Abstract
Background
Infections with intestinal parasites (helminths and intestinal protozoa) are endemic in Southeast Asia and inappropriate management and reuse of wastewater might exacerbate the risk of human infections. In rapidly growing urban settings, little is known about the extent of intestinal parasite infections. We assessed the point-prevalence and risk factors of intestinal parasite infections in population groups differently exposed to wastewater in urban and peri-urban transition zones in Hanoi, the capital of Vietnam.
Methods
A cross-sectional survey was carried out between April and June 2014 in people aged ≥ 18 years at risk of wastewater exposure from To Lich River: workers maintaining wastewater treatment facilities; urban farmers reusing wastewater; and urban dwellers at risk of flooding events. For comparison, two peri-urban population groups living in close proximity to the Red River were chosen: farmers using river water for irrigation purposes; and people living in the same communities. A single stool sample was subjected to Kato-Katz and formalin-ether concentration methods for the diagnosis of helminth and intestinal protozoa infections. A questionnaire was administered to determine risk factors and self-reported signs and symptoms.
Results
A total of 681 individuals had complete data records. Highest point-prevalence rates of intestinal parasite infections were observed for peri-urban farmers (30 %). Hookworm and Trichuris trichiura were the predominant helminth species (25 % and 5 %, respectively). Peri-urban farmers were at higher odds of infection with intestinal parasites than any other groups (adjusted odds ratio 5.8, 95 % confidence interval 2.5 to 13.7). Lack of access to improved sanitation and not receiving deworming within the past 12 months were associated with higher infection risk, while higher educational attainment and socioeconomic status were negatively associated with intestinal parasite infections.
Conclusions
Our results suggest that exposure to wastewater was not directly associated with infection with helminths and intestinal protozoa in different population groups in Hanoi. These findings might be explained by a high level of awareness of health risks and access to safe sanitary infrastructure in urban areas. The high prevalence rates observed in peri-urban farmers call for specific interventions targeting this population group.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1809-6) contains supplementary material, which is available to authorized users.
Keywords: Helminth, Intestinal protozoa, Peri-urban farming, Urban farming, Vietnam, Wastewater
Background
In Southeast Asia, infections with intestinal parasites (e.g. helminths and intestinal protozoa) cause a considerable public health burden [1, 2]. Despite efforts to control morbidity and interrupt transmission, infection with soil-transmitted helminths (Ascaris lumbricoides, hookworm, Strongyloides stercoralis and Trichuris trichiura) are common and show geographic, demographic, socioeconomic and cultural differences within and across countries of Cambodia, Lao People’s Democratic Republic (PDR) and Vietnam [3–5]. In urban areas, socioeconomic development, including improvements in sanitation and water infrastructures are thought to be associated with a decline in the prevalence and intensity of intestinal parasites over the past decades [6–8]. However, in rural areas and deprived urban and peri-urban settings, access to clean water and improved sanitation remains insufficient and is an important risk factor for infections with helminth and intestinal protozoa [9, 10]. Additionally, reuse of wastewater and faeces in agriculture and aquaculture might contribute to the transmission of intestinal parasites [2, 11].
Hanoi, the capital of Vietnam, has undergone considerable economic growth since the end of the Vietnam War in 1975, resulting in a change in lifestyles and increased living standards. Moreover, population growth and rural-urban migration led to an expansion of the city boundaries [12]. Due to rapid urbanization, improved access to health care and awareness campaigns are available (i.e. yearly deworming of school-aged children and hygiene campaigns such as “eating cooked food and drinking boiled water”), which decreased prevalence of intestinal parasitic infections [13]. However, increasing volumes of domestic waste, mixed with chemical and microbial pollutants, have increased the heterogeneity in exposure to such pollutants and pathogens [14, 15]. Especially for urban and peri-urban transition zones around Hanoi, it is crucial to ensure access to basic water and sanitation infrastructures. Moreover, guidance on safe management and reuse of wastewater is needed [6, 7, 16]. It is conceivable that increasing volumes of wastewater might exacerbate the spread of intestinal parasites, enteric bacteria and viruses [16, 17]. Moreover, past extreme weather events, such as heavy rains, jeopardized the proper functionality of Hanoi’s sanitation systems, with likely adverse health outcomes [18].
In urban and peri-urban areas of Hanoi, an estimated 650,000 farmers reuse wastewater in agriculture and aquaculture to supply the 6.7 million people living in the city with fresh vegetables and fish [19]. Use of wastewater comes at low cost for water and nutrients, and hence provides an important livelihood opportunity for farming communities [20]. Of note, lack of sanitation facilities and use of human excreta in such communities were shown to be a major risk factor for intestinal parasite infections. Moreover, diarrhoeal and skin diseases have been associated to occupational contact with wastewater [13, 21–24]. In more rural communities, the occupational exposure to Hanoi’s reused wastewater has also been associated with A. lumbricoides and T. trichiura infections [2]. Thus, it is commonly observed in urban communities that the prevalence rates of intestinal parasitic infections are lower than in peri-urban and rural areas [1]. Over the past decade, a number of studies indicated levels of microbial and chemical pollution above national and international safety standards in the environment [15, 25–28]. Thus, pollution reduction may not be sufficient to allow for safe reuse of wastewater for agriculture and aquaculture [29].
As the city of Hanoi expanded rapidly, with annual population growth rates of up to 3.5 %, timely data on prevalence and risk factors of infection with helminths and intestinal protozoa are needed to understand the effect of urbanization in urban and peri-urban transition zones [12]. Surveys investigating prevalence rates and risk factors for parasitic diseases, diarrhoea, skin and eye infections in the urban and peri-urban environment around Hanoi are dating back to 2005 [13, 21–24]. Such data will help to effectively plan public health interventions and justify investments in sanitary infrastructures [16, 30]. The objective of the present study was to assess the prevalence rates and risks factors for intestinal parasite infections in different population groups exposed to wastewater reuse activities in Hanoi.
Methods
Study design and participants
A cross-sectional survey was conducted between April and June 2014. The study was undertaken in the southern part of Hanoi, along To Lich River (main open storm water and drainage channel of the city) and Red River (natural river stemming from the People's Republic of China that is discharged in the Gulf of Tonkin). These rivers receive most of the city’s wastewater, managed by Hanoi Sewerage and Drainage Company (HSDC). However, water quality differs considerably: while water of the To Lich River is not allowing for the safe reuse of wastewater in agriculture and aquaculture according the World Health Organization (WHO) guidelines, the Red River water quality is within tolerable limits colony forming unit (CFU) total coliforms and Escherichia coli (4.2 × 106 CFU/100 ml and 1.7 × 104 CFU/100 ml, respectively). Helminth eggs were only found in To Lich River (0.1 egg/l), which however is still within the WHO tolerable concentration for safe reuse [16, 29]. Particular emphasis was placed to the wastewater reuse in agriculture and aquaculture in urban and peri-urban transition zones of the districts Hoang Mai and Thanh Tri (geographical coordinates: 21°01'42.5"N, 105°51'15.0"E) (Fig. 1). A detailed description of the study system and water quality of the rivers is published elsewhere [29].
Fig. 1.
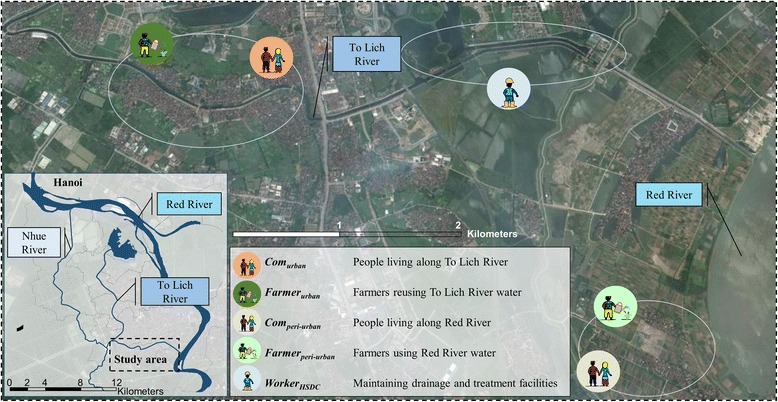
Map of Hanoi showing the study area and the five exposure groups in the Than Tri district. (Map data ©2015 Google)
The study enrolled adults (aged ≥ 18 years) living and working in urban or peri-urban areas in the two districts. According to the level of exposure to wastewater, the study participants were stratified into five population groups: three exposed to wastewater from To Lich River (i-iii); and two comparison groups living along Red River without direct exposure to urban wastewater (iv and v):
-
(i)
“Com urban”, people living in the urban to peri-urban transition zone of Hanoi, in Bang B village or Tam Hiep commune along To Lich River who are potentially exposed to wastewater while flooding events occur during the rainy season. The communities are located in Hoang Mai and Thanh Tri district, respectively (geographical coordinates: 20°57'17.54"N, 105°49'42.48"E), and are prone to rapid demographic transition, industrial development and land use change.
