Summary
Objective
The aim of this study was to determine if the association with adiposity varies by the type (added vs. naturally occurring) and form (liquid vs. solid) of dietary sugars consumed.
Methods
Data from the 10‐year National Heart, Lung, and Blood Institute (NHLBI) Growth and Health Study (n = 2,021 girls aged 9–10 years at baseline; n = 5,156 paired observations) were used. Using mixed linear models, 1‐year changes in sugar intake, body mass index z‐score (BMIz) and waist circumference (WC) were assessed.
Results
The results showed mean daily added sugar (AS) intake: 10.3 tsp (41 g) liquid; 11.6 tsp (46 g) solid and naturally occurring sugar intake: 2.6 tsp (10 g) liquid; 2.2 tsp (9 g) solid. Before total energy adjustment, each additional teaspoon of liquid AS was associated with a 0.222‐mm increase in WC (p = 0.0003) and a 0.002 increase in BMIz (p = 0.003). Each teaspoon of solid AS was associated with a 0.126‐mm increase in WC (p = 0.03) and a 0.001 increase in BMIz (p = 0.03). Adjusting for total energy, this association was maintained only between liquid AS and WC among all and between solid AS and WC among those overweight/obese only. There was no significant association with naturally occurring sugar.
Conclusions
These findings demonstrate to suggest a positive association between AS intake (liquid and solid) and BMI that is mediated by total energy intake and an association with WC that is independent of it.
Keywords: Beverages, obesity, sugar, waist circumference
Introduction
The rapid rise in obesity prevalence in recent years suggests that changing lifestyles are behind this increase. Dietary fats were a focus of obesity treatment and prevention efforts for many years, but more recently, the role of refined carbohydrates, particularly sugars added to foods and beverages, has gained attention. Although added sugar (AS) intake has decreased recently 1, overweight and obesity prevalence among US children rose in parallel to its consumption 2, 3, from the 1970s to the 1990s.
While multiple studies have shown a positive association between adiposity and sugar‐sweetened beverage 4, 5, 6, 7 or total AS intake 4, 8, no known studies have examined the impact of consuming food sources of AS. Similarly, although the nutrient content of fruit juices is similar to that of sugar‐sweetened beverages 9, limited research has been carried out to examine the association between naturally sweet beverages or naturally sweet foods and obesity 8, 10, 11, 12.
Several mechanisms have been proposed to explain why obesity risk may be higher with the increased sugar consumption and why this risk may vary by the type (i.e. added vs. naturally occurring) and the physical form (liquid vs. solid) consumed. First, the most common ASs contain fructose, which has been shown in controlled studies to increase de novo lipogenesis and to contribute to central adiposity 13, 14. Second, the insulin response to regular consumption of foods high in sugars may promote fat storage rather than oxidation of excess calories 15. Third, the nutrient content of naturally sweet foods tends to differ substantially from those high in AS, with naturally sweet foods tending to contain more nutrients such as fibre that are believed to promote healthy weight maintenance 16, 17. Lastly, research suggests that calories consumed in liquid form, as from sugar‐sweetened beverages and 100% fruit juices, may not be fully compensated for with later food intake resulting in lower satiety, excess calorie intake and weight gain 18.
This study sought to evaluate the long‐term effects of dietary sugars, by the type and the physical form consumed, on change in body mass index z‐score (BMIz) and waist circumference (WC) in order to inform the development of intake guidelines.
Methods
Study population
The National Lung, Heart and Blood Institute's Growth and Health Study (NGHS) was a prospective cohort study that enrolled girls from January 1987 to May 1988 at three US study sites. Non‐Hispanic Caucasian (n = 1,166) and African–American (n = 1,213) girls were recruited from each study location. Full inclusion and exclusion criteria have been described elsewhere 19. Briefly, girls were eligible to enrol if they were 9 or 10 years old and both parents self‐identified as either non‐Hispanic African–American or non‐Hispanic White. Participants were followed for 10 years and completed annual physical examinations and survey questionnaires. BMI was assessed annually as was minimum WC (except at baseline). Three‐day food records were collected at every visit except visits 6 and 9.
To examine the association between simultaneous change in intake and change in adiposity, observations were selected in which 1‐year change could be calculated. Because of the data collection schedule, four pairs of observations were available for this analysis: change between visit pairs 2 and 3, 3 and 4, 4 and 5, and 7 and 8 (Figure 1). There were 8,412 pairs of observations from these visits. Pairs of observations were excluded if visits occurred less than 0.8 years or more than 1.2 years apart (n = 1,165), a participant had ever been pregnant (n = 203), missing nutrition information (n = 824), implausible or invalid nutritional intake (foods where the sum of the component carbohydrates was <90% of total carbohydrates, or <650 cal or >4,000 cal d−1, n = 676), missing change in BMI (n = 60), missing change in WC (n = 51) or missing other covariates (n = 320).
Figure 1.
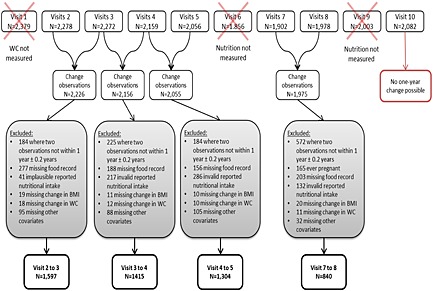
Flow chart of exclusion criteria for analysis of the National Growth and Health Study cohort. BMI, body mass index; WC, waist circumference.
