Abstract
Purpose
To assess patterns of failure in pediatric patients with intracranial germ cell tumors (GCT) treated with intensity-modulated radiation therapy with dose-painting (DP-IMRT).
Methods
Between July 2007 and October 2013, 11 patients with localized GCT, 5 germinomas and 6 non-germinoma germ cell tumors (NGGCT), received definitive treatment with DP-IMRT. Three representative patients were selected for re-planning with (1) whole ventricular irradiation (WVI) with opposed-lateral beams plus IMRT to the primary tumor and (2) sequential-IMRT. These plans were compared to the patients' original DP-IMRT plans for dosimetric analyses.
Results
Four patients with germinoma received RT alone: 45 Gy in 1.8 Gy fractions to the primary tumor and 25 Gy in 1.0 Gy fractions to whole ventricles using a dose-painting plan. One patient with germinoma received a reduced dose of 30.6 Gy to the primary tumor after neoadjuvant chemotherapy. Patients with NGGCT (n=6) underwent multimodality treatment including chemotherapy (n=6) and surgery (n=3). These patients received 54 Gy to the primary tumor and 32.4-36 Gy to the whole ventricles. Dosimetric analyses showed DP-IMRT delivered decreased mean dose to whole brain, temporal lobes, hippocampi, cochleae, and optic nerves. With median follow-up of 4 years, 3-year failure free survival was 100% for patients with germinoma and 67% for patients with NGGCT. One patient with a pineal NGGCT experienced a local recurrence within the high-dose volume while another experienced an isolated biochemical failure.
Conclusions
DP-IMRT is dosimetrically superior to standard IMRT techniques for sparing of normal tissues. Disease control in this small series appears at least comparable to published results.
Introduction
Intracranial germ cell tumors (GCT) are a rare and heterogeneous group of tumors that primarily involve the suprasellar cistern and pineal gland.[1] While pure germinomas have long-term survival rates as high as 93%, non-germinoma germ cell tumors (NGGCT) are associated with worse outcomes and decreased overall survival rates of 68%.[2] Current guidelines suggest localized germinomas can be treated with radiation therapy (RT) alone though multimodality trials are ongoing. Using radiation alone, the standard dose is 45-50 Gy to the primary tumor in conjunction with approximately 24 Gy of whole ventricular irradiation (WVI), producing 88% 5-year control.[2] NGGCT are more aggressive and are generally treated with multimodality treatment including chemotherapy and radiation therapy, and consideration of second look surgery. The boost dose of 54 Gy is higher for NGGCT than for germinomas and the use of craniospinal irradiation (CSI) rather than WVI in localized NGGCT is controversial.
Given the high curability of many GCT and the large treatment volumes often used in these young patients, there are significant concerns regarding long-term RT-related morbidity.[3-4] As such, there are ongoing efforts to explore the feasibility of delivering reduced RT doses to patients with GCT and specifically, to spare patients from CSI. Standard intensity-modulated radiotherapy (IMRT) for whole ventricular irradiation has already been shown to spare a significant amount of normal central nervous tissue for patients with localized GCT as compared to whole brain irradiation (WBI) using opposed-lateral beams.[5-6] However, the use of intensity-modulated radiotherapy with dose-painting (DP-IMRT), a technique characterized by the delivery of non-uniform dose patterns to different target volumes based upon risk, has not been well-studied in pediatric patients with primary central nervous system (CNS) neoplasms.[7] Traditional sequential-IMRT involves sequential dose escalation, where a uniform dose per fraction is used for all target volumes and field sizes are reduced in stages. In contrast, DP-IMRT allows for the delivery of unique doses to different target volumes within each fraction. DP-IMRT, a technique initially described in head and neck patients, has produced excellent local control and decreased toxicity in this population due to more conformal and homogeneous dose distributions.[8] Additionally, recent data examining DP-IMRT in pediatric rhabdomyosarcoma patients similarly demonstrated excellent local control and improved sparing of normal tissues.[9]
In this analysis, we examined patterns of failure in a small series of pediatric patients treated with DP-IMRT for GCT at a single institution. We also selected 3 representative patients to compare dose-volume analyses of 3 separate plans: 1) whole ventricle irradiation (WVI) using opposed lateral beams plus IMRT to the primary tumor; 2) IMRT to the whole ventricles followed by a boost to the primary tumor (sequential-IMRT); and 3) DP-IMRT to simultaneously treat the primary tumor and whole ventricles.
