Abstract
Objectives
To compare contrast-enhanced anatomic imaging to contrast-enhanced tissue characterization (DE-CMR) for left ventricular (LV) thrombus detection.
Background
Contrast echocardiography (echo) detects LV thrombus based on anatomic appearance whereas delayed-enhancement cardiac magnetic resonance (DE-CMR) imaging detects thrombus based on tissue characteristics. DE-CMR has been validated as an accurate technique for thrombus but its utility compared to contrast echo is unknown.
Methods
Multimodality imaging was performed in 121 patients at high-risk for thrombus due to myocardial infarction or heart failure. Imaging included three anatomic imaging techniques for thrombus detection (contrast echo, non-contrast echo, cine-CMR) and a reference of DE-CMR tissue characterization. LV structural parameters were quantified to identify markers for thrombus and predictors of additive utility of contrast-enhanced thrombus imaging.
Results
24 patients had thrombus by DE-CMR. Patients with thrombus had larger infarcts (by DE-CMR), more aneurysms and lower LVEF (by CMR and echo) than those without thrombus. Contrast echo nearly doubled sensitivity (61% vs. 33%, p<0.05) and yielded improved accuracy (92% vs. 82%, p<0.01) vs. non-contrast echo. Patients who derived incremental diagnostic utility from DE-CMR had lower LVEF vs. those in whom non-contrast echo alone accurately assessed thrombus (35±9% vs. 42±14%, p<0.01), with a similar trend for patients that derived incremental benefit from contrast echo (p=0.08). Contrast echo and cine-CMR closely agreed on the diagnosis of thrombus (kappa=0.79, p<0.001). Thrombus prevalence was lower by contrast echo than DE-CMR (p<0.05). Thrombus detected by DE-CMR but not by contrast echo was more likely to be mural in shape or, when apical, small in volume (p<0.05).
Conclusions
Echo contrast in high-risk patients markedly improves detection of LV thrombus, but does not detect a substantial number of thrombi identified by DE-CMR tissue characterization. Thrombi detected by DE-CMR but not by contrast echo are typically mural in shape or small in volume.
Keywords: thrombus, cardiovascular magnetic resonance, echocardiography
Introduction
Accurate detection of left ventricular (LV) thrombus is important as thrombus provides a substrate for embolic events and a rationale for anticoagulation. Both echocardiography (echo) and cardiac magnetic resonance (CMR) imaging can utilize contrast agents to improve thrombus detection. Echo contrast typically improves thrombus detection through cavity opacification, identifying thrombus based on anatomic appearance. CMR uses contrast to identify thrombus based on tissue characteristics related to avascularity. Using the technique of delayed-enhancement (DE) CMR, which is widely employed to discern viable from infarcted myocardium, thrombus can be identified by absence of contrast uptake. As DE-CMR identifies thrombus based on tissue characteristics rather than anatomic appearance, it enables LV thrombus to be delineated from myocardium and chamber cavity irrespective of location or morphology.
DE-CMR has been well-validated as an accurate technique for LV thrombus based on comparisons with pathology findings and clinical embolic events.1,2 In prior studies, DE-CMR has yielded a two to three-fold improvement in thrombus detection versus non-contrast echo.2,3 However, comparative studies have been performed without routine use of echo contrast, which can improve thrombus detection.4 Additionally, indications for contrast use differ between CMR and echo. For CMR, almost all studies include DE-CMR for the primary purpose of distinguishing between viable and infarcted myocardium, meaning that virtually all patients receive contrast. For echo, consensus guidelines recommend that contrast be reserved for non-contrast studies with sub-optimal image quality.5 The utility of a strategy of echo contrast administration based on a-priori clinical risk for thrombus rather than non-contrast echo image quality is unknown. As echo is widely used to screen patients with coronary disease or heart failure at risk for LV thrombus, optimization of diagnostic strategies for thrombus detection is of substantial importance.
The aims of this study were; first, to compare multiple imaging techniques that identify thrombus based on anatomic appearance (non-contrast echo, contrast echo, and cine-CMR) to a reference of DE-CMR tissue characterization; second, to assess whether thrombus detection by anatomic imaging varies in relation to LV geometry or thrombus morphology; and third, to identify imaging markers for LV thrombus in an at-risk population.
Methods
Population
The population consisted of patients at high risk for LV thrombus due to recent myocardial infarction or chronic heart failure who underwent a multimodality imaging protocol at Weill Cornell Medical College between August 2005 and November 2007. Patients were enrolled if CMR and echo were performed within a 7 day interval. All patients had contrast echo performed for the primary indication of LV thrombus assessment; 55% were clinically referred for CMR and 45% were recruited via an ongoing study of post-myocardial infarction thrombus and remodeling. For all patients, imaging was performed in accordance with a pre-defined protocol and interpreted by pre-assigned readers blinded to clinical history and results of other imaging modalities. Patients were enrolled de-novo and none had participated in prior investigations concerning thrombus.1
The imaging protocol consisted of non-contrast echo, contrast echo, cine-CMR, and DE-CMR. Contrast echo was performed irrespective of quality or findings of non-contrast echo. 60.3% of patients had echo and CMR performed on the same day, 32.2% underwent CMR at least one day prior to echo, and 7.4% underwent echo prior to CMR. Clinical data were collected including cardiac risk factors, presence of CAD, and medication regimen. Pathology data (in patients undergoing LV reconstruction surgery within 1 month) were reviewed to verify thrombus as identified at the time of imaging.
