Abstract
Background
Echo-derived linear dimensions offer straightforward indices of right ventricular (RV) structure but have not been systematically compared to RV volumes on cardiac magnetic resonance (CMR).
Methods
Echo and CMR were interpreted among CAD patients imaged via prospective (90%) or retrospective (10%) registries. For echo, American Society of Echocardiography (ASE) recommended RV dimensions were measured in apical 4-chamber (basal RV width, mid RV width, RV length), parasternal long (proximal RV outflow tract [pRVOT]) and short axis (distal RVOT) views. For CMR, RV end-diastolic (RV-EDV) and end-systolic (RV-ESV) volumes were quantified via border planimetry.
Results
272 patients underwent echo and CMR within a narrow interval (0.4±1.0 days); complete acquisition of all ASE dimensions was feasible in 98%. All echo dimensions differed between patients with and without RV dilation on CMR (p<0.05). Basal RV width (r=0.70), pRVOT width (r=0.68), and RV length (r=0.61) yielded highest correlations with RV-EDV on CMR; end-systolic dimensions yielded similar correlations (r=0.68, 0.66, 0.65 respectively). In multivariable regression, basal RV width (regression coefficient 1.96 per mm [CI 1.22–2.70], p<0.001), RV length (0.97[0.56–1.37], p<0.001) and pRVOT width (2.62 [1.79–3.44], p<0.001) were independently associated with CMR RV-EDV[r= 0.80]. RV-ESV was similarly associated with echo dimensions (basal RV width; 1.59 per mm [CI 1.06–2.13], p<0.001) | RV length; 1.00 [0.66–1.34], p<0.001) | pRVOT width; 1.80 [1.22–2.39], p<0.001) [r= 0.79].
Conclusions
RV linear dimensions provide readily obtainable markers of RV chamber size. Proximal RVOT and basal width are independently associated with CMR volumes, supporting use of multiple linear dimensions when assessing RV size on echo.
Keywords: right ventricle, echocardiography, cardiovascular magnetic resonance
Introduction
Abnormal right ventricular (RV) chamber geometry is an established prognostic marker for a broad array of cardiovascular conditions, including patients with coronary artery disease (CAD).[1, 2] Echocardiography (echo) derived linear dimensions are widely used to assess left ventricular (LV) geometry, for which their use has been validated by anatomic correlation and prediction of prognosis.[3–6] However, utility of echo for RV assessment is less certain.[7, 8] Despite known limitations posed by RV geometric complexity, American Society of Echocardiography (ASE) guidelines encompass multiple linear measurements for assessment of RV chamber size, including measurements acquired in apical four chamber, parasternal long, and parasternal short axis views.[3] Relative utility of different echo linear measurements for assessment of RV size is not known.
Cardiac magnetic resonance (CMR) provides excellent endocardial definition that allows RV chamber size to be quantified without geometric assumptions. Prior studies have shown close agreement between CMR results and ex-vivo phantom volumes,[9] and demonstrated CMR measurements of RV structure and function to be reproducible.[10, 11] Echo RV linear measurements have been compared to CMR in prior cohorts. [8, 12, 13] However, insights regarding utility of echo linear dimensions have been limited by methodological issues that have included acquisition of select echo measurements (preventing comparison of individual measurements to one another), small sample size (limiting generalizability of previously reported weak correlations), and prolonged intervals between echo and CMR (an important concern in context of known sensitivity of the RV to loading conditions).
This study examined RV structure and function among a broad cohort of CAD patients undergoing echo and CMR within a narrow interval. In all patients, a uniform echo protocol was performed, which included assessment of RV chamber geometry in standard orientations concordant with consensus guidelines.[3] Study aims were two-fold: (1) to determine feasibility and reproducibility of guideline-recommended RV linear measurements in a diverse CAD cohort, and (2) to compare magnitude of association between different echo-based dimensions and CMR-quantified RV chamber volumes.
Material and Methods
Population
The population comprised CAD patients accrued from separate research registries at Weill Cornell Medical College (WCMC), each of which were focused on multimodality imaging for assessment of ischemic heart disease. Among these patients, 90% (n=246) were accrued prospectively as part of NIH protocols utilizing CMR and echo for CAD associated remodeling (NIH 1R01HL128278-01, K23 HL102249-01) [14], and 10% were accrued via a retrospective registry of patients with chronic obstructive CAD as verified by invasive angiography [15].
For all patients, CMR and echo were performed within 7 days, without interval coronary revascularization between imaging tests. Patients with contra-indications to CMR (e.g. GFR < 30 mL/min/1.73m2, ferromagnetic implants) were excluded from participation. Comprehensive demographic data were collected, including cardiac risk factors, medications, and invasive angiography assigned infarct related artery. This study was conducted with approval of the WCMC institutional review board.
Imaging Protocol
Echo and CMR were each performed using a standardized image acquisition protocol:
Echocardiography
Transthoracic echoes were acquired using commercial equipment (General Electric Vivid-7, Siemens SC2000 [Malvern, PA]). Echo included evaluation of the RV from the parasternal long and short axis and RV focused apical 4-chamber views, as specified in consensus ASE guidelines.[3]
CMR
CMR was performed using 1.5 and 3.0 Tesla scanners (General Electric [Waukesha, WI]). Cine-CMR utilized a steady-state free precession pulse sequence. Images were acquired in standard LV short- and long-axis planes. Short axis images were acquired throughout the RV such that images extended from the pulmonic valve through the RV apex.
RV Chamber Quantification
Echo and CMR were interpreted by experienced physicians (echo - JK | CMR - JWW) using a pre-specified analytic approach for each modality:
Echocardiography
RV linear dimensions were made in orientations concordant with ASE guidelines.[7]
In the apical 4-chamber view, RV width was measured in two locations (1) basal RV width (maximal transverse diameter in the basal one third of RV) and (2) mid RV width (maximal transverse diameter in the middle third of RV, approximately at the level of the papillary muscles). In addition, RV length was measured as maximal distance from the tricuspid annulus to the apex.
In the parasternal long axis view, proximal RV outflow tract (RVOT) width was measured as the maximal distance (perpendicularly oriented) between the RV free wall and septal-aortic junction.
