Scholar]
- 37. Stabile A, Avallone L, Compagnone A, Ansuini V, Bertoni B, Rigante D. Focus on juvenile idiopathic arthritis according to the 2001 Edmonton revised classification from the International League of Associations for Rheumatology: an Italian experience. Eur Rev Med Pharmacol Sci 2006;10:229–34. [PubMed] [Google Scholar]
- 38. Brooks CD. Sulfasalazine for the management of juvenile rheumatoid arthritis. J Rheumatol 2001;28:845–53. [PubMed] [Google Scholar]
- 39. Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long‐term safety in 23,458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Windschall D, Muller T, Becker I, Horneff G. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis‐related arthritis and psoriatic arthritis. Clin Rheumatol 2015;34:61–9. [DOI] [PubMed] [Google Scholar]
- 41. Burgos‐Vargas R, Vazquez‐Mellado J. The early clinical recognition of juvenile‐onset ankylosing spondylitis and its differentiation from juvenile rheumatoid arthritis. Arthritis Rheum 1995;38:835–44. [DOI] [PubMed] [Google Scholar]