All procedures were conducted in accordance with the Institutional Review Board (IRB) at Weill Cornell, which approved the study protocol. All prospectively recruited patients provided informed consent and the IRB approved use of pre-existing data for inclusion in the imaging registry.
Imaging Protocol [full protocol available on-line]
Cardiac Magnetic Resonance
CMR was performed using 1.5T scanners (General Electric Signa). Cine-CMR was performed using a steady-state free precession sequence. Gadolinium was then intravenously administered (0.2 mmol/kg) to patients without contraindications (glomerular filtration rate <30 ml/min/1.73m2).6 DE-CMR was performed 10 minutes thereafter using a segmented inversion recovery sequence.7 Cine- and DE-CMR images were acquired in matching planes. Short axis imaging was contiguous throughout the LV. Long axis images were acquired in two-, three- and four-chamber orientations.
Echocardiography
Echocardiograms were performed using commercial equipment (General Electric Vivid-7 or Siemens Sequoia) by sonographers who had undergone dedicated training concerning the imaging protocol. Non-contrast images were acquired in standard parasternal and apical imaging planes.5 A sonographic contrast agent (Definity, Lantheus Medical Imaging) was then administered in accordance with manufacturer guidelines.8 Non-contrast and contrast-enhanced images were acquired in at least three (two-, three-, four-chamber) apical orientations.
Thrombus Identification
Tissue Characterization
Thrombus was identified on DE-CMR as a mass with tissue characteristics consistent with avascular tissue.1,2 DE-CMR was performed in accordance with the thrombus protocol that has been validated in prior research by our group;1 Standard DE-CMR was performed using an inversion time (TI) tailored to null viable myocardium (297±31msec, range 200–350msec). On standard DE-CMR, thrombus typically had a grey etched appearance compared to black (viable) or white (infarcted) myocardium (Figure 1A, “standard TI”). To further delineate thrombus, additional DE-CMR imaging was performed using a pulse sequence tailored to null avascular tissue.1 By increasing the TI to 600 msec, regions with contrast uptake appear grey and thrombus appears black (Figure 1A, “long TI”). Scanner operators performed standard DE-CMR in all patients and long TI imaging in patients (72%) that tolerated additional breath-holds.
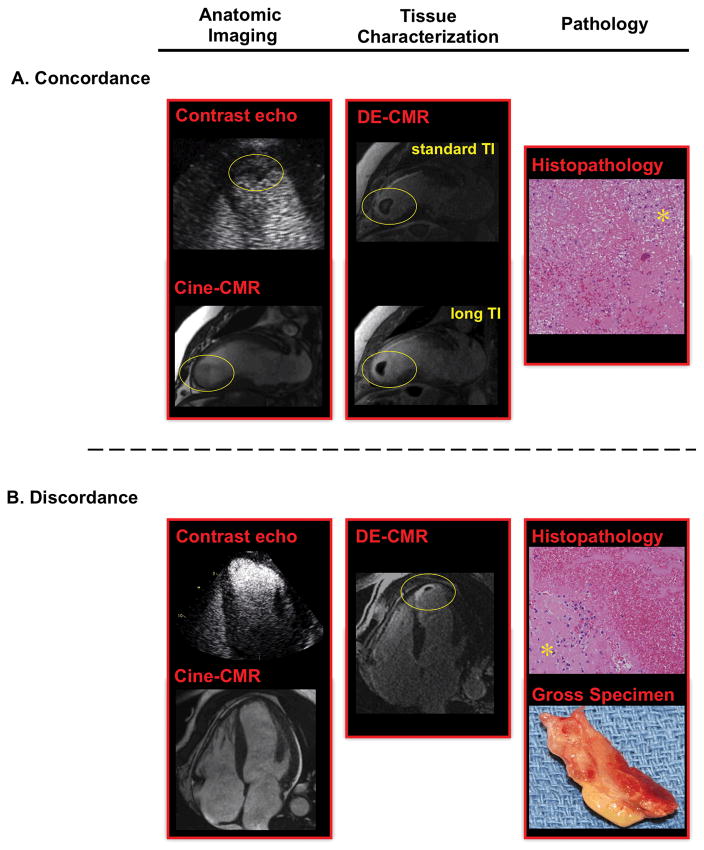
Figure 1. Apical Thrombus by Anatomic and Tissue Characterization Imaging.
(1A) Representative example of apical thrombus (circle) concordantly detected by anatomic imaging (left, contrast echo 4-chamber, cine-CMR 2-chamber) and DE-CMR (center). (1B) Representative example of discordance between anatomic imaging and DE-CMR. DE-CMR identified a small mural thrombus (circle) within the apex. Cine-CMR and contrast echo were interpreted as negative.
For both examples, surgical resection enabled thrombus verification based on histopathology (right, H&E stain, low power), which demonstrated thrombus with associated fibroblasts (asterisk).