In the parasternal short axis (pulmonary bifurcation) view, distal RVOT width was measured as the maximal distance immediately proximal to the pulmonic valve. When pulmonary bifurcation focused view was not available, a non-focused view of the pulmonic valve in short axis was used for approximation of pulmonic valve annulus.
Figure 1 provides representative examples of each RV dimension, which were measured during both end-diastole and end-systole. For the purpose of standardization, measurements in each respective orientation were acquired using the image and cardiac cycle that provided the largest linear dimension.
Figure 1. Echo-Based Linear RV Dimensions.
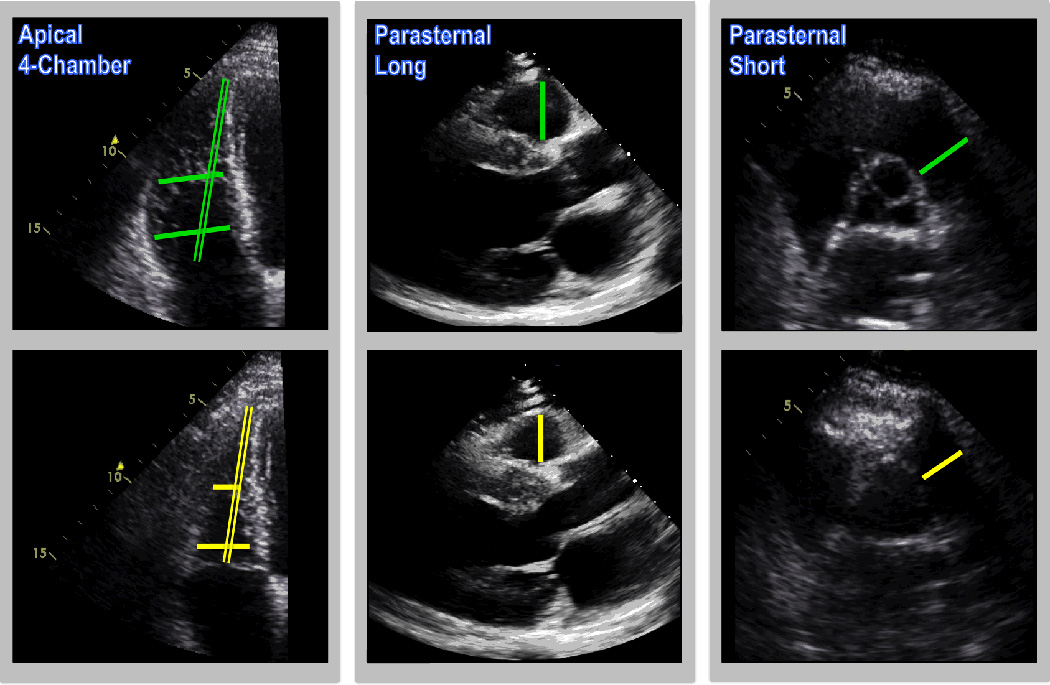
Representative examples of echo-based linear dimensions, as acquired in apical 4-chamber (left), parasternal long (center) and parasternal short (right) axis images [green = diastole, yellow = systole]. Dimensions encompassed both RV width (single lines) and length (double line).
RV systolic function was assessed via TAPSE, S’ and FAC, which were acquired in accordance with consensus guidelines.[3]
CMR
Volumetric quantification was performed using short axis cine-CMR images. Basal and apical image positions were defined in accordance with standard criteria, with the basal RV defined by the image in which the pulmonic valve or valve annulus was visualized, and the apex defined by the distal-most image in which RV myocardium was visualized. End-diastole and end-systole were defined based on the respective frames demonstrating the largest and smallest cavity size. Quantification of end-diastolic (EDV) and end-systolic volume (ESV) was performed using short axis images inclusive of trabeculations and papillary muscle. RV ejection fraction (EF) was calculated based on EDV and ESV. Cine-CMR analysis was performed using a previously validated automated algorithm shown to have excellent agreement with both manual planimetry quantified cardiac chamber size as well as phantom-verified volumes.[9, 16, 17]
Reproducibility
Intra- and inter-reader reproducibility was tested in a random cohort comprising 10% (n=26) of the study population, among whom processing times for both CMR and echo were also recorded. Inter-reader reproducibility was tested via a designated reader (AS) with expertise in both CMR and echo (>2000 exams interpreted annually). Reproducibility datasets for each modality were standardized in relation to initial images with respect cardiac cycle for analysis. Readers were otherwise blinded to clinical history, results of other imaging modalities, and initial measurements. Reproducibility analyses were performed a minimum of 10 days following the initial measurement.
Statistical Methods
Comparisons between groups were made using Student’s t test (expressed as mean ± standard deviation [SD]) for continuous variables. Categorical variables were compared using Chi-square or, when fewer than 5 expected outcomes per cell, Fisher’s exact test. Bivariate correlation coefficients, as well as univariable and multivariable regression analyses were used to evaluate associations between continuous variables: Multivariate modeling was performed via linear regression, for which CMR volumes and echo linear dimensions were both tested as continuous variables. Inter-observer and intra-observer agreement between methods was assessed using the method of Bland and Altman,[18] yielding the mean difference as well as limits of agreement between measurements (mean±1.96 SD). Inter-rater reliability among the two raters was estimated using the intra-class correlation coefficient (ICC), coefficient of variation (CoV, calculated as the standard deviation of the absolute difference between two acquisitions divided by the mean of the repeated acquisitions [expressed as a percentage]), as well as relative difference (RD, calculated as the absolute difference between two acquisitions divided by the mean of the repeated acquisitions [expressed as a percentage]). Statistical calculations were performed using SPSS 22.0 (SPSS Inc. [Chicago, IL]). Two-sided p<0.05 was considered indicative of statistical significance.
Results
Population Characteristics
The population comprised 272 patients with CAD who underwent echo and CMR within a mean interval of 0.4±1.0 days; 94% underwent imaging via both modalities within 1 day. RV dysfunction or dilation on CMR (defined by RVEF<50% or EDV males > 100.9 ml/m2, females > 94.5 ml/m2] concordant with established normative cutoffs) [19] was present in 21% (n=57) of patients: 18% (n=50) of the population had RV systolic dysfunction and 10% (n=26) had RV dilation (7% [n=19] both).