-
(ii)
“Farmer urban”, urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River. A large part of the community members (33 %) are involved in agriculture (e.g. rice, morning glory, neptunia and watercress mainly) or aquaculture activities [31].
-
(iii)
“Worker HSDC”, workers from HSDC maintaining drainage channels and operating the Yen So treatment plants along To Lich River.
-
(iv)
“Com peri-urban”, people living in Duyen Ha commune (comparison group). The commune represents a typical peri-urban community along Red River with poor sanitation and drinking water systems. The commune belongs to Thanh Tri district and is located approximately 5 km from the outskirt of Hanoi (geographical coordinates: 20°55'42.37"N, 105°52'23.32"E).
-
(v)
“Farmer peri-urban”, farmers living in Duyen Ha commune using the irrigation water from Red River (comparison group). About 38 % of the people work in agriculture.
Sample size was calculated by aiming at a power of 95 %, to ensure that a reduction in effective exposure variance by 35 % following confounder adjustment would still leave 80 % power. Our assumptions were that the prevalence of intestinal parasite infections is at least 20 % in Com peri-urban and the odds ratio (OR) of Farmer urban, and Worker HSDC to Com peri-urban is at least 2.5. We also assumed that the final sample size might be reduced by 15 % due to loss to follow-up. Hence, our intended sample size was 1,025 (Com urban, n = 250; Farmer urban, n = 250; Com peri-urban, n = 175; Farmer peri-urban, n = 175; and Worker HSDC, n = 150).
The following inclusion and exclusion criteria were applied. First, households were randomly selected from two separate lists (one for farming and one for all non-farming households in the community) readily available from the communal people committees. All listed households were numbered and the appropriate number selected using a random number list from Excel. All individuals in the selected households were invited to participate in the survey. If they were willing to participate, one person per household (household heads or adults living permanently in the household) was selected for a questionnaire interview at a convenient time at the community health station. Participants were provided with a stool container and asked to return a filled container the day of the interview with her or his own morning stool sample. To select members of Worker HSDC, the HSDC headquarter mobilized and informed the workers and randomly selected them from the existing staff list. Worker HSDC were then invited to come on a fixed day for the interview along with a fresh morning stool sample to the health station of the HSDC the day after the interview.
Procedures
We employed a questionnaire to determine exposure pathways to wastewater, potential confounding factors (e.g. demographic and socioeconomic), risk variables (e.g. water, sanitation, hygiene and occupation) and self-reported signs and symptoms. Our questionnaire had previously been validated in a study in Uganda [32]. The questionnaire was translated into Vietnamese, and further adapted to the Hanoi context and pre-tested among five farmers and five community members not otherwise involved in the current study. Research assistants entered data directly into tablet computers (Samsung Galaxy note 10.1 N8010) via a data entry mask using Open Data Kit (http://opendatakit.org).
Participants were invited to provide a fresh morning stool that was subjected to the Kato-Katz technique (duplicate thick smears, using standard 41.7 mg template) [33] and a formalin-ether concentration technique (FECT) [34] for the diagnosis of helminths (A. lumbricoides, hookworm, T. trichiura and other helminths) and intestinal protozoa (Blastocystis hominis, Chilomastix mesnili, Endolimax nana, Entamoeba coli, Entamoeba histolytica/E. dispar, Entamoeba hartmanni, Giardia intestinalis and Iodamoeba bütschlii). Kato-Katz thick smear and FECT readings were double-entered and cross-checked.
Statistical analysis
Helminth- and intestinal protozo-specific proportions were compared between the five exposure groups, using Pearson’s χ 2 test. Univariate logistic regression was applied to investigate for potential associations between nine dependent variables, i.e. infections with (i) any intestinal parasite; (ii) soil-transmitted helminth; (iii) intestinal protozo; (iv) A. lumbricoides; (v) hookworm; (vi) T. trichiura; (vii) 14-day diarrhoea prevalence; (viii) skin problems; and (ix) eye problems), and 20 independent variables (e.g. exposure groups, sex and age). A measure of socioeconomic status was derived, based on an asset index using principal components analysis (PCA), with participants grouped into four categories, as summarised in Table 1 (most poor, poor, less poor and least poor) [35]. Our multivariate core model included the categorical exposure variables sex, age, educational attainment and socioeconomic status [9, 36]. We then added risk factors that had a P-value lower than 0.2 (using likelihood ratio test) in the univariate analyses. Of note, a univariate or multivariate analysis was only conducted if the number of respective cases was above 50 or 70, respectively.
Table 1.
Demographic and socioeconomic characteristics of the participants enrolled in the cross-sectional survey, stratified by five exposure groups in the Than Tri district, Hanoi, between April and June 2014
| Demographic and socioeconomic characteristics/Exposure groupsa | Com peri-urban | Com urban | Farmer peri-urban | Farmer urban | Worker HSDC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 101 | N = 170 | N = 129 | N = 153 | N = 128 | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Sex | ||||||||||
| Female | 85 | 84.2 | 134 | 78.8 | 105 | 81.4 | 132 | 86.3 | 58 | 45.3 |
| Male | 16 | 15.8 | 36 | 21.2 | 24 | 18.6 | 21 | 13.7 | 70 | 54.7 |
| Age categories (years) (mean ± SD) | 50.0 ± 15.6 | 45.7 ± 14.5 | 48.7 ± 11.1 | 52.6 ± 10.6 | 41.2 ± 10.7 | |||||
| Educational attainment | ||||||||||
| Never went to school | 3 | 3.0 | 7 | 4.1 | 0 | 0.0 | 5 | 3.3 | 0 | 0.0 |
| Primary school | 13 | 12.9 | 16 | 9.4 | 19 | 14.7 | 42 | 27.5 | 2 | 1.6 |
| Secondary school | 47 | 46.5 | 70 | 41.2 | 76 | 58.9 | 76 | 49.7 | 37 | 28.9 |
| Tertiary school | 15 | 14.9 | 59 | 34.7 | 31 | 24.0 | 27 | 17.6 | 73 | 57.0 |
| University degree | 23 | 22.8 | 18 | 10.6 | 3 | 2.3 | 3 | 2.0 | 16 | 12.5 |
| Socioeconomic statusb | ||||||||||
| Most poor | 28 | 27.7 | 31 | 18.2 | 51 | 39.5 | 33 | 21.6 | 12 | 9.4 |
| Poor | 22 | 21.8 | 49 | 28.8 | 42 | 32.6 | 48 | 31.4 | 17 | 13.3 |
| Less poor | 17 | 16.8 | 41 | 24.1 | 25 | 19.4 | 34 | 22.2 | 56 | 43.8 |
| Least poor | 34 | 33.7 | 49 | 28.8 | 11 | 8.5 | 38 | 24.8 | 43 | 33.6 |
| How many people live in your household (mean ± SD) | 4.7 ± 2.0 | 4.6 ± 1.7 | 4.3 ± 1.9 | 5.1 ± 2.6 | 5.3 ± 8.5 | |||||
| Living at the same place (years) (mean ± SD) | 34.4 ± 21.3 | 37.7 ± 19.8 | 37.8 ± 19.5 | 53.3 ± 56.3 | 34.0 ± 14.5 | |||||
aExposure groups: Com peri-urban: people living in the peri-urban commune Duyen Ha, 5 km away from the city along the Red River; Com urban: people living in the urban area of Hanoi, in Bang B village or Tam Hiep commune along the To Lich River and potential exposed to wastewater; Farmer peri-urban: peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; Farmer urban: urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and Worker HSDC: workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
bDerived using principal components analysis (PCA) of the following 11 ownership items: radio, TV, mobile phone, fridge, computer, bicycle, motorbike, car, electricity, running water and latrine
ORs were reported to compare risks. Differences and associations were considered as statistically significant if their P-values were below 0.05 and as indicating a trend if P-values were between 0.05 and 0.1. Statistical analyses were done using STATA version 12.0 (Stata Corporation; College Station, USA).
Results
Among 1,025 people invited, 813 fulfilled our inclusion criteria, provided written informed consent and completed the questionnaire interview (Fig. 2). Stool samples were provided by 718 individuals that were subjected to Kato-Katz thick smear examination. Due to insufficient volumes of stool provided, only 681 of the samples were subjected to FECT. These 681 individuals were considered as the final study cohort, composed of 170 Com urban, 153 Farmer urban, 129, Farmer peri-urban, 128 Worker HSDC and 101 Com peri-urban.
Fig. 2.