For the remainder of this paper, ‘initial’ will refer to the value of a variable at the first time point within a pair of observations, and ‘change’ will refer to the difference of the first time point subtracted from the second time point in a pair of observations.
The proposal for this secondary analysis was reviewed and approved by the Institutional Review Board at Emory University.
Measures of adiposity
At annual physical examinations, height and weight were measured by research staff twice in accordance with standard protocols. A third measurement was taken if the first differed by more than 0.5 cm or 0.3 kg. The closest two of the three measures were used to calculate BMI 19. The 2000 Centers for Disease Control and Prevention (CDC) growth charts were used to determine age‐adjusted and sex‐adjusted BMI z‐scores (BMIz) and to group participants by weight status (normal: BMI < 85th percentile, overweight/obese: BMI ≥ 85th percentile) 20. Minimum WC was measured following breath expiration at all visits except baseline (visit 1). The mean of the repeated measures was used for all analysis 19.
Sugar consumption
Study participants completed 3‐d food records (two weekdays and one weekend day) annually 21, 22. Trained nutritionists reviewed the food records with each girl for clarification of portion sizes and food preparation. Nutrient content was ascribed using the Food Table Version 19 of the Nutrition Coordinating Center (NCC) database. Sugar intake was calculated as the sum of average daily fructose, glucose and sucrose intake consumed. These sugars were attributed to either food (solid) or beverage (liquid) sources and identified as either AS or naturally occurring sugar (NOS). Beverage sources of liquid sugars included soft drinks, energy drinks, fruit juices, sweetened milks and sweetened coffees and teas; intake of solid sugars from food sources was estimated as the difference between total sugar intake and the estimate of sugar intake from beverage sources. NOSs were those consumed in fruits, vegetables and their juices. ASs were defined as all non‐dairy sugars contained in candies, sweetened beverages, sweetened grain products and dairy desserts such as ice cream and pudding. For items such as fruit pies that contained a mixture of added and natural sugars, one‐half of all non‐dairy sugars were assumed to be natural, and the other half were assumed to be added. This assumption has been tested in a previous sensitivity analysis 23. Sugar intake, reported in grams, was converted to teaspoons (tsp) given their common usage and to facilitate comparison with current intake recommendations 24.
Covariates
Race, income and parents' education were obtained from a questionnaire completed by the participants' parents at the baseline visit (visit 1). Maturation stage was assessed annually through visit 6 using a modified Tanner methodology supplemented with areolar stage and was assigned a value of 1–6 19. Physical activity was assessed annually except at visits 6 and 9 with a 3‐d activity diary that coincided with the nutritional diary 25. Participants were given a booklet with 24 activities shown in pictures and were asked to check off boxes indicating the amount of time in 15 min intervals spent doing each per day. These times were summed to estimate total daily physical activity. Percentage of total energy from fat and from carbohydrates other than sugar and fibre was calculated using data from the 3‐d food records. Participants who were missing any of the aforementioned covariates were excluded from the analysis, with the exception of those missing household income. Given the large number with missing income data, a separate category was created for non‐respondents, so they could be retained in the sample.
Statistical analysis
To assess the potential differences between participants who contributed three or four 1‐year observations to those who contributed two or fewer, visit 2 characteristics were compared using t‐tests and chi‐squared tests. In addition, descriptive characteristics were examined by visit to assess general changes over time.
In the main analysis, WC and BMIz were modelled as continuous outcomes. For both measures, the effect of each teaspoon change in sugar intake was examined for total sugars, total AS and total NOS, for four distinct types/forms of sugars: (i) AS from beverages (liquid AS); (ii) AS from foods (solid AS); (iii) NOS from beverages (liquid NOS) and (iv) NOS from foods (solid NOS).
Because each individual could contribute up to four paired observations to the analysis, PROC MIXED was used with a heterogeneous autoregressive R matrix to account for within‐individual correlation. To avoid assumptions of linearity, change in sugars was first modelled in quintiles, and all control covariates were modelled as categorical variables using quartiles 26. For change in sugars, linear trend tests were performed using the median value of each quintile as a continuous variable in the model. As linear trend tests were significant, the final analyses were conducted using sugar intake as a continuous variable. Age was initially included as a non‐linear variable, but because of its lack of significance, it was used as a linear term to preserve degrees of freedom. P‐values <0.05 were considered statistically significant.
Model 1 adjusted for the following: race, initial age, maturation stage (six categories), WC (WC models only), BMIz, quartiles of sugars and quartiles of physical activity; change in quartiles of physical activity and height (WC models only); and current dieting status, baseline parental income (including one category for missing) and parents' education level. Model 2 adjusted for the covariates in model 1 plus quartiles of initial and change in grams of dietary fibre, percentage of energy from fat and percentage of energy from carbohydrates (excluding non‐dairy sugars and fibre). To assess whether or not total energy intake was a mediator between sugar intake and increased adiposity, the third model additionally adjusted for quartiles of initial and change in total energy intake. All models were mutually adjusted for other sugars such that all sugars were accounted for in each model to control for potential confounding.