Table 1 details clinical and imaging characteristics of the population, as well as comparisons between patients with and without RV dilation or dysfunction. As shown, patients with RV structural or functional abnormalities were older, more likely to have had prior MI and prior coronary revascularization (p<0.05 for all). Regarding imaging parameters, patients with RV dilation or dysfunction had larger LV volumes and decreased LV systolic function (all p<0.001), consistent with the concept that post-MI RV and LV structural/functional abnormalities are closely related.
Table 1.
Clinical and Imaging Characteristics
| Overall (n=272) |
RV Dilation or Dysfunction - (n=215) |
RV Dilation or Dysfunction + (n=57) |
P | |
|---|---|---|---|---|
| CLINICAL | ||||
| Age (year) | 59±13 | 57±12 | 63±15 | 0.003 |
| Male gender | 84% (228) | 84% (180) | 84% (48) | 0.93 |
| Body Surface Area | 2.0±0.2 | 1.97±0.24 | 1.97±0.21 | 0.81 |
| Coronary Artery Disease Risk Factors | ||||
| Hypertension | 52% (140) | 47% (100) | 73% (40) | 0.001 |
| Hypercholesterolemia | 53% (142) | 51% (109) | 60% (33) | 0.22 |
| Diabetes Mellitus | 23% (50) | 22% (42) | 31% (8) | 0.30 |
| Tobacco Use | 36% (97) | 35% (75) | 40% (22) | 0.48 |
| Family History | 30% (81) | 30% (65) | 30% (16) | 0.93 |
| Prior Myocardial Infarction | 15% (41) | 10% (22) | 35% (19) | <0.001 |
| Prior Coronary Revascularization | ||||
| Percutaneous Intervention | 17% (47) | 14% (30) | 31% (17) | 0.003 |
| Coronary Artery Bypass Grafting | 6.3% (17) | 2.8% (6) | 20% (11) | <0.001 |
| Cardiovascular Medications | ||||
| Beta-blocker | 93% (250) | 94% (202) | 89% (48) | 0.23 |
| ACE-Inhibitor/Angiotensin Receptor Blocker | 61% (163) | 60% (128) | 65% (35) | 0.48 |
| Loop diuretic | 15% (41) | 7.4% (16) | 46% (25) | <0.001 |
| HMG CoA-Reductase Inhibitor | 91% (246) | 95% (204) | 78% (42) | <0.001 |
| Aspirin | 95% (256) | 98% (211) | 83% (45) | <0.001 |
| Thienopyridine | 82% (221) | 89% (191) | 56% (30) | <0.001 |
|
CARDIAC MORPHOLOGY AND FUNCTION |
||||
| Cardiac Magnetic Resonance | ||||
| Right Ventricle | ||||
| Ejection fraction (%) | 57±11 | 61±6 | 42±9 | <0.001 |
| End-diastolic volume (ml)* | 143±41 (52–321) | 132.0±31.2 (52–203) | 182.9±47.1 (89–321) | <0.001 |
| (ml/m2) | 72±19 (34–161) | 67.0±12.8 (34–101) | 93.2±23.4 (36–161) | <0.001 |
| End-systolic volume (ml)* | 64±32 (16–242) | 51.9±16.9 (16–99) | 107.8±37.9 (52–242) | <0.001 |
| (ml/m2) | 32±16 (11–121) | 26.3±7.5 (11–43) | 55.2±19.5 (21–121) | <0.001 |
| Left Ventricle | ||||
| Ejection fraction (%) | 51±14 | 54±12 | 38±15 | <0.001 |
| End-diastolic volume (ml) | 164±53 | 152.8±40.7 | 203.8±70.5 | <0.001 |
| (ml/m2) | 83±25 | 77.7±18.0 | 103.7±35.9 | <0.001 |
| End-systolic volume (ml) | 86±51 | 72.9±35.3 | 134.0±69.3 | <0.001 |
| (ml/m2) | 44±26 | 37.1±17.3 | 68.6±36.6 | <0.001 |
Data presented as mean ± standard deviation (data in parentheses refer to range for each respective variable)
RV Linear Dimensions
Complete acquisition of all linear dimensions included in ASE guidelines (5 measurements in both end-systole and end-diastole) were obtainable in 98% (266/272) of patients (distal RVOT width not obtainable in 6 patients due to lack of requisite image). Table 2 details inter- and intra-observer reproducibility for both CMR- and echo-derived RV variables. As shown, greatest reproducibility for end-diastolic dimensions was yielded by basal RV width, RV length and proximal RVOT width (relative difference 4.97%, 6.65%, 6.49%, respectively), which were slightly less reproducible than by CMR (4.34%). End-systolic dimensions were less reproducible, paralleling volumetric data by CMR. Image analysis time was shorter for linear measurements via echo (49±15 seconds) than for volumetric segmentation via CMR (90±24 seconds; p<0.001).
Table 2.