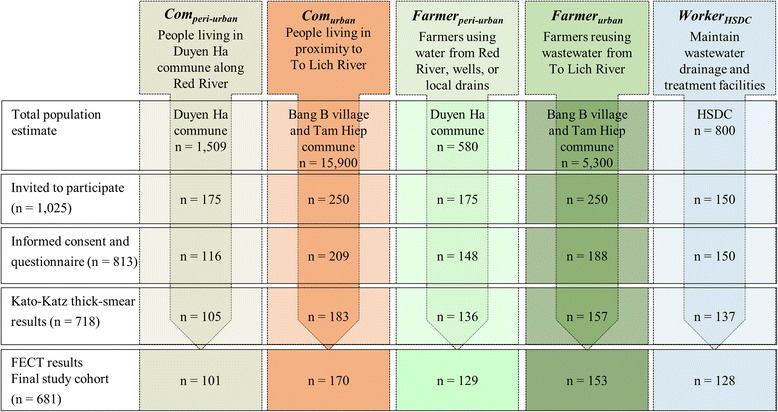
Flow chart indicating the enrolment of study participants and compliance, stratified into exposure groups in the cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014
Table 1 summarises the demographic (sex, age, educational attainment, people per household, living duration at the same place) and socioeconomic characteristics, stratified by the five population groups. In brief, females accounted for 79 % and more in all exposure groups, expect for Worker HSDC (45 %). Most of the participants (>60 %) were aged above 40 years and attended in minimum secondary school. Socioeconomic status was highest in Worker HSDC and Com peri-urban with 34 % classified as “least poor” in both groups. The lowest socioeconomic status was observed in Farmer peri-urban with 40 % classified as “most poor”. On average, between 4.3 and 5.3 people live in a household. Two-thirds of the participants (65 %) reported that they lived in the study area for at least ten years.
Risk factors for intestinal parasite infections, such as perceived exposure to wastewater, access to sanitation, drinking water and bath water and deworming practise are shown in Table 2. Almost 90 % of the participants exposed to wastewater perceived wastewater as polluted water, which causes ill-health and environmental risks (Com urban, Farmer urban and Worker HSDC), while 26 to 28 % Farmer peri-urban perceived no health and environmental risks due to wastewater. Past flooding of the working area was most frequently reported among Farmer urban (39 %) and Worker HSDC (41 %). Overall, 96 % of participants reported to have a toilet at home, whereas 15 % of the Farmer peri-urban had no accesses to sanitation and thus perform open defecation. Self-reported deworming drugs within the past six months ranged between 7 % (Farmer urban) and 13 % (Com peri-urban).
Table 2.
Water, sanitation and hygiene (WASH) specific risk factors of the participants enrolled in a cross-sectional survey, stratified by the five exposure groups in the Than Tri district, Hanoi, between April and June 2014
| Risk factors related to water, sanitation and hygiene/ Exposure groupsa | Com peri-urban | Com urban | Farmer peri-urban | Farmer urban | Worker HSDC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 101 | N = 170 | N = 129 | N = 153 | N = 128 | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Wastewater is … | ||||||||||
| polluted water | 87 | 86.1 | 162 | 95.3 | 111 | 86.0 | 143 | 93.5 | 126 | 98.4 |
| causing health issues | 83 | 82.2 | 149 | 87.6 | 96 | 74.4 | 141 | 92.2 | 126 | 98.4 |
| causing environmental issues | 83 | 82.2 | 149 | 87.6 | 93 | 72.1 | 135 | 88.2 | 127 | 99.2 |
| Exposure to wastewater (water from rivers or lakes around Hanoi) while … | ||||||||||
| flooding of living area | 0 | 0.0 | 5 | 2.9 | 3 | 2.3 | 2 | 1.3 | 17 | 13.3 |
| flooding of working area | 1 | 1.0 | 9 | 5.3 | 21 | 16.3 | 59 | 38.6 | 53 | 41.4 |
| washing clothes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 2 | 1.6 |
| cleaning of a fish pond | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 5 | 3.3 | 15 | 11.7 |
| fishing | 3 | 3.0 | 7 | 4.1 | 3 | 2.3 | 6 | 3.9 | 13 | 10.2 |
| swimming | 1 | 1.0 | 3 | 1.8 | 2 | 1.6 | 0 | 0.0 | 6 | 4.7 |
| Toilet facility at household | ||||||||||
| Flush toilet | 94 | 93.1 | 167 | 98.2 | 108 | 83.7 | 147 | 96.1 | 127 | 99.2 |
| Pit latrine | 6 | 5.9 | 6 | 3.5 | 2 | 1.6 | 6 | 3.9 | 0 | 0.0 |
| No facility (defecation in the open) | 1 | 1.0 | 1 | 0.6 | 19 | 14.7 | 1 | 0.7 | 1 | 0.8 |
| Toilet facility at work | ||||||||||
| Flush toilet | 91 | 90.1 | 150 | 88.2 | 61 | 47.3 | 47 | 30.7 | 39 | 30.5 |
| Pit latrine | 6 | 5.9 | 14 | 8.3 | 4 | 3.1 | 4 | 2.6 | 68 | 53.1 |
| No facility (defecation in the open) | 4 | 4.0 | 6 | 3.5 | 64 | 49.6 | 102 | 66.7 | 21 | 16.4 |
| Household with tap water | 82 | 81.2 | 164 | 96.5 | 102 | 79.1 | 148 | 96.7 | 126 | 98.4 |
| Source of drinking water (multiple answers possible) | ||||||||||
| Bottled water | 40 | 39.6 | 65 | 38.2 | 36 | 27.9 | 41 | 26.8 | 61 | 47.7 |
| Tap water | 60 | 59.4 | 149 | 87.6 | 73 | 56.6 | 136 | 88.9 | 113 | 88.3 |
| Rain water | 14 | 13.9 | 5 | 2.9 | 18 | 14.0 | 9 | 5.9 | 7 | 5.5 |
| Bore hole water | 31 | 30.7 | 7 | 4.1 | 43 | 33.3 | 4 | 2.6 | 1 | 0.8 |
| Source of bathing water (multiple answers possible) | ||||||||||
| Tap water | 73 | 72.3 | 150 | 88.2 | 55 | 42.6 | 70 | 45.8 | 123 | 96.1 |
| Rain water | 6 | 5.9 | 2 | 1.2 | 11 | 8.5 | 18 | 11.8 | 8 | 6.3 |
| Bore hole water | 42 | 41.6 | 16 | 9.4 | 76 | 58.9 | 9 | 5.9 | 12 | 9.4 |
| Well water | 2 | 2.0 | 0 | 0.0 | 4 | 3.1 | 0 | 0.0 | 2 | 1.6 |
| Water from lakes or rivers | 0 | 0.0 | 1 | 0.6 | 5 | 3.9 | 48 | 31.4 | 19 | 14.8 |
| Preventive chemotherapy received in the past | ||||||||||
| < 6 months | 13 | 12.9 | 15 | 8.8 | 15 | 11.6 | 11 | 7.2 | 15 | 11.7 |
| 6 to < 12 months | 9 | 14.1 | 24 | 14,1 | 12 | 9.3 | 12 | 7.8 | 20 | 15.6 |
| > 12 months | 75 | 71.2 | 121 | 71.2 | 96 | 74.4 | 114 | 74.5 | 87 | 68.0 |
| Never took deworming | 4 | 4.0 | 10 | 5.9 | 6 | 5.7 | 16 | 10.5 | 6 | 4.7 |
aExposure groups: Com peri-urban: people living in the peri-urban commune Duyen Ha, 5 km away from the city along the Red River; Com urban: people living in the urban area of Hanoi, in Bang B village or Tam Hiep commune along the To Lich River and potential exposed to wastewater; Farmer peri-urban: peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; Farmer urban: urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and Worker HSDC: workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
Table 3 shows occupational conditions (employment status, working hours, etc.) and protective factors (personal protective equipment) for Farmer peri-urban, Farmer urban and Worker HSDC. While all Worker HSDC reported to be officially contracted, 90 % and 91 % of the Farmer peri-urban and Farmer urban lacked an official employment status, respectively. More than 90 % of all Worker HSDC used different personal equipment (e.g. gloves, boots, uniform) for self-protection against wastewater exposure, while approximately 80 % farmers owned boots and gloves.
Table 3.