Patients and Methods
Patient Population
After approval by the MSKCC Institutional Review Board, medical records of 47 patients with a diagnosis of central nervous system GCT treated at our institution between January 2007 and March 2013 were examined. Excluding patients who received sequential-IMRT and/or CSI, 11 patients who underwent DP-IMRT between July 2007 and October 2013 were identified and selected for study.
Chemotherapy
Though the majority of patients with germinomas were treated with RT alone, a single patient received neoadjuvant carboplatin, cisplatin, and etoposide as per Children's Oncology Group (COG) Protocol ACNS 0232, Regimen B prior to RT. All patients with NGGCT (n=6) received neoadjuvant chemotherapy consisting of alternating cycles of carboplatin/etoposide and ifosfamide/etoposide prior to RT.
Surgery
Three patients, ages 8, 10, and 13, with NGGCT had second look surgeries (SLS) for incomplete radiological responses to chemotherapy. Two SLS were in the pineal region while 1 was in the suprasellar region. In the patient with a suprasellar tumor, SLS revealed only dense fibrous tissue with no evidence of residual tumor while in the two patients with pineal tumors, SLS revealed gross residual tumor.
Radiation Therapy
All patients received radiation therapy (RT) in our department a median of 20 (4-29) weeks after diagnosis. For patients with germinoma undergoing RT alone (n=4), RT began a median of 5 (4-7) weeks after diagnosis.
Treatment planning began with computed tomography (CT) simulation with a custom-made immobilization device. Diagnostic MRI scans were fused for target delineation in all patients. For the primary tumor, the gross tumor volume (GTV) was defined as the extent of disease at diagnosis (pre-chemotherapy). The clinical target volume (CTV) was designed to allow a 1 cm margin beyond the gross tumor volume, and an additional 0.3 cm margin was added to obtain the planning target volume (PTV). For WVI, CTV was comprised of bilateral ventricles with a 0.5 cm expansion to obtain the PTV. In all cases, 6MV photons were used (n=11).
Dose-volume Analysis of Representative Treatment Plan
Two patients with NGGCT of the suprasellar region and 1 patient with germinoma of the pineal region were selected from our series as representative cases. Their treatments were replanned with WVI with opposed-lateral beams plus IMRT to the primary tumor and sequential-IMRT and compared to their original DP-IMRT plans for dosimetric analyses. Mean dose (DM) to critical normal structures was evaluated.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics Version 21 (IBM Corporation, Armonk, NY).
Results
Patient characteristics are shown in Table I. Patients were between the ages of 8 and 21 (median 11) years at diagnosis. Five patients had germinomas and 6 had NGGCT. Five GCT were located in the suprasellar region while 5 were pineal. One patient had a bifocal germinoma with a tumor in both the suprasellar and pineal region, which was not considered disseminated disease.[10] All patients underwent lumbar puncture with cerebrospinal fluid analysis and spinal magnetic resonance imaging (MRI) and had no evidence of disseminated disease at diagnosis.
Table I. Patient Characteristics.
| Patient characteristics | N (%) |
|---|---|
| Total patients | 11 |
| Sex | |
| Males | 6 (55) |
| Females | 5 (45) |
| Median age at diagnosis (in years) | 11 |
| Age range (in years) | 8 - 21 |
| Histology | |
| Germinoma | 5 (45) |
| Non-germinoma Germ Cell Tumor | 6 (55) |
| Site | |
| Suprasellar | 5 (45) |
| Pineal | 5 (45) |
| Suprasellar and pineal | 1 (9) |
| Surgery | 3 (27) |
After a median follow-up of 4 (2-9) years, two patients, both with pineal NGGCT, have experienced a recurrence. One patient experienced a recurrence within the high-dose 54 Gy volume while the other experienced biochemical failure. The other 9 patients (all patients with germinoma (n=6) and 3 of 5 patients with NGGCT) are alive with no evidence of disease in first remission. The patient with locally recurrent NGGCT subsequently underwent additional treatment, but nonetheless died of his disease. The patient who experienced biochemical failure received additional chemotherapy and is currently alive with no evidence of disease.
