Abstract
Treatment advances have increased survival in children with cancer, but subclinical, progressive, irreversible, and sometimes fatal treatment-related cardiovascular effects may appear years later. Cardio-oncologists have identified promising preventive and treatment strategies. Dexrazoxane provides long-term cardioprotection from doxorubicin-associated cardiotoxicity without compromising the efficacy of anticancer treatment. Continuous infusion of doxorubicin is as effective as bolus administration in leukemia treatment, but no evidence has indicated that it provides long-term cardioprotection; continuous infusions should be eliminated from pediatric cancer treatment. Angiotensin-converting enzyme inhibitors can delay the progression of subclinical and clinical cardiotoxicity. All survivors, regardless of whether they were treated with anthracyclines or radiation, should be monitored for systemic inflammation and the risk of premature cardiovascular disease. Echocardiographic screening must be supplemented with screening for biomarkers of cardiotoxicity and perhaps by identification of genetic susceptibilities to cardiovascular diseases; optimal strategies need to be identified. The health burden related to cancer treatment will increase as this population expands and ages.
Keywords: etiology, screening, prevention, treatment
INTRODUCTION
Of the 13.7 million cancer survivors in the United States, nearly 400,000 are survivors of childhood cancer, of whom 20% have survived more than 35 years after diagnosis (1, 2). However, with survival comes vulnerability to adverse health outcomes, including cardiac disease, from the treatments that cured their cancer (3–5).
The leading noncancer-related cause of morbidity and mortality in long-term survivors is cardiovascular disease (4–8). Compared with the general population, survivors are eight times more likely to die from cardiovascular-related disease (9). Compared with sibling controls, they are 15 times more likely to experience heart failure (HF), 10 times more likely to have coronary artery disease, and 9 times more likely to have had a cerebrovascular accident during the first 30 years after cancer diagnosis (4). These risks may persist for at least 45 years after treatment and often increase (10).
Late-occurring cardiotoxicity related to anthracycline treatment is initially subclinical, often progressive, potentially severe, and sometimes fatal (11). More than half of survivors have subclinical cardiac abnormalities 5–10 years after chemotherapy (3, 12, 13).
CARDIOVASCULAR EFFECTS OF CANCER TREATMENTS
Cardiotoxicity related to treatment of childhood cancer can be categorized as acute, early onset, or late onset (14). Less than 1% of children experience acute cardiotoxicity resulting in acute HF when treated with front-line cancer protocols; acute cardiotoxicity may be caused by several anticancer drugs, including anthracyclines or alkylating agents. Acute anthracycline-induced toxicity occurs within one week of starting therapy. It is characterized by transient electrophysiological abnormalities, rare fatal arrhythmias, a pericarditis-myocarditis syndrome, or, rarely, fatal acute left ventricular (LV) dysfunction. These symptoms usually resolve after stopping anthracyclines, but patients who are affected early are at greatest risk for manifesting late cardiotoxicity as survivors (14).
Early-onset cardiotoxicity occurs within the first year of therapy. The damage may persist or progress even after therapy is discontinued. Heart failure and pericardial effusion are examples of early-onset cardiotoxicity. Pericardial effusion is rare but may result in cardiac tamponade in children. As pericardial fluid accumulates, the rise in pressure decreases diastolic filling, which results in decreased cardiac output. Emergency pericardiocentesis may be indicated.
Late-onset anthracycline cardiotoxicity occurs a year or more after therapy (14) and, in addition to long-term cardiac dysfunction, is characterized by impaired myocardial growth. The LV wall is disproportionately small in relation to somatic growth, owing to the loss of functional cardiomyocytes and a reduction in LV mass or LV mass-to-volume ratio (3). Given this difference in growth, the loss of cardiomyocytes progressively increases LV afterload and reduces LV systolic function, which may result in depressed LV contractility and can progress to HF and death.
In survivors who were treated as children, toxicity initially manifests as an atypical form of cardiomyopathy that may have slow or absent progression for many years. Abnormal LV diastolic function is more evident than LV systolic dysfunction. At the completion of anthracycline chemotherapy for childhood cancer, an asymptomatic dilated cardiomyopathy is often measured, which progressively shows restrictive physiology. This is in contrast to survivors who were treated as adults, who tend to present with a dilated cardiomyopathy. Late-onset HF is recalcitrant to usual therapies, and no therapy is of proven benefit.
Several anticancer drugs and ionizing radiation can cause cardiotoxicity. Among the commonly used cardiotoxic drugs are anthracyclines (e.g., doxorubicin, daunorubicin, idarubicin), alkylating agents (e.g., cyclophosphamide, ifosfamide, cisplatin), therapeutic antibodies (e.g., bevacizumab), and tyrosine kinase inhibitors (e.g., imatinib, dasatinib, sunitinib, sorafenib) (Table 1).
Table 1.
Cardiotoxic effects of chemotherapeutic cancer agentsa
| Drug class: agent | Adverse cardiac events |
|---|---|
| Anthracycline: doxorubicin, daunorubicin, epirubicin, idarubicin |
Arrhythmias, pericarditis, myocarditis, HF, LV dysfunction |
| Alkylating agents: busulfan, cisplatin, cyclophosphamide, ifosfamide, nitrosoureas |
Endomyocardial fibrosis, pericarditis, cardiac tamponade, ischemia, MI, hypertension, myocarditis, HF, and arrhythmias |
| Antimetabolites: cytarabine, clofarabine |
Ischemia, chest pain, MI, HF, arrhythmias, pericarditis, pericardial effusions, and hemodynamic abnormalities |
| Antimicrotubules: vinca alkaloids |
Sinus bradycardia, angina, hypotension or hypertension, HF, ischemia, MI, arrhythmias, conduction abnormalities |
| Biological agents: bevacizumab, rituximab |
Hemodynamic abnormalities, LV dysfunction, HF, thromboembolism, angioedema, arrhythmias |
| Tyrosine kinase inhibitors: imatinib, sorafenib, sunitinib, dasatinib |
HF, edema, pericardial effusion, pericarditis, hypertension, arrhythmias, ischemia, prolonged QT interval, chest pain |
| Miscellaneous: asparaginase, arsenic trioxide, all-trans-retinoic acid |
HF, hypotension, MI, electrocardiographic changes, pleural-pericardial effusion, QT prolongation, peripheral edema, bradycardia, ischemia, edema, thromboembolism |
Abbreviations: HF, heart failure; LV, left ventricular; MI, myocardial infarction.