Risk factors related to the occupation of workers and farmers enrolled in the cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014
| Risk factors related to occupation/Exposure groupsa | Farmer peri-urban | Farmer urban | Worker HSDC | |||
|---|---|---|---|---|---|---|
| N = 129 | N = 153 | N = 128 | ||||
| n | % | n | % | n | % | |
| Employed | 13 | 10.1 | 13 | 8.5 | 128 | 100 |
| Retired | 11 | 8.5 | 16 | 10.5 | 0 | 0 |
| Duration worked in the current job (mean ± SD) | 30.3 ± 12.9 | 36.9 ± 13.5 | 15.3 ± 9.1 | |||
| Days worked per week (mean ± SD) | 6.5 ± 1.2 | 5.5 ± 2.1 | 6.2 ± 0.6 | |||
| Hours worked per week (mean ± SD) | 39.8 ± 17.3 | 35.9 ± 23.2 | 50.0 ± 4.0 | |||
| Possession of personal protective equipment | ||||||
| Gloves | 106 | 82.2 | 113 | 73.9 | 117 | 91.4 |
| Boots | 107 | 82.9 | 131 | 85.6 | 110 | 85.9 |
| Uniform/cotton overall | 28 | 21.7 | 11 | 7.2 | 120 | 93.8 |
| Rain coat with boots | 29 | 22.5 | 48 | 31.4 | 120 | 93.8 |
| Rain coat without boots | 36 | 27.9 | 58 | 37.9 | 84 | 65.6 |
| Long sleeves | 97 | 75.2 | 137 | 89.5 | 48 | 37.5 |
| Helmet | 3 | 2.3 | 1 | 0.7 | 117 | 91.4 |
| Soft hat (baseball cap) | 24 | 18.6 | 37 | 24.2 | 7 | 5.5 |
| Vietnamese hat | 111 | 86.0 | 141 | 92.2 | 4 | 3.1 |
| Face mask | 110 | 85.3 | 105 | 68.6 | 121 | 94.5 |
| Application of... | ||||||
| Pesticides | 113 | 87.6 | 117 | 76.5 | nab | |
| Fertilizer | 122 | 94.6 | 146 | 95.4 | na | |
aExposure groups: Farmer peri-urban: peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; Farmer urban: urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and Worker HSDC: workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
bna, not applicable for sanitation workers
The prevalence of infection with any intestinal parasite among Farmer peri-urban, Farmer urban, Com urban, Worker HSDC and Com peri-urban was 30 %, 11 %, 10 %, 10 % and 7 %, respectively (Table 4 and Fig. 3). Only 1 % of the participants was found with multiple intestinal parasitic infections. The highest prevalence of soil-transmitted helminths was found in Farmer peri-urban (25 % for hookworm and 5 % for T. trichiura). Ascaris lumbricoides was only detected in Com urban and Worker HSDC; a prevalence of 2 % and 1 %, respectively. Infections with soil-transmitted helminths were of light intensity [37]. The prevalence of intestinal protozoa was low; only nine infections with B. coli, E. coli and G. intestinalis were found, resulting to an overall prevalence of 1.2 %.
Table 4.
Prevalence and intensity of parasite infections among the participants enrolled in the cross-sectional survey in Hanoi, stratified by five exposure groups in the Than Tri district, Hanoi, between April and June 2014
| Prevalence of infection/Exposure groupsa | Com peri-urban | Com urban | Farmer peri-urban | Farmer urban | Worker HSDC | Chi-square test | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 101 | N = 170 | N = 129 | N = 153 | N = 128 | |||||||
| n | %d | n | %c | n | %c | n | %c | n | %c | P-value | |
| Intestinal parasiteb | 7 | 6.9 | 17 | 10.0 | 39 | 30.2 | 17 | 11.1 | 13 | 10.2 | < 0.001 |
| Soil-transmitted helminthc | 6 | 5.9 | 16 | 9.4 | 39 | 30.2 | 15 | 9.8 | 11 | 8.6 | < 0.001 |
| Intestinal protozoa | 1 | 1.0 | 1 | 0.6 | 2 | 1.6 | 2 | 1.3 | 2 | 1.6 | 0.932 |
| Hookworm | 4 | 4.0 | 6 | 3.5 | 32 | 24.8 | 11 | 7.2 | 5 | 3.9 | < 0.001 |
| Light infection (1–1,999 epg) | 4 | 4.0 | 6 | 3.5 | 32 | 24.8 | 11 | 7.2 | 4 | 3.1 | < 0.001 |
| Moderate infection (2,000–3,999 epg) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 | |
| Trichuris trichiura | 2 | 2.0 | 9 | 5.3 | 7 | 5.4 | 4 | 2.6 | 9 | 7.0 | 0.281 |
| Light infection (1–999 EPG) | 2 | 2.0 | 9 | 5.3 | 7 | 5.4 | 4 | 2.6 | 8 | 6.3 | 0.384 |
| Moderate infection (1,000–9,999 epg) | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 1 | 0.8 | |
| Ascaris lumbricoides | 0 | 0.0 | 2 | 1.2 | 0 | 0 | 0 | 0 | 2 | 1.6 | 0.252 |
| Light infection (1–4,999 epg) | 0 | 0.0 | 2 | 1.2 | 0 | 0 | 0 | 0 | 1 | 0.8 | < 0.001 |
| Moderate infection (5,000–49,999 epg) | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0.0 | < 0.001 |
| Giardia intestinalis | 0 | 0.0 | 1 | 0.6 | 0 | 0 | 0 | 0 | 1 | 0.8 | 0.612 |
| Entamoeba coli | 0 | 0.0 | 1 | 0.6 | 1 | 0.8 | 2 | 1.3 | 1 | 0.8 | 0.833 |
| Entamoeba histolytica/E. dispar | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | na |
| Balantidium coli | 1 | 1.0 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0.403 |
aExposure groups: Com peri-urban: people living in the peri-urban commune Duyen Ha, 5 km away from the city along the Red River; Com urban: people living in the urban area of Hanoi, in Bang B village or Tam Hiep commune along the To Lich River and potential exposed to wastewater; Farmer peri-urban: peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; Farmer urban: urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and Worker HSDC: workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
bIntestinal parasitic infection includes: Ascaris lumbricoides, Trichuris trichiura, hookworm and any intestinal protozoa
cSoil-transmitted helminth infection includes: Ascaris lumbricoides, Trichuris trichiura, hookworm
dPrevalence rate is calculated out of the results of the examination of a single stool sample by means of duplicate Kato-Katz and the formalin-ether concentration method, infection intensity by the examination via duplicate Kato-Katz
Abbreviation: epg, eggs per gram; na, not applicable
Fig. 3.
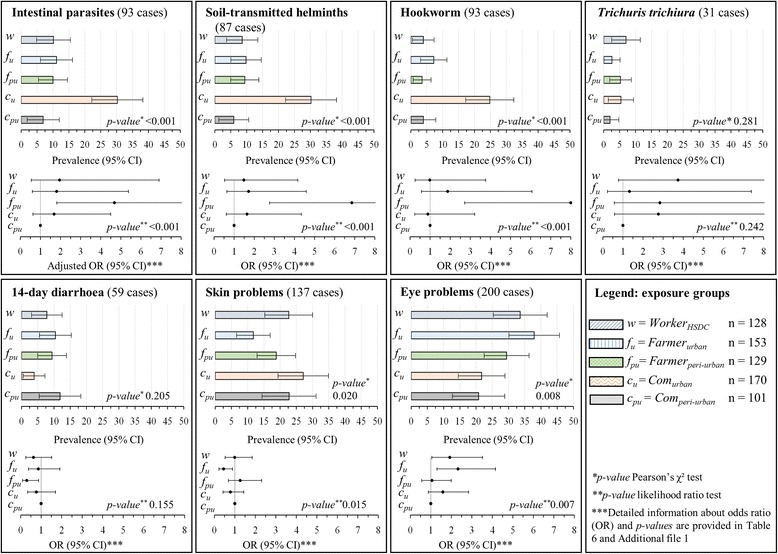
Prevalence rates and adjusted odds ratios (OR) with 95 % confidence intervals (CIs) for infection with any intestinal parasite, soil-transmitted helminth, hookworm, Trichuris trichiura and self-reported diarrhoea, skin problems and eye problems in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. Data for (i)“Com peri-urban” = people living in the peri-urban commune Duyen Ha 5 km away from the city along the Red River; (ii) “Com urban” = people living in the urban area of Hanoi, in Bang B village or Tam Hiep commune along the To Lich River and potential exposed to wastewater; (iii) “Farmer peri-urban” = peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; (iv) “Farmer urban” = urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and (v) “Worker HSDC” = workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
The prevalence of self-reported 14-day diarrhoea was not significantly different between study groups and ranged between 12 % (Com peri-urban) and 4 % (Farmer urban) (Table 5, Fig. 3). However, self-reported rates of skin and eye problems were significantly different between the five exposure groups. General skin problems ranged between 27 % (Farmer peri-urban) and 12 % (Farmer urban). Eye problems were most frequently reported in Farmer urban (38 %), followed by Worker HSDC (34 %) and Com urban (29 %), whereas considerably lower rates of 22 % and 21 % were found in Farmer peri-urban and Com peri-urban.
Table 5.