Radiation Therapy
The majority of patients with germinoma (n=4) received 45 Gy in 1.8 Gy fractions to the primary tumor and 25 Gy in 1.0 Gy fractions to whole ventricles using a dose-painting plan. A single patient with a diagnosis of germinoma received 30.6 Gy in 1.8 Gy fractions to the primary tumor and 25 Gy in 1.5 Gy fractions to whole ventricles using a dose-painting plan after neo-adjuvant chemotherapy. Patients with NGGCT (n=6) received higher fractional and cumulative RT doses than patients with germinoma: 54 Gy in 1.8 Gy fractions to the post-operative bed (n=3) or primary tumor (n=2) and 32.4-36 Gy in 1.1-1.2 Gy fractions to whole ventricles.
Dose-volume Analyses
Three patients from our series, an 8-year-old and a 10-year-old with mixed germ cell tumor arising in the suprasellar region and a 17-year-old with a pineal germinoma, were chosen as representative cases. The 8-year-old underwent an SLS to remove gross tumor after incomplete response to chemotherapy while the 10-year-old experienced a complete response to chemotherapy and thus, did not undergo an SLS. The 17-year-old patient with a germinoma received only RT. Table II compares dose-volume information for critical structures in DP-IMRT, sequential-IMRT, and opposed-laterals WVI plus IMRT primary tumor boost plans for all 3 patients.
Table II. Dose-volume Histogram Statistics for Critical Structures in Representative Patients.
| Organ | Percent Decrease in Mean Dose with DP-IMRT (DP-IMRT vs. Opposed-Laterals Plus IMRT) | Percent Decrease in Mean Dose with DP-IMRT (DP-IMRT vs. Sequential-IMRT) |
|---|---|---|
| Whole Brain | 7-11 | 10-13 |
| Temporal Lobes | 33-36 | 17-21 |
| Hippocampi | 8-11 | 5-12 |
| Brainstem | 3-11 | 6-8 |
| Cochleae | 28-40 | 7-30 |
| Optic Nerves | 15-28 | 16-25 |
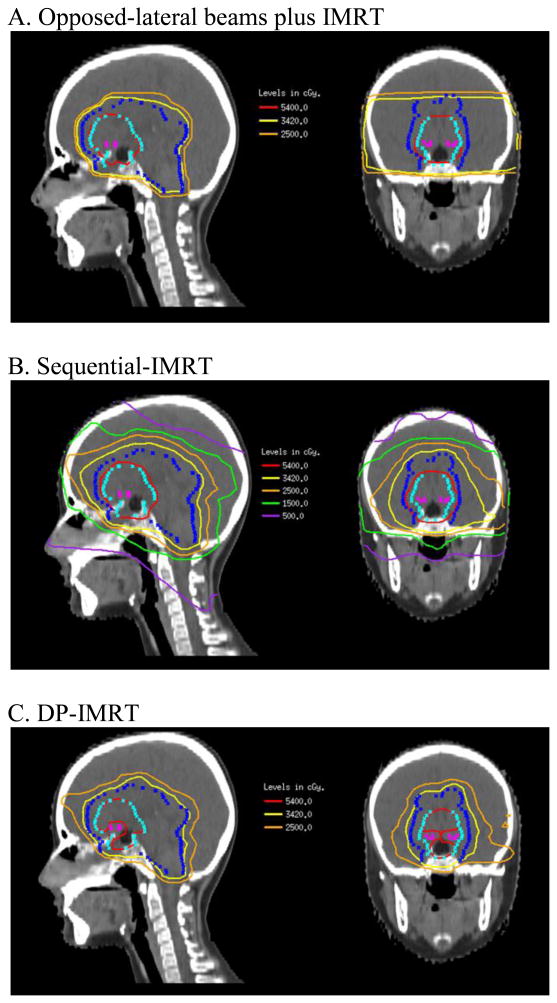
DP-IMRT was able to deliver a lower mean dose (DM) to all at-risk structures, including whole brain, brainstem, cochleae, and optic nerves. As expected, the difference in DM was most dramatic when comparing DP-IMRT to standard WVI with opposed-laterals plus IMRT boost to the primary tumor. For all three patients, DP-IMRT reduced DM to the temporal lobes by 33-36% (10.5-14.9 Gy) when compared to WVI with opposed-laterals. When compared to sequential-IMRT, the difference in DM was less dramatic but nonetheless resulted in a 17-21% (4.8-6.4 Gy) decrease to the temporal lobes. Additionally, DP-IMRT enabled an 11-12% (5.0-6.5 Gy) decrease in DM to the hippocampi for the 2 patients with suprasellar tumors and 5% (1.6-2.6 Gy) decrease for the patient with a pineal tumor when compared to sequential-IMRT. Whole brain generally received 7-10% less in DM when compared to the opposed-laterals plan and 10-13% less in DM when compared to the sequential-IMRT plan. DP-IMRT also resulted in decreased dose to the cochlea and optic nerves in all three patients. Figure 2 shows sample isodose curves in opposed lateral beams plus IMRT, sequential-IMRT, and DP-IMRT plans. The 54.0 Gy (red) and 34.2 Gy (yellow) isodose curves are most conformal with the DP-IMRT plan and show superior sparing of the optic chiasm.