Adapted from Reference 2 with permission.
Anthracyclines
About half of long-term survivors of childhood malignancies received anthracyclines, a class of drugs whose cardiotoxicity has been known for more than 40 years (14). These drugs are used to treat both hematological malignancies and solid tumors. The most commonly hypothesized mechanism for anthracycline-induced cardiotoxicity is the generation of free radicals and super-oxides. Anthracycline-induced loss or damage to a critical number of cardiomyocytes decreases the number of residual myocardial cells, which are required to generate a normal myocardial mass, despite the marked hypertrophy of the remaining cardiomyocytes (3). Cardiomyocyte loss leads to LV wall thinning and, with early to intermediate follow-up during the first six years after anthracycline therapy, to progressive LV dilation. Cardiomyocyte mitochondrial structure and function are particularly affected by anthracycline exposure, and these effects may be persistent (15).
Alkylating Agents
Alkylating agents are used to treat a variety of solid tumors, as well as in conditioning regimens for transplantation. Cyclophosphamide exerts its cardiotoxicity by direct endothelial damage, leading to leakage of plasma proteins and erythrocytes. Abnormal LV wall thickness from interstitial edema and hemorrhage may reduce LV diastolic compliance, creating LV diastolic dysfunction and presenting as restrictive cardiomyopathy (16). With higher doses, myopericarditis may occur. These toxicities usually manifest early, as opposed to the delayed onset of anthracycline-induced cardiotoxicity.
Therapeutic Antibodies
Trastuzumab is primarily used to treat breast cancers (17), but it is used in some children with osteosarcoma whose tumors express the drug’s target, human epidermal growth factor receptor 2 (HER2) (18). Trastuzumab interferes with cellular repair and causes apoptosis. Cardiotoxic side effects include LV systolic dysfunction and HF (19). Additional risk factors for trastuzumab toxicity are concurrent anthracycline treatment and chest radiotherapy (20). In contrast to anthracycline-associated LV systolic dysfunction, trastuzumab-associated dysfunction often reverses after discontinuing the drug, and patients with LV systolic dysfunction may tolerate retreatment with trastuzumab (21).
A study of 41 children and adolescents with metastatic osteosarcoma that overexpressed HER2 found that trastuzumab could be safely given in conjunction with an anthracycline and the cardioprotectant dexrazoxane (22). No patient experienced HF, and biomarker concentrations for cardiotoxicity were normal. Newer anti-HER2 agents, such as lapatinib and pertuzumab, may be less cardiotoxic than trastuzumab (19, 23). Data on using these agents to treat children are emerging (18, 24).
Tyrosine Kinase Inhibitors
Imatinib is a selective inhibitor of multiple tyrosine kinases that inhibits cellular downstream signaling and growth. Imatinib is used to treat chronic myeloid leukemia (CML) and Philadelphia-positive acute lymphoblastic leukemia (ALL) in both adults and children (25). It is rarely associated with HF, perhaps because of its inhibiting effects on cardiomyocytes (26). In a trial of 1,300 patients, 1.7% of adults receiving imatinib experienced HF, but <1% of these occurrences were related to the drug (27). Imatinib cardiotoxicity has not been reported in children (28, 29), but it has occurred in rat myocardium, with myocyte vacuolization, myofibrillar loss, interstitial lymphocytic infiltration, and fibrosis with myofibroblasts and chronic inflammatory cells (30).
Second- and third-generation tyrosine kinase inhibitors (e.g., dasatinib, nilotinib) are being used as front-line agents for CML because of their greater molecular efficacy (31). Ponatinib, which is efficacious against CML in patients with the T315I mutation that renders CML resistant to the other tyrosine kinase inhibitors, is associated with a nearly 12% incidence of serious arterial thrombotic events in adults (32). The vascular toxicity of nilotinib (33) raises concerns about the safety of this class of agents, particularly as front-line agents for treating CML and other cancers (34). The late-occurring toxicities of tyrosine kinase inhibitors, first used two decades ago, remain unknown.
Inhibitors of Vascular Endothelial Growth Factor Receptors
Inhibitors of vascular endothelial growth factor (VEGF) receptors are used to supplement many other antitumor regimens because they change tumor vasculature. As a class, these drugs affect several signaling pathways and share similar cardiovascular side effects, particularly hypertension and, less commonly, cardiomyopathy. Hypertension caused by VEGF receptor inhibitors is usually acute and occurs within 2 weeks of administration (35). In adults with metastatic renal cell carcinoma, hypertension may even increase the anticancer efficacy of sunitinib, an inhibitor that targets multiple growth factor receptors that regulate both tumor angiogenesis and tumor cell survival (36). Drug-related hypertension is often controlled with standard antihypertensive agents, and treatment with VEGF receptor inhibitors rarely needs to be stopped.
Cardiomyopathy from VEGF receptor inhibitors occurs in adults; the incidence of symptomatic HF ranges from 1.5% to 15%, and the incidence of any LV systolic dysfunction ranges from 7% to 28% (37–39). LV systolic dysfunction, like that seen with trastuzumab, is often reversible, and therapy is rarely discontinued. These agents are associated with ischemic cardiac events and arrhythmias, including QT-interval prolongation (39).
Experience in children is limited (40–42). Given that these agents are tested in adults before children, and assuming they are effective, years of follow-up will be required to determine their long-term cardiovascular safety in children. This will be challenging because most trials do not use cardiovascular measures as primary or secondary endpoints, often exclude patients with a history of cardiovascular disease, define cardiotoxicity differently, and often do not include prospective, longer-term monitoring (2).