Effect modification of change in sugars by weight status was tested in all models and determined to be significant only for solid AS and WC. Therefore, results are presented stratified by weight status for WC. All analyses were performed in sas version 9.3 (SAS Institute, Cary, NC, USA).
Results
There were 5,156 pairs of observations for 2,021 unique NGHS study participants (Figure 1). Descriptive characteristics of study participants are presented by visit (Table 1). At visit 2, the first visit used in this analysis, the mean age of participants was 11.0 years, at follow‐up 1, 12.0 years, at follow‐up 2, 13.0 years and at follow‐up 3, 16.0 years. At each visit, there was an approximately even distribution of white and black participants. The percentage of normal weight participants ranged from 65.3% to 70.6% across the visits, and the percentage of overweight participants fluctuated from 12.9% to 17.4%.
Table 1.
Descriptive characteristics of study participants at each observation (n = 5,156)
| Visit 2 (n = 1,597) | Visit 3 (n = 1,415) | Visit 4 (n = 1,304) | Visit 7 (n = 840) | |
|---|---|---|---|---|
| Mean ± SD or n (%) | Mean ± SD or n (%) | Mean ± SD or n (%) | Mean ± SD or n (%) | |
| Age (years) | 11.0 ± 0.57 | 12.0 ± 0.59 | 13.0 ± 0.56 | 16.0 ± 0.54 |
| White participants | 816 (51.1%) | 694 (49.0%) | 667 (51.2%) | 446 (53.1%) |
| Black participants | 781 (48.9%) | 721 (51.0%) | 637 (48.8%) | 394 (46.9%) |
| Weight status | ||||
| Underweight (<5th percentile) | 57 (3.6%) | 45 (3.2%) | 28 (2.1%) | 13 (1.5%) |
| Normal weight (5th–85th percentiles) | 1,074 (67.3%) | 946 (66.9%) | 852 (65.3%) | 593 (70.6%) |
| Overweight (≥85th–95th percentiles) | 239 (15.0%) | 203 (14.3%) | 227 (17.4%) | 108 (12.9%) |
| Obese (≥95th percentile) | 227 (14.2%) | 221 (15.6%) | 197 (15.1%) | 126 (15.0%) |
| Parents' income | ||||
| Missing | 85 (5.3%) | 79 (5.6%) | 69 (5.3%) | 49 (5.8%) |
| $0–$9,999 | 214 (13.4%) | 167 (11.8%) | 145 (11.1%) | 85 (10.1%) |
| $10,000–$19,999 | 192 (12.0%) | 180 (12.7%) | 161 (12.3%) | 96 (11.4%) |
| $20,000–$39,999 | 482 (30.2%) | 429 (30.3%) | 395 (30.3%) | 249 (29.6%) |
| $40,000+ | 624 (39.1%) | 560 (39.6%) | 534 (41.0%) | 361 (43.0%) |
| Parents' education | ||||
| High school or less | 377 (23.6%) | 301 (21.3%) | 263 (20.2%) | 166 (19.8%) |
| 1–3 years post‐high school | 603 (37.8%) | 559 (39.5%) | 513 (39.3%) | 321 (38.2%) |
| College graduate | 617 (38.6%) | 555 (39.2%) | 528 (40.5%) | 353 (42.0%) |
| Puberty stage* | ||||
| 1 (pre‐puberty) | 316 (19.8%) | 49 (3.5%) | 13 (1.0%) | 0 (0.0%) |
| 2 | 601 (37.6%) | 329 (23.3%) | 91 (7.0%) | 0 (0.0%) |
| 3 | 309 (19.3%) | 321 (22.7%) | 166 (12.7%) | 0 (0.0%) |
| 4 | 170 (10.6%) | 215 (15.2%) | 212 (16.3%) | 0 (0.0%) |
| 5 | 190 (11.9%) | 443 (31.3%) | 631 (48.4%) | 0 (0.0%) |
| 6 (post‐menarche) | 11 (0.7%) | 58 (4.1%) | 191 (14.6%) | 840 (100.0%) |
| Dieting to lose weight | ||||
| Always | 99 (6.2%) | 102 (7.2%) | 101 (7.7%) | 83 (9.9%) |
| Sometimes | 356 (22.3%) | 349 (24.7%) | 388 (29.8%) | 261 (31.1%) |
| Never | 1142 (71.5%) | 964 (68.1%) | 815 (62.5%) | 496 (59.0%) |
| Initial physical activity | 474.1 ± 438.6 | 490.0 ± 383.5 | 446.9 ± 348.2 | 335.7 ± 299.3 |
| Change in physical activity | 22.7 ± 475.0 | −53.3 ± 418.5 | −37.4 ± 342.2 | −57.1 ± 303.4 |
| Change in height (cm) | 6.4 ± 2.17 | 4.7 ± 2.60 | 3.2 ± 2.35 | 0.4 ± 0.87 |
Maturation stage was not collected after visit 6; thus, a maturation stage of 6 was imputed for all participants in visit 7.
SD, standard deviation.
There were 1,113 participants who had three or four pairs of observations and 1,266 participants who had two or fewer pairs of observations in this analysis (Supporting Information Table 1). There were several significant differences between these two groups. Participants with two or fewer pairs of observations were more likely to be black, be of lower socioeconomic status as measured by both parents' income and education level and be more physically active. The percentage of energy from fat was higher among those with two or fewer pairs of observation, and the distribution of puberty stages also differed slightly.