Self-reported health outcomes experienced in the last two weeks before the interview among the participants enrolled in a cross-sectional survey stratified by five exposure groups in the Than Tri district, Hanoi, between April and June 2014
| Self-reported health issues over the past 2 weeks/ Exposure groupa | Com peri-urban | Com urban | Farmer peri-urban | Farmer urban | Worker HSDC | Chi-square test | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 101 | N = 170 | N = 129 | N = 153 | N = 128 | |||||||
| n | % | n | % | n | % | n | % | n | % | P-value | |
| Diarrhoea | |||||||||||
| 14-day prevalence | 12 | 11.9 | 16 | 9.4 | 5 | 3.9 | 16 | 10.5 | 10 | 7.8 | 0.205 |
| 7-day prevalence | 10 | 9.9 | 11 | 6.5 | 4 | 3.1 | 12 | 7.8 | 7 | 5.5 | 0.279 |
| Number of episodes (14 days) | |||||||||||
| 1 | 9 | 8.9 | 12 | 7.1 | 5 | 3.9 | 12 | 7.8 | 6 | 4.7 | 0.411 |
| 2 | 0 | 0.0 | 3 | 1.8 | 0 | 0.0 | 1 | 0.7 | 3 | 2.3 | |
| 3 | 2 | 2.0 | 0 | 0.0 | 0 | 0.0 | 2 | 1.3 | 1 | 0.8 | |
| 4 | 1 | 1.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Eye problems (one or more symptoms) | 21 | 20.8 | 50 | 29.4 | 28 | 21.7 | 58 | 37.9 | 43 | 33.6 | 0.008 |
| Eye irritation | 8 | 7.9 | 6 | 3.5 | 10 | 7.7 | 23 | 15.0 | 32 | 25.0 | < 0.001 |
| Sensitivity to light | 2 | 2.0 | 1 | 0.6 | 0 | 0.0 | 5 | 3.3 | 3 | 2.3 | 0.172 |
| Other eye problems | 11 | 10.9 | 45 | 26.4 | 18 | 14.0 | 41 | 26.8 | 13 | 10.2 | 0.352 |
| Skin problems (one or more symptoms) | 23 | 22.8 | 32 | 18.8 | 35 | 27.1 | 18 | 11.8 | 29 | 22.7 | 0.024 |
| Skin irritation | 3 | 3.0 | 5 | 2.9 | 6 | 4.7 | 2 | 1.3 | 13 | 10.2 | 0.004 |
| Itching | 21 | 20.8 | 22 | 12.9 | 29 | 22.5 | 10 | 6.5 | 18 | 14.1 | 0.001 |
| Other skin problems | 0 | 0.0 | 10 | 5.9 | 3 | 2.3 | 10 | 6.5 | 5 | 4.7 | 0.402 |
| Other self-reported signs and symptoms | |||||||||||
| Headache | 38 | 37.6 | 69 | 40.6 | 68 | 52.7 | 84 | 54.9 | 50 | 39.1 | 0.006 |
| Fever | 7 | 6.9 | 8 | 4.7 | 9 | 7.0 | 10 | 6.5 | 4 | 3.1 | 0.591 |
| Abdominal pain | 27 | 26.7 | 40 | 23.5 | 39 | 30.2 | 42 | 27.5 | 26 | 20.3 | 0.398 |
| Acute coughing | 25 | 24.8 | 46 | 27.1 | 39 | 30.2 | 44 | 28.8 | 40 | 31.3 | 0.822 |
| Chronic coughing | 5 | 5.0 | 15 | 8.8 | 1 | 0.8 | 14 | 9.2 | 2 | 1.6 | 0.002 |
| Chest pain | 13 | 12.9 | 30 | 17.6 | 23 | 17.8 | 30 | 19.6 | 18 | 14.1 | 0.582 |
| Loss of weight | 14 | 13.9 | 16 | 9.4 | 14 | 10.9 | 17 | 11.1 | 5 | 3.9 | 0.113 |
| Nausea | 12 | 11.9 | 16 | 9.4 | 7 | 5.4 | 15 | 9.8 | 5 | 3.9 | 0.125 |
| Vomiting | 2 | 2.0 | 3 | 1.8 | 2 | 1.6 | 3 | 2.0 | 1 | 0.8 | 0.941 |
| Vomiting of blood | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 0.485 |
| Muscle pain | 19 | 18.8 | 43 | 25.3 | 32 | 24.8 | 52 | 34.0 | 33 | 25.8 | 0.097 |
| Back pain | 48 | 47.5 | 80 | 47.1 | 77 | 59.7 | 102 | 66.7 | 45 | 35.2 | < 0.001 |
| Joint pain | 30 | 29.7 | 74 | 43.5 | 68 | 52.7 | 91 | 59.5 | 29 | 22.7 | < 0.001 |
| Injuries | 3 | 3.0 | 8 | 4.7 | 5 | 3.9 | 8 | 5.2 | 5 | 3.9 | 0.922 |
aExposure groups: Com peri-urban: people living in the peri-urban commune Duyen Ha, 5 km away from the city along the Red River; Com urban: people living in the urban area of Hanoi, in Bang B village or Tam Hiep commune along the To Lich River and potential exposed to wastewater; Farmer peri-urban: peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; Farmer urban: urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and Worker HSDC: workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
Farmer peri-urban had the highest adjusted odds of intestinal parasitic infection compared to the other groups (aOR 5.3, 95 % CI: 2.1–13.7) (Table 6 and Fig. 3). Higher educational attainment and socioeconomic status were negatively associated with parasitic infections, though without statistical significance. Lack of access to toilet at home and not being dewormed for more than 12 months showed an almost significant positive association with intestinal parasitic infection (aOR 3.1, 95 % CI: 0.9–11.0 and aOR 2.5, 95 % CI: 0.9–7.0, respectively). By means of univariate regression analysis, higher odds for intestinal parasite infections were observed by at least a factor of 1.7 for all exposure groups when compared to Farmer peri-urban (Fig. 3 and Additional file 1: Tables S1-S6). For hookworm infections, increased risks were observed among Farmer peri-urban and Farmer urban (OR 8.0, 95 % CI: 2.7–23.5 and 1.9, 95 % CI: 2.7–6.1, respectively). For T. trichiura infection, highest risks were observed in Worker HSDC (OR 3.7, 95 % CI: 0.8–17.7). Risks for eye problems were highest in participants with exposure to wastewater; Farmer urban, Com urban and Worker HSDC (OR of 2.3, 95 % CI: 1.5–1.9, respectively). No trend for a difference in risk between the exposure groups was observed for 14-day diarrhoea prevalence.
Table 6.
Results of univariate and multivariate logistic regression analysis for total parasitic infections (Ascaris lumbricoides, Trichuris trichiura, hookworm and intestinal protozoa) in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014
| Intestinal parasitic infectiona
(total population, N = 681; infections 13.6 %, n = 93) |
Infections | Univariate logistic regressionc | Multivariate logistic regressionc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95 % CI | P-valued | aOR | 95 % CI | P-valued | ||||
| Exposure groupb | Com peri-urban | 101 | 6.9 | 1.00 | < 0.001 | 1.00 | |||||
| Com urban | 170 | 10.0 | 1.49 | 0.60 | 3.73 | 0.392 | 1.61 | 0.61 | 4.22 | 0.333 | |
| Farmer peri-urban | 129 | 30.3 | 5.82 | 2.48 | 13.68 | < 0.001 | 5.30 | 2.05 | 13.69 | 0.001 | |
| Farmer urban | 153 | 11.1 | 1.68 | 0.67 | 4.21 | 0.269 | 1.72 | 0.60 | 4.91 | 0.314 | |
| Worker HSDC | 128 | 10.2 | 1.52 | 0.58 | 3.96 | 0.393 | 2.11 | 0.71 | 6.24 | 0.179 | |
| Sex | Male | 166 | 12.1 | 1.00 | |||||||
| Female | 512 | 14.6 | 0.84 | 0.49 | 1.42 | 0.511 | 0.77 | 0.42 | 1.41 | 0.395 | |
| Age | 1.02 | 1.01 | 1.04 | 0.001 | 1.01 | 1.00 | 1.03 | 0.122 | |||
| Educational attainment | Never went to school | 15 | 20.0 | 1.00 | 0.035 | ||||||
| Primary school | 92 | 16.3 | 0.78 | 0.20 | 3.10 | 0.723 | 0.67 | 0.15 | 3.03 | 0.604 | |
| Secondary school | 306 | 17.0 | 0.82 | 0.22 | 3.00 | 0.763 | 0.68 | 0.16 | 2.96 | 0.605 | |
| Tertiary school | 205 | 8.8 | 0.39 | 0.10 | 1.49 | 0.167 | 0.33 | 0.07 | 1.62 | 0.173 | |
| Higher education | 63 | 7.9 | 0.34 | 0.07 | 1.64 | 0.181 | 0.51 | 0.09 | 3.01 | 0.459 | |
| Socioeconomic status | Most poor | 155 | 18.6 | 1.00 | 0.114 | ||||||
| Poor | 178 | 11.8 | 0.61 | 0.33 | 1.12 | 0.110 | 0.89 | 0.44 | 1.82 | 0.754 | |
| Less poor | 173 | 15.6 | 0.84 | 0.47 | 1.50 | 0.552 | 1.80 | 0.86 | 3.74 | 0.116 | |
| Least poor | 175 | 9.7 | 0.49 | 0.26 | 0.93 | 0.030 | 1.07 | 0.48 | 2.39 | 0.868 | |
| Number of people per household | 0.90 | 0.79 | 1.01 | 0.067 | 0.93 | 0.82 | 1.05 | 0.250 | |||
| Toilet facility at home | Yes | 661 | 12.9 | 1.00 | |||||||
| No | 20 | 40.0 | 5.14 | 2.10 | 12.57 | < 0.001 | 3.12 | 0.88 | 11.03 | 0.078 | |
| Toilet facility at work | Yes | 458 | 12.1 | 1.00 | |||||||
| No | 195 | 17.4 | 1.54 | 0.96 | 2.48 | 0.076 | 0.87 | 0.47 | 1.60 | 0.653 | |
| Wastewater cause health issues | No | 86 | 22.1 | 1.00 | |||||||
| Yes | 595 | 12.4 | 0.50 | 0.28 | 0.88 | 0.016 | 0.74 | 0.39 | 1.40 | 0.352 | |
| Flooding of living area | No | 654 | 13.9 | 1.00 | |||||||
| Yes | 27 | 7.4 | 0.49 | 0.12 | 2.13 | 0.344 | |||||
| Flooding of working area | No | 538 | 13.4 | 1.00 | |||||||
| Yes | 143 | 14.7 | 1.11 | 0.66 | 1.88 | 0.687 | |||||
| Drinking tap water | No | 150 | 14.0 | 1.00 | |||||||
| Yes | 531 | 13.6 | 0.96 | 0.57 | 1.63 | 0.890 | |||||
| Drinking rain water | No | 628 | 13.4 | 1.00 | |||||||
| Yes | 53 | 17.0 | 1.32 | 0.62 | 2.81 | 0.464 | |||||
| Drinking bore hole water | No | 595 | 12.9 | 1.00 | |||||||
| Yes | 86 | 18.6 | 1.54 | 0.85 | 2.78 | 0.155 | 0.91 | 0.41 | 2.01 | 0.808 | |
