Abstract
Evidence‐based and patient‐centred health care movements have each enhanced the discussion of how health care might best be delivered, yet the two have evolved separately and, in some views, remain at odds with each other. No clear model has emerged to enable practitioners to capitalize on the advantages of each so actual practice often becomes, to varying degrees, an undefined mishmash of each. When faced with clinical uncertainty, it becomes easy for practitioners to rely on formulas for care developed explicitly by expert panels, or on the tacit ones developed from experience or habit. Either way, these tendencies towards ‘cookbook’ medicine undermine the view of patients as unique particulars, and diminish what might be considered patient‐centred care. The sequence in which evidence is applied in the care process, however, is critical for developing a model of care that is both evidence based and patient centred. This notion derives from a paradigm for knowledge delivery and patient care developed over decades by Dr. Lawrence Weed. Weed's vision enables us to view evidence‐based and person‐centred medicine as wholly complementary, using computer tools to more fully and reliably exploit the vast body of collective knowledge available to define patients’ uniqueness and identify the options to guide patients. The transparency of the approach to knowledge delivery facilitates meaningful practitioner–patient dialogue in determining the appropriate course of action. Such a model for knowledge delivery and care is essential for integrating evidence‐based and patient‐centred approaches.
Keywords: evidence‐based medicine, medical informatics, patient‐centred care
It is now almost assumed that clinical decisions will be ‘evidence based’, so ensconced is the concept in the narratives of clinical care and education. Just what that means in practice, however, is another question. Evidence‐based medicine (EBM) gives primacy to evidence and knowledge derived from clinical and science research, while de‐emphasizing the role of idiosyncratic experiences, intuitions and judgments of practitioners in the clinical setting. Over several decades, the EBM paradigm [1, 2, 3] has underpinned the generation of thousands of guidelines and protocols designed to provide recommendations to clinicians regarding how best to manage patients with various conditions.
The limitations of the EBM paradigm also have become more apparent over time [2, 3, 4, 5, 6, 7]. Some suggest that EBM is at odds with another parallel movement towards care that is ‘patient centred’ that has arisen, in part at least, from concerns that scientific and technological advance contributes to more impersonal, fragmented clinical practice [8, 9, 10, 11, 12, 13, 14]. Rather than giving primacy to knowledge derived from research, the patient‐centred care (PCC) paradigm operates within a humanistic framework that considers what emerges from clinical interactions – for example, values, preferences and aspirations – as equally critical in the patient care process.
More and more acknowledge the need to join EBM and PCC in clinical practice, but clear models that bring them together have largely been absent. After briefly describing key difficulties EBM poses with respect to PCC, this essay suggests that complementarity between the two can be found within the alternative paradigm proffered by Dr. Lawrence Weed, father of the problem‐oriented medical record and originator of the problem‐knowledge coupling approach to clinical decision support [15, 16, 17, 18]. In this view, the sequence involved in the application of evidence in the patient care process is critical to this complementarity – that is, using collective knowledge embodied in external tools to clearly define patients’ unique complexity before activating clinical experience, intuition, and judgment in meaningful dialogue with patients. The paper concludes by suggesting that there is a significant discrepancy between how knowledge currently is applied (or not applied) to patient care versus how it could be applied if computer tools were deployed in a systematic way. As the discrepancy becomes more apparent, leaders in health care might look to the vision Weed has championed for decades.
EBM and PCC
While EBM's view that scientific evidence trumps practitioners’ experiences and intuition has moderated somewhat in light of recent criticisms, in everyday practice, it becomes easy to consider evidence‐based guidelines as overly prescriptive [7, 14, 19, 20]. The conditions under which clinical practices function pose real challenges to PCC. Clinical circumstances often are increasingly complex and uncertain, making it easy for practitioners to turn to established protocols and to perceive patients in stereotypic, formulaic ways [20]. Pay‐for‐performance and other financial incentives encourage adherence to guidelines, while threats of litigation deter deviations from them [7, 9]. A risk‐averse, ‘cookbook’ approach to care becomes a safer path; defaulting to established guidelines shifts clinical decision making from the ‘consultation room’ to the ‘professional association’ [8].