The dietary patterns of the girls in the study sample are summarized by visit in Table 2. Average daily sugar consumption by sugar type and form is illustrated in Figure 2. The mean consumption of liquid AS increased steadily over time, from 9.1 tsp at visit 2 to 12.1 tsp at visit 7 (1 tsp = 4 g). In contrast, consumption of the other types of sugar remained relatively stable over time. Mean consumption of solid AS hovered around 11 tsp, mean liquid NOS stayed between 2.4 and 3.0 tsp and mean intake of solid NOS was approximately 2.0 tsp across visits. Intake of AS did not differ between overweight or obese girls and normal weight girls; both consumed 17.7–17.8% of their total energy intake as AS. There was a small but significant difference in the consumption of NOS with those normal weight consuming more than those overweight or obese, 7.3% vs. 6.7%, respectively.
Table 2.
Nutritional characteristics of study participants at each observation (n = 5,156)
| Visit 2 (n = 1,597) | Visit 3 (n = 1,415) | Visit 4 (n = 1,304) | Visit 7 (n = 840) | |||||
|---|---|---|---|---|---|---|---|---|
| Initial | Change | Initial | Change | Initial | Change | Initial | Change | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Total energy intake (kcal) | 1,899.3 ± 650.15 | 56.1 ± 750.4 | 1,946.6 ± 664.7 | −68.1 ± 730.0 | 1,871.0 ± 654.1 | −27.5 ± 690.9 | 1,851.2 ± 622.5 | −44.3 ± 604.4 |
| Total non‐dairy sugar (tsp) | 25.8 ± 12.9 | 1.4 ± 14.9 | 27.2 ± 13.0 | −0.7 ± 14.3 | 26.3 ± 12.5 | 0.1 ± 13.6 | 28.0 ± 12.6 | 0.6 ± 14.5 |
| Added non‐dairy sugar (tsp) | 21.0 ± 11.8 | 1.3 ± 13.7 | 22.3 ± 12.0 | −0.1 ± 13.3 | 22.1 ± 11.5 | −0.3 ± 12.7 | 22.6 ± 11.7 | 0.7 ± 13.5 |
| Liquid added (tsp) | 9.1 ± 6.8 | 0.9 ± 7.9 | 10.2 ± 7.3 | 0.6 ± 8.4 | 10.7 ± 7.4 | 0.6 ± 8.6 | 12.1 ± 9.1 | 1.0 ± 10.9 |
| Solid added (tsp) | 11.8 ± 8.4 | 0.4 ± 10.4 | 12.1 ± 8.3 | −0.7 ± 9.6 | 11.4 ± 7.9 | −0.8 ± 9.0 | 10.5 ± 7.2 | −0.4 ± 8.0 |
| Natural non‐dairy sugar (tsp) | 4.8 ± 4.5 | 0.0 ± 5.4 | 4.9 ± 4.7 | −0.6 ± 5.5 | 4.3 ± 4.4 | 0.3 ± 5.2 | 5.3 ± 5.8 | −0.1 ± 6.5 |
| Liquid natural (tsp) | 2.6 ± 3.3 | 0.1 ± 4.3 | 2.7 ± 3.7 | −0.2 ± 4.4 | 2.4 ± 3.3 | 0.4 ± 4.3 | 3.0 ± 4.6 | −0.1 ± 5.4 |
| Solid natural (tsp) | 2.2 ± 2.8 | −0.1 ± 3.3 | 2.2 ± 2.6 | −0.4 ± 3.1 | 1.9 ± 2.5 | 0.0 ± 2.9 | 2.4 ± 3.0 | 0.1 ± 3.5 |
| Fibre (g) | 11.4 ± 4.9 | 0.6 ± 6.12 | 12.1 ± 5.3 | −0.7 ± 5.91 | 11.4 ± 5.2 | −0.2 ± 5.95 | 11.8 ± 5.2 | −0.4 ± 5.49 |
| Total fat (% of energy) | 35.6 ± 5.71 | −0.3 ± 7.0 | 35.3 ± 5.74 | −0.4 ± 7.1 | 34.8 ± 5.99 | −0.1 ± 7.3 | 33.2 ± 7.24 | −1.1 ± 7.8 |
| All other carbs (% of energy) | 14.6 ± 3.2 | −0.2 ± 4.0 | 14.3 ± 3.2 | 0.1 ± 3.9 | 14.4 ± 3.2 | −0.3 ± 3.8 | 14.1 ± 3.3 | 0.1 ± 4.0 |
SD, standard deviation.
Figure 2.
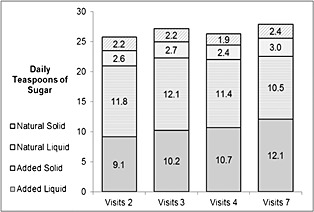
Average daily consumption of non‐dairy sugars in teaspoons by type, physical form and visit in the National Growth and Health Study cohort (n = 5,156).
In the first set of models, we examined the change in BMIz with total sugar intake, total intake of AS and NOS and with AS and NOS grouped by form (liquid vs. solid). Change in total sugars was positively associated with BMIz (Table 3): after adjustment for demographics, activity and nutritional components in model 2, each teaspoon increase in total sugars was associated with a 0.002 increase in BMIz (95% confidence interval (CI) 0.001, 0.002, p = 0.0004). After adjusting for energy intake in model 3, this estimate was attenuated and became non‐significant.