In accordance with established criteria,1,3 thrombus was distinguished from myocardial infarction with microvascular obstruction (MVO) based on: (a) surrounding structures (MVO encompassed by hyperenhanced myocardium); (b) appearance (MVO within myocardium, thrombus adjacent to LV cavity and typically involves abrupt endocardial transitions); (c) stability of size on consecutive DE-CMR images (MVO shrinks from peripheral contrast fill-in, thrombus size stable).
Anatomic Imaging
For cine-CMR and echo, thrombus was diagnosed based on anatomic appearance and defined as an LV mass that was distinguishable from papillary muscles, trabeculae, chordal structures, technical artifact, or tangential views of the LV wall.1,9 Thrombus was echodense on echocardiography, and signal intensity was similar to myocardium on cine-CMR.
Data Analysis
Thrombus Assessment
Each modality was interpreted during a separate reading session by an experienced physician (CMR or echo AHA/ACC level III) who was blinded to the results of other modalities and clinical history. Inter-observer reproducibility was assessed by having a second reader independently re-interpret each modality for 30 randomly selected patients. All echoes were reviewed for image quality, which was graded using a scale combining scores for endocardial border definition (1=poor, 2=fair, 3=excellent) and cavity artifacts (1= present/obscuring full assessment, 2=present/not preventing interpretation, 3=absent)
Thrombus volume, morphology, and location were scored on DE-CMR; Volume was quantified by planimetry. Morphology was classified as mural (borders concave, similar to surrounding endocardial contours) or intracavitary (borders protruding into the cavity, distinct from surrounding endocardial contours).1,10 Location was classified as apical if localized to the LV apex or apical segments of other LV walls.
Imaging Markers
CMR and echo indices were measured to determine relationships with thrombus. Non-contrast echo quantified LVEF and chamber size based on linear dimensions.5 Cine-CMR quantified LVEF and chamber volumes based on planimetry. Regional contractility and infarction were scored using a 17-segment model. Segmental function was graded on cine-CMR (0=normal contraction; 1=mild hypokinesia; 2=moderate hypokinesia; 3=severe hypokinesia; 4=akinesia; 5=dyskinesia). Cine-CMR and echo were scored for presence of LV aneurysms. DE-CMR was scored for infarct size based on transmural extent of hyperenhancement (0=none; 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%). Global infarct size was calculated by summing segmental scores weighted by the midpoint of the range of hyperenhancement and dividing by the total number of segments.11
Statistical Methods
Continuous variables were compared using Student’s t test with Levene’s test for equality of variance to confirm homogeneity. Variables were also tested for skewness and kurtosis; comparisons between non-normally distributed data (thrombus volumes) were based on logarithmic transformation. Limits of agreement (1.96 times the standard deviation of between-method differences) were calculated. Categorical variables were compared using Chi-square or Fisher’s exact test. Diagnostic test performance and thrombus prevalence were compared using McNemar’s test with exact binomial probability calculations. Magnitude of agreement between tests was measured using the kappa statistic (κ). Univariate and multivariable logistic regression analyses were performed to evaluate associations between imaging parameters and thrombus. Two-sided p<0.05 was considered indicative of statistical significance. Analyses were performed using SPSS 12.0 (Chicago, Illinois).
Results
Population
The population consisted of 121 patients that underwent CMR and echo within a mean interval of 0.8±1.4 days. Both modalities demonstrated advanced LV dysfunction (LVEF difference 0.5±6.9% p=0.42; limits of agreement −13.2% to 14.2%).
DE-CMR Thrombus
DE-CMR identified LV thrombus in 24/121 patients (20%). Pathology verification of thrombus was available in three patients, all of whom had thrombus by DE-CMR. As shown in Table 1, patients with thrombus did not differ significantly from those without thrombus in age, gender or CAD risk factors. However, patients with thrombus had more severe LV dysfunction as measured by NYHA class (p=0.007) and the imaging parameters of LVEF (p≤0.001) or aneurysmal dilation by echo (p=0.09) or cine-CMR (p=0.01). Patients with thrombus also had larger total and transmural infarct size as measured by DE-CMR (p≤0.001).
Table 1.
