Abstract
Background
Concerns remain regarding the cancer risk associated with perinatal antiretroviral (ARV) exposure among infants. No excessive cancer risk has been found in short-term studies.
Methods
Children born to HIV-infected women (HIV-exposed) in New Jersey from 1995 to 2008 were identified through the Enhanced HIV/AIDS Reporting System and cross-referenced with data from the New Jersey State Cancer Registry to identify new cases of cancer among children who were perinatally exposed to ARV. Matching of individuals in the Enhanced HIV/AIDS Reporting System to the New Jersey State Cancer Registry was conducted based on name, birth date, Social Security number, residential address, and sex using AutoMatch. Age- and sex-standardized incidence ratio (SIR) and exact 95% confidence intervals (CIs) were calculated using New Jersey (1979–2005) and US (1999–2009) cancer rates.
Results
Among 3087 children (29,099 person-years; median follow-up: 9.8 years), 4 were diagnosed with cancer. Cancer incidence among HIV-exposed children who were not exposed to ARV prophylaxis (22.5 per 100,000 person-years) did not differ significantly from the incidence among children who were exposed to any perinatal ARV prophylaxis (14.3 per 100,000 person-years). Furthermore, the number of cases observed among individuals exposed to ARV did not differ significantly from cases expected based on state (SIR = 1.21; 95% CI: 0.25 to 3.54) and national (SIR = 1.27; 95% CI: 0.26 to 3.70) reference rates.
Conclusions
Our findings are reassuring that current use of ARV for perinatal HIV prophylaxis does not increase cancer risk. We found no evidence to alter the current federal guidelines of 2014 that recommend ARV prophylaxis of HIV-exposed infants.
Keywords: HIV, cancer, perinatal, children, antiretroviral, ARV, exposed
INTRODUCTION
Antiretroviral (ARV) prophylaxis for prevention of mother-to-child HIV transmission was recommended by the US Public Health Service for all HIV-infected pregnant women and their infants in 1994.1 Initially, the regimen for the woman and the infant included only zidovudine, a nucleoside reverse-transcriptase inhibitor (NRTI), but by the late 1990s, combination ARV therapy during pregnancy was recommended for women.2 Between 1994 and 2010, approximately 100,000 US infants were prenatally exposed to ARV; in the same time frame, it is estimated that more than 25,000 cases of perinatally acquired HIV infection were prevented that would have occurred in the absence of ARV prophylaxis.3
NRTIs are usually important components of ARV prophylaxis regimens. NRTI binds nuclear and mitochondrial DNA and thus inhibits DNA polymerase-γ, which is responsible for mitochondrial DNA replication. Therefore, since the time of the first recommendation for neonatal prophylaxis, there have been concerns about short- and long-term risks of NRTI use. Observed adverse events have included hematologic toxicities (anemia4–9 and leukopenia4–6,8,10–12), which are usually transient and infrequently of clinical significance. Lactic acidemia, likewise transient and usually insignificant, has been reported as well.13–16 More significant mitochondrial dysfunction was originally reported in small numbers of ARV exposees in France17 and was identified on the basis of symptoms in the Pediatric AIDS Clinical Trials Group (PACTG) 219/219C in 20 of 1037 children (prevalence 1.9%) born between 1991 and 2002.18 However, symptomatic mitochondrial dysfunction was not found to be associated with deaths of HIV-exposed uninfected children in a large collaborative cohort in the United States.19
Nuclear binding of NRTI has raised the possibility of cancer and remains a concern because of its potential occurrence over a longer time frame. There were no cases of cancer identified in the first years of follow-up of infant participants in PACTG 07620 or in a collaborative study from the Women and Infants Transmission Study and PACTG 219.21 Larger studies with longer follow-up of HIV-exposed, ARV-exposed HIV-uninfected children found either no cases of cancer22 or rates of cancer statistically indistinguishable from the rate in the general population.23,24 To investigate cancer risk over a longer period, we conducted a match between New Jersey's surveillance data among HIV-exposed, ARV-exposed children and the State Cancer Registry.