Most would agree that caregivers should always show certain qualities that characterize PCC: ‘compassion, empathy, and responsiveness to the needs, values, and expressed preferences of the individual patient’ [13]. In this regard, ‘all medicine should be patient‐centred’ [8]. Perhaps, it only needs to be highlighted because of the severity of the threats to it and the pervasive ‘impersonal’ care that often characterizes everyday clinical medicine. Apart from involving compassion, empathy and the recognition of patients’ preferences in decision making, optimal decision making also would require that the full range of evidence is consistently delivered and considered in the care of patients. Yet, the volume of evidence that might bear on a particular problem far exceeds what practitioners, left to their own devices, can consider. The options available for patients are limited to those the practitioner knows and recalls, reducing patient choice and the opportunities for their values to be realized.
While several deficiencies in EBM are now widely recognized, practitioners are still expected to fully consider what clinical and basic science research might offer in the care of patients. Complementarity between EBM and PCC is required for either to be fully actualized [3, 11]. EBM's incompleteness lies in having inadequately articulated where the evidence of EBM belongs in the clinical care process. How do you apply general knowledge to ‘unique’ individual patients? As suggested below, ‘the sequence in which evidence is considered is crucial’ [17].
Partitioning EBM and PCC: evidence‐based inputs to inform patient‐centred decisions
Consistent, high‐quality decision making that is both evidence based and assures patient centredness requires that a systematic approach be taken to assure that the information that forms the basis for each decision is of high quality. The sequence used for the deployment of evidence is critical to this, establishing the foundation for patient‐centred decision making. In his various writings, Weed describes such a system [15, 16, 17, 18]. As outlined in Fig. 1, it would (1) develop computer tools to define what data to collect from patients with a particular problem; (2) disseminate those tools to clinical practices to collect data from patients, and then (3) use the tools to align patient‐specific findings with diagnostic or management options. The output of this process for each patient would inform (4) the patient–provider dialogue and the judgment exercised in decision making. Each step is described in greater detail below.
Figure 1.
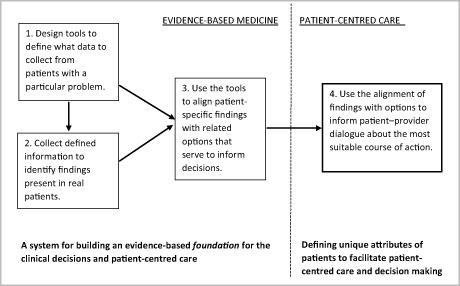
Partitioning EBM and PCC.
Step 1: using collective knowledge to identify what information to collect
Learning the clinically relevant details of each patient's case is an essential first step in deciding how best to address the problem presented, and the first order of business when it comes to providing evidence‐informed, patient‐centred care. Tradition assumes that experts will reliably produce a thorough patient database on which to base decisions going forward: ‘the expert makes a rapid initial differential diagnosis through intuition, and then uses a more selective history, examination and set of tests to rule in or rule out particular possibilities’ [7]. Yet, the assertion is based more on faith than on reason and evidence. Doctors naturally vary in terms of their background, clinical experience and expertise. Further, all are prone to the flaws, biases and memory limitations that characterize human decision making [21, 22, 23, 24, 25]. The human mind is inadequate for undertaking the information processing task required to thoroughly and reliably delineate the various dimensions of the patient's problem. If the thoroughness and quality of the initial work‐up is variable, the quality of the decisions they are based on will be as well.
Defining standards for the collection of data, embodying them in information tools, and disseminating them for use across clinical practices, can alleviate this difficulty. Expert teams can assess the research evidence to identify the possible diagnostic or management options known to be associated with the problem (not just those that are common in the general population), along with the positive findings that would enable one to discriminate among them. The task would involve assessing the costs and value of gathering a particular piece of information. Collecting information from patients about the problem history would cost little; expensive or invasive tests would be requested only if they yield information that is critical for discrimination among options. The information determined to be most relevant and cost‐effective would define that standard for the patient work‐up.
