Abstract
Aims/Hypothesis
Insulin activates the insulin receptor (IR) protein tyrosine kinase and downstream phosphatidylinositol-3-kinase (PI3K)/Akt signalling in muscle to promote glucose uptake. The IR can serve as a substrate for the protein tyrosine phosphatases (PTP) 1B and TCPTP, which share a striking 74% sequence identity in their catalytic domains. PTP1B is a validated therapeutic target for the alleviation of insulin resistance in type 2 diabetes. PTP1B dephosphorylates the IR in liver and muscle to regulate glucose homeostasis, whereas TCPTP regulates IR signalling and gluconeogenesis in the liver. In this study we have assessed for the first time the role of TCPTP in the regulation of IR signalling in muscle.
Methods
We generated muscle-specific TCPTP-deficient (MCK-Cre;Ptpn2lox/lox) mice and assessed the impact on glucose homeostasis and muscle IR signalling in chow versus high fat fed mice.
Results
Blood glucose and insulin levels, insulin and glucose tolerances and insulininduced muscle IR activation and downstream PI3K/Akt signalling remained unaltered in chow fed MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice. In addition, body weight, adiposity, energy expenditure, insulin sensitivity and glucose homeostasis were not altered in high fat fed MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice.
Conclusions
These results indicate that TCPTP deficiency in muscle has no effect on insulin signalling and glucose homeostasis and does not prevent the development of high fat diet-induced insulin resistance. Thus, despite their high degree of sequence identity, PTP1B and TCPTP differentially contribute to IR regulation in muscle. Our results are consistent with these two highly related PTPs having distinct contributions to IR regulation in different tissues.
Keywords: Diabetes, glucose homeostasis, insulin resistance, insulin signalling, muscle, protein tyrosine phosphatase, PTP, PTP1B, TCPTP
INTRODUCTION
Type 2, or non-insulin-dependent diabetes accounts for 90–95% of cases of diabetes and is characterized by insulin resistance and hyperglycemia. Insulin resistance manifests as diminished insulin responsiveness in insulin’s key target tissues including liver, fat and muscle and is associated with aging, obesity and a sedentary lifestyle [1, 2]. Insulin is secreted by the pancreas in response to an increase in blood glucose levels and suppresses glucose production in the liver whilst promoting glucose uptake into muscle and adipose tissue. Insulin exerts its effects by binding to the insulin receptor (IR) on the surface of cells. Binding of insulin activates the protein tyrosine kinase (PTK) activity of the intracellular β-subunit, resulting in autophosphorylation and the phosphorylation of substrates such as insulin receptor substrate (IRS)-1 [1, 3, 4]. Phosphotyrosyl residues on IRS-1 serve as docking sites for the recruitment of SH2 domain-containing proteins; these include the adaptor protein Grb-2 and the p85 regulatory subunit of phosphatidylinositol-3-kinase (PI3K), which activate the Ras/Mitogen-activated protein kinase (MAPK) and PI3K/Akt cascades to elicit the metabolic and mitogenic actions of insulin [1, 3, 4]. In particular, PI3K signalling and Akt2 are essential for the translocation/docking of the GLUT4 glucose transporter on the plasma membrane in muscle and fat [1, 2, 5–7]. Although the precise molecular mechanism(s) that underlie the development of insulin resistance remain unclear, one widely accepted mechanism involves the impairment of PI3K recruitment to IRS-1 [1, 2]. Hence, strategies that enhance IR activation and downstream signalling may be effective in alleviating insulin resistance.
Protein tyrosine phosphatases (PTPs) [8, 9] are key regulators of IR signalling, dephosphorylating and inactivating the IR within minutes of stimulation [10]. The prototypic family member PTP1B (encoded by PTPN1) dephosphorylates the IR β subunit Y1162/Y1163 activation loop autophosphorylation site to attenuate insulin signalling in vivo [11–16]. Mice with a global deficiency in PTP1B (Ptpn1−/−) exhibit enhanced insulin sensitivity, associated with increased IR activation and downstream IRS-1 tyrosine phosphorylation in liver and muscle [12–16]. PTP1B’s direct contribution to IR regulation in muscle has been substantiated by the generation of muscle-specific PTP1B deficient (MCK-Cre;Ptpn1lox/lox) mice [16]. MCK-Cre; Ptpn1lox/lox mice exhibit improved insulin sensitivity and glucose tolerance associated with elevated muscle IR activation and signalling [16]. Moreover, MCK-Cre; Ptpn1lox/lox mice are protected from the development of insulin resistance induced by a high fat diet [16]. Conversely, overexpression of PTP1B in muscle causes insulin resistance [17] and PTP1B expression and/or activity may be increased in muscle and adipose tissue of insulin-resistant humans and rodents [18–23]. PTP1B’s prominent role in IR regulation and glucose homeostasis has engendered considerable interest from the pharmaceutical industry for the development of PTP1B inhibitors for the treatment of type 2 diabetes [22, 24–26].
Several other tyrosine-specific PTPs have also been implicated in the attenuation of insulin signalling in muscle. Like PTP1B, LAR (Leukocyte common antigen–related) PTP expression may be increased in muscle from insulin-resistant rodents/humans and transgenic overexpression of LAR in muscle suppresses insulin signalling and glucose uptake and promotes whole body insulin resistance [18, 19, 27–30]. Recent studies have also shown that IR activation and signalling are elevated in PTPε-deficient differentiated myoblasts, but its role in glucose homeostasis in vivo remains unknown [31].
Previously, we showed that the IR can also serve as a bona fide substrate for the phosphatase TCPTP (encoded by PTPN2) [8, 11, 32, 33]. In particular, we reported that IR activation and signalling were enhanced in TCPTP-deficient fibroblasts and in HepG2 hepatoma cells after TCPTP knockdown [32, 33]. Moreover, we reported that TCPTP knockdown in PTP1B-deficient MEFS further enhanced IR activation, consistent with the two PTPs acting in a coordinated manner to attenuate insulin signalling [11]. PTP1B and TCPTP are also similarly oxidised by reactive oxygen species in fibroblasts in response to insulin [33]. The concerted oxidation of PTP1B and TCPTP suggests that both enzymes coordinately attenuate IR signalling [9, 34, 35]. Finally, we have shown that TCPTP may also regulate insulin signalling and glucose homeostasis in vivo [36]. We reported that insulin signalling was elevated in hepatocytes isolated from TCPTP heterozygous mice (Ptpn2+/−) and that high fat fed Ptpn2+/− mice were protected from the development of hyperglycemia [36]. The prevention of hyperglycemia in high fat fed Ptpn2+/− mice could be attributed to decreased hepatic gluconeogenesis and glucose production associated with enhanced STAT3 phosphorylation, as well as prolonged hepatic insulin-induced PI3K/Akt signalling [36]. Previous studies have shown that PTP1B-deficiency in liver also enhances IR activation and the insulin-induced suppression of hepatic glucose production [37]. Thus, the combinatorial inhibition of PTP1B and TCPTP in liver might represent an effective strategy for the prevention of fasting hyperglycemia in patients with type 2 diabetes.