Methodological Reproducibility
| Intra-observer Reproducibility | Inter-observer Reproducibility | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Intraclass Correlation Coefficient |
Limits of Agreement |
Coefficient of Variation (%) |
Relative Difference (%) |
Mean ± SD | Intraclass Correlation Coefficient |
Limits of Agreement |
Coefficient of Variation (%) |
Relative Difference (%) |
|
| Echo | ||||||||||
| Diastole | ||||||||||
| Basal RV width (mm) | −0.4 ± 2.3 | 0.89 | −4.9 to 4.1 | 3.60 | 4.97 | −0.5 ± 2.4 | 0.87 | −5.1 to 4.2 | 4.21 | 4.37 |
| Mid RV width (mm) | 1.4 ± 3.3 | 0.81 | −5.1 to 8.0 | 7.72 | 9.81 | 2.6 ± 4.1 | 0.63 | −5.5 to 10.6 | 10.83 | 14.93 |
| RV length (mm) | −0.8 ± 6.8 | 0.69 | −14.2 to 12.6 | 5.34 | 6.65 | −0.8 ± 5.1 | 0.78 | −10.9 to 9.2 | 4.36 | 4.74 |
| Proximal RVOT width (mm) | 0.7 ± 3.0 | 0.82 | −5.3 to 6.6 | 6.69 | 6.49 | −0.1 ± 2.6 | 0.89 | −5.1 to 4.9 | 5.59 | 5.79 |
| Distal RVOT width (mm) | 1.0 ± 3.1 | 0.74 | −5.0 to 7.0 | 8.78 | 9.61 | −2.2 ± 4.1 | 0.69 | −10.3 to 5.9 | 13.44 | 11.89 |
| Systole | ||||||||||
| Basal RV width (mm) | 0.8 ± 3.8 | 0.67 | −6.7 to 8.3 | 8.53 | 12.06 | −0.5 ± 3.4 | 0.75 | −7.2 to 6.1 | 8.50 | 10.44 |
| Mid RV width (mm) | 1.2 ± 3.9 | 0.61 | −6.5 to 8.8 | 13.56 | 17.52 | 0.6 ± 3.3 | 0.68 | −5.9 to 7.1 | 14.29 | 12.51 |
| RV length (mm) | −0.9 ± 7.2 | 0.60 | −15.0 to 13.1 | 6.81 | 7.78 | −0.5 ± 3.7 | 0.85 | −7.9 to 6.8 | 2.71 | 4.69 |
| Proximal RVOT width (mm) | 0.3 ± 3.3 | 0.81 | −6.2 to 6.8 | 8.98 | 10.66 | −0.1 ± 4.4 | 0.67 | −8.5 to 8.6 | 16.03 | 9.10 |
| Distal RVOT width (mm) | 1.8 ± 3.0 | 0.79 | −4.0 to 7.7 | 13.90 | 15.98 | −1.3 ± 3.3 | 0.75 | −7.8 to 5.3 | 16.20 | 14.88 |
| CMR | ||||||||||
| End-diastolic volume (ml) | −1.1 ± 5.9 | 0.99 | −12.8 to 10.5 | 2.32 | 4.34 | −4.0 ± 7.9 | 0.98 | −19.6 to 11.5 | 4.10 | 5.48 |
| End-systolic volume (ml) | −3.5 ± 4.6 | 0.98 | −12.5 to 5.5 | 7.64 | 7.64 | −1.8 ± 5.7 | 0.97 | −13.0 to 9.4 | 7.80 | 8.32 |
Table 3 compares echo linear dimensions stratified by the combined partition of RV dilation or dysfunction on CMR (left), as well as each of the two individual parameters. As shown, all RV linear dimensions were larger among patients with CMR-evidenced RV dilation (p<0.05 for all). Regarding RV systolic dysfunction, fractional shortening as measured in each linear plane was lower among patients with reduced EF (<50%) defined by CMR (p<0.05 for all).
Table 3.
Linear Dimensions Stratified by CMR-Evidenced RV Dilation or Dysfunction
| RV Dilation or Dysfunction - (n=215) |
RV Dilation or Dysfunction + (n=57) |
P | RV dysfunction - (n=222) |
RV dysfunction + (n=50) |
P | RV dilation - (n=246) |
RV dilation + (n=26) |
P | |
|---|---|---|---|---|---|---|---|---|---|
|
Right Ventricle | |||||||||
| End Diastolic Dimensions | |||||||||
| Basal RV width (mm) | 35.7±5.2 | 43.7±7.3 | <0.001 | 35.9±5.4 | 43.9±7.3 | <0.001 | 36.3±5.7 | 47.1±6.2 | <0.001 |
| Mid RV width (mm) | 26.3±5.8 | 31.9±7.0 | <0.001 | 26.4±6.0 | 32.0±6.8 | <0.001 | 26.7±5.9 | 35.0±6.7 | <0.001 |
| RV length (mm) | 78.5±8.2 | 89.5±9.0 | <0.001 | 78.9±8.4 | 89.5±9.3 | <0.001 | 79.7±8.8 | 91.5±9.6 | 0.001 |
| Proximal RVOT width (mm) | 31.9±4.7 | 37.7±5.7 | <0.001 | 32.1±4.9 | 37.3±5.9 | <0.001 | 32.5±5.0 | 39.2±5.5 | <0.001 |
| Distal RVOT width (mm) | 24.3±4.5 | 27.1±4.6 | <0.001 | 24.3±4.6 | 27.1±4.4 | <0.001 | 24.5±4.5 | 28.4±4.9 | <0.001 |
| End Systolic Dimensions | |||||||||
| Basal RV width (mm) | 25.0±4.9 | 33.7±7.0 | <0.001 | 25.2±5.1 | 34.2±6.7 | <0.001 | 25.8±5.6 | 36.2±6.8 | <0.001 |
| Mid RV width (mm) | 16.5±4.5 | 23.0±5.9 | <0.001 | 16.6±4.6 | 23.3±6.0 | <0.001 | 17.0±4.8 | 25.7±5.8 | <0.001 |
| RV length (mm) | 66.9±7.6 | 78.5±8.6 | <0.001 | 67.2±7.7 | 78.8±9.0 | <0.001 | 68.1±8.3 | 81.1±8.8 | <0.001 |
| Proximal RVOT width (mm) | 23.2±4.4 | 29.9±5.9 | <0.001 | 23.5±4.6 | 29.6±6.2 | <0.001 | 23.9±4.9 | 31.4±5.7 | <0.001 |
| Distal RVOT width (mm) | 17.1±3.9 | 19.7±4.7 | <0.001 | 17.1±3.9 | 19.9±4.7 | <0.001 | 17.2±3.9 | 21.4±5.3 | 0.001 |
| Fractional Shortening | |||||||||
| Basal RV (%) | 29.9±8.9 | 23.1±7.9 | <0.001 | 29.9±9.0 | 22.2±7.0 | <0.001 | 29.0±9.1 | 23.6±8.0 | 0.004 |
| Mid RV (%) | 36.9±12.4 | 27.9±9.5 | <0.001 | 36.7±12.3 | 27.4±9.6 | <0.001 | 35.9±12.4 | 26.7±8.6 | <0.001 |
| RV length (%) | 14.7±6.2 | 12.2±5.2 | 0.006 | 14.6±6.2 | 12.0±4.9 | 0.005 | 14.4±6.0 | 11.2±5.9 | 0.01 |
| Proximal RVOT (%) | 27.3±7.5 | 20.9±7.8 | <0.001 | 27.0±7.6 | 21.0±7.7 | <0.001 | 26.5±7.7 | 20.0±8.0 | <0.001 |
| Distal RVOT (%) | 29.7±7.7 | 27.7±8.5 | 0.09 | 29.8±7.6 | 27.0±8.7 | 0.03 | 29.7±7.6 | 25.4±9.4 | 0.009 |
|
Left Ventricle | |||||||||
| End-diastolic diameter (mm) | 56.4±4.8 | 59.2±7.6 | 0.01 | 56.4±4.8 | 59.6±7.8 | 0.007 | 56.7±5.2 | 59.3±8.2 | 0.13 |
| End-systolic diameter (mm) | 42.0±6.1 | 48.4±10.5 | <0.001 | 42.0±6.1 | 49.3±10.4 | <0.001 | 42.8±6.9 | 48.5±11.7 | 0.02 |
| Fractional shortening (%) | 25.8±6.6 | 19.2±9.2 | <0.001 | 25.8±6.7 | 18.2±8.7 | <0.001 | 25.0±7.1 | 19.2±10.3 | 0.009 |
RV Volumes
Table 4 reports correlations between CMR-quantified RV chamber volumes and echo-quantified RV dimensions. Echo dimensions in all planes correlated significantly with CMR RV volumes (p<0.001 for all). Regarding end-diastolic volume, greatest magnitude of correlation was observed for basal RV width (r=0.70), proximal RVOT width (r=0.68) and RV length (r=0.61). Similar correlations were observed for corresponding end-systolic dimensions (r=0.68, 0.66, 0.65 respectively). Table 4 also demonstrates that proximal RVOT fractional shortening (measured in parasternal long axis) was the linear parameter that yielded the greatest correlation with RVEF (r=0.38, p<0.001). Echo-quantified fractional area change (FAC) (r=0.55, p<0.001) as well as TAPSE (r=0.48, p<0.001) yielded slightly higher correlations with CMR-quantified RVEF.