Population Characteristics
| Overall (n=121) | DE-CMR + Thrombus (n=24) | DE-CMR − Thrombus (n=97) | P | |
|---|---|---|---|---|
| CLINICAL | ||||
| Age (year) | 61.2 ± 13.3 | 58.0 ± 14.6 | 61.9 ± 12.9 | 0.19 |
| Male gender | 77% (93) | 83% (20) | 75% (73) | 0.40 |
| New York Heart Association Class | 2.0 ± 0.8 | 2.5 ± 0.8 | 1.8 ± 0.8 | 0.007 |
| Coronary Artery Disease Risk Factors | ||||
| Hypertension | 67% (81) | 67% (16) | 67% (65) | 0.97 |
| Hypercholesterolemia | 88% (107) | 92% (22) | 88% (85) | 0.73 |
| Diabetes Mellitus | 33% (40) | 42% (10) | 31% (30) | 0.32 |
| Tobacco Use | 33% (40) | 38% (9) | 32% (31) | 0.61 |
| Family History | 21% (25) | 29% (7) | 19% (18) | 0.27 |
| Coronary Artery Disease | 98% (118) | 100% (24) | 97% (94) | 1.0 |
| Prior Myocardial Infarction | ||||
| Any history prior to CMR | 83% (100) | 79% (19) | 84% (81) | .56 |
| Within 2 months prior to CMR† | 69% (84) | 71% (17) | 69% (67) | .87 |
| Coronary Revascularization | ||||
| Percutaneous Intervention | 59% (71) | 42% (10) | 63% (61) | 0.06* |
| Coronary Artery Bypass Grafting | 10% (12) | 8% (2) | 10% (10) | 1.0 |
| Atrial Fibrillation | 6% (7) | - | 7% (7) | .34 |
| Lifetime History of Cerebrovascular Event | ||||
| Cerebrovascular Accident (CVA) | 11% (13) | 13% (3) | 10% (10) | 0.72 |
| Transient Ischemic Attack (TIA) | 3% (4) | - | 4% (4) | 0.58 |
| CVA or TIA | 12% (15) | 13% (3) | 12% (12) | 1.0 |
| Cardiovascular Medications | ||||
| Beta-blocker | 78% (94) | 79% (19) | 77% (75) | 0.85 |
| ACE-Inhibitor or Angiotensin Receptor Blocker | 67% (81) | 79% (19) | 64% (62) | 0.16 |
| Loop diuretic | 17% (20) | 21% (5) | 16% (15) | 0.55 |
| HMG CoA-Reductase Inhibitor | 79% (96) | 88% (21) | 77% (75) | 0.40 |
| Antithrombotic Medications | ||||
| Aspirin | 85% (103) | 96% (23) | 83% (80) | 0.12 |
| Warfarin | 9% (11) | 4% (1) | 10% (10) | 0.69 |
| Thienopyridines | 44% (53) | 33% (8) | 46% (45) | 0.25 |
| ECHOCARDIOGRAPHY | ||||
| LV Function and Morphology | ||||
| Ejection fraction (%) | 40.4 ± 13.1 | 33.6 ± 8.9 | 42.0 ± 13.5 | 0.001 |
| End-diastolic diameter (cm) | 5.9 ± 0.7 | 6.1 ± 1.0 | 5.9 ± 0.6 | 0.39 |
| End-systolic diameter (cm) | 4.8 ± 0.9 | 5.1 ± 1.1 | 4.7 ± 0.8 | 0.03 |
| Aneurysm present | 13% (16) | 25% (6) | 10% (10) | 0.09* |
| CARDIAC MAGNETIC RESONANCE | ||||
| LV Function and Morphology | ||||
| Ejection fraction (%) | 39.9 ± 13.9 | 30.3 ± 11.0 | 42.0 ± 13.6 | < 0.001 |
| Wall motion score index | 1.6 ± 0.9 | 2.2 ± 0.8 | 1.5 ± 0.9 | 0.001 |
| End-diastolic volume (ml) | 182.9 ± 72.7 | 212.5 ± 113.4 | 176.3 ± 58.7 | 0.16 |
| End-systolic volume (ml) | 116.4 ± 72.7 | 157.0 ± 113.3 | 107.2 ± 56.7 | 0.06* |
| Myocardial mass (gm) | 166.1 ± 55.7 | 192.4 ± 65.9 | 160.2 ± 51.6 | 0.01 |
| Aneurysm present | 12% (15) | 29% (7) | 8% (8) | 0.01 |
| LV Infarction | ||||
| % LV with transmural infarction‡ | 17.2 ± 13.3 | 24.8 ± 12.5 | 15.3 ± 12.8 | 0.001 |
| % LV infarct size | 17.6 ± 10.5 | 24.6 ± 8.6 | 15.9 ± 10.2 | < 0.001 |
| Anterior wall infarction | 69% (83) | 92% (22) | 63% (61) | 0.007 |
| Inferior or lateral wall infarction | 63% (76) | 71% (17) | 61% (59) | 0.36 |
Boldface type indicates p<0.05
Indicates p<0.1
Mean 3.1±1.7 weeks prior to CMR
Calculated based on aggregate # segments with transmural extent of infarction > 50%
Multivariable analyses were performed to identify imaging parameters associated with LV thrombus. Separate models were used to examine whether transmural myocardial infarction by DE-CMR was an independent marker for thrombus after adjustment for measures of chamber function and morphology derived from either cine-CMR or echo. When considering only CMR parameters (Table 2A), transmural infarct size was an independent marker for thrombus after adjustment for LVEF and aneurysmal dilation. A similar relationship was seen when substituting echo-derived LVEF in the multivariate model, although echo-identified LV aneurysm was not an independent marker for thrombus (Table 2B). Both models demonstrated that the relationship between thrombus and infarction was continuous, such that likelihood of thrombus increased in proportion to infarct size with an approximate 40% increase in relative risk for every 10% increment in transmural infarction.
Table 2.