Radiation Therapy
Cancer survivors treated with radiation therapy are particularly vulnerable to chronic pericardial disease; premature coronary artery disease; atherosclerosis; cardiomyopathy, including LV systolic and diastolic dysfunction (restrictive cardiomyopathy) leading to HF; valvular disease; and conduction abnormalities, all of which may appear decades after treatment (43).
Adams and coauthors (44) assessed long-term cardiovascular status in 48 survivors of childhood and adolescent Hodgkin’s lymphoma who had received mediastinal radiation (median dose of 40 Gy) and were asymptomatic at the time of evaluation. All but one survivor had cardiac abnormalities, including restrictive cardiomyopathy-like structural findings (decreased LV dimension and mass without increased LV wall thickness); 20 had marked valvular defects; 36 had conduction defects, including persistent tachycardia and autonomic dysfunction; and 14 had reduced peak oxygen uptake during exercise, a possible marker of subclinical HF.
Historically, cranial radiation has been used to treat the central nervous system in children with ALL. In one study, survivors who had received cranial radiation had decreased LV mass and LV dimension, probably secondary to postradiation hypothalamic and pituitary dysfunction, including growth hormone deficiency (45). Even if the survivors of childhood cancer had not received cardiotoxic chemotherapy, radiation therapy increased their risk for cardiac abnormalities, atherosclerotic disease, and systemic inflammation.
In a study of 201 survivors of childhood cancer, the 45 who had received radiotherapy but not cardiotoxic chemotherapy had decreases in LV mass and LV wall thickness that were similar to those in survivors who had also received cardiotoxic chemotherapy (46). Radiation-related cardiotoxicity, like anthracycline-related cardiotoxicity, progresses gradually and may remain latent for decades.
RISK FACTORS FOR CARDIOTOXICITY
The degree and progression of anthracycline-related toxicity vary widely among individuals, suggesting a genetic predisposition and the presence of modifiable and nonmodifiable risk factors (Table 2) (47).
Table 2.
Risk factors for anthracycline-related cardiotoxicitya
| Risk factor | Comment | References |
|---|---|---|
| Cumulative anthracycline dose | Cumulative doses >300 mg/m2 are associated with significantly elevated long-term risk | 3, 12, 96–98 |
| Time after therapy | The incidence of clinically important cardiotoxicity increases progressively over decades | 3, 7, 12, 97 |
| Rate of anthracycline administration | Continuous infusion not cardioprotective in children | 7, 78 |
| Individual anthracycline dose | Higher individual doses are associated with increased late cardiotoxicity, even when cumulative doses are limited; no dose is risk-free | 12, 61, 97 |
| Type of anthracycline | Liposomal encapsulated preparations may reduce cardiotoxicity. Data on anthracycline analogues and differences in cardiotoxicity are conflicting | 85, 86, 99 |
| Radiation therapy | Cumulative cardiac radiation dose >30 Gy before or concomitant with anthracycline treatment; as little as 5 Gy increases the risk | 7, 14, 98, 100 |
| Concomitant therapy | Trastuzumab, cyclophosphamide, bleomycin, vincristine, amsacrine, and mitoxantrone, among others, may increase susceptibility or toxicity | 99, 100 |
| Preexisting cardiac risk factors | Hypertension; ischemic, myocardial, and valvular heart disease; prior cardiotoxic treatment | 99 |
| Personal health habits | Smoking; consumption of alcohol, energy drinks, stimulants, prescription and illicit drugs | 7 |
| Comorbidities | Diabetes, obesity, renal dysfunction, pulmonary disease, endocrinopathies, electrolyte and metabolic abnormalities, sepsis, infection, pregnancy, viruses, elite athletic participation, low vitamin D concentrations | 7, 46, 51, 95, 99 |
| Age | Both young (<1 year) and advanced age at treatment are associated with elevated risk | 3, 7, 97, 98 |
| Sex | Females are at greater risk than males | 61, 97 |
| Complementary therapies | More information needs to be collected to assess risk | 7 |
| Additional factors | Trisomy 21; African American ancestry | 96 |
Adapted from Reference 47 with permission from BMJ Publishing Group Ltd.
A high cumulative dose of anthracyclines is the most important risk factor for late cardiac compromise. The risk is 11 times higher with a cumulative dose of >300 mg/m2 than it is with a dose of <300 mg/m2 (11, 48). However, because cardiac damage can occur at doses of <240 mg/m2 (49), there is no “safe” dose of anthracyclines.
Additional risk factors for cardiac abnormalities in cancer survivors include female sex, trisomy 21, younger age at treatment, longer follow-up after treatment, concomitant treatments (such as radiation therapy and trastuzumab), and the presence of preexisting cardiovascular disease and comorbidities (high blood pressure, obesity, physical inactivity) (47). However, some patients appear to be more vulnerable than others, independent of these risk factors.
The overall prevalence of obesity in survivors is similar to that in the general population (50), although survivors treated with cranial radiation are at greater risk for obesity (45). Obesity is a serious health issue for survivors whose cardiovascular system might not be able to compensate for associated events, such as ischemia or atherosclerotic disease. Obesity, among other risk factors, also puts survivors of pediatric cancer at risk for metabolic cardiovascular disease. The median Pathobiological Determinants of Atherosclerosis in Youth score, a composite score of risk factors for cardiovascular disease, was measured in a diverse group of survivors (51). Based on traditional risk factors for cardiovascular disease as assessed by the PDAY score, more than half of survivors had at least 2.2 times greater odds of currently having an advanced coronary artery lesion than an individual of similar age and sex without risk factors for cardiovascular disease. However, the mean survivor score was not statistically different from the mean score of sibling controls. This risk was not strongly associated with a specific cancer type, cancer treatment, or marker of endocrine function, but there was a trend toward an association with physical inactivity and possibly with cranial irradiation (51).
Physical inactivity is among the most important modifiable risk factors associated with cardiovascular disease and other risks for atherosclerosis, such as metabolic syndrome. Survivors of childhood cancer more often report having an inactive lifestyle and are less likely to meet physical activity guidelines than their siblings or age- and sex-matched controls (52). Miller and coauthors (53) found that older age, a higher percentage of body fat, prior methotrexate exposure, and unusually high or low LV mass were associated with lower maximum oxygen consumption in survivors.