METHODS
Children born to HIV-infected women (HIV-exposed) in New Jersey from 1995 to 2008 were identified through the Enhanced HIV/AIDS Reporting System and cross-referenced with data from the New Jersey State Cancer Registry to identify new cases of cancer among children who were exposed to ARV prenatally, during birth, or postnatally. Matching was based on name, birth date, Social Security number, address of residence, and sex. AutoMatch is a commercially available software that implements probabilistic record linkage methodology for matching records under conditions of uncertainty.25 The resulting linked data set was updated with vital records from the New Jersey Office of Vital Statistics and Registry to determine deaths among the sample. Sample size, follow-up time, and cancer incidence were determined for all HIV-exposed infants, HIV-exposed infants with no ARV exposure, HIV-exposed infants with any ARV exposure, and HIV-exposed infants with prenatal ARV exposure.
A child exposed to ARV at any stage was considered “exposed,” while “no exposure” was recorded if “no” was indicated for all questions in the Enhanced HIV/AIDS Reporting System that indicated ARV receipt. Follow-up was censored at cancer diagnosis, death, or end of the study on December 31, 2010. Age- and sex-standardized incidence ratio (SIR) was calculated by comparing observed cases with expected cases. Expected cases were calculated using the method described by Scklo and Nieto26 and performed separately based on New Jersey cancer rates between 1979 and 200527 and US cancer rates between 1999 and 2009.28 Exact confidence intervals (CIs) for SIR were calculated directly from the Poisson distribution29–31 using an alpha of 0.05. Chi-square was used to test associations between demographic variables and ARV receipt. HIV-infected children were excluded from the analysis because of the known risk of cancer associated with HIV infection.
This analysis was approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey and given a nonresearch determination for purposes of internal review by the Human Protections Administrator of the New Jersey Department of Health and Senior Services.
RESULTS
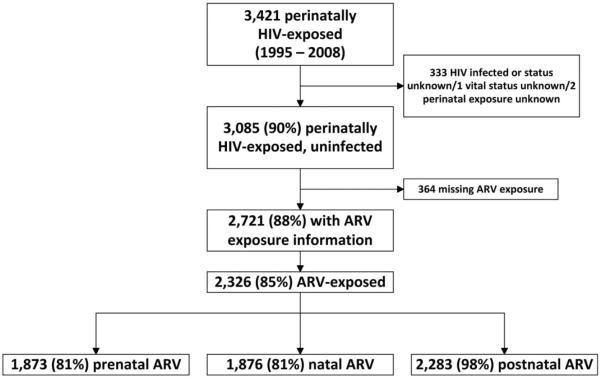
Between 1995 and 2008, 3421 children were born to HIV-infected women in New Jersey. Of these perinatally HIV-exposed children, 90% were not infected with HIV, resulting in 3085 exposed uninfected children. Of the 2721 (88%) children with information on ARV exposure, 2326 (85%) received ARV prophylaxis to prevent the establishment of HIV infection. Among these 2326 children, 81% received ARV prophylaxis prenatally, 81% during birth, and 98% postnatally (Fig. 1). Follow-up time for all HIV-exposed uninfected children totaled 29,099 person-years; median follow-up time per child was 9.8 years (range: <1 to 16 years).
FIGURE 1.
Flowchart of study sample and ARV exposure type.
Perinatally HIV-exposed uninfected children were evenly distributed across sex categories with 52% being male (Table 1). The majority of children were non-Hispanic black (69%), followed by Hispanic (19%), and non-Hispanic white (10%). Seventy-three percent of children were delivered at full term. As of December 31, 2010, 54 children (2%) had been reported to the New Jersey Office of Vital Statistics and Registry as deceased. A smaller proportion of non-Hispanic blacks were perinatally exposed to ARV (84%) than children of other races/ethnicities (range: 88%–90%). Those who had been reported to the State as deceased had a significantly lower proportion of ARV exposure than those who were not reported as deceased (70% vs. 86%; P = 0.0015).
TABLE 1.