Skeletal muscle accounts for the majority of glucose disposal following a meal and insulin resistance in muscle is an early event in the pathogenesis of type 2 diabetes. Therefore, the inhibition of PTPs such as PTP1B that attenuate IR activation and signalling might be particularly effective in alleviating insulin resistance. Given the established potential for PTP1B and TCPTP to act together in the attenuation of IR signalling in fibroblasts and hepatocytes [32, 33, 36], we asked whether TCPTP might also regulate the insulin response and glucose homeostasis in muscle.
METHODS
Antibodies and reagents
Human insulin was from Sigma-Aldrich (St Louis, MO, USA) and the rat insulin RIA kit from Linco Research (St Charles, MO, USA). Rabbit α-phospho-Y1162/1163-IR from Biosource International (Camarillo, CA, USA), α-phospho-S473-Akt, α–phospho-Y705-STAT3 and α-Akt and mouse α-STAT3 were from Cell Signaling (Beverly, MA, USA). Mouse α-IR β subunit from Santa Cruz (Santa Cruz, CA, USA), α-tubulin (Ab-5) from Sigma-Aldrich (St Louis, MO, USA) and α-TCPTP (6F3) from Medimabs (Montréal, Québec, Canada).
Mice
We maintained mice on a 12 h light-dark cycle in a temperature-controlled high barrier facility (ARL, Monash University, Victoria, Australia) with free access to food and water according to NHMRC Australian Code of Practice for the Care and Use of Animals. MCK-Cre mice have been described previously [16] and have been backcrossed onto the C57BL/6 background for 6 generations. A detail description of the construct design and the generation of Ptpn2lox/+ mice will be provided elsewhere [38]. Briefly, the targeting construct incorporated loxP sites flanking exons 5 and 6 and a neomycin-resistance cassette flanked by FRT sites. The linearised construct was electroporated into Bruce 4 (C57BL/6J) ES cells and G418 resistant colonies identified by Southern blot analysis. Correctly targeted clones were injected into BALB/c blastocysts. High percentage male chimeras were mated with C57BL/6J mice to produce Ptpn2lox/+ offspring and then bred with FLPe mice (C57BL/6) to excise the neomycin-resistance cassette. We used aged-matched and sex-matched mice for all experiments. Mice were fed a standard chow (19% protein, 4.6% fat and 4.8% crude fibre) or a high fat diet (23% fat; 45% of total energy from fat; SF04-027; Specialty Feeds, Glen Forest, WA, Australia) as indicated.
Metabolic measurements
Insulin and glucose tolerance tests were performed as described previously [39]. Fed and fasted blood were collected by retro-orbital bleeding and blood glucose plasma insulin levels determined as described previously [39]. To measure oxygen consumption and energy expenditure, food intake and ambulatory activity mice were acclimated for 24 h and then monitored for 48 h in an environmentally controlled Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH, USA) fitted with indirect open circuit calorimetry and food consumption and activity monitors. Respiratory exchange ratios (VCO2/VO2) were calculated from the gas exchange data. Data were averaged for 2 dark and two light cycles.
2-Deoxy-D-glucose uptake assay
Soleus muscles dissected from 8 week old mice were incubated in oxygenated (95% O2/5% CO2) Krebs-Henseleit buffer supplemented with 0.1% BSA, 8 mmol/l mannitol, 2 mmol/l pyruvate plus 10 µmol/l 2-deoxy-D-glucose for 20 min and 2-deoxy-D-[1,2 3H]-glucose (1.85 × 104 Bq/ml; GE Healthcare, Mount Prospect, IL, USA) uptake determined for 10 min in the presence or absence of 10 nmol/l insulin (Actrapid, Nova Nordisk, Bagsværd, Denmark) as described previously [40, 41].
Cell culture
Primary mouse myoblasts were isolated from the muscle of 2–4 week old mice and cultured in Ham’s F10 medium containing 20% (v/v) heat inactivated FBS, antibiotics [100 U/ml penicillin and 100 µg/ml streptomycin] and 2.5 ng/ml human basic FGF as described previously [39]. Mouse myoblasts were differentiated into multinucleated myotubes in DME medium containing 5% (v/v) horse serum (Sigma-Aldrich, MO, USA) plus antibiotics for 5 days and then serum-starved for 6 h and stimulated with insulin. Rat L6 myoblasts (ATCC) were cultured in DME containing 20% (v/v) heat inactivated FBS plus antibiotics. Where indicated L6 myoblasts were serum starved for 4 h, pre-incubated with PTP1B inhibitor [compound II; [42]] and/or TCPTP inhibitor [compound 8; [43]] for 1 h and then stimulated with 1 nmol/l insulin.
Biochemical analyses
Mouse tissues were dissected and immediately frozen in liquid N2. Tissues were homogenized in 10–20 volumes of ice cold RIPA lysis buffer and clarified by centrifugatBenjaminion as described previously [39]. Cells were lysed in RIPA buffer and clarified by centrifugation (16,000 × g for 5 min at 4°C). Tissue and cell lysates were resolved by SDS-PAGE, transferred onto Immobilon-P transfer membrane (Millipore, Billerica, MA, USA) and immunoblotted as described previously [44]. Lox/lox and MTKO tissue and cell lysates, or cells treated with vehicle control or inhibitor, were resolved on the same SDS-PAGE gel, or otherwise transferred onto the same transfer membrane for immunoblotting and development.
Statistical Analyses
All data were presented as mean ± SEM and statistical significance determined using the Student’s t test, or repeated-measures 2-way ANOVA. A p value of less than 0.05 was considered significant.
