Abstract
Histoplasma capsulatum (Hc) exists in the soil and is capable of adapting to the shift in environment during infection to ensure survival. Yeast encounter a restrictive host environment low in nutrients such as zinc. In this study we functionally analyzed a putative zinc regulated transporter, HcZrt2, in zinc limiting conditions by complementation of HcZrt2 and gene knockdown through RNA interference (RNAi). Complementation analysis demonstrated HcZrt2's ability to functionally replace the characterized Saccharomyces cerevisiae zinc plasma membrane transporters Zrt1 and Zrt2 in zinc deficient medium. Gene silencing revealed that HcZrt2 is essential for growth in zinc deficient medium and plays a role in zinc accumulation. Fungal burden was reduced in mice infected with HcZrt2 silenced strains compared to a control strain. Sixty-seven percent of mice infected with a lethal dose of HcZrt2-RNAi#1 survived, and 100% of mice infected with HcZrt2-RNAi#2 withstood lethal infection. Our data suggest that HcZrt2 is a vital part of zinc homeostasis and essential for the pathogenesis of histoplasmosis.
Keywords: Histoplasma capsulatum, zinc deficiency, TPEN, RNAi
Introduction
Zinc, an essential divalent micronutrient, is the second most abundant transition metal in organisms after iron and is involved in many cellular processes.1 It is a strong Lewis acid with flexible coordination geometry making it an ideal structural cofactor of enzymes such as alcohol dehydrogenase, RNA polymerase, and Cu/Zn superoxide dismutase.2 Zinc influences intracellular and intercellular signaling.3 In addition, the integrity of cell membranes is also maintained by the formation of bridges between zinc and lipid molecules.4
Zinc acquisition is accomplished by sensing availability of free zinc ions and employing plasma membrane transporters to traffic zinc to the cytosol. Eukaryotic zinc transporters of the Zrt, Irt-like Protein (ZIP) family were first characterized in Saccharomyces cerevisiae based on sequence similarity to Irt1, an iron transporter in Arabidopsis thaliana.5 Zinc homeostatic mechanisms have been well studied in S. cerevisiae. Zinc levels are sensed by Zap1p, a zinc sensing transcription factor, which regulates zinc transporters. S. cerevisiae Zrt1 is a high affinity zinc transporter that imports zinc from the environment when cytosolic zinc is critically low.6 In contrast, Zrt2 is a low affinity transporter that functions to import zinc when zinc concentrations are replete.7 In addition, Zrt3 mobilizes zinc from the vacuole, a zinc storage site, into the zinc limiting cytosol.8 Yeasts are still viable in Zrt1/Zrt2/Zrt3 mutants suggesting that zinc is also taken in through secondary zinc transporters, Fet4 and Pho84.8
Zinc homeostatic mechanisms exist in pathogenic fungi such as Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, and Cryptococcus gattii which encounter low zinc environments within the host.9–13 The zinc transporters orthologous to ScZrt1 and ScZrt2 are not only important for survival in vitro but also help combat the zinc limited conditions of the host. H. capsulatum also resides in a zinc limited environment within macrophages but is able to survive.14 Zinc transporters have not been characterized in H. capsulatum. Furthermore, it is not known if zinc transporters play a role in virulence. In this study we sought to characterize a zinc transporter in H. capsulatum. We report that HcZrt2 encodes a zinc transporter similar to ZIP family transporters. We suppressed expression of HcZrt2 and demonstrated that zinc accumulation decreases. HcZrt2 is vital for growth in zinc deficient medium and virulence of H. capsulatum.