Characteristics of Perinatally HIV-Exposed Uninfected Children by Any ARV Prophylaxis Exposure, New Jersey, 1995–2008
| Total |
Any ARV Exposure |
||||
|---|---|---|---|---|---|
| Variable | n | Col, % | n | Row, % | P * |
| Total | 2721 | 100 | 2326 | 86 | — |
| Cancer diagnosis | |||||
| Yes | 4 | <1 | 3 | 75 | 0.5514 |
| No | 2717 | >99 | 2323 | 86 | — |
| Sex | |||||
| Male | 1413 | 52 | 1202 | 85 | 0.5220 |
| Female | 1308 | 48 | 1124 | 86 | — |
| Race | |||||
| Non-Hispanic black | 1876 | 69 | 1574 | 84 | 0.0057 |
| Non-Hispanic white | 281 | 10 | 253 | 90 | — |
| Hispanic, all races | 517 | 19 | 457 | 88 | — |
| Other | 47 | 2 | 42 | 89 | — |
| Neonatal status | |||||
| Full term | 1938 | 73 | 1675 | 86 | 0.0975 |
| Premature† | 718 | 27 | 603 | 84 | — |
| Vital status (as of December 31, 2010) | |||||
| Dead | 54 | 2 | 38 | 70 | 0.0015 |
| Alive | 2667 | 98 | 2288 | 86 | — |
Chi-square for any ARV exposure.
Less than 37 weeks' gestation.
Among the 3085 perinatally HIV-exposed uninfected children born between 1995 and 2008, 4 were diagnosed with cancer (Hodgkin nodular sclerosis, acute myeloid leukemia, hepatocellular carcinoma, and pleuropulmonary blastoma) (Table 2). Three of the diagnoses (75%) were among males, and all diagnoses were among non-Hispanic blacks. Three of the 4 children with cancer diagnoses were exposed to ARV prophylaxis at some point before, during, or after birth. The age at cancer diagnosis ranged from <1 to 7 years.
TABLE 2.
Listing of Cancer Diagnoses Among Perinatally HIV-Exposed Uninfected Individuals Born From 1995 Through 2008, New Jersey, 2010
| Exposed to ARV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Sex | Race | Birth Year | Primary Site | Histology | Age at Cancer Diagnosis, yrs | Before Birth | During Birth | After Birth | Vital Status |
| 1 | Male | NH black | 1996 | Lymph nodes of multiple regions | Hodgkin nodular sclerosis | 7 | Yes | Unknown | Yes | Alive |
| 2 | Male | NH black | 1997 | Myeloproliferative | Acute myeloid leukemia | <1 | Yes | Yes | Yes | Alive |
| 3 | Male | NH black | 1999 | Liver | Hepatocellular carcinoma | 5 | Yes | Yes | Yes | Alive |
| 4 | Female | NH black | 2001 | Lower lobe, bronchus, or lung | Pleuropulmonary blastoma | 2 | No | No | Unknown | Alive |
NH, nan-Hispanic.
The incidence of all cancer types identified among all HIV-exposed uninfected children was 13.7 per 100,000 person-years (95% CI: 3.7 to 35.2). Cancer incidence among HIV-exposed children who were not exposed to ARV prophylaxis [22.5 per 100,000 person-years (95% CI: 0.6 to 125.3)] did not differ significantly from the incidence among children who were exposed to any ARV prophylaxis [14.3 per 100,000 person-years (95% CI: 3.0 to 41.9)] or who were exposed to ARV prophylaxis prenatally [18.1 per 100,000 person-years (95% CI: 3.7 to 52.7)], during birth [12.3 per 100,000 person-years (95% CI: 1.5 to 44.3)], or postnatally [14.7 per 100,000 person-years (95% CI: 3.0 to 42.8)] (Table 3).
TABLE 3.
Cancer Incidence Rate Among HIV-Exposed Children by ARV Exposure Type, New Jersey, 1995–2010
| Cancer, n | Exposed, n | Follow-up Time, person-years | Incidence* | LL† | UL† | |
|---|---|---|---|---|---|---|
| All HIV-exposed | 4 | 3085 | 29,099 | 13.7 | 3.7 | 35.2 |
| HlV-exposed, no ARV exposure | 1 | 395 | 4444 | 22.5 | 0.6 | 125.3 |
| HIV-exposed, any perinatal ARV exposure | 3 | 2326 | 20,909 | 14.3 | 3.0 | 41.9 |
| HIV-exposed, prenatal ARV exposure | 3 | 1873 | 16,610 | 18.1 | 3.7 | 52.7 |
| HIV-exposed, intrapartum ARV exposure | 2 | 1876 | 16,308 | 12.3 | 1.5 | 44.3 |
| HIV-exposed, postnatal ARV exposure | 3 | 2283 | 20,464 | 14.7 | 3.0 | 42.8 |
Per 100,000 person-years.
95% CI.
LL, lower limit; UL, upper limit.