Survivors with a family history of premature cardiovascular disease and genetic susceptibilities to cardiovascular diseases might also be at increased risk of cardiotoxicity. Individual variation in the risk of anthracycline-related cardiotoxicity at a given dose suggests a genetic predisposition (54–58). Mutations of the hemochromatosis gene (HFE) that are associated with hereditary hemochromatosis can interfere with iron metabolism and lead to iron overload, thus increasing the susceptibility to anthracycline-related cardiotoxicity (54, 59). Based on the population frequency of carriers of the HFE C282Y mutation, 10% of children with ALL are carriers and the risk of doxorubicin-related myocardial injury is nine times higher in these carriers when compared with noncarriers (54). Patients who are homozygous for the G allele of the CBR3 gene, which encodes carbonyl reductase 3, are also at increased risk of cardiomyopathy (56).
PREVENTING CARDIOTOXICITY
Dexrazoxane decreases oxygen free radicals through intracellular iron chelation, thereby decreasing tissue damage (60). Its approval by the US Food and Drug Administration (FDA) for use as a cardioprotectant is currently limited to women with metastatic breast cancer who have received a cumulative dose of anthracycline of 300 mg/m2 or higher. According to the FDA’s approval statement, dexrazoxane has reduced the intermediate or surrogate endpoints of cardiotoxicity in several randomized trials of children (61, 62). In August 2014, dexrazoxane was designated by the FDA an orphan drug for “prevention of cardiomyopathy for children and adolescents 0 through 16 years of age treated with anthracyclin” (62a).
In 206 children with ALL, dexrazoxane prevented or reduced cardiac injury, as reflected by fewer episodes of elevated serum concentrations of cardiac troponin T (cTnT), without compromising the antileukemic efficacy of doxorubicin (63). Longer-term follow-up of 134 of the 206 children produced echocardiographic evidence that dexrazoxane was cardioprotective (Figure 1) (61). Other studies have found dexrazoxane to be cardioprotective in children treated with doxorubicin for T cell ALL and lymphoma (64), in children with osteosarcoma (22) whose treatment also included the known cardiotoxic drug trastuzumab, and in children with osteosarcoma treated with doxorubicin and dose escalations up to a cumulative dose of 600 mg/m2 (65). Dexrazoxane had greater long-term cardioprotective effects in girls than in boys, particularly with respect to changes in the LV end-diastolic thickness-to-dimension ratio, a marker of pathological LV remodeling (61).
Figure 1.
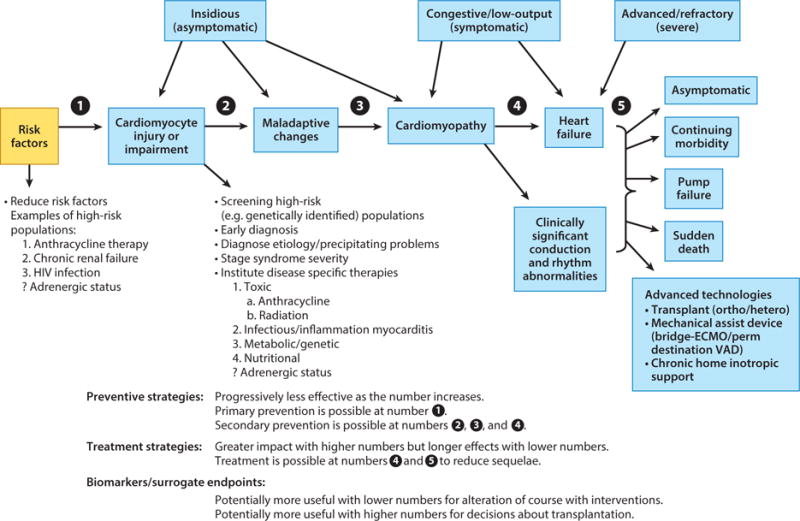
Stages in the course of pediatric ventricular dysfunction. These stages can be monitored by echocardiographic measurements of left ventricular structure and function in combination with concentrations of validated cardiac biomarkers. Risk factors and high-risk populations for ventricular dysfunction are highlighted where preventive or early therapeutic strategies may be effective. Determining the cause of dysfunction may suggest cause-specific therapies. The circled numbers 1–5 indicate points of preventive or therapeutic interventions and where biomarker measurements may be helpful. Abbreviations: ECMO, Extracorporeal Membrane Oxygenation; VAD, Ventricular Assist Device. Figure reprinted from Reference 101 with permission from Oxford University Press.
Despite evidence suggesting the cardioprotective effects of dexrazoxane, in the United States only 2% of children with acute myeloid leukemia received dexrazoxane between 1999 and 2009 (66). Further, notwithstanding the initial conflicting data (67, 68) that have not been substantiated with longer follow-up (69), several trials have found no association between dexrazoxane and an increased risk of secondary malignancies (64, 65, 70, 71) or decreased oncological efficacy (61, 73). In 553 children with high-risk ALL who received dexrazoxane as a cardioprotectant, only one additional malignant neoplasm was found (72). This finding contrasts with that from a randomized trial by the Pediatric Oncology Group, which found 8 second malignancies among 239 children with Hodgkin’s lymphoma who had received dexrazoxane (74). This difference in findings likely results from differences in the underlying malignancy and other concurrent therapies. Finally, in a large multicenter randomized trial, dexrazoxane provided long-term cardioprotection without compromising oncological efficacy in 205 children treated with doxorubicin for high-risk ALL (61). An ongoing Children’s Oncology Group study is following children from two Hodgkin’s lymphoma trials and one T cell ALL clinical trial (74–76) to determine whether dexrazoxane exposure is associated with longer-term effects on cardiac outcomes and to update the data on second cancers and overall mortality (National Clinical Trial #01790152) (77). In a pooled analysis of these three trials involving children treated with doxorubicin for ALL or Hodgkin’s lymphoma (low- to intermediate-stage or advanced disease), dexrazoxane was not associated with a differential risk of mortality or relapse (77).