Figure 2.
Sample isodose curves in opposed-lateral plus IMRT, sequential-IMRT, and DPIMRT plans.
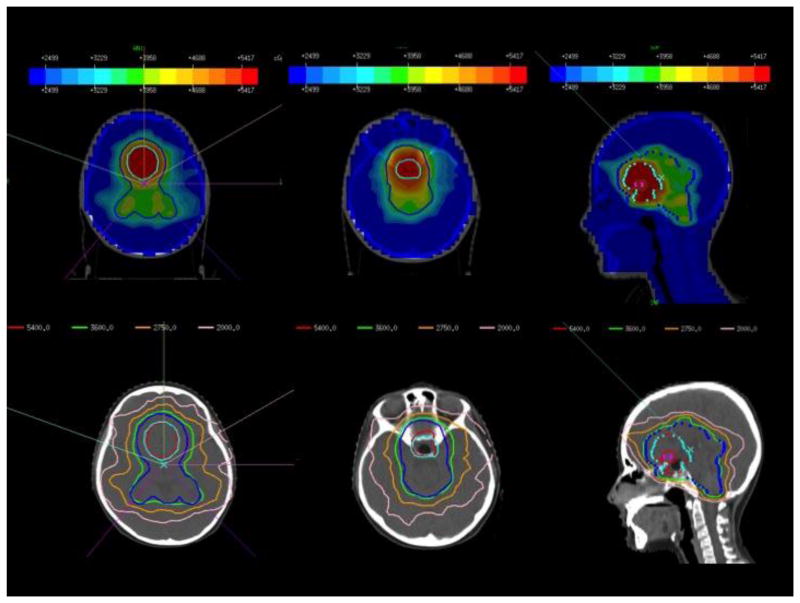
Representative images from a sample DP-IMRT plan for a patient with NGGCT are shown in Figure 3. In this plan, 6 coplanar beams and 1 non-coplanar beam were used to create a maximally conformal plan. In general, at our institution, 6 to 7 fields, some non-coplanar, are used to create a homogeneous dose distribution.
Figure 3.
Representative axial and sagittal images from a sample DP-IMRT plan for a patient with NGGCT. Six coplanar and 1 non-coplanar beams were used to increase conformality and dose homogeneity.
Toxicity
Patients tolerated RT with mild acute toxicity. All patients had grade 1-2 fatigue and alopecia in the radiation portals during RT. Additionally, 6 patients had grade 1-2 nausea that was managed with ondansetron. A single patient with germinoma developed gelastic seizures after 2 fractions of RT and was placed on Keppra prophylaxis that continue for 2 months post-radiation.
No patients had fatigue or somnolence post-treatment. One 13-year-old with a suprasellar germinoma experienced mild nausea without vomiting that persisted until 6 months after RT completion and was symptomatically managed with ondansetron as needed. While all patients experienced temporary epilation in the radiotherapy fields, all had complete hair re-growth.