| Bathing with tap water | No | 90 | 16.7 | 1.00 | |||||||
| Yes | 591 | 13.2 | 1.69 | 0.71 | 4.00 | 0.232 | |||||
| Bathing with rain water | No | 647 | 13.3 | 1.00 | |||||||
| Yes | 34 | 20.6 | 1.31 | 0.80 | 2.13 | 0.278 | |||||
| Bathing with bore hole water | No | 514 | 12.8 | 1.00 | |||||||
| Yes | 167 | 16.2 | 1.27 | 0.15 | 10.97 | 0.830 | |||||
| Preventive chemotherapy received in the past | < 6 months | 69 | 7.2 | 1.00 | 0.038 | ||||||
| 6 to <12 months | 77 | 6.5 | 0.89 | 0.25 | 3.21 | 0.857 | 0.83 | 0.20 | 3.42 | 0.798 | |
| <12 months | 493 | 15.6 | 2.37 | 0.92 | 6.08 | 0.073 | 2.53 | 0.92 | 6.95 | 0.072 | |
| Never took deworming | 42 | 14.3 | 2.13 | 0.61 | 7.48 | 0.237 | 1.87 | 0.48 | 7.25 | 0.363 | |
aIntestinal parasitic infection includes: Ascaris lumbricoides, Trichuris trichiura, hookworm and any intestinal protozoa
bExposure groups: Com peri-urban: people living in the peri-urban commune Duyen Ha, 5 km away from the city along the Red River; Com urban: people living in the urban area of Hanoi, in Bang B village or Tam Hiep commune along the To Lich River and potential exposed to wastewater; Farmer peri-urban: peri-urban farmers living in Duyen Ha commune using the irrigation water from Red River, wells or local drains, which are not contaminated with the city’s wastewater; Farmer urban: urban farmers living in Bang B village or Tam Hiep commune reusing wastewater from To Lich River; and Worker HSDC: workers from Hanoi Sewerage and Drainage Company (HSDC) maintaining drainage channels and operating the Yen So treatment plants
c P-values were obtained from likelihood ratio tests. The core of the multivariate model included exposure group, sex, age, educational attainment, socioeconomic status and number of people per household. In addition, all risk factors with a P-value < 0.2 in the univariate analyses were included into the multivariate regression analysis (as indicated in the table)
d P-values were obtained from likelihood ratio tests overall P-value of the respective categorical variable are indicated in italic letters
Discussion
We report prevalence rates of, and risk factors for, intestinal parasite infections in urban and peri-urban communities that are at different levels of exposure to the wastewater reuse system in Hanoi, Vietnam. The highest prevalence of intestinal parasite infections was observed in peri-urban farmers (30 %), whereas lower prevalences (< 11 %) were found in urban farmers reusing wastewater, workers who maintain the wastewater channels and common urban and peri-urban community members. Hookworm was the predominate soil-transmitted helminth with an overall prevalence of 25 % in peri-urban farmers. Peri-urban farmers were at a significantly higher odds of intestinal parasite infection compared to other groups (aOR 5.3, 95 % CI: 2.1–13.7). The considerable risk for intestinal parasite infection in this group might be explained, at least partially, by a reported lack of access to toilet facility at home and a general lack of awareness towards the health risk in regard to wastewater among peri-urban farmers. Moreover, it was striking that 72 % of all participants reported to not having received deworming within the past 12 months before the study.
The observed differences between rural and peri-urban communities, especially in farmers, are in line with previous reports from studies in Asia and other parts of the world, indicating that urbanization is related to a decline of intestinal parasites [1, 6]. We found that at least one third of the peri-urban inhabitants rely on bore hole water as source for drinking or bathing and that 15 % of the peri-urban inhabitants had no access to toilet facilities at their home. Our findings support the conclusions of Do and colleagues who conducted a cross-sectional survey in Yen So commune in Hanoi in 2002 that revealed similar risk of intestinal parasite infections among urban farmers handling wastewater compared to peri-urban farmers [38]. However, prevalence rates of species-specific soil-transmitted helminths were considerably higher across all participants [A. lumbricoides (21.6 %), T. trichiura (9.8 %) and hookworm (21.8 %)] in [38], as compared to prevalences of 0.4 %, 4.4 % and 8.4 %, respectively, observed in our study. These considerably lower rates might suggest that the various improvements due to education and socioeconomic development in face of urbanization helped to bring down the prevalence of intestinal parasites over the last decade. Another reason is that people in many parts of Southeast Asia are being targeted by preventive chemotherapy against soil-transmitted helminthiasis and other neglected tropical diseases [39, 40]. The low prevalence of A. lumbricoides and T. trichiura infections correlates with concentrations of < 1 egg/l found in the environment and the presumed low infection risk of A. lumbricoides and T. trichiura [29]. However, the absence of hookworm eggs does not correlate with the respective prevalence in the exposure groups, especially in peri-urban farmers [29]. This may be explained by the fact that only hookworm eggs in water were assessed, while larval stages and eggs in soil or sediments were not [41]. Another reason for hookworm transmission could be open defecation, which is mainly practised by peri-urban farmers, due to a lack of access to toilet facilities at home and at work [9]. Overall, the prevalence of intestinal protozoa detected in the current study (< 2 %) was considerably lower than what has been reported from rural communities along Nhue River in Hanam province [11]. However, other intestinal protozoa species that were not detected by our diagnostic approach, such as Cryptosporidium spp. and Cyclospora spp., may be of importance [42]. The higher prevalence of diarrhoea, skin and eye diseases in farmers and workers exposed to wastewater compared to other groups is in line with reports from other studies conducted around Hanoi and along sanitation chains of urban and peri-urban settings [23, 24, 32]. Hence, further risk profiling such as quantitative microbial risk assessment (QMRA) or chemical risk assessments should be pursued for specific causative hazards (i.e. pathogenic bacteria, viruses and toxic chemicals, such as heavy metals, pesticides and fertilizers).
Our study has several limitations. First, the general attendance was lower than anticipated, and hence, we did not achieve the intended sample size. Results must be interpreted with caution. Secondly, most of the participants were females aged 40 years and above. Hence, our sample is not representative of the general population. However, it is representative for Hanoi’s farmers as farming activities in urban and peri-urban communities are indeed mostly carried out by older women [43]. Thirdly, a single stool sample was examined, and hence, the point-prevalence rates of helminth and intestinal protozoa infections were underestimated [44]. In order to increase the sensitivity and to have a more precise understanding of the diversity of pathogenic organisms, multiple stool samples and a suite of highly sensitive diagnostic approaches such as polymerase chain reaction (PCR) or a metagenomics approach should be considered [45, 46]. Fourthly, since this study only reflects one point in time, i.e. the rainy season, we may have missed seasonal outbreaks of typhoid, cholera and other diseases. More generally, there might be seasonal patterns of intestinal parasite infections, not captured by our study design [47–49]. Finally, it has been shown that self-reported disease outcomes (e.g. diarrhoea, skin and eye problems) are prone to reporting bias. Hence, longitudinal monitoring of diarrhoea incidence by well-trained health personnel are warranted to obtain a more accurate understanding [50].