RESULTS
Generation and characterisation of mice with a muscle-specific deletion of TCPTP
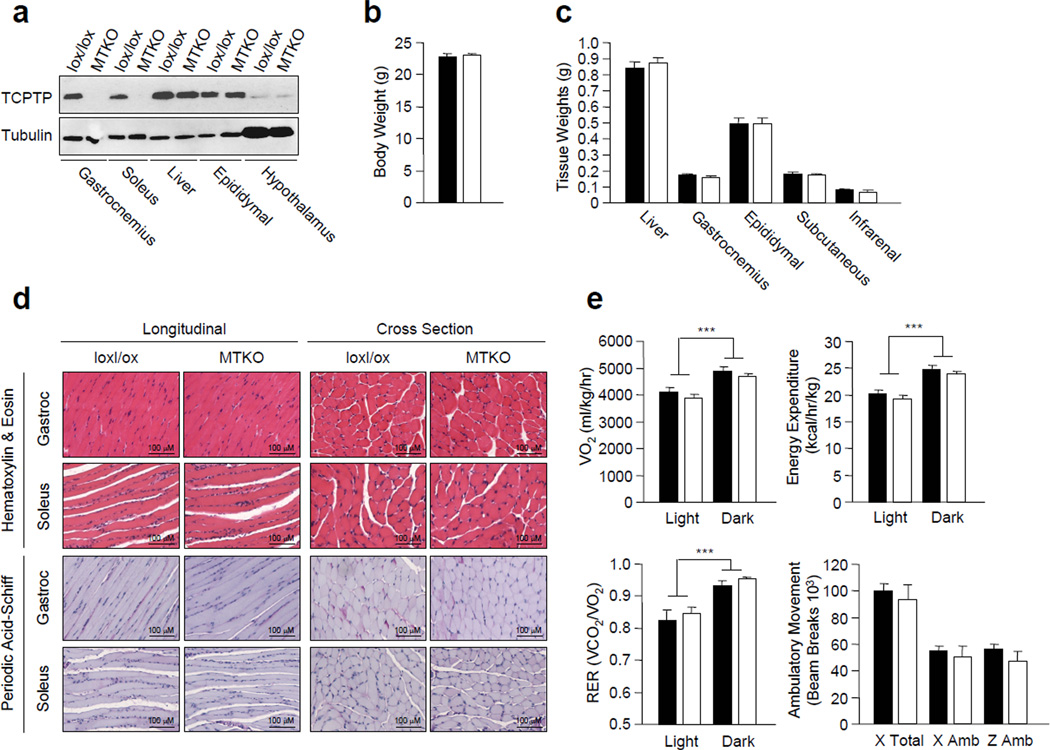
To ask if TCPTP has a role in the regulation of insulin receptor signalling in muscle, we generated a floxed allele of Ptpn2 (loxP sites flanking exons 5 and 6) by gene targeting in Bruce 4 (C57BL/6J) embryonic stem cells. Ptpn2lox/lox (C57BL/6) mice were crossed with mice expressing the Cre recombinase under the control of the muscle creatine kinase promoter (MCK-Cre; C57Bl/6) to excise Ptpn2 in muscle. TCPTP expression, as determined by immunoblot analysis, was deleted in the gastrocnemius and soleus muscles of the MCK-Cre;Ptpn2lox/lox mice, but not in other insulin responsive tissues such as liver, fat and hypothalamus (Fig. 1a).
Figure 1. Generation and characterisation of muscle-specific TCPTP-knockout mice.
(a) TCPTP protein levels in Ptpn2lox/lox control (lox/lox) and MCK-Cre; Ptpn2lox/lox muscle-specific TCPTP knockout (MTKO) mice as determined by immunoblotting. (b) Body weights in 8 week-old male lox/lox and MTKO mice. (c) Tissue weights in 8–10 week-old male lox/lox and MTKO mice. (d) Gastrocnemius and soleus muscle from lox/lox and MTKO mice were isolated and processed for histology (hematoxylin & eosin and periodic acid-schiff). (e) Oxygen consumption, energy expenditure and respiratory exchange ratios in the light and dark cycles in 8–10 week-old lox/lox and MTKO mice were determined by indirect calorimetry. Ambulatory activity (x and z axes) was also determined. Results shown are mean ± SE. ***p<0.001 for light cycle versus dark cycle. Black bars, lox/lox, n=7; white bars, MTKO, n=7.
At 8 weeks of age, no differences in body weight were noted between MCK-Cre; Ptpn2lox/lox and Ptpn2lox/lox male mice (Fig. 1b). Consistent with this, corresponding tissue weights, including gastrocnemius muscle, were similar in both groups (Fig. 1c). No overt differences in muscle development or glycogen content (as assessed by hematoxylin & eosin and periodic acid-schiff staining) were evident in MCK-Cre;Ptpn2lox/lox mice (Fig. 1d). Oxygen consumption, energy expenditure (as assessed by indirect calorimetry) and respiratory exchange ratios (RER; a measure of fat and carbohydrate utilisation) were unaltered between MCK-Cre;Ptpn2lox/lox and Ptpn2lox/lox male mice, suggesting that TCPTP deficiency in muscle had no impact on whole body energy homeostasis and fuel utilisation (Fig. 1e). Moreover, ambulatory activity was not affected in mice lacking TCPTP in muscle when compared with Ptpn2lox/lox littermates (Fig. 1e).
Insulin sensitivity and glucose homeostasis are not altered in muscle-specific TCPTP-deficient mice
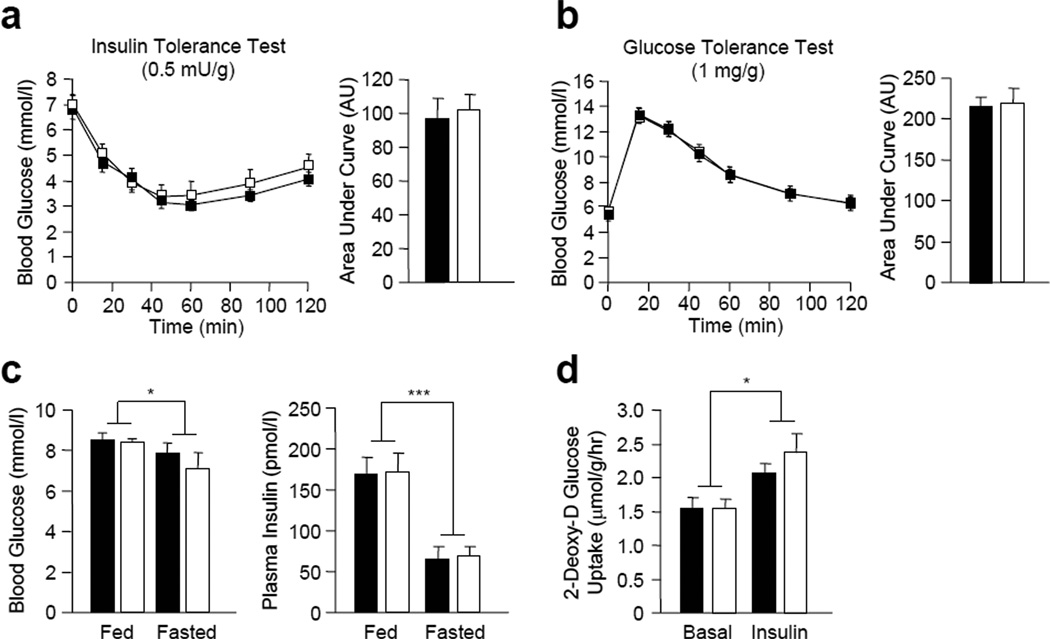
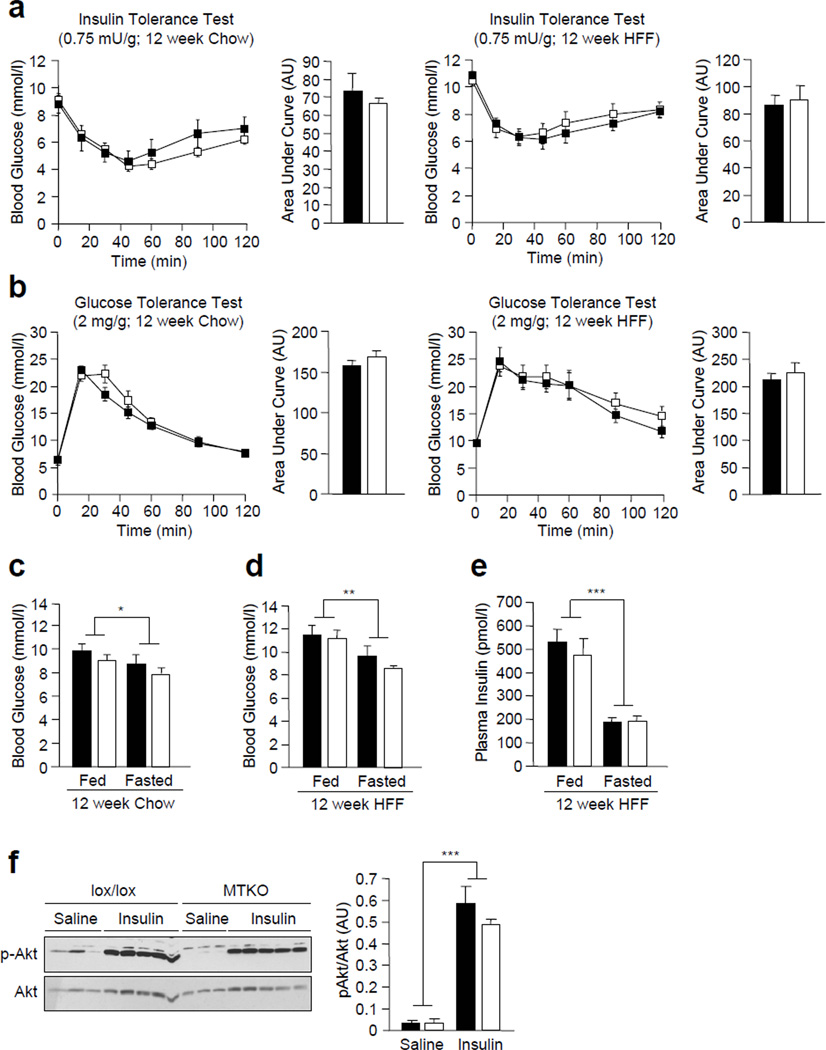