Three patients underwent formal psycho-educational evaluation for assessment of cognitive and memory function at 15, 18, and 27 months post completion of treatment. One patient with NGGCT who underwent chemotherapy, RT, and SLS had a Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) Full Scale IQ at the 90th percentile and Working Memory Index in the superior range while the other patient who underwent the same treatment had a WISC-IV Full Scale IQ at the 30th percentile and a Working Memory Index in the average range. Of note, the second patient had previously been diagnosed with ADD and Mood Disorder two years prior to undergoing treatment for NGGCT. A third patient with germinoma received formal psycho-educational evaluation as a 17 year old and thus was tested using the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV). He scored at the 55th percentile and his Working Memory Index was in the average range. For the remaining patients, no new deficits have been documented at routine follow-up, but formal testing has not yet been completed.
Clinical Outcomes
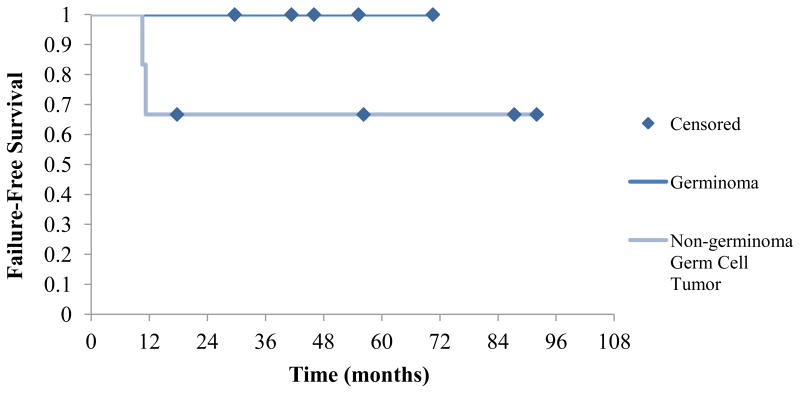
Three-year local failure-free and event-free survival for all patients was 83% (Figure 1): 100% for patients with germinoma and 67% for patients with NGGCT. Overall 5-year survival was also 100% for patients with germinoma and 75% for patients with NGGCT. One 13-year-old boy with an alpha-fetoprotein-positive NGGCT of the pineal region experienced local failure within the high dose-volume 3 months post completion of RT. He subsequently died of his disease. Additionally, an 11-year-old boy with NGGCT of the pineal region was noted to have an elevated serum alpha-fetoprotein level despite an MRI showing interval contraction of the tumor. He was subsequently taken for resection of presumed recurrent disease but pathology revealed no evidence of disease. He was followed closely and given rising serum alpha-fetoprotein levels, was determined to have experienced biochemical failure 5 months post completion of RT. He is currently alive with no evidence of disease. No patients with germinoma experienced local failures or in-field failures within the treated ventricular system.
Figure 1. Failure-free survival in patients with germinoma versus non-germinoma germ cell tumors.
Discussion
Both sequential-IMRT and WVI using opposed-lateral beams followed by an IMRT boost to the primary tumor are accepted standard-of-care radiation therapy techniques for GCT and produce excellent local control rates for GCT.[2-3] However, given the high curability of germinomas and the increased risk of late toxicity in pediatric patients, efforts directed at limiting dose to non-target structures must be pursued. DP-IMRT, which has been shown to produce more conformal dose distributions that result in better sparing of critical structures in adult head-and-neck and pediatric rhabdomyosarcoma patients, is one example of a brain-sparing technique that can be applied to pediatric patients with intracranial GCT.[5, 8, 10]
In this small series, DP-IMRT was able to produce outstanding 100% local failure-free survival in patients with germinoma and 67% local failure-free survival in patients with NGGCT, rates that appear at least comparable to those produced by sequential-IMRT and WVI with opposed-laterals plus IMRT boost to the primary tumor. Additionally, there were no failures seen within the treated ventricular system, though dose-painting technique resulted in a slightly decreased biological equivalent dose (BED) secondary to smaller fraction size. The lower BED may help to minimize the risk of late effects.
Further, DP-IMRT was significantly able to reduce the dose to normal structures including whole brain, temporal lobes, and hippocampi without sacrificing in-field control. DP-IMRT was also able to significantly reduce dose to cochlea and optic nerves, though this is less important as the whole ventricular dose did not reach the dose threshold for these structures. Finally, DP-IMRT was well tolerated among young patients who had only mild acute toxicity.