Table 3.
Estimated 1‐year change in BMI z‐score due to 1‐tsp (4 g) increase in total, added and natural, liquid and solid sugars in NGHS (n = 5,156)
| Model 1: demographics and PA | Model 2: model 1 and nutrition | Model 3: model 2 and total energy intake | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (95% CI) | p‐value | Estimate | (95% CI) | p‐value | Estimate | (95% CI) | p‐value | |
| Sugar type/form | |||||||||
| Total sugars | 0.001 | (0.000, 0.002) | 0.002 | 0.002 | (0.001, 0.002) | 0.0004 | 0.001 | (0.000, 0.002) | 0.16 |
| Added (total) | 0.001 | (0.000, 0.002) | 0.002 | 0.002 | (0.001, 0.002) | 0.0003 | 0.001 | (0.000, 0.002) | 0.13 |
| Added liquid | 0.001 | (0.000, 0.002) | 0.02 | 0.002 | (0.001, 0.003) | 0.003 | 0.001 | (0.000, 0.002) | 0.10 |
| Added solid | 0.001 | (0.000, 0.002) | 0.06 | 0.001 | (0.000, 0.003) | 0.03 | 0.000 | (−0.001, 0.002) | 0.58 |
| Natural (total) | 0.0003 | (−0.002, 0.002) | 0.73 | 0.001 | (−0.001, 0.003) | 0.50 | 0.0003 | (−0.002, 0.002) | 0.79 |
| Natural liquid | −0.001 | (−0.003, 0.002) | 0.64 | −0.0001 | (−0.002, 0.002) | 0.95 | −0.001 | (−0.003, 0.002) | 0.60 |
| Natural solid | 0.002 | (−0.001, 0.005) | 0.24 | 0.002 | (−0.001, 0.006) | 0.22 | 0.002 | (−0.001, 0.006) | 0.23 |
All models were mutually adjusted for each type of sugar consumption. Model 1 adjusted for race, initial age, initial BMI, initial puberty stage, parents' income, parents' education, dieting status, initial and change in physical activity and baseline sugars. Model 2 additionally adjusted for initial and change in grams of fibre, percentage of energy from fat and percentage of energy from other carbohydrates. Model 3 additionally adjusted for initial and change in total energy intake.
BMI, body mass index; CI, confidence interval; NGHS, National Growth and Health Study.
Change in intake of total AS was also associated with change in BMIz in models 1 and 2. In model 2, 1‐tsp increase in total AS intake was associated with a 0.002 increase in BMIz (95% CI 0.001, 0.002, p = 0.0003). After adjustment for energy intake in model 3, this association was no longer significant. The estimates for solid and liquid ASs were similar to each other in both models 2 and 3 and followed the same pattern as total AS of significance before adjustment for total energy and non‐significance after. There were no associations between total NOS, liquid NOS or solid NOS and BMIz in any of the models. There was no evidence of a significant interaction between sugar intake and BMIz by weight status.
Similarly, in the second set of models, we examined the change in intake of sugars with change in WC. Given the significant interaction observed between weight status and solid AS intake, results are presented stratified by initial weight status (Table 4). Adjusting for covariates as specified, change in total sugar intake was positively associated with WC in models 1 and 2. In model 2, each teaspoon increase in sugars was associated with a 0.154‐mm increase in WC (95% CI 0.071, 0.237, p = 0.0003). After adjusting for energy intake in model 3, the estimate attenuated to 0.086 mm and became non‐significant (95% CI −0.016, 0.187, p = 0.10).
Table 4.
Estimated change in WC (in millimetre) due to 1‐tsp (4 g) increase in added and natural, liquid and solid sugars, in NGHS, stratified by weight status (n = 5,156)
| Model 1: demographics and PA | Model 2: model 1 and nutrition | Model 3: model 2 and total energy intake | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (95% CI) | p‐value | Estimate | (95% CI) | p‐value | Estimate | (95% CI) | p‐value | |
| Total sugars | 0.106 | (0.035, 0.176) | 0.003 | 0.154 | (0.071, 0.237) | 0.0003 | 0.086 | (−0.016, 0.187) | 0.10 |
| Total added sugars | 0.130 | (0.054, 0.205) | 0.0008 | 0.179 | (0.093, 0.265) | <0.0001 | 0.107 | (0.002, 0.212) | 0.046 |
| Total NO sugars | 0.051 | (−0.130, 0.232) | 0.58 | 0.096 | (−0.100, 0.293) | 0.34 | 0.064 | (−0.136, 0.264) | 0.53 |
| Stratified by weight status | |||||||||
| Added liquid | |||||||||
| Normal | 0.169 | (0.048, 0.289) | 0.006 | 0.235 | (0.108, 0.361) | 0.0003 | 0.164 | (0.026, 0.303) | 0.02 |
| Overweight/obese | 0.216 | (0.032, 0.400) | 0.02 | 0.280 | (0.091, 0.468) | 0.004 | 0.207 | (0.009, 0.404) | 0.04 |
| Added solid | |||||||||
| Normal | 0.164 | (0.045, 0.284) | 0.007 | 0.197 | (0.068, 0.327) | 0.003 | 0.107 | (−0.038, 0.252) | 0.15 |
| Overweight/obese | 0.327 | (0.135, 0.519) | 0.001 | 0.357 | (0.159, 0.556) | 0.0004 | 0.267 | (0.058, 0.476) | 0.01 |
| Natural liquid | |||||||||
| Normal | 0.128 | (−0.116, 0.371) | 0.31 | 0.185 | (−0.064, 0.434) | 0.15 | 0.127 | (−0.127, 0.381) | 0.33 |
| Overweight/obese | 0.222 | (−0.162, 0.606) | 0.26 | 0.271 | (−0.116, 0.658) | 0.17 | 0.205 | (−0.186, 0.596) | 0.30 |
| Natural solid | |||||||||
| Normal | 0.082 | (−0.255, 0.420) | 0.63 | 0.106 | (−0.258, 0.470) | 0.57 | 0.103 | (−0.261, 0.467) | 0.58 |