In step 1, choices surrounding what information to collect from patients – the first, crucial step in clinical problem solving – can be determined in a deliberate fashion before any patient enters a clinic. Importantly, this removes the effects of human flaws, biases and memory limitations, and ensures that all relevant options are consistently considered. The investment in building the knowledge infrastructure would be substantial, but, once developed, maintaining and disseminating the knowledge tools would be comparatively low.
Step 2: collecting information from real patients – defining their unique complexity
Embodying these choices in external information tools would enable their wide dissemination and use in clinical practice. Step 2 involves using the tools to collect relevant data from actual patients. This has been shown to work successfully in primary care, employing principles of high reliability organizations [26, 27]. Most patients can readily complete problem‐specific online questionnaires to provide a comprehensive picture of the history of the problem and submit it in advance of the office visit; in‐office accommodations can be made for those unable to. This also enables patients to more fully prepare for their appointment, and to make a more considered assessment of their situation in completing their problem history than would be feasible during the office visit. Where appropriate, relevant laboratory data is obtained and submitted in advance of the office visit as well. Even greater efficiencies in the data collection phase will likely emerge as computer interfaces become more sophisticated along with the savviness of the patients who use them. The rise of mobile apps offers additional promise for enhancing individuals’ role in their own health and health care [28]. Notwithstanding real questions about the thoroughness and credibility of the knowledge network upon which the recommendations are made [29], this reflects consumers’ central interest in managing their own health, along with their willingness and ability to monitor and take charge of it.
By using the patient as a resource prior to the actual visit, considerably more information about the problem can be collected at little or no cost to the practice. During the visit, positive findings can be reviewed and physical findings gathered by the practitioner or an assistant; practitioners can add their own observations and impressions to the history if deemed necessary. Standardizing the process of data collection in this way enables a consistent, well‐defined and comprehensive characterization of the patients’ present a problem. Presumably, the results also would be situated within the medical record, offering a larger context of the patient's situation. The complex uniqueness of each patient is better shown when a broad range of data is collected during the initial work‐up, rather than the narrower range of information that the practitioner is able to gather within the confines of the office visit. It circumvents problems associated with human reasoning mentioned above, and limits arbitrary variation in what data are collected. Patients become involved earlier in a more efficient care process, and can be confident in the thoroughness with which their problem is being managed.
Step 3: aligning patient‐specific findings with collective knowledge
While standardizing data collection in this way helps ensure that available knowledge is used to delineate the complexity of each case, by itself, this complexity can overwhelm decision making. Identifying the appropriate diagnostic or management options amid the greater abundance of patient‐specific findings can appear to exacerbate the information overload that now burdens decisions. What should be done with the patient's data once it is collected?
In Weed's paradigm, the collective knowledge used to determine what information to gather to differentiate among possible diagnostic or management options also serves to identify what subset of options to consider for the individual patient by linking the positive findings to one or more of the guidance options. If clusters of positive findings endorse a particular option, then that option is identified as worth considering. Where the match between findings and options is weak or nil, then these options would fall from consideration. This narrows the range of options to consider to those that account for one or several patient‐specific findings.
This is the basis for the ‘knowledge coupling’ process Weed describes [16, 17] whereby details of the patient's problem become automatically linked or ‘coupled’ with available knowledge. This process serves to identify the inputs to decisions – the subset of diagnostic or management options endorsed by the configuration of findings that define the patients’ unique situation. Descriptions of these options and the pros and cons associated with them serve to further guide the decision‐making process. When collective knowledge becomes embodied in computer tools, that knowledge can readily be disseminated and used to generate a new form of evidence‐based medicine where guidance options worth considering and the patient‐specific findings that endorse them serve to inform the decision‐making process.