For patients with germinoma, we believe dose-painting to be an effective brain-sparing technique that can be used to reduce unwanted dose to normal structures in developing pediatric patients without sacrificing local control. For patients with localized NGGCT, who had 67% local failure-free survival, DP-IMRT as a brain-sparing technique can be pursued in the setting of appropriate multimodality treatment. Perhaps there is a role for dose-escalation to the primary tumor in NGGCT patients since one patient experienced local failure within the high dose-volume.
Finally, though our patients had local failure-free survival rates that compared favorably with published data, we would not advocate for further treatment volume reduction. While data have shown that it is acceptable to use WVI instead of whole brain irradiation (WBI), further decreasing the size of the treatment field may lead to increased recurrence.[6] In a recently published analysis of patterns of failure in patients with germinoma, WVI produced better outcomes than focal RT alone, even in patients with limited disease and complete response to neo-adjuvant chemotherapy.[11] Similarly, patients with NGGCT had an increased rate of failure when focal RT alone was used.[12] Ongoing multimodality studies seek to define the role of chemotherapy to allow RT volume and dose reduction for germinoma.
In addition to ensuring delivery of sufficient RT to prevent local and ventricular recurrence, it is equally important to balance and minimize the risk of late effects. Pediatric GCT patients treated with chemoradiation are at risk of a variety of late effects including neuroendocrine dysfunction, ototoxicity, neurocognitive dysfunction and less frequently, secondary malignancies. With modern day treatment strategies of modest chemotherapy and reduced-dose and volume irradiation, long-term neurocognitive dysfunction is rare.[13] However, neuroendocrine dysfunction is more common and recent data have shown that even low radiation doses can result in impaired growth and final height if patients are not followed carefully and treated appropriately for growth hormone deficiency.[14] As such, it is integral to reduce unwanted dose to normal structures when possible. DP-IMRT, which allows for better sparing of non-target structures without compromising treatment fields, may be a valuable technique in the treatment of pediatric GCT patients.
Similarly, excellent dosimetry has also been reported with proton radiotherapy. Previously published data comparing the use of proton therapy to sequential-IMRT suggest that 3D-conformal proton therapy (3D-CPT) and intensity modulated proton therapy (IMPT) are able to further decrease the mean dose to the temporal lobes and whole brain with excellent local control. Of note, IMPT using fine pencil beams achieves greater temporal lobe sparing than 3D-CPT. Interestingly, proton therapy did not spare the hippocampal region.[15] More recent data also show proton therapy is able to achieve lower mean doses to the temporal lobes and whole brain than sequential-IMRT.[16]
A limitation in our series and the study of all GCT is small sample size. Additionally, other limitations include retrospective methodology, a heterogeneous patient population, and a small number of events. Given the widespread availability of IMRT technology and data from this series and other studies examining the merits of DP-IMRT, a prospective multi-institutional study with a larger patient population would be ideal to further explore brain-sparing DP-IMRT for GCT patients. Similarly, as the availability of proton therapy increases, it would also be worthwhile to compare DP-IMRT and proton therapy for GCT patients.