In a multicenter randomized trial of children with high-risk ALL, cardioprotection and event-free survival were similar in patients who had received doxorubicin as a continuous infusion or as a bolus infusion (78). Both groups had similar LV function and structure values. Ten-year event-free survival did not differ significantly between groups (83% and 78% for continuous and bolus, respectively). Additional studies found similar results in patients 5 to 7 years after treatment (79, 80). In the absence of cardioprotection or improvement in oncologic efficacy, we recommend that continuous infusion not be used in children because it increases hospital stays and costs as well as the risks of thromboembolic events and mucositis (81). Despite a lack of evidence for cardioprotection, continuous infusion of anthracycline is still part of some pediatric treatment protocols for cardioprotection.
Several studies have reported that angiotensin-converting enzyme (ACE) inhibitors reduce the incidence of clinical HF in adults with subclinical ventricular dysfunction (82). For example, among adult cancer patients who had elevated concentrations of cardiac troponin I (cTnI) immediately after anthracycline chemotherapy, those treated early with the ACE inhibitor enalapril experienced less late cardiotoxicity than did control patients (69). Data are limited about the late occurrence of clinically important cardiovascular effects in these children as they age.
In 18 survivors of childhood cancer, during the first 6 years of enalapril therapy, LV dimension, afterload, fractional shortening, and mass progressively improved, but these improvements were lost 6 to 10 years after beginning enalapril treatment (83). Mean LV wall thickness deteriorated throughout the study, as did LV contractility and systolic blood pressure. After 6 years on ACE inhibitor therapy, all 6 patients in HF at the start of therapy had either died or undergone heart transplantation, suggesting that the enalapril-induced improvements in LV structure and function were transient (83). Likewise, in a randomized double-blind placebo-controlled trial of 135 long-term survivors of childhood cancer with at least one cardiac abnormality identified any time after anthracycline exposure, those receiving enalapril had lower LV wall stress in the first year after therapy, and the reduction was maintained during the 5-year study; treatment did not influence exercise performance (84).
Treatment with ACE inhibitors delays, but does not prevent, the progression of subclinical and clinical cardiotoxicity in survivors (83). This finding emphasizes the importance of primary prevention, including using lower cumulative doses of anthracyclines, less cardiotoxic anthracycline analogues, and cardioprotective agents (85, 86).
SCREENING AND SURVEILLANCE FOR CARDIOTOXICITY
Currently, there are no evidence-based guidelines for cardiovascular monitoring in survivors of childhood cancer. Echocardiography, the most frequently used modality, lacks sufficient sensitivity and specificity to detect early cardiac injury.
Serum cardiac biomarkers are increasingly used to evaluate cardiotoxicity during and after chemotherapy. In a study of children with high-risk ALL (87), elevated serum concentrations of cTnT during the first 90 days of anthracycline treatment were significantly associated with reduced LV end-diastolic posterior wall thickness, reduced LV mass, and increased LV remodeling 4 years after therapy. This finding is particularly important because FDA guidelines call for using dexrazoxane only after patients have received greater than 300 mg/m2 of anthracyclines. Similarly, in this same study, elevated serum concentrations of N-terminal probrain natriuretic peptide (NT-proBNP), which may indicate increasing LV wall stress, during the first 90 days of therapy were also associated with abnormal LV thickness-to-dimension ratios 4 years later, suggesting pathological LV remodeling. NT-proBNP concentrations were elevated in 89% of children before treatment and in 48% after treatment. Overall, NT-proBNP concentrations were elevated in more children than were cTnT concentrations, a result that might identify patients at risk for cardiotoxicity prior to developing cardiomyocyte injury and predict late LV remodeling. High-sensitivity C-reactive protein assays used to assess concentrations during anthracycline therapy did not identify children with late cardiotoxicity. Currently, only serum concentrations of cTnT and NT-proBNP measured during active chemotherapy have been validated as surrogate endpoints for late cardiotoxicity in long-term survivors (87).
Cardiac magnetic resonance imaging is also being increasingly used in pediatric cardiology to identify late cardiotoxicity occurring with abnormal longitudinal LV strain and regional strain despite a normal LV ejection fraction, and with a higher mean extracellular volume that is consistent with diffuse fibrosis. However, the predictive value for late outcomes is unknown.
TREATING CARDIOTOXICITY
Treatments for anthracycline-related cardiomyopathy depend on LV preload and LV afterload abnormalities, and on the progression of cardiac fibrosis. The goal of early treatment is to prevent pathological LV remodeling using drugs that target LV preload (diuretics) and LV afterload (ACE inhibitors or angiotensin-receptor blockers) (Figure 1). The beneficial effects of ACE inhibitors for survivors of childhood cancer are transient.
Effective tailored, precision therapies for anthracycline-associated HF have not yet been developed. Because anthracycline-related structural changes progress from dilated cardiomyopathy to a restrictive-like cardiomyopathy, it is critically important to understand the type of cardiomyopathy causing HF in these patients. Many treatments appropriate for HF caused by dilated cardiomyopathy are inappropriate for HF caused by restrictive cardiomyopathy. Symptomatic patients may benefit from tailored, precision therapy based on hemodynamic monitoring or from heart transplantation. Carvedilol, a nonselective β-blocker, improves LV function and mortality in adults (88, 89), but its effects in survivors of childhood cancer are unknown. In survivors of childhood cancer, HF can progress rapidly to functional impairment that is refractory to drug therapy. In this situation, mechanical support may be considered.
For patients who do not respond well to all other cardiac treatments, heart transplantation is an option for end-stage anthracycline-induced cardiomyopathy (90). In 17 children (mean age at cancer diagnosis of 6 years) who underwent heart transplantation at a median of 9.2 years after cancer diagnosis, 5 of them underwent transplantation within 5 years from the end of treatment. Cancer recurred in 1 patient. All patients survived 1 year; 16 survived 2 years; and 10 survived at least 5 years (91).