Imaging Markers for LV Thrombus*
| 2A. Cardiac Magnetic Resonance (Function + Remodeling + Infarction) | |||
|---|---|---|---|
| χ2 = 23.1, p < 0.001
| |||
| Variable | Odds Ratio | 95% Confidence Interval | P |
| LV Ejection Fraction† (cine-CMR) | 1.63 | 1.17 – 2.11 | 0.007 |
| LV Aneurysm (cine-CMR) | 4.43 | 1.25 – 15.69 | 0.02 |
| LV with transmural infarction‡ (DE-CMR) | 1.39 | 1.01 – 1.79 | 0.04 |
| 2B. Echo (Function + Remodeling) + DE-CMR (Infarction) | |||
|---|---|---|---|
| χ2 = 17.0, p = 0.001
| |||
| Variable | Odds Ratio | 95% Confidence Interval | P |
| LV Ejection Fraction† (echo) | 1.58 | 1.11 – 2.07 | 0.01 |
| LV Aneurysm (echo) | 2.53 | 0.56 – 7.50 | 0.28 |
| LV with transmural infarction‡ (DE-CMR) | 1.43 | 1.04 – 1.83 | 0.03 |
Indices calculated using DE-CMR as the standard for LV thrombus
Per 10 point decrement in LV ejection fraction
Per 10% LV myocardium with transmural infarction
Diagnostic Performance of Anatomical Imaging
Table 3 reports diagnostic performance of anatomical imaging techniques compared to a reference of DE-CMR. Echo contrast markedly improved diagnostic performance for thrombus detection in this high-risk population. Sensitivity improved nearly two-fold vs. non-contrast echo (p<0.05), resulting in substantially higher accuracy (p<0.01). Similar to contrast echo, cine-CMR provided markedly higher sensitivity and accuracy vs. non-contrast echo (p≤0.001). There were no significant differences in diagnostic indices between contrast echo and cine-CMR, with strong agreement between modalities for the diagnosis of thrombus (κ=0.79, p<0.001). Diagnostic performance of echo was similar between patients grouped according to differences in testing sequence between CMR and echo as evidenced by non-significant differences in accuracy of contrast echo (p=0.37) and non-contrast echo (p=0.11) between groups.
Table 3.
Diagnostic Performance of Anatomic Imaging for LV Thrombus*
| Sensitivity | Specificity | Accuracy | Positive Predictive Value | Negative Predictive Value | |
|---|---|---|---|---|---|
| Non-Contrast Echo | 33% (8/24) | 94% (91/97) | 82% (99/121) | 57% (8/14) | 85% (91/107) |
| Contrast Echo§ | 61% (14/23)† | 99% (96/97) | 92% (110/120)‡ | 93% (14/15) | 91% (96/105) |
| Cine-CMR | 79% (19/24)§ | 99% (96/97) | 95% (115/121)§ | 95% (19/20) | 95% (96/101) |
Indices calculated using DE-CMR as the standard for LV thrombus
p < 0.05 (vs. non-contrast echo),
p < 0.01 (vs. non-contrast echo),
p ≤ 0.001 (vs. non-contrast echo)
One patient (with thrombus by non-contrast echo, cine-CMR, and DE-CMR) could not undergo the contrast echo component of the protocol due to contraindications to the echo contrast agent in the interim between study consent and imaging (decompensated heart failure)8.
Among the patent subgroup that underwent re-interpretation of images, inter-observer agreement (reported as proportion of concordant reads and kappa value) was good for non-contrast echo (25/30; κ=0.52, p=0.003) and excellent for contrast echo (29/30; kκ=0.87), cine-CMR (29/30; kκ=0.91), and DE-CMR (30/30; kκ=1.00) (all p<0.001).
Predictors of Improved Contrast-Echo Thrombus Detection
Qualitative and Structural Parameters
Multiple parameters were examined for markers that could identify patients who derived incremental diagnostic benefit from echo contrast. Table 4 reports image quality parameters for contrast and non-contrast echo in the overall population (white columns) and in patients with thrombus detected or missed by each echo technique (grey columns). In the total population, echo contrast improved endocardial border definition, cavity delineation, and overall image quality vs. non-contrast echo (p<0.001). However, echo performance was not associated with reader-assigned quality, with similar values for all qualitative parameters between contrast and non-contrast echoes that missed thrombus vs. those that detected DE-CMR evidenced thrombus (p=NS). Similar relationships were demonstrated when comparing image quality scores between non-contrast echoes that independently detected thrombus and those in which contrast use provided incremental utility for thrombus detection (p=NS).
Table 4.
Echo Image Quality in Relation to LV Thrombus Detection*
| Image Quality‡ | Endocardial Border Definition | LV Cavity Delineation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Overall Population† | Pts with Thrombus Detected | Pts with Thrombus Missed | P | Overall Population† | Pts with Thrombus Detected | Pts with Thrombus Missed | P | Overall Population† | Pts with Thrombus Detected | Pts with Thrombus Missed | P | |
| Non-Contrast Echo | 4.66±1.45 | 4.13±1.73 | 4.06±1.39 | 0.92 | 2.31±0.77 | 2.00±0.93 | 2.06±0.68 | 0.85 | 2.34±0.76 | 2.13±0.83 | 2.00±0.82 | 0.73 |
| Contrast Echo | 5.44±0.89 | 5.21±0.89 | 4.56±1.24 | 0.15 | 2.61±0.59 | 2.50±0.52 | 2.22±0.67 | 0.28 | 2.83±0.42 | 2.71±0.47 | 2.33±0.71 | 0.13 |
Indices calculated using DE-CMR as the standard for LV thrombus
p<0.001 for all comparisons between contrast and non-contrast echo
Aggregate scale comprised of border definition and cavity delineation scores.