To investigate whether TCPTP deficiency in muscle improves insulin sensitivity and whole body glucose clearance, we performed insulin tolerance tests (ITTs) and glucose tolerance tests (GTTs) in 8 week-old MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice. No differences in ITTs (Fig. 2a) or GTTs (Fig. 2b) were evident in MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice. Consistent with these results we found no significant differences in fed and fasted blood glucose levels, or fed and fasted plasma insulin levels in MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice (Fig. 2c). In addition, no differences were evident in the uptake of 2-deoxy-D glucose uptake into MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox soleus muscle ex vivo in response to insulin (Fig. 2d). These results indicate that muscle-specific TCPTP-deficiency in chow-fed lean mice does not alter insulin sensitivity and glucose homeostasis.
Figure 2. Glucose homeostasis in MTKO mice.
(a) 8–10 week-old lox/lox and MTKO male mice were fasted and insulin tolerance tests (ITTs; 0.5 insulin mU/g body weight) or (b) glucose tolerance tests (GTTs; 1 mg glucose/g body weight) performed. Areas under ITT or GTT curves were determined. (c) Fed and fasted blood glucose and plasma insulin levels in 8–10 week-old lox/lox and MTKO male mice were determined. (d) 2-Deoxy-D-[3H]-glucose uptake assays of control and insulin-stimulated soleus explant from lox/lox and MTKO mice. Results shown are means ± SE.. *p<0.05 and ***p<0.001 for fed versus fasted. Black bars, lox/lox, n=7; white bars, MTKO, n=7.
Insulin signalling is not altered by TCPTP-deficiency in muscle
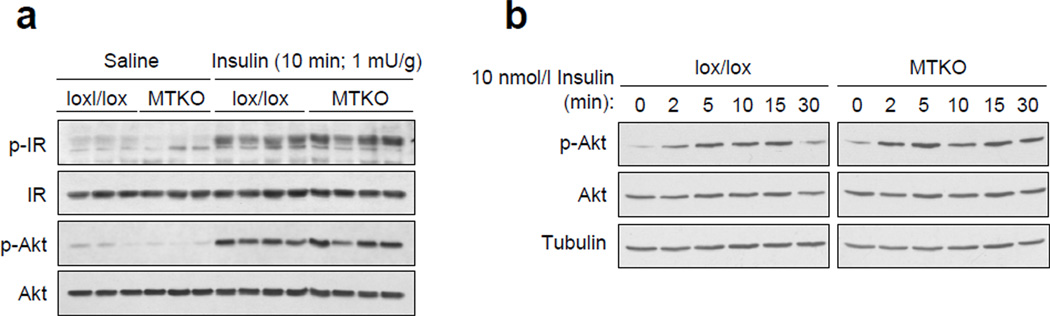
Next, we determined whether TCPTP deficiency alters insulin signalling in muscle (Fig. 3). First, we determined whether IR activation and signalling were altered by TCPTP deficiency in gastrocnemius muscle after bolus insulin administration (1 mU/g, 10 min; intraperitoneal). IR activation and downstream PI3K/Akt signalling were assessed by immunoblot analysis using antibodies to the IR β-subunit Y1162/Y1163 and Akt Ser-473 phosphorylation sites respectively. No differences in basal or insulin-induced IR activation and PI3K/Akt signalling were evident in muscle in vivo (Fig. 3a). Second, we isolated myoblasts from MCK-Cre; Ptpn2lox/lox versus Ptpn2lox/lox mice and assessed insulin-induced signalling in the corresponding differentiated myotubes (Fig. 3b); the differentiation of myoblasts into myotubes, as assessed by the expression of MyoD and myogenin, was unaltered by TCPTP-deficiency (data not shown). We found that insulin (1–10 nmol/l)-induced signalling (0–30 min), as assessed by Akt Ser-473 phosphorylation, was not altered by TCPTP-deficiency in myotubes in vitro (Fig. 3b; data not shown). Taken together, these results indicate that TCPTP-deficiency does not alter insulin signalling in muscle.
Figure 3. Insulin signalling in MTKO mice and muscle cells.