| Overweight/obese | 0.034 | (−0.481, 0.548) | 0.90 | 0.049 | (−0.483, 0.581) | 0.86 | 0.038 | (−0.493, 0.570) | 0.89 |
Models were mutually adjusted to account for all types of sugar. Interaction by weight status was only significant for added solid sugars (p < 0.01 in all models). Model 1 adjusted for race, initial age, initial BMI, initial WC, initial puberty stage, parents' income, parents' education, dieting status, initial and change in physical activity, change in height and baseline sugars. Model 2 additionally adjusted for initial and change in grams of fibre, percentage of energy from fat and percentage of energy from other carbohydrates. Model 3 additionally adjusted for initial and change in total energy intake.
BMI, body mass index; CI, confidence interval; NGHS, National Growth and Health Study; NO, naturally occurring; WC, waist circumference.
The results examining the unique impact of added vs. NOS on WC were mostly consistent with the findings for BMIz. In nearly all models, both liquid and solid ASs were associated with increased WC, but there was no significant association, in any of the models, between NOS consumption and WC. In contrast to the BMIz analysis, the association with WC was maintained after adjustment for total energy. Each additional teaspoon of liquid AS was associated with a WC increase of 0.164 mm (95% CI 0.026, 0.303, p = 0.04) among normal weight individuals and an increase of 0.207 mm (95% CI 0.009, 0.404, p = 0.02) among those overweight or obese. In the same model, each additional teaspoon of solid AS was associated with a significant increase in WC 0.267 mm (95% CI 0.058, 0.476, p = 0.01) among those overweight or obese, but there was no association among those normal weight (estimate, 0.107 mm, 95% CI −0.038, 0.252, p = 0.15).
Discussion
Our results support the findings of others who have shown a positive association between dietary sugars and adiposity 5 while at the same time providing new insight into how this association varies by the type and form of the sugars consumed. We demonstrated that increased intake of total sugars and total AS, whether consumed in foods or beverages, was associated with increased BMIz but that this association was attenuated and no longer significant when controlling for total energy. This suggests that it is the corresponding increase in calories consumed and not an independent effect of the sugars themselves that results in an increase in total adiposity.
In contrast, for central adiposity, we found the effect of the type and form of sugars to be more pronounced. The positive association of liquid AS with WC was independent of total energy intake and was demonstrated for those normal weight and for those overweight/obese. For solid AS consumption, the association with WC was significant prior to controlling for total energy intake regardless of weight status but was maintained only among those overweight/obese when total energy was added to the model.
Consumption patterns of liquid and solid NOSs differed substantially from those of AS, averaging approximately 20% of their AS equivalent. While the estimated change in WC for liquid NOS was similar to those for liquid AS, these did not reach statistical significance in any of the models. There was no suggestion of a positive association between WC and NOS consumed in foods.
Although there have been numerous studies examining sugar‐sweetened beverage intake and obesity, few studies have examined the association between total AS intake or AS consumed in foods and adiposity, particularly among children and adolescents. Similarly, there has been little study of the impact of NOS in foods or beverages. Nicklas et al. found no cross‐sectional association between total AS and any of the nine measures of adiposity, including WC, BMI and BMIz, in adolescents in the National Health and Nutrition Examination Survey (NHANES) 2003–2006 8. Two longitudinal studies have examined food and beverage sources of AS and their associations with adiposity, with differing results. A study of 630 Canadian children ages 8–10 years found no associations between either solid or liquid AS and 2‐year change in BMI or WC 27. In the Cardiovascular Risk in Young Finns Study, an increase in sugar‐sweetened beverages but not other sweets from childhood to adulthood was associated with increased odds of overweight as an adult among women but not men 28.
Research examining fruit juice consumption and obesity risk has shown mixed results with some studies showing a positive association among children 29, 30, others demonstrating no association 31, 32 and one suggesting that the risk may vary by a child's baseline weight status 12. Among adults, a study using national survey data found a U‐shaped association between quintiles of fruit juice consumption and WC and BMI after adjustment for demographic factors 10. One large prospective study among adults demonstrated greater weight gain with higher juice consumption 33.