Regarding LV quantitative parameters, non-contrast echoes that correctly identified or excluded thrombus had less depressed systolic function compared to echoes that were discordant with DE-CMR. As shown in Table 5, both cine-CMR and echo demonstrated that LVEF was lower (p<0.05) among patients with non-contrast echoes that were discordant with the DE-CMR diagnosis of thrombus. Similarly, echo-evidenced LVEF tended to be lower in pts in whom contrast echo improved thrombus assessment vs. those in whom non-contrast echo alone accurately assessed thrombus (34.2±9.9% vs. 41.2±13.3%, p=0.08). There were no significant differences in clinical parameters between patients with thrombus detected and those with thrombus missed by non-contrast echo.
Table 5.
LV Geometry and Function in Relation to Echo Performance
| NC-Echo Concordance with DE- | NC-Echo Discordance with DE- | P | |
|---|---|---|---|
| Echo | |||
|
| |||
| Ejection fraction (%) | 41.6±13.5 | 34.5±9.2 | 0.005 |
| End-systolic diameter (cm) | 4.7±0.9 | 5.0±0.9 | 0.11 |
| End-diastolic diameter (cm) | 5.9±0.6 | 6.0±0.8 | .46 |
| Aneurysmal Dilation | 12.1% | 18.2% | .49 |
|
| |||
| Cine-CMR | |||
|
| |||
| Ejection fraction (%) | 41.5±13.8 | 32.5±12.1 | 0.007 |
| End-systolic volume (ml) | 111.1±69.7 | 141.6±82.4 | 0.08* |
| End-diastolic volume (ml) | 179.4±70.2 | 199.5±83.5 | 0.25 |
| Aneurysmal Dilation | 9.1% | 27.3% | 0.03 |
Boldface type indicates p<0.05
Indicates p<0.1
Thrombus Morphology
Despite the marked overall improvement in thrombus detection yielded by a routine strategy of contrast use, 39% of DE-CMR evidenced thrombi were not detected by contrast echo, resulting in lower overall prevalence (13%) compared to DE-CMR (20%; p=0.02); Whereas DE-CMR detected 14 of the 15 thrombi detected by contrast echo (sensitivity 93% based on echo reference), contrast echo identified 14 of 23 thrombi (61%) detected by DE-CMR. Impaired thrombus detection was not specific to contrast echo but was also evident for anatomical imaging by CMR, as demonstrated by the fact that 21% of thrombi were missed by cine-CMR. Figures 1B and 2 provide examples of patients with thrombus detected by DE-CMR but missed by either contrast echo or cine-CMR.
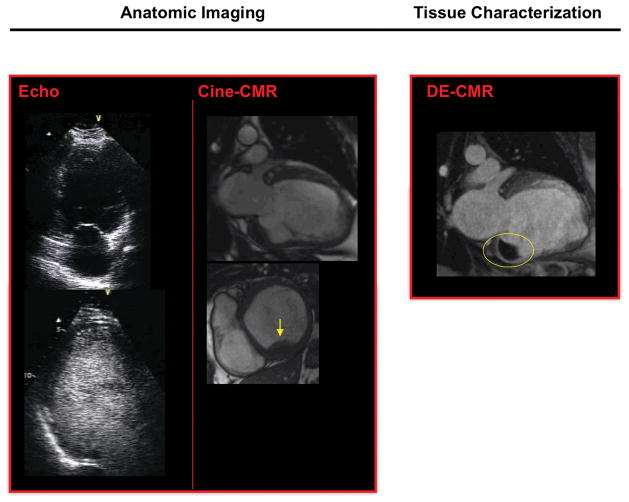
Figure 2. Non-Apical Thrombus Despite Negative Anatomic Imaging.
Representative example of non-apical thrombus detection by DE-CMR despite negative anatomic imaging. Non-contrast and contrast echo (left, 2-chamber view) were negative for thrombus. Cine-CMR (middle) identified thrombus, attributable to acquisition of both short and long axis images. DE-CMR (right, 2-chamber view) identified a large mural thrombus (circle) adjacent to the basal inferior wall.
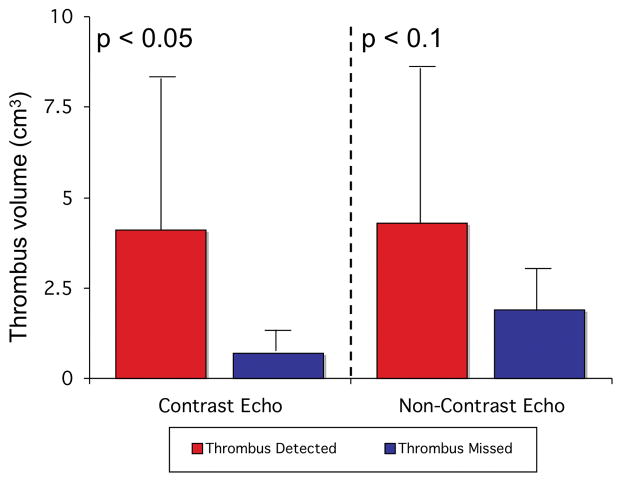
Detection of thrombus by anatomic imaging varied according to location, type, and size. Overall, 75% of thrombi were apical in location and 67% were intracavitary in shape. Apical and non-apical thrombi were similar in size (3.2±4.0cm3 vs. 5.4±7.4cm3, p=0.7). While apical thrombi missed by contrast echo were, on-average, less than 20% as large as those detected (0.8±0.6cm3 vs. 4.3±4.5cm3, p=0.02), non-apical thrombi missed by contrast echo varied widely in size with thrombi missed (7.3±8.7cm3, range 0.2 – 19.2cm3) occasionally larger than those detected (1.5±1.1cm3, 0.8 – 2.3cm3) (p=0.7). As shown in Figure 3, a similar trend was observed for apical thrombi missed vs. detected by non-contrast echo (2.0±2.7cm3 vs. 4.6±5.1cm3, p=0.097). Thrombus shape also modified detection by anatomic imaging, with mural thrombus less likely to be detected by contrast echo and cine-CMR (both p<0.05). Half (3/6) of all non-apical thrombi were mural in shape.