Growth hormone therapy is also used to treat cardiotoxicity. In one study of 34 survivors of childhood cancer who had somatic growth deficiency, growth hormone therapy improved LV structure and blood pressure, but these improvements were lost 4 years after therapy had been discontinued. Growth hormone replacement therapy did not ameliorate progressive LV dysfunction (92).
CONCLUSIONS
Advances in treatment have increased survival in children with cancer, but their cardiovascular-related health burden will increase as this population grows and ages (46, 51, 53, 87, 93, 94). The developing field of pediatric cardio-oncology helps clinicians identify and understand the damage to the cardiovascular system caused by cancer treatment. Optimal monitoring strategies and preventive treatments still need to be identified. Treatment-related cardiovascular effects may appear decades after treatment and are often progressive and irreversible. Thus, screening, preventing, or reducing treatment-related cardiovascular damage by using agents such as dexrazoxane, screening for risk factors, and implementing serial cardiac monitoring are important for survivors of childhood cancer.
SUMMARY POINTS.
Cardioprotection with dexrazoxane reduced anthracycline cardiotoxicity without compromising antitumor efficacy or increasing the risk of second malignancies in randomized clinical trials of children with ALL, Hodgkin’s lymphoma, lymphoblastic lymphoma, and osteogenic sarcoma (7, 70, 87).
In survivors of childhood high-risk ALL, continuous infusion of doxorubicin provided no advantage over bolus infusion in terms of long-term cardioprotection or event-free survival (78).
All survivors of childhood cancer are at increased risk of cardiotoxicity, regardless of therapy, suggesting that all survivors should be screened for premature cardiovascular disease (46).
Survivorship guidelines should address cardiovascular concerns, including the risk of atherosclerotic disease and systemic inflammation, in both anthracycline-exposed and unexposed survivors (46). Screening guidelines should include specific recommendations for survivors who did not receive cardiotoxic treatments, and future investigations of new or existing treatments should consider cardiotoxicity (12, 46, 94). Detecting decrements in LV fractional shortening alone is unlikely to identify all survivors at risk of premature cardiovascular disease from all causes (7, 46).
Serum biomarkers of cardiomyopathy and cardiomyocyte injury or death that are measured during therapy can predict late cardiotoxicity, allowing studies to determine whether these markers can be used to tailor anticancer therapy and to determine whether overall outcomes are improved. Developing a comprehensive panel of biomarkers to assess cardiac damage in survivors should be a research priority. Monitoring N-terminal pro-brain natriuretic peptide concentrations during therapy might predict which children are at higher risk of cardiotoxicity, allowing treatment to be modified before cardiomyocyte damage is irreversible (87).
Survivorship clinics can use low-cost cardiac risk calculators to identify the survivors who are at greatest risk of premature, symptomatic cardiovascular disease and to implement specific preventive strategies (46, 51, 95).
The impact of survivorship might also include lifestyle behaviors, such as physical inactivity, that mediate risk-factor profiles for cardiovascular risk (53, 94, 95).
The burden of cardiotoxicity related to cancer treatment will increase as this population expands and ages (7, 51, 53, 87, 94).
Acknowledgments
DISCLOSURE STATEMENT
V.F. is supported by funding from The Michael Garil Fund. S.L. was a paid consultant to The Clinigen Group to help organize the expert panel on cardio-oncology in Newark, NJ in July 2014. S.L. was also supported in part by grants from the National Institutes of Health (HL072705, HL078522, HL053392, CA127642, CA068484, HD052104, AI50274, HD052102, HL087708, HL079233, HL004537, HL087000, HL007188, HL094100, HL095127, and HD80002), the Children’s Cardiomyopathy Foundation, the Women’s Cancer Association of the University of Miami, the Lance Armstrong Foundation, the STOP Children’s Cancer Foundation, the Scott Howard Fund, and the Michael Garil Fund.
Contributor Information
Steven E. Lipshultz, Email: slipshultz@med.wayne.edu.
Vivian I. Franco, Email: vfranco@med.wayne.edu.
Tracie L. Miller, Email: tmiller2@med.miami.edu.
Steven D. Colan, Email: steven.colan@cardio.chboston.org.
Stephen E. Sallan, Email: stephen_sallan@dfci.harvard.edu.
LITERATURE CITED
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: Am. Cancer Soc; 2013. [Google Scholar]
- 2.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10:697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–15. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–81. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 8.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–15. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 9.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–72. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 10.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–79. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 11.Nysom K, Holm K, Lipsitz SR, et al. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16:545–50. doi: 10.1200/JCO.1998.16.2.545. [DOI] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 13.Kremer LC, van der Pal HJ, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–29. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 14.Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44:600–6. doi: 10.1002/pbc.20352. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Miller TL, Gerschenson M, et al. Effect of dexrazoxane on impaired mitochondrial structure and function in doxorubicin-treated childhood ALL survivors. J Clin Oncol. 2012;30(Suppl):9530. Abstr. [Google Scholar]
- 16.Taniguchi I. Clinical significance of cyclophosphamide-induced cardiotoxicity. Intern Med. 2005;44:89–90. doi: 10.2169/internalmedicine.44.89. [DOI] [PubMed] [Google Scholar]
- 17.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 18.Albanell J, Montagut C, Jones ET, et al. A phase I study of the safety and pharmacokinetics of the combination of pertuzumab (rhuMab 2C4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2726–31. doi: 10.1158/1078-0432.CCR-07-1980. [DOI] [PubMed] [Google Scholar]
- 19.De Keulenaer GW, Doggen K, Lemmens K. The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res. 2010;106:35–46. doi: 10.1161/CIRCRESAHA.109.205906. [DOI] [PubMed] [Google Scholar]