Table 4.
Echo-Quantified RV Parameters in Relation to CMR Quantified RV Volumes and Function
| Correlation Coefficient (r) | P | Regression Equation | |
|---|---|---|---|
|
Diastolic Chamber Size | |||
| Linear parameters | |||
| Basal RV width | 0.70 | <0.001 | y = 4.33x − 19.0 |
| Mid RV width | 0.60 | <0.001 | y = 3.80x + 38.3 |
| RV length | 0.61 | <0.001 | y = 2.60x − 67.7 |
| Proximal RVOT width | 0.68 | <0.001 | y = 5.10x − 26.0 |
| Distal RVOT width * | 0.43 | <0.001 | y = 3.76x + 48.5 |
| Area (4-chamber) | 0.60 | <0.001 | y = 4.47x + 58.8 |
|
Systolic Chamber Size | |||
| Linear parameters | |||
| Basal RV width | 0.68 | <0.001 | y = 3.43x − 28.3 |
| Mid RV width | 0.64 | <0.001 | y = 3.74x − 3.1 |
| RV length | 0.65 | <0.001 | y = 2.31x − 96.2 |
| Proximal RVOT width | 0.66 | <0.001 | y = 3.87x − 31.7 |
| Distal RVOT width * | 0.46 | <0.001 | y = 3.51x + 1.2 |
| Area (4-chamber) | 0.67 | <0.001 | y = 4.80x + 18.0 |
|
Contractile Function | |||
| Fractional Shortening | |||
| Basal RV width | 0.31 | <0.001 | y = 0.36x + 46.8 |
| Mid RV width | 0.31 | <0.001 | y = 0.26x + 47.9 |
| RV length | 0.13 | 0.04 | y = 0.22x + 54.0 |
| Proximal RVOT width | 0.38 | <0.001 | y = 0.50x + 44.1 |
| Distal RVOT width | 0.22 | <0.001 | y = 0.29x + 48.8 |
| Fractional area change | 0.55 | <0.001 | y = 0.47x + 33.4 |
| RVS’ | 0.36 | <0.001 | y = 1.86x + 35.3 |
| TAPSE | 0.48 | <0.001 | y = 12.52x + 31.5 |
Unobtainable in 6 patients (2%) due to absence of distal RVOT image acquisition
Figure 2 stratifies representative echo dimensions in relation to population-based quartiles of CMR-quantified RV chamber volumes. As shown, end-diastolic and end-systolic dimensions increased stepwise in relation to RV volumetric quartiles (p<0.001). For example, end-diastolic proximal RVOT width was 33% higher among patients in the highest (38.0±4.6mm), compared to the lowest (28.5±3.8mm) quartile of RV end-diastolic volume. Similarly, basal RV width was 37% higher in the highest (43.4±6.3mm) vs. lowest (31.7±4.4mm) RV volumetric quartiles.
Figure 2. Echo Linear Dimensions in Relation to CMR RV Volume.

Echo-quantified basal RV width (mean ± standard deviation), as measured at (2A) end-diastole (green) and (2B) end-systole (yellow), in relation to population-based quartiles of RV chamber volume on CMR. Echo-quantified proximal RVOT width (mean ± standard deviation), as measured at (2C) end-diastole (green) and (2D) end-systole (yellow), in relation to population-based quartiles of RV chamber volume on CMR. Note that both echo linear measurements demonstrated stepwise increments in relation to CMR volumetric data (p<0.001 for trend).
Independent Markers of RV Volume
Multivariable linear regression was used to determine whether specific echo dimensions were independently associated with RV volume. Models were constructed by including dimensions from each orientation (parasternal long and short axis, 4 chamber length and width). For orientations in which two dimensions were measured (i.e. 4-chamber width [basal and mid]) models included the dimension that most strongly correlated with RV volume in univariable analysis.
Table 5 reports regression models for both RV end-diastolic (5A) and end-systolic (5B) volumes. Both models demonstrate that proximal RVOT width, basal RV width, and RV length were each independently associated with RV volume (p<0.001), whereas distal RVOT width was not (p=NS). Of note, the relationship between RV dimensions and volumes was continuous: applied clinically, the observed regression coefficient for end-diastolic proximal RVOT width (2.62 ml per mm [CI 1.79–3.44]; p<0.001) would indicate that a 4 mm increase in echo-quantified diameter would correspond to a 10 ml increase in RV chamber volume.
Table 5.