The number of cases observed among individuals exposed to ARV at any time did not differ significantly from cases expected based on state (SIR = 1.21; 95% CI: 0.25 to 3.54) and national (SIR = 1.27; 95% CI: 0.26 to 3.70) reference rates. Furthermore, the observed number of cancer cases among those exposed to ARV prophylaxis at each stage, prenatal, intrapartum, and postnatal, did not differ significantly from cases expected based on state and national rates (Table 4).
TABLE 4.
SIRs for Cancer Diagnosed Among HIV-Exposed Uninfected Children, New Jersey, 1995–2010
| Exposure Type | Observed Cases | Expected Cases (State Reference)* | SIR† | LI‡ | UL‡ |
|---|---|---|---|---|---|
| Any ARV exposure | 3 | 2.5 | 1.21 | 0.25 | 3.54 |
| Prenatal ARV exposure | 3 | 3.1 | 0.96 | 0.20 | 2.81 |
| Intrapartum ARV exposure | 2 | 2.4 | 0.83 | 0.10 | 2.99 |
| Postnatal ARV exposure | 3 | 3.0 | 0.99 | 0.20 | 2.88 |
| Exposure Type | Observed Cases | Expected Cases (National Reference)§ | SIR† | LL‡ | UL‡ |
|---|---|---|---|---|---|
| Any ARV exposure | 3 | 2.4 | 1.27 | 0.26 | 3.70 |
| Prenatal ARV exposure | 3 | 3.0 | 1.01 | 0.21 | 2.94 |
| Intrapartum ARV exposure | 2 | 2.3 | 0.87 | 0.10 | 3.13 |
| Postnatal ARV exposure | 3 | 2.9 | 1.03 | 0.21 | 3.01 |
Expected cases determined based on New Jersey cancer rates among children <19 years of age from 1979 to 2005.
Standardized incidence rate (age and sex standardization).
95% CI.
Expected cases determined based on US cancer rates among children <19 years of age from 1999 to 2009.
LL, lower limit; UL, upper limit.
DISCUSSION
In this investigation, 16 years of cancer registry and HIV case surveillance data were reviewed to assess the risk of cancer among HIV-exposed uninfected children who were perinatally exposed to ARV prophylaxis. This study period represents the longest median follow-up time of any cancer study among HIV-exposed uninfected children and is the second largest in terms of total follow-up. No elevated cancer risk was found among children who were exposed to ARV prophylaxis at each or any time before, during, or after birth, when compared with HIV-exposed children who were not perinatally exposed to ARV prophylaxis. Moreover, the number of cases observed in this investigation among those who were exposed to ARV prophylaxis at each or any time before, during, or after birth did not differ from state or national cancer incidence rates of children aged from <1 to 19 years.
The results of this study are consistent with those of previous research. The incidence of cancer in HIV-exposed uninfected children is comparable with that in the French National Perinatal Study (18.8/100,000 person-years)23 and in PACTG 219C in the United States (12.7/100,000 person-years).24 These 2 studies, respectively, followed study participants for 53,052 person-years (median: 5.4 years/person) and 7871 person-years (median: 3.1 years/person). Furthermore, earlier studies in the United States, a follow-up study of PACTG 076 (median follow-up: 4.2 years/person)20 and a collaborative study including data from the Women and Infants Transmission Study and PACTG 219 (follow-up: 14.5 and 38.3 months/person, respectively, and total follow-up of 1111 person-years),21 found no cancers. Likewise, a study in the United Kingdom that linked data from the National Study of HIV in Pregnancy and Childhood to records of the National Health Service found no cases over a period of 7013 person-years (median: 2.5 years/person, range: 1.0–5.0).22
There are limitations in this study that should be considered when interpreting these findings. Data on ARV exposure were incomplete; of the perinatally HIV-exposed uninfected children, 12% were missing information on ARV exposure. The effect of these missing data on our findings is unknown. However, those with missing ARV information were not different from those with ARV information with respect to cancer diagnosis, death, sex, or race. Second, cancer diagnoses and deaths that occurred outside New Jersey, or were not recorded in state records, were not identified in our analyses. Therefore, we cannot quantify loss to follow-up of individuals who moved or sought care outside New Jersey, which could have resulted in an underestimate of incidence. Furthermore, data on the exact timing and duration of intrauterine exposure to ARV were not available; thus, we were unable to quantify a child's exposure to ARV. Finally, this is an investigation of HIV-exposed children born in the state of New Jersey, thus, these findings should not be generalized beyond this population.