This study was subject to some important limitations. First, attrition was significant, as two consecutive visits were needed to assess 1‐year change. Half (53%) of participants contributed two or fewer observations to the analysis, of a possible four pairs of observations. These participants were more likely to be black, have lower parent income and educational attainment and be more physically active. Second, the type of sugars (added vs. naturally occurring) was not available on the nutrient database and therefore had to be estimated by the investigators after the nutritional information was abstracted, introducing the possibility of measurement error. The results of a prior sensitivity analysis indicated that the results were robust to small alterations in this methodology 23. Third, consumption of natural sugars was low for most observations: 40.1% had no natural liquid sugar consumption and 4.9% had no natural solid sugar consumption at an initial visit. This may have limited our ability to detect the associations between natural sugars and adiposity. However, fruit juice consumption among NGHS participants (42 kcal d−1) closely matched that of female adolescents in NHANES in 1999–2004 (39 kcal d−1) 34. Fourth, there may be systematic underreporting of sugary foods and beverages, particularly among those who reported low energy intake 35. We attempted to control for this by including dieting status in the models, as dieters tend to underreport their energy intake 36. Finally, because our study included only women aged 9–18 years, the generalizability of our results may be limited.
There were also many strengths of the present study. First, this analysis used data collected longitudinally to relate change in sugar consumption to change in adiposity. Compared with a cross‐sectional design, this enables stronger inferences about causality and establishes a temporal association between exposure and the outcome. Second, the 3‐d food record is one of the most reliable methods available for assessing dietary intake in observational epidemiology studies and has been shown to be valid among children and adolescents 21, 25. Availability of data on total diet allowed for an analysis of all forms of non‐dairy sugars while controlling for total energy and other nutrient intake. We were also able to control for other potential confounders, including physical activity. Third, we were able to assess changes in adiposity using WC and BMIz; many studies of adolescents have only used BMI. Fourth, although NGHS began in 1987, consumption of AS by NGHS participants closely matched that of NHANES adolescents in 2007–2008, at around 17% of energy intake 1. Finally, this study enrolled approximately equal numbers of non‐Hispanic white and non‐Hispanic black girls from three study sites across the USA, which increases the generalizability of these results.
In conclusion, our results suggest that the association between dietary sugars and adiposity varies by the type and form of the sugars consumed. While consumption of ASs appears to have a unique and independent effect on central adiposity, the association between AS and BMI (a measure of total adiposity) is mediated by total energy intake. NOSs in foods and beverages do not appear to increase adiposity at the levels consumed by study participants. Further research is needed to determine if NOSs impact adiposity at higher levels of consumption and to elucidate the mechanisms by which AS consumption results in increased central adiposity.
Conflict of Interest Statement
No conflict of interest was declared.
Authors' contributions
A. K. L. and J. A. W. developed the study plan, and A. K. L. and R. C. performed statistical analysis. A. K. L. and J. A. W. prepared the first draft, and all authors reviewed and approved subsequent versions.
Supporting information
Supplemental Table 1. Baseline descriptive characteristics of individuals with 3 or 4 change observations compared to 2 or fewer change observations, n=2,379.
Supporting info item
Acknowledgements
This manuscript was prepared using NGHS Research Materials obtained from the NHLBI Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the NGHS or the NHLBI.
This research was funded in part by Children's Healthcare of Atlanta.
Lee, A. K. , Chowdhury, R. , and Welsh, J. A. (2015) Sugars and adiposity: the long‐term effects of consuming added and naturally occurring sugars in foods and in beverages. Obesity Science & Practice, 1: 41–49. doi: 10.1002/osp4.7.
References
- 1. Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011; 94: 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jolliffe D. Extent of overweight among US children and adolescents from 1971 to 2000. Int J Obes Relat Metab Disord 2004; 28: 4–9. [DOI] [PubMed] [Google Scholar]
- 3. Popkin BM, Nielsen SJ. The sweetening of the world's diet. Obes Res 2003; 11: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 4. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta‐analyses of randomised controlled trials and cohort studies 2013. [DOI] [PubMed]
- 5. Malik VS, Pan A, Willett WC, Hu FB. Sugar‐sweetened beverages and weight gain in children and adults: a systematic review and meta‐analysis. Am J Clin Nutr 2013; 98: 1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebbeling CB, Feldman HA, Chomitz VR, Antnelli TA, Gortmaker S, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2013; 367: 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar‐free or sugar‐sweetened beverages and body weight in children. N Engl J Med 2012; 367: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 8. O'Neil CE, Nicklas TA, Zanovec M, Fulgoni VL. III Diet quality is positively associated with 100% fruit juice consumption in children and adults in the United States: NHANES 2003–2006. Nutr J 2011; 10: 1475–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welsh JA, Cunningham SA. The role of added sugars in pediatric obesity. Pediatr Clin North Am 2011; 58: 1455–1466, xi. [DOI] [PubMed] [Google Scholar]