Despite these limitations, our findings raise a number of important issues. First, even though the risk of parasite infection was relatively low, other pathogenic organisms such as viruses or bacteria may be transmitted directly or indirectly via the crops and fish produced with wastewater, which may give rise to diarrhoea, skin and eye diseases as reported by the participants of our study [16]. Secondly, even though we found low prevalence in adults, intestinal parasite infections may be a health issue in school-aged children in these settings, as children may play in agriculture fields or swim in ponds fed with wastewater. This is underlined by a study published in 2004, which detected a high prevalence rate in schoolchildren (77 %), particularly T. trichiura (67 %) and A. lumbricoides (34 %), in the area around Hanoi [51]. Thirdly, integrated strategies to control or eliminate intestinal parasitic infections in such urban and peri-urban transition zones are needed [52, 53]. For example, adapted risk analysis frameworks and transmission assessment surveys of intestinal parasitic infections to break transmission cycles and approach local elimination of intestinal parasitic infections [54].
Conclusions
Taken together, our results suggest that peri-urban farmers are at higher risk of intestinal parasitic infections than their urban counterparts, even though exposure to highly contaminated wastewater is less common. Peri-urban communities, located only 5 km away from the urban area have limited access to improved sanitation and lack awareness towards health risks of exposure to contained water, which is associated with a high prevalence of intestinal parasitic infections. We recommend further quantitative risk assessments of microbial and chemical hazards and transmission assessment surveys of intestinal parasite infections, diarrhoeal, skin and eye diseases. Hence, there is a need for the implementation of control strategies to break transmission cycles, approach local elimination of parasitic infections and reduce risk for diarrhoea in urban and peri-urban transition zones in Hanoi and other cities in Southeast Asia.
Acknowledgements
Our special thanks go to all the study participants for actively participating in the survey. We thank our study team, namely: Nguyen Thanh Hien, Nguyen Duy Tien, Le Thi Huyen Trang and Pierre Schneeberger for all their efforts in data collection. Thanks are given to the staff of the Department of Parasitology at the National Institute of Malaria, Parasitology and Entomology (NIMPE), National Institute of Hygiene and Epidemiology (NIHE), National Institute of Veterinary Research (NIVR) who performed stool examinations. We also thank the staff of the health stations of Tam Hiep and Duyen Ha communes as well as community members and the workers and staffs of the Hanoi Sewerage and Drainage Company (HSDC) for their kind cooperation and participation in the study. We appreciate the institutional involvement of the Center for Public Health and Ecosystem Research (CENPHER), Hanoi School of Public Health. We are grateful to our project partners from the resource recovery and reuse project; namely, the International Water Management Institute (Colombo, Sri Lanka); the World Health Organization (Geneva, Switzerland); the International Centre for Water Management Services (Willisau, Switzerland); and the Department of Water and Sanitation in Developing Countries, Swiss Federal Institute of Aquatic Science and Technology (Dübendorf, Switzerland) for their valuable inputs. This study received financial support from the Swiss Agency for Development and Cooperation.
Funding
Funding was received from the Swiss Agency for Development and Cooperation (SDC).
Authors’ contributions
All authors contributed to the study design. SF and PPD managed the study. SF and MSW drafted the manuscript. All authors contributed to redrafting the paper. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was approved by the institutional research commission of the Swiss Tropical and Public Health Institute (Swiss TPH; Basel, Switzerland; reference no. FK#106). Ethical approval was obtained from the ethics committee of the cantons of Basel-Stadt and Basel-Landschaft (EKBB; reference no. 137/13) and the Hanoi School of Public Health (Hanoi, Vietnam; reference no. 010/2014/YTCC-HD3). This study is registered with the clinical trial registry ISRCTN (identifier: ISRCTN13601686).
All participants were informed about the purpose, procedures, and the potential risk and benefits of the study and they were invited to sign a written informed consent. Those with informed consent were assigned a unique identifier. In case of illiteracy, thumb-print and signature of a witness was requested. Results were communicated to participants and those found infected with soil-transmitted helminths were treated according to national guidelines with a single oral dose of albendazole (400 mg). Participants found infected with intestinal protozoa were referred to a local health centre.
Additional file
Univariate logistic regression models for intestinal parasitic infections and self-reported signs. Table S1. Results of univariate logistic regression analysis for soil-transmitted helminth infections (Ascaris lumbricoides, Trichuris trichiura and hookworm) in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. Table S2. Results of univariate logistic regression analysis for Trichuris trichiura infections in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. Table S3. Results of univariate logistic regression analysis for hookworm infections in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. Table S4. Results of univariate logistic regression analysis for self-reported 14-days diarrhoea in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. Table S5. Results of univariate logistic regression analysis for self-reported skin problems in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. Table S6. Results of univariate logistic regression analysis for self-reported eye problems in a cross-sectional survey in the Than Tri district, Hanoi, between April and June 2014. (DOCX 106 kb)
Contributor Information
Samuel Fuhrimann, Email: samuel.fuhrimann@unibas.ch.
Mirko S. Winkler, Email: mirko.winkler@unibas.ch
Phuc Pham-Duc, Email: phucnihe@gmail.com.
Dung Do-Trung, Email: dotrungdung.nimpe.vn@gmail.com.
Christian Schindler, Email: christian.schindler@unibas.ch.
Jürg Utzinger, Email: Juerg.utzinger@unibas.ch.
Guéladio Cissé, Email: gueladio.cisse@unibas.ch.
References
- 1.Jex AR, Lim YAL, Bethony JM, Hotez PJ, Young ND, Gasser RB. Soil-transmitted helminths of humans in Southeast Asia: towards integrated control. Adv. Parasitol. 2011;74:231–65. doi: 10.1016/B978-0-12-385897-9.00004-5. [DOI] [PubMed] [Google Scholar]
- 2.Pham-Duc P, Nguyen-Viet H, Hattendorf J, Zinsstag J, Phung-Dac C, Zurbrügg C, et al. Ascaris lumbricoides and Trichuris trichiura infections associated with wastewater and human excreta use in agriculture in Vietnam. Parasitol. Int. 2013;62:172–80. doi: 10.1016/j.parint.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Forrer A, Vounatsou P, Sayasone S, Vonghachack Y, Bouakhasith D, Utzinger J, et al. Risk profiling of hookworm infection and intensity in southern Lao People’s Democratic Republic using Bayesian models. PLoS Negl. Trop. Dis. 2015;9:e0003486. doi: 10.1371/journal.pntd.0003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karagiannis-Voules D-A, Odermatt P, Biedermann P, Khieu V, Schär F, Muth S, et al. Geostatistical modelling of soil-transmitted helminth infection in Cambodia: do socioeconomic factors improve predictions? Acta Trop. 2015;141:204–12. doi: 10.1016/j.actatropica.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Utzinger J, Brattig NW, Leonardo L, Zhou X-N, Bergquist R. Progress in research, control and elimination of helminth infections in Asia. Acta Trop. 2015;141:135–45. doi: 10.1016/j.actatropica.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Utzinger J, Keiser J. Urbanization and tropical health: then and now. Ann. Trop. Med. Parasitol. 2006;100:517–33. doi: 10.1179/136485906X97372. [DOI] [PubMed] [Google Scholar]
- 7.Rydin Y, Bleahu A, Davies M, Dávila JD, Friel S, De Grandis G, et al. Shaping cities for health: complexity and the planning of urban environments in the 21st century. Lancet. 2012;379:2079–108. doi: 10.1016/S0140-6736(12)60435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Y-S, Zhou X-N, Utzinger J, Vounatsou P. Bayesian geostatistical modelling of soil-transmitted helminth survey data in the People’s Republic of China. Parasit. Vectors. 2013;6:359. doi: 10.1186/1756-3305-6-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt W-P. The elusive effect of water and sanitation on the global burden of disease. Trop. Med. Int. Health. 2014;19:522–7. doi: 10.1111/tmi.12286. [DOI] [PubMed] [Google Scholar]
- 11.Pham-Duc P, Nguyen-Viet H, Hattendorf J, Zinsstag J, Dac Cam P, Odermatt P. Risk factors for Entamoeba histolytica infection in an agricultural community in Hanam province, Vietnam. Parasit. Vectors. 2011;4:102. doi: 10.1186/1756-3305-4-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GSO . Multiple indicator cluster survey 2011. Hanoi: General Statistical Office Vietnam; 2011. [Google Scholar]
- 13.Do TT, Mølbak K, Phung DC, Dalsgaard A. Helminth infections among people using wastewater and human excreta in peri-urban agriculture and aquaculture in Hanoi, Vietnam. Trop. Med. Int. Health. 2007;12 Suppl 2:82–90. doi: 10.1111/j.1365-3156.2007.01945.x. [DOI] [PubMed] [Google Scholar]
- 14.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 15.Ingvertsen ST, Marcussen H, Holm PE. Pollution and potential mobility of Cd, Ni and Pb in the sediments of a wastewater-receiving river in Hanoi, Vietnam. Environ. Monit. Assess. 2013;185:9531–48. doi: 10.1007/s10661-013-3271-7. [DOI] [PubMed] [Google Scholar]
- 16.WHO . WHO guidelines for the safe use of wastewater, excreta and greywater. Volume I-IV. Geneva: World Health Organization; 2006. [Google Scholar]
- 17.Strande L, Ronteltap M, Brdjanovic D. Faecal sludge management: systems approach for implementation and operation. 1. London: IWA Publishing; 2014. [Google Scholar]
- 18.Bich TH, Quang LN, Ha LTT, Hanh TTD, Guha-Sapir D. Impacts of flood on health: epidemiologic evidence from Hanoi, Vietnam. Glob. Health Action. 2011;4:6356. doi: 10.3402/gha.v4i0.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raschid-sally L, Jayakody P. Drivers and characteristics of wastewater agriculture in developing countries: results from a global assessment. Colombo: International Water Management Institute; 2008. [Google Scholar]
- 20.Drechsel P, Qadir M, Wichelns D. Wastewater: economic asset in an urbanizing world. London: Springer; 2015.