(a) 8–10 week-old lox/lox and MTKO male mice were fasted for 4 h and injected with saline or insulin (1 mU/g, 10 min; IP) and gastrocnemius muscle extracted and processed for immunoblot analysis with antibodies to the phosphorylated (Y1162/Y1163) and activated IR β subunit (p-IR) and Ser-473 phosphorylated Akt (p-Akt) and then total IR and Akt. (b) Myoblasts from lox/lox and MTKO mice were differentiated, serum starved and stimulated with 1 nM insulin and processed for immunoblot analysis as indicated. Results shown in a–b are representative of three independent experiments.
Body weight and glucose homeostasis are not altered in high fat fed (HFF) muscle-specific TCPTP-deficient mice
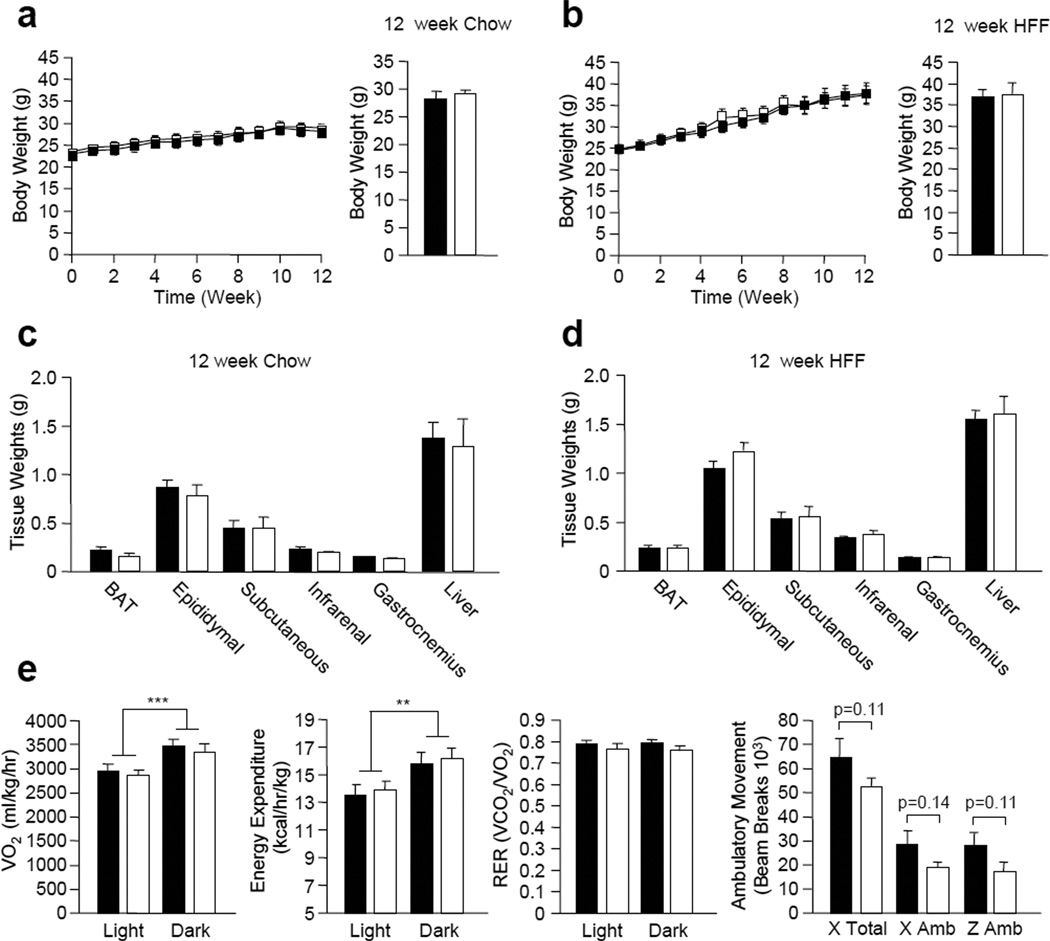
Although insulin sensitivity was unaltered in chow fed MCK-Cre;Ptpn2lox/lox mice, it is possible that differences might only be evident when mice are fed a high fat diet to render them insulin resistant. For example, mice with a muscle-specific deficiency in PTEN, a PTP superfamily member with lipid phosphatase activity that attenuates PI3K signalling, do not exhibit any overt differences in insulin sensitivity unless challenged with a high fat diet [9, 45]. Therefore, we next examined whether muscle-specific TCPTP deficiency could enhance insulin sensitivity and prevent high fat diet-induced weight gain and the development of insulin resistance. 8–10 week-old MCK-Cre;Ptpn2lox/lox and Ptpn2lox/lox male mice were fed either a normal chow (4.6% fat) or a high fat (23% fat) diet for 12 weeks. No differences in weight gain or adiposity were evident in either chow fed or high fat fed (HFF) MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice (Fig. 4a–d). Similarly no differences in oxygen consumption, energy expenditure or fuel utilisation (RER) were noted in HFF MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice (Fig. 4e; data not shown). There was a trend for lower ambulatory activity in HFF MCK-Cre;Ptpn2lox/lox mice (Fig. 4e), but this was not statistically significant (p=0.11).
Figure 4. Body weight, adiposity and energy homeostasis in MTKO mice.
8–10 week-old male lox/lox and MTKO male mice were either (a) fed a normal chow diet (n=6 per genotype), or (b) high fat fed (HFF; n=7 per genotype) for 12 weeks and weekly body weights measured. Tissue weights in (c) chow fed (n=6 per genotype) and (d) HFF (n=7 per genotype) lox/lox and MTKO mice. (e) Oxygen consumption, energy expenditure, respiratory exchange ratios and ambulatory activity were determined in HFF lox/lox and MTKO mice (n=6 per genotype). Results shown in a–d are means ± SE. **p<0.01 and ***p<0.001 for light cycle versus dark cycle. Black bars/squares, lox/lox; white bars/squares, MTKO.
To determine whether muscle-specific TCPTP deficiency could attenuate the development of high fat diet-induced insulin resistance we performed insulin and glucose tolerances tests. No significant differences in insulin sensitivity or glucose tolerance were observed in 8 week-old MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox male mice that were fed a chow or a high fat diet for 12 weeks (Fig. 5a and b). In addition, fed and fasted blood glucose levels were similar between MCK-Cre;Ptpn2lox/lox and Ptpn2lox/lox mice on either diet (Fig. 5c and d). Moreover, fed and fasted plasma insulin levels were unaltered in HFF MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice (Fig. 5e). Finally, insulin-induced muscle Akt Ser-473 phosphorylation was not different in HFF MCK-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice (Fig. 5f). Taken together, these results indicate that TCPTP deficiency in muscle has no overt effect on insulin signalling and glucose homeostasis and does not prevent the development of insulin resistance associated with high fat feeding.