The reduction of mother-to-child transmission of HIV in the United States is a major public health success, and ARV prophylaxis is an integral part of the mother-to-child transmission prevention cascade.32 Cancer incidence in HIV-exposed but uninfected ARV-exposed children was not significantly different from incidence in the general population of children and adolescents less than 19 years of age. However, because of the evolving spectrum of ARV usage by pregnant women, further monitoring of the risk of cancer and of other health outcomes associated with ARV exposure is needed to ensure that ARV medications are safe for mothers and children. Our data are reassuring that current use of ARV for perinatal prophylaxis does not increase cancer risk. We found no evidence to alter the current federal guidelines of 2014 that recommend ARV prophylaxis of HIV-exposed infants.33
Acknowledgments
Supported by the Centers for Disease Control and Prevention and the New Jersey Department of Health.
Footnotes
Presented at the International AIDS Conference, July 23, 2012, Washington, DC (Abstract: MOPE 063).
The authors have no funding or conflicts of interest to disclose.
This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy.
REFERENCES
- 1.Centers for Disease Control and Prevention Recommendations of the U.S. Public Health Service Task Force on the use of zidovudine to reduce perinatal transmission of human immunodeficiency virus. MMWR Recomm Rep. 1994;43:1–20. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Public Health Service Task Force recommendations for the use of antiretroviral drugs in pregnant women infected with HIV-1 for maternal health and for reducing perinatal HIV-1 transmission in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–30. [PubMed] [Google Scholar]
- 3.Taylor AW, Little KM, Zhang X, et al. Estimated Perinatal Antiretroviral Exposures, Cases Prevented and Infected Infants in the Era of Antiretroviral Prophylaxis in the United States; Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. Abstract: T-103. [Google Scholar]
- 4.Bunders M, Thorne C, Newell ML. European Collaborative Study. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS. 2005;19:1071–1079. doi: 10.1097/01.aids.0000174454.63250.22. [DOI] [PubMed] [Google Scholar]
- 5.Le Chenadec J, Mayaux MJ, Guihenneuc-Jouyaux C, et al. Enquete Perinatale Francaise Study Group. Perinatal antiretroviral treatment and hematopoiesis in HIV-uninfected infants. AIDS. 2003;17:2053–2061. doi: 10.1097/00002030-200309260-00006. [DOI] [PubMed] [Google Scholar]
- 6.Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 2001;285:2083–2093. doi: 10.1001/jama.285.16.2083. [DOI] [PubMed] [Google Scholar]
- 7.Mussi-Pinhata MM, Freimanis L, Yamamoto AY, et al. Infectious disease morbidity among young HIV-1-exposed but uninfected infants in Latin American and Caribbean countries: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Pediatrics. 2007;119:e694–e704. doi: 10.1542/peds.2006-1856. [DOI] [PubMed] [Google Scholar]
- 8.Pacheco SE, McIntosh K, Lu M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the Women and Infants Transmission Study. J Infect Dis. 2006;194:1089–1097. doi: 10.1086/507645. [DOI] [PubMed] [Google Scholar]
- 9.Witt MD, Lewis RJ, Rieg G, et al. Predictors of the isolated hepatitis B core antibody pattern in HIV-infected and -uninfected men in the multicenter AIDS cohort study. Clin Infect Dis. 2013;56:606–612. doi: 10.1093/cid/cis908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae WH, Wester C, Smeaton LM, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS. 2008;22:1633–1640. doi: 10.1097/QAD.0b013e328307a029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Collaborative Study Levels and patterns of neutrophil cell counts over the first 8 years of life in children of HIV-1-infected mothers. AIDS. 2004;18:2009–2017. doi: 10.1097/00002030-200410210-00005. [DOI] [PubMed] [Google Scholar]
- 12.Feiterna-Sperling C, Weizsaecker K, Buhrer C, et al. Hematologic effects of maternal antiretroviral therapy and transmission prophylaxis in HIV-1-exposed uninfected newborn infants. J Acquir Immune Defic Syndr. 2007;45:43–51. doi: 10.1097/QAI.0b013e318042d5e3. [DOI] [PubMed] [Google Scholar]