Step 4: human judgment and patient‐centred practice – deciding among relevant options
No two patients present the same configuration of findings, and, thus, no alignment between findings and options would be identical from one patient to the next. Capturing patient ‘uniqueness’ in this way is virtually the opposite of what ‘cookbook’ approach medicine would entail. Using collective knowledge to consistently identify the clinically relevant findings for each patient (steps 1 and 2) is a central attribute of any practice claiming to be patient centred. Aligning detailed findings with relevant guidance options (step 3) then serves as the input to informed and meaningful dialogue between the practitioner and the patient contemplating next steps. Of course, practitioners must account for the subtle cues patients give off during the encounter, and consider them in the interpretation of the findings. When combined with an organized medical record, the details of the patient's problem provide a broad context for considering the pertinent diagnostic or management options presented. Informed and meaningful patient engagement in the consultation is a central tenet of patient‐centred care [12, 13, 14, 19].
Only for the occasional ‘textbook’ case will a set of findings unequivocally support a single diagnostic or management option, in which case the decision essentially makes itself. The most critical task for patient care – deciding what the appropriate next step would be with evidence to back it up – remains comparatively uncomplicated. Even with such straightforward cases, however, using this stepwise process is instructive for patients. It enables them to gain a clearer picture of the relationships between the details of their unique situation and the diagnostic or management protocol that is indicated, along with the possible side effects and other warnings that might be associated with it. Appreciating the basis for the decision made, and the evidence that supports it, can enhance the meaningfulness of the dialogue, as well as the patient's engagement with and confidence in the decision made [30].
Often enough, however, such a clear picture may not emerge, and two or more diagnostic or management options will present themselves as plausible. The lack of clarity in this regard should not be construed as a deficiency in the process but, rather, suggests the difficulties faced when attempting to match general knowledge to particular cases. Medical practice remains a ‘science of particulars’ so an imperfect alignment between generalizations derived from research and the specific findings of unique patients should be unsurprising [31, 32, 33, 34]. This is where the ‘art’ of medicine becomes truly expressed – where practitioners draw upon their experience, communication skills, judgment and ‘wisdom’ to elicit patients’ values and preferences to help them navigate the ambiguities and tradeoffs associated with two or more plausible options. This process facilitates the engagement of patients in the decision‐making process, insofar as they wish to be included. This dialogue, with its transparent foundation, serves to enable an outcome that is ‘meaningful and valuable’ to the patient [14].
Conclusion
The EBM paradigm aimed to de‐emphasize the role knowledge based on intuition and idiosyncratic clinical experience plays in clinical decision making, but left unquestioned how personal judgment might limit what research evidence is used in the care of patients. The human mind is an unreliable conduit for knowledge delivery, and our reliance on human judgment to decide what knowledge and evidence to use in the process prematurely confines the deployment of knowledge to what the unaided mind can manage rather than what the problem requires. The gap between what evidence is applicable and what is actually applied to a particular problem becomes more and more significant as the body of knowledge continues to grow and change [35]. Moreover, this gap diminishes how patient‐centred health care can be. Not only do patients ultimately gain only partial access to clinical knowledge that might be relevant to their situation, but the basis for clinical recommendations remains largely obscured from them. This stifles meaningful dialogue and genuine participation. In contrast, partitioning the problem‐solving process as suggested above would introduce a systematic approach to knowledge delivery, bring transparency to care, facilitate meaningful dialogue and encourage patient participation in their health and health care.
The deficiencies of EBM illustrate a wider disorder in how health care knowledge is delivered in the care of patients. For decades, Weed has described this disorder as resulting from the largely unquestioned reliance on the unaided mind to deliver knowledge to patients. So far, however, little has changed. For now, the disorder often is obscured by the status and cultural authority that the medical profession holds, and the trust that patients place in the doctors on whom they depend. Further, reluctance to define and routinely apply standards to the care of patients also makes evaluating quality difficult, and so shields practitioners from scrutiny.