Multivariable Regression for Right Ventricular Chamber Volume
| A. RV End-Diastolic Volume | |||
|---|---|---|---|
| Correlation Coefficient =0.80; p<0.001 | |||
| Variable | Regression Coefficient (95% Confidence Interval) |
P | Partial Correlation |
| Basal RV width (mm) | 1.96 (1.22 – 2.70) | <0.001 | 0.31 |
| RV length (mm) | 0.97 (0.56 – 1.37) | <0.001 | 0.28 |
| Proximal RVOT width (mm) | 2.62 (1.79 – 3.44) | <0.001 | 0.36 |
| Distal RVOT width (mm) | 0.56 (−0.19– 1.30) | 0.14 | 0.09 |
| B. RV End-Systolic Volume | |||
|---|---|---|---|
| Correlation Coefficient =0.79; p<0.001 | |||
| Variable | Regression Coefficient (95% Confidence Interval) |
P | Partial Correlation |
| Basal RV width (mm) | 1.59 (1.06 – 2.13) | <0.001 | 0.34 |
| RV length (mm) | 1.00 (0.66 – 1.34) | <0.001 | 0.34 |
| Proximal RVOT width (mm) | 1.80 (1.22 – 2.39) | <0.001 | 0.35 |
| Distal RVOT width (mm) | 0.60 (−0.07 – 1.27) | 0.08 | 0.11 |
Diagnostic Performance for RV Dilation
Echo quantified linear dimensions were tested with regard to diagnostic test performance for RV end-diastolic chamber dilation as defined by CMR. As shown in Table 6A, application of linear cutoffs encompassed in ASE guidelines yielded reasonable diagnostic performance for discriminating between patients with and without RV dilation defined by CMR, which were similar for proximal RVOT width (sensitivity 81%, specificity 75%) and basal RV width (81%, 84%), but slightly lower for RV length (77%, 67%) – negative predictive value for all ASE linear cutoffs was high (≥94%) but positive predictive value low (20–39%). ROC analysis was used to further test whether BSA adjustment yielded improved diagnostic performance for linear variables. As shown in Table 6B, BSA-adjusted linear RV dimensions yielded good overall performance in relation to CMR-defined chamber dilation (AUC 0.77–0.87, all p<0.001): Applying a cutoff optimized for sensitivity and specificity (minimum 80%) showed proximal RVOT width, basal RV width, and RV length to yield similar results to un-indexed ASE cutoffs[3].
Table 6.
Diagnostic Performance of Echo RV Linear Dimensions for Right Ventricular Chamber Dilation on CMR*
| A. ASE Guideline Recommended Linear Cutoffs | ||||||
|---|---|---|---|---|---|---|
| Cutoff (mm) |
Sensitivity | Specificity | Accuracy | PPV | NPV | |
| Proximal RVOT width | 35 | 81% | 75% | 75% | 25% | 97% |
| Basal RV width | 41 | 81% | 84% | 84% | 36% | 98% |
| Mid RV width | 35 | 46% | 92% | 88% | 39% | 94% |
| RV length | 83 | 77% | 67% | 68% | 20% | 96% |
| Distal RVOT width | 27 | 68% | 80% | 79% | 27% | 96% |
| B. Cohort-derived Linear Cutoffs** | |||||||
|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Cutoff (mm/m2) |
Sensitivity | Specificity | Accuracy | PPV | NPV | |
| Proximal RVOT width | 0.86 (0.79–0.93); p<0.001 | 18.7 | 77% | 82% | 82% | 31% | 97% |
| Basal RV width | 0.87 (0.78–0.95); p<0.001 | 22.4 | 77% | 89% | 87% | 42% | 97% |
| Mid RV width | 0.82 (0.73–0.92); p<0.001 | 16.7 | 77% | 84% | 83% | 33% | 97% |
| RV length | 0.78 (0.69–0.87); p<0.001 | 45.8 | 54% | 82% | 79% | 24% | 94% |
| Distal RVOT width | 0.77 (0.66–0.88); p<0.001 | 14.2 | 72% | 82% | 81% | 29% | 97% |
RV dilation assessed at end-diastole on both echo and CMR
optimized for sensitivity using a minimum specificity cutoff of 80%
Discussion
This is the largest study to test all RV linear dimensions encompassed in ASE guidelines as markers of abnormal RV chamber size on CMR. There are several key findings: (1) Among a broad cohort of patients with CAD, complete RV linear dimensions were obtainable in nearly all patients (98%). Data acquisition was rapid (49±15 seconds); end-diastolic dimensions yielded good reproducibility, which was slightly less than that of CMR. (2) All echo-derived RV linear dimensions were larger among patients with CMR-evidenced RV dilation (p<0.001). Echo dimensions in all planes correlated significantly with RV end-diastolic and end-systolic volumes (p<0.001). Greatest magnitude of correlation was observed for basal 4 chamber RV width (r=0.70), proximal RVOT width (r=0.68), and 4-chamber RV length (r=0.61). Similar correlations were observed for corresponding end-systolic dimensions (r=0.68, 0.66, 0.65 respectively). (3) In multivariable regression analysis, echo dimensions in apical 4-chamber and parasternal long axis views were each independently associated with RV volume measured at end-diastole and end-systole. (4) RV linear cutoffs yielded reasonable diagnostic performance for assessment of CMR-defined RV chamber dilation. ASE guideline recommended cutoffs and derived BSA-indexed cutoffs yielded high negative predictive value (94–98%) but low positive predictive value (maximum 42%). Applied clinically, these data indicate that whereas linear dimensions within normative reference ranges can effectively exclude RV dilation, abnormal results should be interpreted cautiously with integration of other dimensions and/or confirmation via volumetric imaging such as 3D echo or CMR.
Quantification of RV size has been shown to reduce intra-observer variability as compared to qualitative assessment and is thus strongly recommended in ASE consensus guidelines.[3, 20] However, RV linear measurement by 2D echo is known to be challenging due to RV geometric complexity as well as absence of specific landmarks for optimization of RV imaging. The anterior and retrosternal location of the RV, as well as its thin-walled architecture, can compromise RV assessment in particular imaging planes, the nature of which can vary on a case-by-case basis (e.g. due to patient-specific artifacts). In the context of these challenges, ASE guidelines recommend that linear measurements be made in multiple orientations.[3] However, the feasibility and incremental value of each linear dimension has not been methodically studied, thus calling to question the utility of multiple measurements in routine clinical practice. To address this knowledge gap, our protocol tested both end-diastolic and end-systolic linear dimensions, so as to assess their relative utility for evaluation of RV size (measured by the reference of CMR). It is important to recognize that while RV contractile function would be expected to influence echo-quantified end-systolic diameter, it would also influence CMR-quantified RV end-systolic volume. Our findings are consistent with this concept, demonstrating that correlations between echo linear dimensions and CMR chamber volumes were generally of similar magnitude at end-diastole and end-systole.