- 21.Do TT, Mølbak K, Cam PD, Dalsgaard A. Incidence of and risk factors for skin ailments among farmers working with wastewater-fed agriculture in Hanoi, Vietnam. Trans. R. Soc. Trop. Med. Hyg. 2007;101:502–10. doi: 10.1016/j.trstmh.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Do TT, Bui TTH, Mølbak K, Phung DC, Dalsgaard A. Epidemiology and aetiology of diarrhoeal diseases in adults engaged in wastewater-fed agriculture and aquaculture in Hanoi, Vietnam. Trop. Med. Int. Health. 2007;12 Suppl 2:23–33. doi: 10.1111/j.1365-3156.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 23.Hien BTT, Do TT, Scheutz F, Phung DC, Mølbak K, Dalsgaard A. Diarrhoeagenic Escherichia coli and other causes of childhood diarrhoea: a case-control study in children living in a wastewater-use area in Hanoi, Vietnam. J. Med. Microbiol. 2007;56:1086–96. doi: 10.1099/jmm.0.47093-0. [DOI] [PubMed] [Google Scholar]
- 24.Anh VT, van der Hoek W, Ersbøll AK, Van TN, Tuan ND, Cam PD, et al. Dermatitis among farmers engaged in peri-urban aquatic food production in Hanoi, Vietnam. Trop. Med. Int. Health. 2007;12 Suppl 2:59–65. doi: 10.1111/j.1365-3156.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TT, Dalsgaard A. Water used to moisten vegetables is a source of Escherichia coli and protozoan parasite contamination at markets in Hanoi, Vietnam. J. Water Health. 2014;12:896–900. doi: 10.2166/wh.2014.145. [DOI] [PubMed] [Google Scholar]
- 26.Marcussen H, Ha LTT, Polprasert C, Holm PE. Contents and mass balances of cadmium and arsenic in a wastewater-fed fish pond of Hoang Mai, Hanoi, Vietnam. Environ. Sci. Health. 2012;47:2246–53. doi: 10.1080/10934529.2012.707546. [DOI] [PubMed] [Google Scholar]
- 27.Holm PE, Marcussen H, Dalsgaard A. Fate and risks of potentially toxic elements in wastewater-fed food production systems-the examples of Cambodia and Vietnam. Irrig. Drain. Syst. 2010;24:127–42. doi: 10.1007/s10795-009-9086-6. [DOI] [Google Scholar]
- 28.Kuroda K, Nakada N, Hanamoto S, Inaba M, Katayama H, Do AT, et al. Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci. Total Environ. 2015;506-507:287–98. doi: 10.1016/j.scitotenv.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Fuhrimann S, Pham-Duc P, Tram NT, Hoang H, Dung DT, Ngoc P, et al. Microbial contamination and health risks due to wastewater use in urban farming in Hanoi, Vietnam. Sci. Total Environ. 2016;566-567:1014–22. doi: 10.1016/j.scitotenv.2016.05.080. [DOI] [PubMed] [Google Scholar]
- 30.WHO . Sanitation safety planning: manual for safe use and disposal of wastewater, greywater and excreta. Geneva: World Health Organization; 2015. [Google Scholar]
- 31.Lan A, Nguyen H, Nguyen V, Yamaji E. Wastewater reuse in Thanh Tri district, Hanoi suburb, Vietnam. Hanoi: Hanoi University of Civil Engineering; 2012. [Google Scholar]
- 32.Fuhrimann S, Winkler MS, Kabatereine NB, Tukahebwa EM, Halage AA, Rutebemberwa E, et al. Risk of intestinal parasitic infections in people with different exposures to wastewater and fecal sludge in Kampala, Uganda: a cross-sectional study. PLoS Negl. Trop. Dis. 2016;10:e0004469. doi: 10.1371/journal.pntd.0004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 34.Utzinger J, Botero-Kleiven S, Castelli F, Chiodini PL, Edwards H, Köhler N, et al. Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin. Microbiol. Infect. 2010;16:267–73. doi: 10.1111/j.1469-0691.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- 35.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–32. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 36.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001620. doi: 10.1371/journal.pmed.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO . Helminth control in school-age children. Geneva: World Health Organization; 2011. [Google Scholar]
- 38.Do TT, van der Hoek W, Cam PD, Vinh KT, Van Hoa N, Dalsgaard A. Low risk for helminth infection in wastewater-fed rice cultivation in Vietnam. J. Water Health. 2006;4:321–31. doi: 10.2166/wh.2006.013. [DOI] [PubMed] [Google Scholar]
- 39.WHO Soil-transmitted helminthiases: number of children treated in 2013. Wkly. Epidemiol. Rec. 2015;90:89–96. [PubMed] [Google Scholar]
- 40.WHO. Global programme to eliminate lymphatic filariasis: progress report, 2014. Wkly. Epidemiol. Rec. 2015;90:489–504. [PubMed]
- 41.WHO . Integrated guide to sanitary parasitology. Geneva: World Health Organization; 2004. [Google Scholar]
- 42.Nguyen TT, Hoang LMN, Cam PD, Chung PT, Fyfe MW, Isaac-Renton JL, et al. Cyclospora spp. in herbs and water samples collected from markets and farms in Hanoi, Vietnam. Trop. Med. Int. Health. 2008;13:1415–20. doi: 10.1111/j.1365-3156.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 43.Pham-Duc P, Nguyen-Viet H, Hattendorf J, Cam PD, Zurbrügg C, Zinsstag J, et al. Diarrhoeal diseases among adult population in an agricultural community Hanam province, Vietnam, with high wastewater and excreta re-use. BMC Public Health. 2014;14:978. doi: 10.1186/1471-2458-14-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl. Trop. Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker SL, Vogt J, Knopp S, Panning M, Warhurst DC, Polman K, et al. Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect. Dis. 2013;13:1–21. doi: 10.1186/1471-2334-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneeberger PHH, Becker SL, Pothier JF, Duffy B, N’Goran EK, Beuret C, et al. Metagenomic diagnostics for the simultaneous detection of multiple pathogens in human stool specimens from Côte d’Ivoire: a proof-of-concept study. Infect. Genet. Evol. 2016;40:389–97. doi: 10.1016/j.meegid.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 47.Bwire G, Malimbo M, Maskery B, Kim YE, Mogasale V, Levin A. The burden of cholera in Uganda. PLoS Negl. Trop. Dis. 2013;7:e2545. doi: 10.1371/journal.pntd.0002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, et al. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob. Health. 2014;2:e570–80. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 49.Neil KP, Sodha SV, Lukwago L, O-Tipo S, Mikoleit M, Simington SD, et al. A large outbreak of typhoid fever associated with a high rate of intestinal perforation in Kasese district, Uganda, 2008-2009. Clin. Infect. Dis. 2012;54:1091–9. doi: 10.1093/cid/cis025. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt WP, Arnold BF, Boisson S, Genser B, Luby SP, Barreto ML, et al. Epidemiological methods in diarrhoea studies: an update. Int. J. Epidemiol. 2011;40:1678–92. doi: 10.1093/ije/dyr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uga S, Hoa NTV, Thuan LK, Noda S, Fujimaki Y. Intestinal parasitic infections in schoolchildren in a suburban area of Hanoi, Vietnam. Southeast Asian J. Trop. Med. Public Health. 2005;36:1407–11. [PubMed] [Google Scholar]
- 52.WHO . Global strategic framework for integrated vector management. Geneva: World Health Organization; 2004. [Google Scholar]
- 53.Nguyen-Viet H, Zinsstag J, Schertenleib R, Zurbrügg C, Obrist B, Montangero A, et al. Improving environmental sanitation, health, and well-being: a conceptual framework for integral interventions. Ecohealth. 2009;6:180–91. doi: 10.1007/s10393-009-0249-6. [DOI] [PubMed] [Google Scholar]
- 54.WHO . Assessing the epidemiology of soil-transmitted helmiths drunig a transmission assessment survey in the global programme for the elimination of lymphatic filariasis. Geneva: World Health Organization; 2015. [Google Scholar]