Despite the vast and expanding corpus of knowledge available to address patients’ problems, we still rely on structures for knowledge delivery that took root during the Progressive Era over a century ago and long before computer tools were available to process and deliver information. Even though patients and consumers increasingly access knowledge and information to assist in the management of their own health, we still rely on century‐old institutional arrangement that rest on the view that science is too complex and inaccessible for the layperson [36]. But as the deficiencies in the current approach to knowledge delivery become more and more glaring, patients, clinicians and leaders in health care will increasingly seek alternative approaches to knowledge delivery. Reflecting Weed's broad vision of knowledge delivery, the partitioning of EBM and PCC as described here exploits the strengths of both approaches – using electronic tools to deliver relevant knowledge while enabling practitioners to deliver patient‐centred care.
References
- 1. Guyatt, G. , Cairnes, J. , Churchill, D. , et al (1992) Evidence‐based medicine: a new approach to teaching the practice of medicine. Journal of the American Medical Association, 268 (17), 2420–2425. [DOI] [PubMed] [Google Scholar]
- 2. Eddy, D. M. (2005) Evidence‐based medicine: a unified approach. Health Affairs, 24 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- 3. Wyer, P. C. & Silva, S. A. (2009) Where is the wisdom? I – a conceptual history of evidence‐based medicine. Journal of Evaluation in Clinical Practice, 15 (6), 891–898. [DOI] [PubMed] [Google Scholar]
- 4. Tonelli, M. R. (1998) The philosophical limits of evidence‐based medicine. Academic Medicine, 73 (12), 1234–1240. [DOI] [PubMed] [Google Scholar]
- 5. Tonelli, M. R. (2006) Integrating evidence into clinical practice: an alternative to evidence‐based approaches. Journal of Evaluation in Clinical Practice, 12 (3), 248–256. [DOI] [PubMed] [Google Scholar]
- 6. Tonelli, M. R. (2010) The challenge of evidence in clinical medicine. Journal of Evaluation in Clinical Practice, 16 (2), 384–389. [DOI] [PubMed] [Google Scholar]
- 7. Greenhalgh, T. , Howick, J. & Maskrey, N. (2014) Evidence based medicine: a movement in crisis? BMJ (Clinical Research Ed.), 348, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bensing, J. (2000) Bridging the gap: the separate worlds of evidence‐based medicine and patient‐centered medicine. Patient Education and Counseling, 39 (1), 17–25. [DOI] [PubMed] [Google Scholar]
- 9. Hartzband, P. & Groopman, J. (2009) Keeping the patient in the equation – humanism and health care reform. New England Journal of Medicine, 361 (6), 554–555. [DOI] [PubMed] [Google Scholar]
- 10. Miles, A. (2009) On a medicine of the whole person: away from scientistic reductionism and towards the embrace of the complex in clinical practice. Journal of Evaluation in Clinical Practice, 15 (6), 941–949. [DOI] [PubMed] [Google Scholar]
- 11. Miles, A. & Mezzich, J. E. (2011) The care of the patient and the soul of the clinic: person‐centered medicine as an emergent model of modern clinical practice. The International Journal of Person Centered Medicine, 1 (2), 207–222. [Google Scholar]
- 12. Silva, S. A. & Wyer, P. C. (2009) Where is the wisdom? II – evidence‐based medicine and the epistemological crisis in clinical medicine. Exposition and commentary on Djulbegovic, B., Guyatt, G. H. & Ashcroft, R. E. (2009) Cancer Control, 16, 158–168. Journal of Evaluation in Clinical Practice, 15 (6), 899–906. [DOI] [PubMed] [Google Scholar]
- 13. Institute of Medicine (2001) Crossing the Quality Chasm: A New Health System for the Twenty‐First Century. Washington: National Academy Press. [Google Scholar]
- 14. Epstein, R. M. & Street, R. L. (2011) The Values and Value of Patient‐Centered Care. The Annals of Family Medicine. 9 (2), 100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weed, L. L. (1969) Medical Records, Medical Education, and Patient Care: The Problem‐Oriented Record as a Basic Tool. Cleveland, OH: Press of Case Western Reserve University. [Google Scholar]
- 16. Weed, L. L. (1991) Knowledge Coupling: New Premises and New Tools for Medical Care and Education. New York: Springer‐Verlag. [Google Scholar]
- 17. Weed, L. L. & Weed, L. (2011) Medicine in Denial. Creative Commons Attribution 3.0 Unreported License.