Regarding clinical feasibility of our approach, it is important to note that 98% of echoes provided necessary data for complete measurement of all ASE recommended linear dimensions. Analysis was rapid (mean processing time < 1 minute), supporting the concept that our approach for RV quantification is feasible and adds minimal time to standard echo interpretation. Intra-observer reproducibility was high for basal RV width, proximal RVOT width, and RV length – each of which was independently associated with CMR quantified RV chamber volumes. Consistent with our results among an adult CAD population, a recent neonatal study has shown echo-quantified linear dimensions to be feasible (obtainable in 94% of exams) and yield good reproducibility when measured in a standardized manner.[21] We believe that our systematic approach of acquiring the largest linear dimension for each orientation yielded data uniformity, supporting widespread clinical application.
Among the broad cohort of patients with CAD studied, correlations between CMR volumes and echo dimensions were strongest for measurements acquired in apical 4-chamber and parasternal long axis views. Proximal RVOT width (measured in parasternal long-axis) and basal RV width (measured in apical 4 chamber) yielded near equivalent correlations with CMR volumes supporting the notion that RV linear dimensions in conventional echo orientations provide meaningful information regarding RV geometry. On the other hand, it is important to recognize the limits of linear dimensions: Maximum correlations in our dataset (r=0.68–0.70) suggest that echo-based linear dimensions account for less than 50% of variance in CMR quantified RV EDV. While it is conceivable that a truncated range of chamber volumes among our cohort may have reduced correlations, it is also likely that our data reflects the fact that singular linear indices are imprecise markers of RV dilation. Beyond individual parameters tested in this study, it is important to note that moderate correlations between echo and CMR indices might have been stronger were equivalent (3D) data compared between modalities. Linear indices reflect regional RV remodeling, and differences between modalities would be expected to cube when transposing data derived from a single plane in relation to a multiplanar 3D volume. Despite this, our observed moderate correlations and high negative predictive value yielded by routine echo support the notion that global RV chamber dilation alters regional RV dimensions, such that linear dimensions quantified on 2D echo can be used to stratify likelihood of RV chamber dilation and thereby identify patients in whom more sophisticated volumetric imaging (via 3D echo or CMR) approaches for RV assessment are most warranted.
Our data sheds new light on prior studies that have tested relationships between echo-derived RV linear measurements and CMR-derived RV volumes. For example, Kjaergaard et al, studying 34 patients, reported that proximal RV width yielded similar correlations with RV end-diastolic volume (r=0.58) as was observed in our study (r=0.68).[8] However, this prior report only tested a single linear dimension, prohibiting comparison of different RV dimensions. Similarly, Prakken et al. tested two linear dimensions (4 chamber basal RV and long-axis proximal RVOT widths) and reported that each correlated with CMR end-diastolic volumes to a similar magnitude (r=0.80, 0.56 respectively) to that observed in our study.[12] However, this prior study did not test whether or not each linear dimension provided additive value for assessment of RV chamber size, as was shown in multivariable linear regression analyses performed in the current study. Finally, Lai et al tested all 5 ASE encompassed measurements among a cohort of 31 normative controls and 56 patients with congenital heart disease.[13] In this study, echo and CMR correlations varied widely (r=0.15–0.73) possibly reflecting highly specific changes in RV geometry in the context of different congenital conditions. Moreover, substantial time interval between echo and CMR (up to 6 months) may have affected results in context of known impact of variable loading conditions on RV chamber size.
It is important to recognize that our protocol tested conventional 2D echo rather than newly available approaches such as real time three-dimensional echo (RT3DE). While RT3DE has been shown to provide reproducible and accurate RV volumes compared to CMR [22–24], widespread clinical application has been limited by logistical and technical challenges. 3D echo requires dedicated full volume image acquisition and post processing, both of which require technical proficiency for accurate measurement of RV volumes. Emerging tools such as 3D knowledge based reconstruction (KBR) show promise for improving data accuracy and reducing processing time[25], although this technique is not widely available in current clinical practice. Pending broader availability of advanced echo 3D acquisition methods and reliable processing algorithms, our data support use of readily obtainable RV linear dimensions as a straightforward means of rapidly assessing RV chamber geometry.
Study Limitations
Several limitations should be noted. First, whereas this study evaluated a broad cohort of CAD patients, variability in RVEF was small and global RV dysfunction (EF<50%) was uncommon (18%) – thereby limiting our ability to test linear echo indices as markers of RV systolic performance. Second, while our population included a substantial number of post-myocardial infarction (MI) patients, standardized assessment was not performed for clinical or hemodynamic evidence of RV infarction, or precise location of proximal infarct location. Third, this study examined abnormal RV in patients with CAD, and thus it is not certain as to how linear dimensions perform among patients with non-ischemic cardiomyopathies. However, alterations in RV chamber geometry would be expected to be global in context of non-ischemic processes that affect the RV and thus even better suited for assessment by linear dimensions than CAD-related RV remodeling (in which RV injury is often localized) and thus may either under or overestimate global RV abnormality depending on whether linear indices include affected RV segments. It is also important to note that the same population was used for both the derivation of BSA-indexed cutoffs as well as testing of its diagnostic performance. This approach represents a “best case scenario” and may not reflect actual test performance of linear indices applied in clinical practice. Finally, whereas the large majority of patients in our cohort (90%) were prospectively imaged as part of imaging research protocols examining CAD related remodeling, a minority (10%) were accrued retrospectively – yielding uncertainty as to whether results would have differed were all patients to have been derived from a singular prospective cohort. Future studies are needed to address these issues.
In conclusion, the current study demonstrates that RV linear dimensions encompassed in ASE guidelines provide readily obtainable markers of RV chamber volume. Parasternal long-axis RVOT width and 4-chamber RV basal diameter are independently associated with CMR volumes, supporting use of multiple linear dimensions when assessing RV size on echo.