Figure 3. Thrombus Size in Relation to Echo Detection.
Apical thrombi detected by contrast echo were larger than those missed, with a similar trend for non-contrast echo (data shown as mean±SD).
Discussion
This study, the first to simultaneously compare contrast and non-contrast echo to DE-CMR for LV thrombus, provides several new observations: First, among a high-risk population, thrombus detection was markedly improved by a uniform strategy of contrast-enhanced imaging. Patients who derived incremental benefit from DE-CMR or contrast echo had lower LVEF than those in whom non-contrast echo alone accurately assessed thrombus. Second, despite the marked improvement in diagnostic performance yielded by echo contrast, DE-CMR detected more thrombi than did contrast echo. Contrast echo limitations were not modality-specific as evidenced by the fact that cine-CMR missed 21% of DE-CMR evidenced thrombi, with cine-CMR and contrast echo less likely to detect mural thrombus. Third, infarct size by DE-CMR was an independent marker for thrombus even after controlling for LVEF and aneurysmal dilation.
A major aim of our investigation was to compare an established technique of thrombus tissue characterization by DE-CMR1,2 to multiple techniques that identify thrombus based on anatomic appearance. Two-thirds of DE-CMR evidenced thrombi were not detected by non-contrast echo whereas contrast-echo improved sensitivity nearly two-fold. While it is possible that interval resolution of thrombus may have contributed to differences in test performance between echo and CMR, the interval between modalities was short (0.8±1.4 days) and the additive value of contrast-enhanced imaging was demonstrated for echo and CMR, supporting the concept that improved thrombus detection was related to contrast-enhancement.
When interpreting these results, it is important to recognize that there was no uniform gold standard for thrombus and that there are inherent limitations to using any given imaging method as a reference standard. However, our study was predicated on prior research that used pathology and clinical data to validate DE-CMR as a highly accurate technique for thrombus.1,2 Consistent with our findings, prior studies have reported that DE-CMR improves thrombus detection vs. echo. In a study that included 12 patients with DE-CMR evidenced thrombus, Mollet et al reported that echo detected thrombus in 42%.3 Srichai et al,2 studying patients with uniform pathology verification of thrombus, reported that sensitivity of echo was 23% vs. 88% for CMR. However, both studies may have understated echo capabilities; In the former, comparisons were exclusively made to non-contrast echo. In the latter, CMR with uniform gadolinium use was retrospectively compared to echo with only occasional use of echo contrast.
A central aspect of our protocol involved uniform echo contrast use irrespective of diagnostic findings or image quality of non-contrast echo. Our results demonstrate that routine administration of echo contrast and dedicated imaging protocols can improve thrombus detection in at-risk patients. Our findings are especially pertinent in the context of recent product labeling changes concerning risks of ultrasound contrast agents8 and ensuing debate regarding the risks of echo contrast and future of widespread use in the context of these labeling changes.12,13 While prior studies have reported that echo contrast improves LV thrombus detection, use has almost always been reserved for selected patients with suboptimal non-contrast echo quality.4,14 Our results demonstrate that while echo contrast improved image quality in the overall population, image quality alone did not predict improved thrombus assessment. For example, among patients with thrombus by DE-CMR, image quality scores did not differ between non-contrast echoes that detected thrombus and those in which in which contrast use provided additive benefit. This can be partially explained by our observation that thrombus can be present in areas other than the LV apex and thereby missed despite high quality apical imaging. The marked improvement in thrombus assessment yielded by echo contrast calls into question consensus guidelines that primarily recommend contrast use based on non-contrast echo image quality.5
Our findings demonstrate that LV function can be useful for guiding imaging strategies for thrombus; Non-contrast echoes that were discordant with DE-CMR had lower LVEF than those that were concordant with DE-CMR. Similarly, patients that derived incremental diagnostic utility from echo contrast tended to have lower LVEF than those in who non-contrast echo alone accurately diagnosed thrombus. LVEF was predictive as measured by either echo or cine-CMR despite variance between the two modalities, with differences possibly attributable to the fact that cine-CMR employed planimetry whereas echo employed linear measurements. Irrespective of differences between modalities, our findings confirm a well-established association between contractile dysfunction and thrombus,1,9 with the incremental utility of contrast-enhanced imaging paralleling higher thrombus prevalence with impaired LVEF. While DE-CMR provided improved thrombus detection vs. both contrast and non-contrast echo, optimization of echo protocols for thrombus remains important for situations in which echo is used to assess ventricular function, or in which DE-CMR is not available or is contraindicated. As our results demonstrate improved thrombus detection by DE-CMR, it is reasonable to expect that the advantage of tissue characterization is not modality-specific. Perfusion echo can identify thrombus based on tissue characteristics15 and our findings suggest that this approach may provide added benefit compared to contrast echo solely for LV cavity opacification.