- 20.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–26. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 22.Ebb D, Meyers P, Grier H, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2545–51. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez EA, Koehler M, Byrne J, et al. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc. 2008;83:679–86. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 24.Fouladi M, Stewart CF, Blaney SM, et al. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28:4221–27. doi: 10.1200/JCO.2010.28.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematol Am Soc Hematol Educ Program. 2010;2010:368–76. doi: 10.1182/asheducation-2010.1.368. [DOI] [PubMed] [Google Scholar]
- 26.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 27.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–37. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 28.Bond M, Bernstein ML, Pappo A, et al. A phase II study of imatinib mesylate in children with refractory or relapsed solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2008;50:254–58. doi: 10.1002/pbc.21132. [DOI] [PubMed] [Google Scholar]
- 29.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a Children’s Oncology Group study. J Clin Oncol. 2009;27:5175–81. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman EH, Knapton A, Rosen E, et al. A multifaceted evaluation of imatinib-induced cardiotoxicity in the rat. Toxicol Pathol. 2011;39:1091–106. doi: 10.1177/0192623311419524. [DOI] [PubMed] [Google Scholar]
- 31.Pavey T, Hoyle M, Ciani O, et al. Dasatinib, nilotinib and standard-dose imatinib for the first-line treatment of chronic myeloid leukaemia: systematic reviews and economic analyses. Health Technol Assess. 2012;16(42):1–277. doi: 10.3310/hta16420. [DOI] [PubMed] [Google Scholar]
- 32.US Food Drug Admin. FDA Drug Safety Communication: FDA asks manufacturer of the leukemia drug Iclusig (ponatinib) to suspend marketing and sales. US Food Drug Admin; Washington DC: 2013. http://www.fda.gov/Drugs/DrugSafety/ucm373040.htm. [Google Scholar]
- 33.Tefferi A. Nilotinib treatment-associated accelerated atherosclerosis: When is the risk justified? Leukemia. 2013;27:1939–40. doi: 10.1038/leu.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groarke JD, Cheng S, Moslehi J. Cancer-drug discovery and cardiovascular surveillance. N Engl J Med. 2013;369:1779–81. doi: 10.1056/NEJMp1313140. [DOI] [PubMed] [Google Scholar]
- 35.Bair SM, Choueiri TK, Moslehi J. Cardiovascular complications associated with novel angiogenesis inhibitors: emerging evidence and evolving perspectives. Trends Cardiovasc Med. 2013;23:104–13. doi: 10.1016/j.tcm.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–19. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19:1613–18. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 39.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–12. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 40.Dubois SG, Shusterman S, Ingle AM, et al. Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: a Children’s Oncology Group study. Clin Cancer Res. 2011;17:5113–22. doi: 10.1158/1078-0432.CCR-11-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inaba H, Rubnitz JE, Coustan-Smith E, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29:3293–300. doi: 10.1200/JCO.2011.34.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widemann BC, Kim A, Fox E, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children’s Oncology Group Phase I Consortium report. Clin Cancer Res. 2012;18:6011–22. doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boivin JF, Hutchison GB, Lubin JH, Mauch P. Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer. 1992;69:1241–47. doi: 10.1002/cncr.2820690528. [DOI] [PubMed] [Google Scholar]
- 44.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 45.Landy DC, Miller TL, Lipsitz SR, et al. Cranial irradiation as an additional risk factor for anthracycline cardiotoxicity in childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr Cardiol. 2013;34:826–34. doi: 10.1007/s00246-012-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipshultz SE, Landy DC, Lopez-Mitnik G, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30:1050–57. doi: 10.1200/JCO.2010.33.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–33. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 48.Kremer LC, van Dalen EC, Offringa M, et al. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19:191–96. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 49.Trachtenberg BH, Landy DC, Franco VI, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32:342–53. doi: 10.1007/s00246-010-9878-3. [DOI] [PubMed] [Google Scholar]
- 50.Messiah SE, Arheart KL, Lopez-Mitnik G, et al. Ethnic group differences in cardiometabolic disease risk factors independent of body mass index among American youth. Obesity. 2013;21:424–28. doi: 10.1002/oby.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landy DC, Miller TL, Lopez-Mitnik G, et al. Aggregating traditional cardiovascular disease risk factors to assess the cardiometabolic health of childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Am Heart J. 2012;163:295–301.e2. doi: 10.1016/j.ahj.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–94. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller AM, Lopez-Mitnik G, Somarriba G, et al. Exercise capacity in long-term survivors of pediatric cancer: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Pediatr Blood Cancer. 2013;60:663–68. doi: 10.1002/pbc.24410. [DOI] [PubMed] [Google Scholar]
- 54.Lipshultz SE, Lipsitz SR, Kutok JL, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119:3555–62. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–95. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 56.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1415–21. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Visscher H, Ross CJ, Rassekh SR, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–28. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 58.Visscher H, Ross CJ, Rassekh SR, et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013;60:1375–81. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 59.Miranda CJ, Makui H, Soares RJ, et al. Hfe deficiency increases susceptibility to cardiotoxicity and exacerbates changes in iron metabolism induced by doxorubicin. Blood. 2003;102:2574–80. doi: 10.1182/blood-2003-03-0869. [DOI] [PubMed] [Google Scholar]
- 60.Lipshultz SE. Dexrazoxane for protection against cardiotoxic effects of anthracyclines in children. J Clin Oncol. 1996;14:328–31. doi: 10.1200/JCO.1996.14.2.328. [DOI] [PubMed] [Google Scholar]
- 61.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomized, multicentre trial. Lancet Oncol. 2010;11:950–61. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14:362–72. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 62a.US Food and Drug Administration. Orphan drug designations and approvals. http://www.accessdata.fda.gov/scripts/opdlisting/oopd/OOPD_Results_2.cfm?_Index_Number=441314. Accessed Sep. 2014.