Acknowledgments
Sources of Funding: NIH 1R01HL128278-01 (JWW), K23 HL102249-01 (JWW)
Abbreviations
- ASE
American Society of Echocardiography
- CAD
coronary artery disease
- CI
confidence interval
- CMR
cardiac magnetic resonance
- CoV
coefficient of variation
- Echo
echocardiography
- ESV
end-systolic volume
- EDV
end-diastolic volume
- EF
ejection fraction
- FAC
fractional area change
- ICC
intra-class correlation coefficient
- LOA
limits of agreement
- LV
left ventricle
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- RD
relative difference
- RV
right ventricle
- RVOT
right ventricle outflow tract
Footnotes
Conflicts of Interests Disclosure: None
References
- 1.Anavekar NS, Skali H, Bourgoun M, Ghali JK, Kober L, Maggioni AP, et al. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study) Am J Cardiol. 2008;101:607–612. doi: 10.1016/j.amjcard.2007.09.115. [DOI] [PubMed] [Google Scholar]
- 2.Antoni ML, Scherptong RW, Atary JZ, Boersma E, Holman ER, van der Wall EE, et al. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ Cardiovasc Imaging. 2010;3:264–271. doi: 10.1161/CIRCIMAGING.109.914366. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 5.Devereux RB, Roman MJ, Palmieri V, Liu JE, Lee ET, Best LG, et al. Prognostic implications of ejection fraction from linear echocardiographic dimensions: the Strong Heart Study. Am Heart J. 2003;146:527–534. doi: 10.1016/S0002-8703(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 6.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 7.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 8.Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, Hassager C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr. 2006;7:430–438. doi: 10.1016/j.euje.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Codella NC, Weinsaft JW, Cham MD, Janik M, Prince MR, Wang Y. Left ventricle: automated segmentation by using myocardial effusion threshold reduction and intravoxel computation at MR imaging. Radiology. 2008;248:1004–1012. doi: 10.1148/radiol.2482072016. [DOI] [PubMed] [Google Scholar]
- 10.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Shors SM, Fung CW, Francois CJ, Finn JP, Fieno DS. Accurate quantification of right ventricular mass at MR imaging by using cine true fast imaging with steady-state precession: study in dogs. Radiology. 2004;230:383–388. doi: 10.1148/radiol.2302021309. [DOI] [PubMed] [Google Scholar]
- 12.Prakken NH, Teske AJ, Cramer MJ, Mosterd A, Bosker AC, Mali WP, et al. Head-to-head comparison between echocardiography and cardiac MRI in the evaluation of the athlete's heart. Br J Sports Med. 2012;46:348–354. doi: 10.1136/bjsm.2010.077669. [DOI] [PubMed] [Google Scholar]
- 13.Lai WW, Gauvreau K, Rivera ES, Saleeb S, Powell AJ, Geva T. Accuracy of guideline recommendations for two-dimensional quantification of the right ventricle by echocardiography. Int J Cardiovasc Imaging. 2008;24:691–698. doi: 10.1007/s10554-008-9314-4. [DOI] [PubMed] [Google Scholar]
- 14.Weinsaft JW, Kim J, Medicherla CB, Ma CL, Codella NC, Kukar N, et al. Echocardiographic Algorithm for Post-Myocardial Infarction LV Thrombus: A Gatekeeper for Thrombus Evaluation by Delayed Enhancement CMR. JACC Cardiovasc Imaging. 2015 doi: 10.1016/j.jcmg.2015.06.017. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Medicherla CB, Ma CL, Feher A, Kukar N, Geevarghese A, et al. Association of Right Ventricular Pressure and Volume Overload with Non-Ischemic Septal Fibrosis on Cardiac Magnetic Resonance. PLoS One. 2016;11:e0147349. doi: 10.1371/journal.pone.0147349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Codella NC, Cham MD, Wong R, Chu C, Min JK, Prince MR, et al. Rapid and accurate left ventricular chamber quantification using a novel CMR segmentation algorithm: a clinical validation study. J Magn Reson Imaging. 2010;31:845–853. doi: 10.1002/jmri.22080. [DOI] [PubMed] [Google Scholar]
- 17.Kawaji K, Codella NC, Prince MR, Chu CW, Shakoor A, LaBounty TM, et al. Automated segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging. 2009;2:476–484. doi: 10.1161/CIRCIMAGING.109.879304. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 19.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H, et al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Ling LF, Obuchowski NA, Rodriguez L, Popovic Z, Kwon D, Marwick TH. Accuracy and interobserver concordance of echocardiographic assessment of right ventricular size and systolic function: a quality control exercise. J Am Soc Echocardiogr. 2012;25:709–713. doi: 10.1016/j.echo.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Jain A, Mohamed A, El-Khuffash A, Connelly KA, Dallaire F, Jankov RP, et al. A comprehensive echocardiographic protocol for assessing neonatal right ventricular dimensions and function in the transitional period: normative data and z scores. J Am Soc Echocardiogr. 2014;27:1293–1304. doi: 10.1016/j.echo.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Grewal J, Majdalany D, Syed I, Pellikka P, Warnes CA. Three-dimensional echocardiographic assessment of right ventricular volume and function in adult patients with congenital heart disease: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2010;23:127–133. doi: 10.1016/j.echo.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Leibundgut G, Rohner A, Grize L, Bernheim A, Kessel-Schaefer A, Bremerich J, et al. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr. 2010;23:116–126. doi: 10.1016/j.echo.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 24.van der Zwaan HB, Helbing WA, McGhie JS, Geleijnse ML, Luijnenburg SE, Roos-Hesselink JW, et al. Clinical value of real-time three-dimensional echocardiography for right ventricular quantification in congenital heart disease: validation with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2010;23:134–140. doi: 10.1016/j.echo.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Wheeler M, Leipsic J, Trinh P, Raju R, Alaamri S, Thompson CR, et al. Right Ventricular Assessment in Adult Congenital Heart Disease Patients with Right Ventricle-to-Pulmonary Artery Conduits. J Am Soc Echocardiogr. 2015;28:522–532. doi: 10.1016/j.echo.2014.11.016. [DOI] [PubMed] [Google Scholar]