Materials and methods
Strains and culture conditions
All strains used in this study are described in Table 1. H. capsulatum strains utilized in this study were derived from the ATCC Histoplasma capsulatum strain G217B (ATCC No. 26032). H. capsulatum was grown in liquid or solid Histoplasma Macrophage Medium (HMM), at 37°C and 5% CO2. Solid medium contained 0.8% Agarose (Lonza Seakem ME, Pittsburgh, PA, USA) and supplemented with 25 μM FeSO4. For analysis of growth or gene expression in zinc deficient media, N, N, N′, N′-tetrakis 2-pyridylmethyl ethane-1, 2-diamine (TPEN) was added to HMM liquid culture. S. cerevisiae ZHY3 was received from Dr. David Eide (Madison, Wisconsin) and was routinely grown in yeast extract-peptone-dextrose complex medium at 30°C. For zinc deficient medium, yeasts were grown in yeast nitrogen base with ammonium sulfate, (without amino acids, dextrose, without Cu, Zn, Fe, Mn added; purchased from MP Biomedicals). For complete synthetic medium, amino acid supplement without uracil, dextrose, 10 μM Fe3+, 2 μM Cu2+, and 2.3 μM Manganese were added. To chelate zinc either 10 μM TPEN or 1 mM ethylenediaminetetraacetic acid (EDTA) was added with Zn2+ as indicated. For zinc deficient medium with EDTA, 207.5 μM Magnesium, 1 mM Ca, 20 mM sodium citrate (buffered at pH 4.2) were supplemented. Growth rate was determined by measuring the absorbance of cell suspensions at 590 nm (A590).
Table 1.
Strains and plasmids.
| Genotype | |
|---|---|
| Histoplasma capsulatum strains | |
| G217B | Wild type |
| UC7 | G217B (ura5-, PCBP1-gfp+) |
| HcZrt2-RNAi | G217B (ura5-, PCBP1-gfp+) & pCR186-Zrt2 |
| RNAi-vector control | G217B (ura5-, PCBP1-gfp+) & pCR186 |
| Saccharomyces cerevisiae strains | |
| ZHY3 | MATα ade6 can1 his3 leu2 trp1 ura3 zrt1::LEU2 zrt2::HIS3 |
| ZHY3-ScZrt1 | MATα ade6 can1 his3 leu2 trp1 ura3 zrt1::LEU2 zrt2::HIS3 & pRS416+ ScZrt1 |
| ZHY3-HcZrt2 | MATα ade6 can1 his3 leu2 trp1 ura3 zrt1::LEU2 zrt2::HIS3 & pRS416+ HcZrt2 |
| Plasmids | |
| pCR186 | PaURA5, PH2B, gfp-RNAi |
| pCR186-Zrt2 | PaURA5, PH2B, gfp-Zrt2-RNAi |
| pRS416 | CEN, Pura3-URA3, Plac-LacZa |
| pRS416+Zrt2 | CEN, Pura3-URA3, PScZrt1-HcZrt2 |
| pRS416+Zrt1 | CEN, Pura3-URA3, PScZrt1-ScZrt1 |
Note: PaURA5- Podospora anserine URA5 gene, Pura3- Ura3 gene promoter, gfp- green fluorescent protein, PCB1-calcium binding promoter, PH2B- Histone H2B promoter, PSCZRT1- S. cerevisiae Zrt1 promoter, CEN-centromere.
Bioinformatic analysis of HcZrt2
The putative coding sequence of HcZrt2 was identified by BLAST analysis of the HistoBase database http://histo.ucsf.edu/ using the S. cerevisiae Zrt1. Amino acid sequences of S. cerevisiae Zrt1 orthologs were aligned and phylogenetic tree generated using DNAMAN Version 5.2.9. Observed divergency and 1000 bootstrap trials were applied for phylogenetic analysis. HcZrt2 was identified as a ZIP family protein based on ZIP family characteristics.15 Secondary structure of H. capsulatum Zrt2 was predicted using HMMTOP http://www.enzim.hu/hmmtop/ (Prediction of transmembrane helices and topology of proteins) Version 2.