Figure 5. Glucose homeostasis in MTKO mice fed a high fat diet.
8–10 week-old male lox/lox and MTKO male mice were fed either a normal chow diet or HFF for 12 weeks. (a) Mice were fasted and insulin tolerance tests (ITTs; 0.75 insulin mU/g body weight) or (b) glucose tolerance tests (GTTs; 2 mg glucose/g body weight) were performed. Areas under ITT or GTT curves were determined (n=7 per genotype). (c–d) Fed and fasted blood glucose levels in chow fed (n=6 per genotype) and HFF (n=7 per genotype) lox/lox and MTKO male mice. (e) Fed and fasted plasma insulin levels in HFF lox/lox and MTKO male mice (n=7 per genotype). (f) HFF mice were fasted for 4 h and injected with saline or insulin (1 mU/g; IP) and gastrocnemius muscle extracted and processed for immunoblot analysis with antibodies to p-Akt, Akt and tubulin. Results shown are means ± SE. *p<0.05, **p<0.01 and ***p<0.001 for fed versus fasted. Black bars/squares, lox/lox; white bars/squares, MTKO.
TCPTP and PTP1B are not redundant in insulin signalling
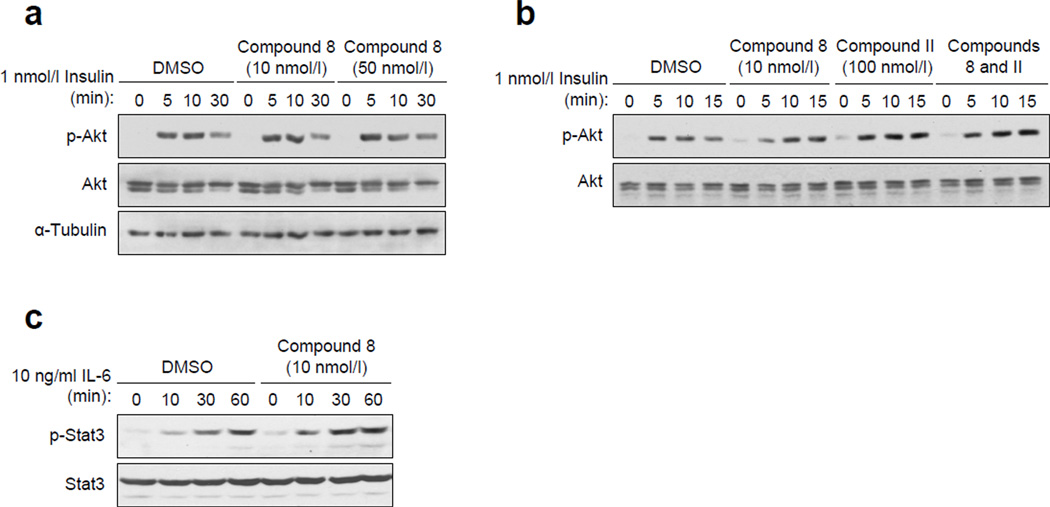
The unaltered insulin signalling in TCPTP deficient muscle might be due to a functional redundancy with PTP1B. To explore this possibility we asked whether the combined inhibition of TCPTP and PTP1B in muscle cells might result in synergistic or additive effects on insulin signalling (Fig. 6a). To inhibit TCPTP and/or PTP1B we took advantage of the highly selective inhibitors compound 8 (Ki for TCPTP of 4.3 nmol/l; [43]) and compound II (Ki for PTP1B of 26 nmol/l; [46]) respectively. Consistent with previous studies implicating PTP1B in the regulation of insulin signalling in muscle [11–16], we that found PTP1B, but not TCPTP inhibition enhanced insulin-induced PI3K/Akt signalling in rat L6 myoblasts (Fig. 6a and b). TCPTP inhibition with compound 8 resulted in increased phosphorylation of STAT3 (Fig. 6c), a bona fide TCPTP substrate [8, 36]. However, no additive effects on insulin signalling (as assessed by Akt Ser-473 phosphorylation) were evident in L6 cells when both PTP1B and TCPTP were inhibited (Fig. 6a). Therefore these results indicate that TCPTP does not regulate insulin-induced PI3K/Akt signalling in muscle cells, even when PTP1B is inhibited.
Figure 6. PTP1B but not TCPTP inhibition in muscle cells enhances insulin signalling.
(a–b) L6 myoblasts were serum starved and pre-incubated with 10–50 nmol/l compound 8 (TCPTP inhibitor), 100 nmol/l compound II (PTP1B inhibitor), or both for 1 h and then stimulated with 1 nmol/l insulin for the indicated times and processed for immunoblot analysis antibodies to p-Akt, Akt and tubulin. (c) L6 cells were pretreated with vehicle control of 10 nmol/l compound 8 and stimulated with 10 ng/ml IL-6 for the indicated time and processed for immunoblot analysis with antibodies to p-STAT3 and STAT3. Results shown in a–c are representative of three independent experiments.
DISCUSSION
Although we have shown previously that the IR can serve as a substrate for TCPTP [8, 11, 32, 33, 36], in this study we report that TCPTP is redundant in IR signalling in muscle. In contrast to PTP1B deficiency, which enhances insulin signalling and glucose uptake in muscle and protects mice from the development of insulin resistance [12, 13, 16], we found that TCPTP-deficiency in muscle had no overt effect on glucose homeostasis.
The catalytic domains of PTP1B and TCPTP share a high degree of primary (72% identity; 86% similarity) and tertiary structural similarity and have similar active sites. In particular, both PTPs have a second phosphotyrosine-binding pocket that allows for the selective recognition of tandem phosphorylated substrates [47, 48] such as the IR PTK [11, 32, 47, 49], JAK PTKs (JAK1-3 and TYK2) [49] and the MET receptor PTK [50]. However despite their similarity, TCPTP and PTP1B exhibit a high degree of substrate selectivity. For example, PTP1B can dephosphorylate JAK2, but not JAK1/3, whereas TCPTP dephosphorylates JAK1/3, but not JAK2 [49, 51, 52]. These differences in PTP1B versus TCPTP substrate selectivity are associated with inherent differences in PTP catalytic domain substrate specificity, as well as differences in tissue distribution and subcellular localisation. PTP1B is ubiquitous, whereas TCPTP is most abundant in the hematopoietic compartment [8]. Moreover, PTP1B is targeted to the endoplasmic reticulum by a hydrophobic C–terminus, whereas TCPTP is expressed as two variants, with one being targeted to the endoplasmic reticulum and the other to the nucleus [8]; both TCPTP variants are expressed in muscle [36].