- 10. Periera MA, Fulgoni VL. Consumption of 100% fruit juice and risk of obesity and metabolic syndrome: findings from the National Health and Nutrition Examination Survey 1999–2004. J Am Coll Nutr 2010; 29: 625–629. [DOI] [PubMed] [Google Scholar]
- 11. Nicklas TA, O'Neil CE, Kleinman R. Association between 100% juice consumption and nutrient intake and weight of children aged 2 to 11 years. Arch Pediatr Adolesc Med 2008; 162: 557–565. doi:10.1001/archpedi.162.6.557 [DOI] [PubMed] [Google Scholar]
- 12. Welsh JA, Cogswell ME, Rogers S, et al Overweight among low‐income preschool children associated with the consumption of sweet drinks: Missouri, 1999–2002. Pediatrics 2005; 115: e223–e229. [DOI] [PubMed] [Google Scholar]
- 13. Stanhope KL. Role of fructose‐containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med 2012; 63: 329–343. [DOI] [PubMed] [Google Scholar]
- 14. Pollock NK, Bundy V, Kanto W, et al Greater fructose consumption is associated with cardiometabolic risk markers and visceral adiposity in adolescents. J Nutr 2012; 142: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludwig DS. Dietary glycemic index and obesity. J Nutr 2000; 130: 280S–283S. [DOI] [PubMed] [Google Scholar]
- 16. Marriott BP, Olsho L, Hadden L, Connor P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003–2006. Crit Rev Food Sci Nutr 2010; 50: 228–258. [DOI] [PubMed] [Google Scholar]
- 17. Forshee RA, Storey ML. The role of added sugars in the diet quality of children and adolescents. J Am Coll Nutr 2001; 20: 32–43. [DOI] [PubMed] [Google Scholar]
- 18. DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord 2000; 24: 794–800. [DOI] [PubMed] [Google Scholar]
- 19. The National Heart L, and Blood Institute Growth and Health Study Research Group . Obesity and cardiovascular disease risk factors in Black and White girls: the NHLBI Growth and Health Study. Am J Public Health 1992; 82: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yon BA, Johnson RK, Stickle TR. School children's consumption of lower‐calorie flavored milk: a Plate Waste Study. J Acad Nutr Diet 2012; 112: 132–136. [DOI] [PubMed] [Google Scholar]
- 21. Crawford PB, Obarzanek E, Morrison J, Sabry Z. Comparative advantage of 3‐day food records over 24‐hour recall and 5‐day food frequency validated by observation of 9‐ and 10‐year‐old girls. J Am Diet Assoc 1994; 94: 626–630. [DOI] [PubMed] [Google Scholar]
- 22. Crawford PB, Obarzanek E, Schreiber GB, et al The effects of race, household income, and parental education on nutrient intakes of 9‐ and 10‐year‐old girls. NHLBI Growth and Health Study. Ann Epidemiol 1995; 5: 360–368. [DOI] [PubMed] [Google Scholar]
- 23. Lee AK, Binongo JN, Chowdhury R, et al Consumption of less than 10% of total energy from added sugars is associated with increasing HDL in females during adolescence: a longitudinal analysis. J Am Heart Assoc 2014; 3: e000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson RK, Appel LJ, Brands M, et al Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009; 120: 1011–1020. [DOI] [PubMed] [Google Scholar]
- 25. Obarzanek E, Schreiber GB, Crawford PB, et al Energy intake and physical activity in relation to indexes of body fat: the National Heart, Lung, and Blood Institute Growth and Health Study. Am J Clin Nutr 1994; 60: 15–22. [DOI] [PubMed] [Google Scholar]
- 26. Willett WC. Nutritional Epidemiology, 3rd edn Oxford University Press: UK, 2012. [Google Scholar]
- 27. Wang J, Light K, Henderson M, et al Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr 2014; 144: 81–86. [DOI] [PubMed] [Google Scholar]
- 28. Nissinen K, Mikkilä V, Männistö S, et al Sweets and sugar‐sweetened soft drink intake in childhood in relation to adult BMI and overweight. The Cardiovascular Risk in Young Finns Study. Public Health Nutr 2009; 12: 2018–2026. [DOI] [PubMed] [Google Scholar]
- 29. Faith MS, Dennison BA, Edmunds LS, Stratton HH. Fruit juice intake predicts increased adiposity gain in children from low‐income families: weight status‐by‐environment interaction. Pediatrics 2006; 118: 2066–2075. [DOI] [PubMed] [Google Scholar]
- 30. Dennison BA, Rockwell HL, Baker SL. Excess fruit juice consumption by preschool‐aged children is associated with short stature and obesity. Pediatrics 1997; 99: 15–22. [PubMed] [Google Scholar]
- 31. Davis JN, Koleilat M, Shearrer GE, Whaley SE. Association of infant feeding and dietary intake on obesity prevalence in low‐income toddlers. Obesity 2014; 22: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 32. Beck AL, Tschann J, Butte NF, Penilla C, Greenspan LC. Association of beverage consumption with obesity in Mexican American children. Public Health Nutr 2014; 17: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan A, Malik VS, Hao T, et al Changes in water and beverage intake and long‐term weight changes: results from three prospective cohort studies. Int J Obes 2013; 37: 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar‐sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics 2008; 121: e1604–e1614. [DOI] [PubMed] [Google Scholar]
- 35. Krebs‐Smith SM, Graubard BI, Kahle LL, et al Low energy reporters vs others: a comparison of reported food intakes. Eur J Clin Nutr 2000; 54: 281–287. [DOI] [PubMed] [Google Scholar]
- 36. Field AE, Austin SB, Taylor CB, et al Relation between dieting and weight change among preadolescents and adolescents. Pediatrics 2003; 112: 900–906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Baseline descriptive characteristics of individuals with 3 or 4 change observations compared to 2 or fewer change observations, n=2,379.
Supporting info item