Acknowledgments
P30 CA008748 (associated via MyNCBI).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 2.Haas-Kogan DA, Missett BT, Wara WM, Donaldson SS, Lamborn KR, Prados MD, Fisher PG, Huhn SL, Fisch BM, Berger MS, Le QT. Radiation therapy for intracranial germ cell tumors. Int J Radiat Oncol Biol Phys. 2003;56:511–518. doi: 10.1016/s0360-3016(02)04611-4. [DOI] [PubMed] [Google Scholar]
- 3.Wolden SL, Wara WM, Larson DA, Prados MD, Edwards MS, Sneed PK. Radiation therapy for primary intracranial germ-cell tumors. Int J Radiat Oncol Biol Phys. 1995;32:943–949. doi: 10.1016/0360-3016(95)00067-9. [DOI] [PubMed] [Google Scholar]
- 4.Galloway TJ, Indelicato DJ, Amdur RJ, Swanson EL, Smith AA, Marcus RB., Jr Second tumors in pediatric patients treated with radiotherapy to the central nervous system. Am J Clin Oncol. 2012;35:279–83. doi: 10.1097/COC.0b013e318210f533. [DOI] [PubMed] [Google Scholar]
- 5.Chen MJ, Santos AD, Sakuraba RK, Lopes CP, Goncalves VD, Weltman E, Ferrigno R, Cruz JC. Intensity-modulated and 3D-conformal radiotherapy for whole-ventricular irradiation as compared with conventional whole-brain irradiation in the management of localized central nervous system germ cell tumors. Int J Radiat Oncol Biol Phys. 2010;76:608–614. doi: 10.1016/j.ijrobp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Roberge D, Kun LE, Freeman CR. Intracranial germinoma: on whole-ventricular irradiation. Pediatr Blood Cancer. 2005;44:358–362. doi: 10.1002/pbc.20257. [DOI] [PubMed] [Google Scholar]
- 7.Tanderup K, Olsen DR, Grau C. Dose painting: art or science? Radiother Oncol. 2006;79(3):245–248. doi: 10.1016/j.radonc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Dogan N, King S, Emami B. Assessment of different IMRT boost delivery methods on target coverage and normal-tissue sparing. Int J Radiat Oncol Biol Phys. 2003;57:1480–1491. doi: 10.1016/s0360-3016(03)01569-4. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Dharmarajan KV, Wexler LH, La Quaglia MP, Happersett L, Wolden SL. Intensity Modulated Radiation Therapy with Dose Painting to Treat Rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2012;84(3):e371–7. doi: 10.1016/j.ijrobp.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Weksberg D, Shibamoto Y, Paulino A. Bifocal intracranial germinoma: A retrospective analysis of treatment outcomes in 20 patients and review of the literature. Int J Radiat Oncol Biol Phys. 2012;82:1341–1351. doi: 10.1016/j.ijrobp.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Paximadis P, Hallock A, Bhambhani K, Chu R, Sood S, Wang Z, Konski A. Patterns of failure in patients with primary intracranial germinoma treated with neoadjuvant chemotherapy and radiotherapy. Pediatr Neurol. 2012;47:162–166. doi: 10.1016/j.pediatrneurol.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Kim JW, Kim WC, Cho JH, Kim DS, Shim KW, Lyu CJ, Won SC, Suh CO. A multimodal approach including craniospinal irradiation improves the treatment outcome of high-risk intracranial nongerminomatous germ cell tumors. Int J Radiat Oncol Biol Phys. 2012;84(3):625–31. doi: 10.1016/j.ijrobp.2011.12.077. [DOI] [PubMed] [Google Scholar]
- 13.O'Neil S, Ji Lingyun, Buranahirun C, Azoff J, Dhall G, Khatua S, Patel S, Panigrahy A, Borchert M, Sposto R, Finlay J. Neurocognitive outcomes in pediatric and adolescent patients with central nervous system germinoma treated with a strategy of chemotherapy followed by reduced-dose and volume irradiation. Pediatr Blood Cancer. 2011;57:669–673. doi: 10.1002/pbc.23146. [DOI] [PubMed] [Google Scholar]
- 14.Odagiri K, Omura M, Hata M, Aida N, Niwa T, Ogino I, Kigasawa H, Ito S, Adachi M, Inoue T. Treatment outcomes, growth height, and neuroendocrine functions in patients with intracranial germ cell tumors treated with chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(3):632–8. doi: 10.1016/j.ijrobp.2011.12.084. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald SM, Trofimov A, Safai S, Adams J, Fullerton B, Ebb D, Tarbell NJ, Yock TI. Proton radiotherapy for pediatric central nervous system germ cell tumors: early clinical outcomes. Int J Radiat Oncol Biol Phys. 2011;79:121–129. doi: 10.1016/j.ijrobp.2009.10.069. [DOI] [PubMed] [Google Scholar]
- 16.Park J, Park Y, Lee SU, Kim T, Choi YK, Kim JY. Differential dosimetric benefit of proton beam therapy over intensity modulated radiotherapy for a variety of targets in patients with intracranial germ cell tumors. Radiat Oncol. 2015;10:135. doi: 10.1186/s13014-015-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]