In keeping with the two phosphatases exhibiting differences in substrate specificity, we have shown previously that PTP1B and TCPTP can function cooperatively in the same cell to regulate the intensity and duration of IR activation and signalling [11, 32, 33]. Similarly, PTP1B and TCPTP work in concert to regulate MET receptor phosphorylation [50] and PTP1B and TCPTP regulate STAT6 phosphorylation in the cytoplasm and nucleus respectively [53]. Thus, PTP1B and TCPTP can function in a coordinated manner for the temporal and spatial control of cellular signalling. Both TCPTP [36] and PTP1B [37] appear to be required for IR regulation in the liver. In other studies, we have shown that chow-fed liver-specific TCPTP-deficient mice exhibit enhanced glucose homeostasis (unpublished observation). So why then might TCPTP be redundant in IR activation and signalling in muscle? On possibility is that relative TCPTP expression might be decreased and/or PTP1B expression increased in muscle, but this does not appear to be the case [36], [37]; indeed PTP1B levels are lower in muscle when compared to liver [37]. Moreover, we have shown in this study that TCPTP-deficiency does not enhance insulin signalling even when PTP1B is inhibited, arguing against a functional redundancy due to the presence of PTP1B.
The evidence for PTP1B regulating IR activation and signalling in muscle is compelling [12, 13, 16]. In particular, insulin sensitivity is increased in chow fed muscle-specific PTP1B knockout mice and muscle IR and IRS-1 phosphorylation are elevated in the fasted state [16]. Consistent with this, our studies indicate that PTP1B inhibition in muscle cells can enhance insulin signalling. However, recent studies have indicated that PTP1B, but not TCPTP may affect the endosomal sorting of activated receptor PTKs through the dephosphorylation of proteins such as STAM2 [54, 55]. Therefore, one possibility is that the effects of PTP1B on insulin signalling may be indirect and attributable to altered endosomal sorting of the IR. In this case, PTPs other than PTP1B and TCPTP may be responsible for the direct dephosphorylation of the IR in response to insulin. One candidate may be PTPε, which translocates to the IR in response to insulin [31]. Another candidate is SHP-1, the global deletion of which results in a dramatic improvement in insulin sensitivity and glucose tolerance and a significant enhancement in insulin-induced IR and IRS phosphorylation in muscle [56].
The inhibition of PTP1B for the enhancement of insulin responsiveness and the alleviation of insulin resistance remains a rational therapeutic approach for the treatment of type 2 diabetes [24, 25]. However, PTP1B inhibitors in preclinical development also variably inhibit TCPTP [24, 25]. One question that has arisen from our previous work has been whether the inadvertent inhibition of TCPTP by PTP1B inhibitors in muscle might contribute to the enhancement of insulin sensitivity; our current studies suggest that this is unlikely. Additional studies are required to determine if TCPTP has a role in other insulin responsive tissues such as fat, where PTP1B may be redundant in regulating the insulin response [13, 14].
ACKNOWLEDGMENTS
We thank Christine Yang and Teresa Tiganis for technical support. This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (to T.T. and M.J.W.) and the National Institutes of Health [Z.-Y.Z (RO1 CA126937), B.G.N. (R37 CA49152)] and funds from the Ontario Ministry of Health and Long Term Care and the Princess Margaret Hospital Foundation (to B.G.N.); T.T. and M.J.W. are NHMRC Research Fellows and B.G.N. a Canada Research Chair (Tier I).
Abbreviations
- GTT
Glucose tolerance test
- HFF
High fat fed
- IR
insulin receptor
- IRS
insulin receptor substrate
- ITT
insulin tolerance test
- LAR
leukocyte common antigen–related
- MAPK
mitogen-activated protein kinase
- (PI3K)
phosphatidylinositol-3-kinase
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
Footnotes
Duality of Interest: Zhong-Yin Zhang is a co-founder of Aarden Pharmaceuticals and Chairman of its scientific advisory board. There is no other duality of interest associated with this manuscript.
Author contributions: T.L.M., S.G., B.J.W, S.Z., Z.-Y.Z. contributed to the design of experiments and/or interpretation of data and provided intellectual input; K.L., M.J.W and B.G.N helped design experiments and/or interpreted data and contributed to the drafting and revision of the article; T. T. conceived and designed the study, interpreted data and drafted and revised the manuscript.
REFERENCES
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 3.Bevan P. Insulin signalling. J Cell Sci. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- 4.White MF. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 5.Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 2008;7:348–356. doi: 10.1016/j.cmet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Bai L, Wang Y, Fan J, et al. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 8.Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A, Dube N, Gu F, Tremblay ML. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur J Biochem. 2002;269:1050–1059. doi: 10.1046/j.0014-2956.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 11.Galic S, Hauser C, Kahn BB, et al. Coordinated Regulation of Insulin Signaling by the Protein Tyrosine Phosphatases PTP1B and TCPTP. Mol Cell Biol. 2005;25:819–829. doi: 10.1128/MCB.25.2.819-829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 13.Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 15.Haj FG, Zabolotny JM, Kim YB, Kahn BB, Neel BG. Liver specific protein-tyrosine phosphatase 1B (PTP1B) Re-expression alters glucose homeostasis of PTP1B−/−mice. J Biol Chem. 2005 doi: 10.1074/jbc.M413240200. [DOI] [PubMed] [Google Scholar]
- 16.Delibegovic M, Bence KK, Mody N, et al. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol. 2007;27:7727–7734. doi: 10.1128/MCB.00959-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zabolotny JM, Haj FG, Kim YB, et al. Transgenic overexpression of protein-tyrosine phosphatase 1B in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action. J Biol Chem. 2004;279:24844–24851. doi: 10.1074/jbc.M310688200. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad F, Azevedo JL, Cortright R, Dohm GL, Goldstein BJ. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J Clin Invest. 1997;100:449–458. doi: 10.1172/JCI119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad F, Considine RV, Goldstein BJ. Increased abundance of the receptor-type protein-tyrosine phosphatase LAR accounts for the elevated insulin receptor dephosphorylating activity in adipose tissue of obese human subjects. J Clin Invest. 1995;95:2806–2812. doi: 10.1172/JCI117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad F, Goldstein BJ. Alterations in specific protein-tyrosine phosphatases accompany insulin resistance of streptozotocin diabetes. Am J Physiol. 1995;268:E932–E940. doi: 10.1152/ajpendo.1995.268.5.E932. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy BP, Ramachandran C. Protein tyrosine phosphatase-1B in diabetes. Biochem Pharmacol. 2000;60:877–883. doi: 10.1016/s0006-2952(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein BJ. Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J Clin Endocrinol Metab. 2002;87:2474–2480. doi: 10.1210/jcem.87.6.8641. [DOI] [PubMed] [Google Scholar]