- 13.Alimenti A, Burdge DR, Ogilvie GS, et al. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr Infect Dis J. 2003;22:782–789. doi: 10.1097/01.inf.0000086400.93257.74. [DOI] [PubMed] [Google Scholar]
- 14.Ekouevi DK, Toure R, Becquet R, et al. Serum lactate levels in infants exposed peripartum to antiretroviral agents to prevent mother-to-child transmission of HIV: Agence Nationale de Recherches Sur le SIDA et les Hepatites Virales 1209 study, Abidjan, Ivory Coast. Pediatrics. 2006;118:e1071–e1077. doi: 10.1542/peds.2006-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giaquinto C, De Romeo A, Giacomet V, et al. Lactic acid levels in children perinatally treated with antiretroviral agents to prevent HIV transmission. AIDS. 2001;15:1074–1075. doi: 10.1097/00002030-200105250-00023. [DOI] [PubMed] [Google Scholar]
- 16.Noguera A, Fortuny C, Munoz-Almagro C, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114:e598–e603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 17.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 18.Brogly SB, Abzug MJ, Watts DH, et al. Birth defects among children born to human immunodeficiency virus-infected women: pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J. 2010;29:721–727. doi: 10.1097/INF.0b013e3181e74a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulterys M, Nesheim S, Abrams EJ, et al. Lack of evidence of mitochondrial dysfunction in the offspring of HIV-infected women. Retrospective review of perinatal exposure to antiretroviral drugs in the Perinatal AIDS Collaborative Transmission Study. Ann N Y Acad Sci. 2000;918:212–221. doi: 10.1111/j.1749-6632.2000.tb05491.x. [DOI] [PubMed] [Google Scholar]
- 20.Culnane M, Fowler M, Lee SS, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 21.Hanson IC, Antonelli TA, Sperling RS, et al. Lack of tumors in infants with perinatal HIV-1 exposure and fetal/neonatal exposure to zidovudine. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:463–467. doi: 10.1097/00042560-199904150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Hankin C, Lyall H, Peckham C, et al. Monitoring death and cancer in children born to HIV-infected women in England and Wales: use of HIV surveillance and national routine data. AIDS. 2007;21:867–869. doi: 10.1097/QAD.0b013e3280b01822. [DOI] [PubMed] [Google Scholar]
- 23.Benhammou V, Warszawski J, Bellec S, et al. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. AIDS. 2008;22:2165–2177. doi: 10.1097/QAD.0b013e328311d18b. [DOI] [PubMed] [Google Scholar]
- 24.Brogly S, Williams P, Seage GR, III, et al. In utero nucleoside reverse transcriptase inhibitor exposure and cancer in HIV-uninfected children: an update from the Pediatric AIDS Clinical Trials Group 219 and 219C cohorts. J Acquir Immune Defic Syndr. 2006;41:535–536. doi: 10.1097/01.qai.0000194735.66322.d9. [DOI] [PubMed] [Google Scholar]
- 25.Jaro MA. Probabilistic linkage of large public health data files. Stat Med. 1995;14:491–498. doi: 10.1002/sim.4780140510. [DOI] [PubMed] [Google Scholar]
- 26.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2nd ed. Jones and Bartlett Publishers; Sudbury, MA: 2007. [Google Scholar]
- 27.Roche LM, Agovino PK, Niu X, et al. Childhood Cancer in New Jersey, 1979-2005. Cancer Epidemiology Services, New Jersey Department of Health and Senior Services; New Jersey: 2008. 2008. [Google Scholar]
- 28.United States Cancer Statistics: 1999-2009 Incidence United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute. 2011 Available at: http://wonder.cdc.gov/cancer-v2009.html. Accessed May 6, 2013.
- 29.Re: A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1991;133:212–214. doi: 10.1093/oxfordjournals.aje.a115863. [DOI] [PubMed] [Google Scholar]
- 30.Daly L. Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Comput Biol Med. 1992;22:351–361. doi: 10.1016/0010-4825(92)90023-g. [DOI] [PubMed] [Google Scholar]
- 31.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131:373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 32.Stoto MA, Almario DA, McCormick MC. Reducing the Odds: Preventing Perinatal Transmission of HIV in the United States. National Academy Press; Institute of Medicine: 1999. [PubMed] [Google Scholar]
- 33.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed May 6, 2013.