It is important to recognize that this population was at high pre-test risk for thrombus, as reflected by the fact that 20% had thrombus by DE-CMR. Among this high-risk population, transmural infarct size was a marker for thrombus even after controlling for LVEF or aneurysmal dilation. This finding is consistent with our prior study showing that infarct size by DE-CMR was associated with thrombus among a diverse heart failure cohort.1 However, in our prior study, echo predictors were not evaluated. In this study, thrombus was associated with transmural infarct size even after controlling for LVEF and aneurysm measured by either echo or cine-CMR, demonstrating that infarcted myocardium is an independent marker for thrombus irrespective of the modality used to assess LV function/geometry.
Several limitations should be recognized. First, breath-held DE-CMR can be difficult for some patients and this can affect tolerance of DE-CMR. Free-breathing alternatives, including navigator or single-shot DE-CMR, were not tested. Our protocol was limited to segmented DE-CMR in order to evaluate one validated method for thrombus tissue characterization and provide a uniform comparison with echo. Second, while our study compared diagnostic performance of anatomic and tissue characterization imaging, the clinical implications of thrombus detected by DE-CMR but missed by echo are not established.
In conclusion, these findings demonstrate that DE-CMR is useful for LV thrombus detection among at-risk patients. Thrombus was associated with infarct size – an additional parameter provided by DE-CMR. While echo diagnostic performance was markedly improved by a strategy of routine contrast use, prevalence was lower than by DE-CMR, especially for mural thrombus and small apical thrombus. Cine-CMR and contrast echo demonstrated similar performance and strong agreement, suggesting that limitations were not modality-specific but rather attributable to intrinsic features of imaging techniques that identify LV thrombus based on anatomic appearance without assessment of tissue properties.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by a Doris Duke Clinical Scientist Development Award (JWW), the Michael J. Wolk Foundation, and Lantheus Medical Imaging.
Footnotes
Conflicts of Interest: This work was partially supported by Lantheus Medical Imaging (echo contrast manufacturer).
References
- 1.Weinsaft J, Kim H, Shah DJ, et al. Detection of Left Ventricular Thrombus by Delayed-Enhancement CMR: Prevalence and Markers in Patients with Systolic Dysfunction. J Am Coll Cardiol. 2008;52:148–57. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathologic characteristics of left ventricular thrombus: A comparison of contrast enhanced magnetic resonance imaging, transthoracic echocardiography and transesophageal echocardiography with surgical or pathological validation. American Heart Journal. 2006;152:75–84. doi: 10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106:2873–6. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 4.Thanigaraj S, Schechtman KB, Perez JE. Improved echocardiographic delineation of left ventricular thrombus with the use of intravenous second-generation contrast image enhancement. J Am Soc Echocardiogr. 1999;12:1022–6. doi: 10.1016/s0894-7317(99)70097-0. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Biereg M, Devereux RM, et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed June 1, 2007];New US Food and Drug Administration Information on Gadolinium-Containing Contrast Agents. Available at http://www.fda.gov/cder/drug/infopage/gcca/default.htm.
- 7.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–23. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed October 20, 2007];New US Food and Drug Administration prescribing information for Definity approved. 2007 Oct 10; Available at http://www.fda.gov/cder/foi/label/2007/021064s007lbl.pdf.
- 9.Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305:297–302. doi: 10.1056/NEJM198108063050601. [DOI] [PubMed] [Google Scholar]
- 10.Domenicucci S, Chiarella F, Bellotti P, Bellone P, Lupi G, Vecchio C. Long-term prospective assessment of left ventricular thrombus in anterior wall acute myocardial infarction and implications for a rational approach to embolic risk. Am J Cardiol. 1999;83:519–24. doi: 10.1016/s0002-9149(98)00906-0. [DOI] [PubMed] [Google Scholar]
- 11.Sievers B, Elliott MD, Hurwitz LM, et al. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007;115:236–44. doi: 10.1161/CIRCULATIONAHA.106.635409. [DOI] [PubMed] [Google Scholar]
- 12.Main ML, Goldman JH, Grayburn PA. Thinking outside the “box” - the ultrasound contrast contraversy. Journal of the American College of Cardiology. 2007;50:2434–7. doi: 10.1016/j.jacc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Kusnetzky LL, Khalid A, Khumri TM, Moe TG, Jones PG, Main ML. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent: results in 18,671 consecutive studies. J Am Coll Cardiol. 2008;51:1704–6. doi: 10.1016/j.jacc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Castello R, Bella JN, Rovner A, Swan J, Smith J, Shaw L. Efficacy and time-efficiency of a “sonographer-driven” contrast echocardiography protocol in a high-volume echocardiography laboratory. Am Heart J. 2003;145:535–41. doi: 10.1067/mhj.2003.164. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick JN, Wong T, Bednarz JE, et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412–9. doi: 10.1016/j.jacc.2003.09.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.