- 18. Weed, L. L. & Weed, L. (2014) Diagnosing diagnostic failure. Diagnosis, 1 (1), 13–17. [DOI] [PubMed] [Google Scholar]
- 19. Djulbegovic, B. & Guyatt, G. H. (2014) Evidence‐based practice is not synonymous with delivery of uniform health care. Journal of the American Medical Association, 312 (13), 1293–1294. [DOI] [PubMed] [Google Scholar]
- 20. Timmermans, S. & Mauck, A. (2005) The promises and pitfalls of evidence‐based medicine. Health Affairs, 24 (1), 18–28. [DOI] [PubMed] [Google Scholar]
- 21. Tversky, A. & Kahneman, D. (1974) Judgment under uncertainty: heuristics and biases. Science, 185, 1124–1131. [DOI] [PubMed] [Google Scholar]
- 22. Elstein, A. S. , Shulman, L. S. & Spraska, S. A. (1978) Medical Problem Solving and Analysis of Clinical Reasoning. Cambridge, MA: Harvard University Press. [Google Scholar]
- 23. Dawes, R. (1988) Rational Choice in an Uncertain World. New York: Harcourt Brace Jovanovich. [Google Scholar]
- 24. Groopman, J. (2007) How Doctors Think. New York: Houghton Mifflin Company. [Google Scholar]
- 25. Kahneman, D. (2011) Thinking, Fast and Slow. Toronto: Doubleday Canada. [Google Scholar]
- 26. Burger, C. (2010) The use of problem‐knowledge couplers in a primary care practice. The Permanente Journal, 14 (1), 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weaver, R. R. (2015) Seeking high reliability in primary care: leadership, tools, and organization. Health Care Management Review, 40 (3), 183–192. [DOI] [PubMed] [Google Scholar]
- 28. Lupton, D. & Jutel, A. (2015) ‘It's like having a physician in your pocket!’ A critical analysis of self‐diagnosis smartphone apps. Social Science & Medicine, 133, 128–135. [DOI] [PubMed] [Google Scholar]
- 29. Buijink, A. W. G. , Visser, B. J. & Marshall, L. (2013) Medical apps for smartphones: lack of evidence undermines quality and safety. Evidence‐based Medicine, 18 (3), 90–92. [DOI] [PubMed] [Google Scholar]
- 30. Weaver, R. R. (2003) Informatics tools and medical communication: patient perspectives of ‘knowledge coupling’ in primary care. Health Communication, 15 (1), 59–78. [DOI] [PubMed] [Google Scholar]
- 31. Gorovitz, S. & MacIntyre, A. (1975) Toward a theory of medical fallibility. Hastings Center Report, 5 (6), 13–23. [PubMed] [Google Scholar]
- 32. Cassell, E. (1986) Toward a science of particulars. Hastings Center Report, October, 12–14. [Google Scholar]
- 33. McWhinney, J. R. (1989) An acquaintance with particulars. Family Medicine, 21 (4), 296–298. [PubMed] [Google Scholar]
- 34. Sturmberg, J. P. , Martin, C. M. & Katerndahl, D. A. (2014) Systems and complexity thinking in the general practice literature: an integrative, historical narrative review. The Annals of Family Medicine, 12 (1), 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weaver, R. R. (2002) Resistance to computer innovation: knowledge coupling in clinical practice. ACM SIGCAS Computers and Society, 32 (1), 16–21. [Google Scholar]
- 36. Starr, P. (1982) The Social Transformation of American Medicine: The Rise of a Sovereign Profession and the Making of a Vast Industry. New York: Basic Books, Inc. [Google Scholar]