- 23.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson TO, Ermolieff J, Jirousek MR. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat Rev Drug Discov. 2002;1:696–709. doi: 10.1038/nrd895. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today. 2007;12:373–381. doi: 10.1016/j.drudis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Swarbrick MM, Havel PJ, Levin AA, et al. Inhibition of protein tyrosine phosphatase-1B with antisense oligonucleotides improves insulin sensitivity and increases adiponectin concentrations in monkeys. Endocrinology. 2009;150:1670–1679. doi: 10.1210/en.2008-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto N, Feener EP, Zhang WR, Goldstein BJ. Insulin receptor protein-tyrosine phosphatases. Leukocyte common antigen-related phosphatase rapidly deactivates the insulin receptor kinase by preferential dephosphorylation of the receptor regulatory domain. J Biol Chem. 1992;267:13811–13814. [PubMed] [Google Scholar]
- 28.Kulas DT, Zhang WR, Goldstein BJ, Furlanetto RW, Mooney RA. Insulin receptor signalling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J Biol Chem. 1995;270:2435–2438. doi: 10.1074/jbc.270.6.2435. [DOI] [PubMed] [Google Scholar]
- 29.Zhang WR, Li PM, Oswald MA, Goldstein BJ. Modulation of insulin signal transduction by eutopic overexpression of the receptor-type protein-tyrosine phosphatase LAR. Mol Endocrinol. 1996;10:575–584. doi: 10.1210/mend.10.5.8732688. [DOI] [PubMed] [Google Scholar]
- 30.Zabolotny JM, Kim YB, Peroni OD, et al. Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc Natl Acad Sci U S A. 2001;98:5187–5192. doi: 10.1073/pnas.071050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aga-Mizrachi S, Brutman-Barazani T, Jacob AI, Bak A, Elson A, Sampson SR. Cytosolic protein tyrosine phosphatase-epsilon is a negative regulator of insulin signalling in skeletal muscle. Endocrinology. 2008;149:605–614. doi: 10.1210/en.2007-0908. [DOI] [PubMed] [Google Scholar]
- 32.Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, et al. Regulation of Insulin Receptor Signaling by the Protein Tyrosine Phosphatase TCPTP. Mol Cell Biol. 2003;23:2096–2108. doi: 10.1128/MCB.23.6.2096-2108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK. Regulation of Insulin Signaling through Reversible Oxidation of the Protein-tyrosine Phosphatases TC45 and PTP1B. J Biol Chem. 2004;279:37716–37725. doi: 10.1074/jbc.M404606200. [DOI] [PubMed] [Google Scholar]
- 34.Tiganis T. Reactive oxygen species and insulin resistance: the good, the bad and the ugly. Trends Pharmacol Sci. 2011;32:82–89. doi: 10.1016/j.tips.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Rhee SG. Cell signalling. H2O2, a necessary evil for cell signalling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima A, Loh K, Galic S, et al. T-cell protein tyrosine phosphatase attenuates STAT3 and insulin signalling in the liver to regulate gluconeogenesis. Diabetes. 2010;59:1906–1914. doi: 10.2337/db09-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delibegovic M, Zimmer D, Kauffman C, et al. Liver-Specific Deletion of Protein-Tyrosine Phosphatase 1B (PTP1B) Improves Metabolic Syndrome and Attenuates Diet-Induced ER Stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loh K, Fukushima A, Zhang X, et al. Elevated Hypothalamic TCPTP in Obesity Contributes to Cellular Leptin Resistance. Cell Metab. 2011 doi: 10.1016/j.cmet.2011.09.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loh K, Deng H, Fukushima A, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen PA, Wang W, Marshall BA, Holloszy JO, Mueckler M. Dissociation of GLUT4 translocation and insulin-stimulated glucose transport in transgenic mice overexpressing GLUT1 in skeletal muscle. J Biol Chem. 1998;273:18173–18179. doi: 10.1074/jbc.273.29.18173. [DOI] [PubMed] [Google Scholar]
- 41.Lau P, Fitzsimmons RL, Pearen MA, Watt MJ, Muscat GE. Homozygous staggerer (sg/sg) mice display improved insulin sensitivity and enhanced glucose uptake in skeletal muscle. Diabetologia. 54:1169–1180. doi: 10.1007/s00125-011-2046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie L, Lee SY, Andersen JN, et al. Cellular effects of small molecule PTP1B inhibitors on insulin signalling. Biochemistry. 2003;42:12792–12804. doi: 10.1021/bi035238p. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Chen L, Luo Y, Gunawan A, Lawrence DS, Zhang ZY. Acquisition of a potent and selective TC-PTP inhibitor via a stepwise fluorophore-tagged combinatorial synthesis and screening strategy. J Am Chem Soc. 2009;131:13072–13079. doi: 10.1021/ja903733z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiganis T, Bennett AM, Ravichandran KS, Tonks NK. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol Cell Biol. 1998;18:1622–1634. doi: 10.1128/mcb.18.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wijesekara N, Konrad D, Eweida M, et al. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol Cell Biol. 2005;25:1135–1145. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie L, Zhang YL, Zhang ZY. Design and characterization of an improved protein tyrosine phosphatase substrate-trapping mutant. Biochemistry. 2002;41:4032–4039. doi: 10.1021/bi015904r. [DOI] [PubMed] [Google Scholar]
- 47.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for recognition and dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Molecular Cell. 2000;6:1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 48.Iversen LF, Moller KB, Pedersen AK, et al. Structure determination of T cell protein tyrosine phosphatase. J Biol Chem. 2002;20:20. doi: 10.1074/jbc.M200567200. [DOI] [PubMed] [Google Scholar]
- 49.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–453. doi: 10.1016/s0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 50.Sangwan V, Paliouras GN, Abella JV, et al. Regulation of the Met receptor-tyrosine kinase by the protein-tyrosine phosphatase 1B and T-cell phosphatase. J Biol Chem. 2008;283:34374–34383. doi: 10.1074/jbc.M805916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myers MP, Andersen JN, Cheng A, et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- 52.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 53.Lu X, Malumbres R, Shields B, et al. PTP1B is a negative regulator of interleukin 4-induced STAT6 signalling. Blood. 2008;112:4098–4108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stuible M, Tremblay ML. In control at the ER: PTP1B and the down-regulation of RTKs by dephosphorylation and endocytosis. Trends Cell Biol. 20:672–679. doi: 10.1016/j.tcb.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 55.Stuible M, Abella JV, Feldhammer M, et al. PTP1B targets the endosomal sorting machinery: dephosphorylation of regulatory sites on the endosomal sorting complex required for transport component STAM2. J Biol Chem. 285:23899–23907. doi: 10.1074/jbc.M110.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubois MJ, Bergeron S, Kim HJ, et al. The SHP-1 protein tyrosine phosphatase negatively modulates glucose homeostasis. Nat Med. 2006;12:549–556. doi: 10.1038/nm1397. [DOI] [PubMed] [Google Scholar]