- 63.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–53. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 64.Asselin B, Devidas M, Zhou T, et al. Cardioprotection and safety of dexrazoxane (DRZ) in children treated for newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) or advanced stage lymphoblastic leukemia (T-LL) J Clin Oncol. 2012;30(Suppl):9504. doi: 10.1200/JCO.2015.60.8851. Abstr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kopp LM, Bernstein ML, Schwartz CL, et al. The effects of dexrazoxane on cardiac status and second malignant neoplasms (SMN) in doxorubicin-treated patients with osteosarcoma (OS) J Clin Oncol. 2012;30(Suppl):9503. Abstr. [Google Scholar]
- 66.Walker DM, Fisher BT, Seif AE, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013;60:616–20. doi: 10.1002/pbc.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipshultz SE, Lipsitz SR, Orav EJ. Dexrazoxane-associated risk for secondary malignancies in pediatric Hodgkin’s disease: a claim without compelling evidence. J Clin Oncol. 2007;25:3179. doi: 10.1200/JCO.2007.11.8778. author reply 3180. [DOI] [PubMed] [Google Scholar]
- 68.Hellmann K. Dexrazoxane-associated risk for secondary malignancies in pediatric Hodgkin’s disease: a claim without evidence. J Clin Oncol. 2007;25:4689–90. doi: 10.1200/JCO.2007.12.6888. author reply 90–91. [DOI] [PubMed] [Google Scholar]
- 69.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–81. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 70.Barry EV, Vrooman LM, Dahlberg SE, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008;26:1106–11. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 70a.Lipshultz SE, Franco VI, Sallan SE et al. Dexrazoxane for reducing anthracycline-related cardiotoxicity in children with cancer: an update of the evidence. Prog Pediatr Cardiol. 2014 doi: 10.1016/j.ppedcard.2014.09.007. [DOI] [Google Scholar]
- 71.Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47:1373–79. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the Children’s Oncology Group. Leukemia. 2010;24:355–70. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lipshultz SE, Franco VI, Sallan SE, et al. Dexrazoxane for reducing anthracycline-related cardiotoxicity in children with cancer: an update of the evidence. Prog Pediatr Cardiol. 2014;36:39–49. [Google Scholar]
- 74.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 75.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59:1259–65. doi: 10.1002/pbc.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114:2051–59. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chow EJ, Asselin B, Schwartz CL, et al. Late mortality and relapse after dexrazoxane (DRZ) treatment: an update from the Children’s Oncology Group (COG) J Clin Oncol. 2014;32(Suppl):10024. Abstr. [Google Scholar]
- 78.Lipshultz SE, Miller TL, Lipsitz SR, et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics. 2012;130:1003–11. doi: 10.1542/peds.2012-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levitt GA, Dorup I, Sorensen K, Sullivan I. Does anthracycline administration by infusion in children affect late cardiotoxicity? Br J Haematol. 2004;124:463–68. doi: 10.1111/j.1365-2141.2004.04803.x. [DOI] [PubMed] [Google Scholar]
- 80.Gupta M, Steinherz PG, Cheung NK, Steinherz L. Late cardiotoxicity after bolus versus infusion anthracycline therapy for childhood cancers. Med Pediatr Oncol. 2003;40:343–47. doi: 10.1002/mpo.10298. [DOI] [PubMed] [Google Scholar]
- 81.Lipshultz SE, Giantris AL, Lipsitz SR, et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol. 2002;20:1677–82. doi: 10.1200/JCO.2002.20.6.1677. [DOI] [PubMed] [Google Scholar]
- 82.SOLVD Investig. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 83.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20:4517–22. doi: 10.1200/JCO.2002.12.102. [DOI] [PubMed] [Google Scholar]
- 84.Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–28. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 85.Wouters KA, Kremer LC, Miller TL, et al. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561–78. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 86.van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;2005(1):CD003917. doi: 10.1002/14651858.CD003917.pub2. [DOI] [PubMed] [Google Scholar]
- 87.Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–49. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (prevention of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J Am Coll Cardiol. 2013;61(23):2355–62. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 89.Sliwa K, Norton GR, Kone N, et al. Impact of initiating carvedilol before angiotensin-converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure. J Am Coll Cardiol. 2004;44:1825–30. doi: 10.1016/j.jacc.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 90.Ewer MS, Yeh ET. Cancer and the Heart. New York: BC Decker Inc; 2006. [Google Scholar]
- 91.Ward KM, Binns H, Chin C, et al. Pediatric heart transplantation for anthracycline cardiomyopathy: cancer recurrence is rare. J Heart Lung Transplant. 2004;23:1040–45. doi: 10.1016/j.healun.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 92.Lipshultz SE, Vlach SA, Lipsitz SR, et al. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics. 2005;115:1613–22. doi: 10.1542/peds.2004-1004. [DOI] [PubMed] [Google Scholar]
- 93.Lipshultz SE, Franco VI, Cochran TR. Cardiotoxicity in childhood cancer survivors: a problem with long-term consequences in need of early detection and prevention. Pediatr Blood Cancer. 2013;60:1395–96. doi: 10.1002/pbc.24597. [DOI] [PubMed] [Google Scholar]
- 94.Steiner R. Increasing exercise in long-term survivors of pediatric cancer and their siblings: should treatment be a family affair? Pediatr Blood Cancer. 2013;60:529–30. doi: 10.1002/pbc.24465. [DOI] [PubMed] [Google Scholar]
- 95.Miller TL, Lipsitz SR, Lopez-Mitnik G, et al. Characteristics and determinants of adiposity in pediatric cancer survivors. Cancer Epidemiol Biomark Prev. 2010;19:2013–22. doi: 10.1158/1055-9965.EPI-10-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krischer JP, Epstein S, Cuthbertson DD, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–52. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 97.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–43. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 98.van der Pal HJ, van Dalen EC, Hauptmann M, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–55. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 99.Barry E, Alvarez JA, Scully RE, et al. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8:1039–58. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 100.Giantris A, Abdurrahman L, Hinkle A, et al. Anthracycline-induced cardiotoxicity in children and young adults. Crit Rev Oncol Hematol. 1998;27:53–68. doi: 10.1016/s1040-8428(97)10007-5. [DOI] [PubMed] [Google Scholar]
- 101.Lipshultz SE, Wilkinson JD. Beta-adrenergic adaptation in idiopathic dilated cardiomyopathy: differences between children and adults. Eur Heart J. 2014;35:10–12. doi: 10.1093/eurheartj/ehs402. [DOI] [PubMed] [Google Scholar]