RNA isolation and quantitative real time PCR
RNA was extracted from yeast-phase strains grown in liquid culture using the MasterPure Yeast RNA extraction Kit (Epicentre, Madison, WI, USA) and protocol. The RNA quality and quantity was assessed using the NanoDrop® ND1000 spectrophometer (Wilmington, DE). The cDNA was generated using 1 μg of RNA, gene specific primers and Superscript First Strand reverse transcriptase for RT-PCR (Invitrogen, Carlsbad, CA, USA). Gene specific primers used were Zrt2: ATGCGCCATCAATTCCACTAAAGC. The cDNA was diluted 1:20, and 5 μl was used for each reaction. A standard curve was generated from cDNA made from an RNA pool of all samples tested. To make the standards the pool cDNA was diluted 1:50, and three additional 1:5 serial dilutions were made. SYBR green qPCR mastermix (Applied Biosystems) was used for detection and quantitative RT-PCR was performed using the Applied Biosystems 7500 Real-time PCR system (Applied Biosystems Grand Island, NY). Primer used were Zrt25′ sense primer: TCCTCGAATTCGGCATCATT and Zrt23′ antisense primer: ATGGAAATTGGAGTAGAGAGGC and Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) sense primer: ATTGGGCGTATTGTCTTCC and Gapdh3′ antisense primer TTGAGCATGTAGGCAGCATA. The data were collected using the comparative Ct method with Gapdh housekeeping gene used as a standard.
H. capsulatum macrophage assay
In sum, 1 × 105 bone marrow-derived macrophages (BMDM) were harvested from male C57BL/6 mice (Charles River, Frederick, Maryland) and cultured using 2 μg/ml of (GM-CSF) Granulocyte-macrophage colony stimulating factor (Miltenyi Biotec, Auburn, Ca) and placed in 96-well plates and kept at 37°C in a CO2 incubator. Macrophages were infected with 5 × 104 mid-log phase H. capsulatum yeast cells in DMEM (Dulbecco's Modified Eagle Medium, Gibco, NY) at multiplicity of infection (MOI) of .5 yeasts per macrophage. After 2 hours non-phagocytosed yeasts were removed by washing the monolayer with medium 3×. To determine the number of yeasts phagocytosed, a portion of the infected cells were lysed in sterile water and placed in 37°C for 5 minutes. To determine viability of yeasts within macrophages, the monolayer was only washed with medium 3× after 2 hours and supplemented with fresh medium an additional 24 and 48 hours. Nonphagocytosed yeasts were then removed by washing the monolayer with medium, then lysed. Lysates were serially diluted and plated onto HMM plates.
Construction of plasmids used for S. cerevisiae transformation
The ZHY3 strain was transformed with the centromeric plasmids pHcZrt2 and pScZrt1 and is described in Table 1. These plasmids expressed either HcZrt2 or ScZrt1 under the transcriptional control of the S. cerevisiae Zrt1 promoter. To construct these plasmids, the S. cerevisiae Zrt1 promoter was the coding regions of H. capsulatum Zrt2 and S. cerevisiae Zrt1 genes were obtained by PCR and cloned into TOPO TA. Primers used for PCR were: S. cerevisiae Zrt15′ sense primer: CACCATGGGCAACGTTACTACG and S. cerevisiae Zrt13′ antisense primer ATAAGCCCACTTACCGATCAAAGCC and H. capsulatum Zrt25′ sense primer: GCCATGGTACCTTCCGCTTCT and H. capsulatum Zrt23′ antisense primer: TAAAGCCCAATTTCCCAACAGGG. Plasmids were sequenced to ensure fidelity of the cloned gene. PCR products were digested and cloned into pRS416 with 2kb nucleotides upstream the S. cerevisiae Zrt1 gene, making the pHcZrt2 and pScZrt1 plasmids. ZHY3 strains transformed with pHcZrt2 and pScZrt1 were Ura+ strains. ZHY3 were also transformed with pRS416 with only the ScZrt1 promoter as the negative control. Transformation was carried according to the lithium acetate procedure.16 Transformed strains were selected on ura- dropout medium supplemented with 1 mM Zn2+.
Inductively coupled plasma-mass spectrometry
Elemental detection was accomplished using an Agilent 8800 (Agilent Technologies, Santa Clara, CA). The instrument was equipped with a microconcentric nebulizer (Glass Expansion, Pocasset, MA), a Scott double channel spray chamber (cooled to 2°C), a shielded torch, two quadrupole mass analyzers separated by an octopole collision-reaction cell with helium gas pressurization (purity of 99.999%) and an electron multiplier for detection. The following isotopes were analyzed 24Mg, 43Ca, 44Ca, 55Mn, 56Fe, 57Fe, 59Co, 63Cu, 65Cu, 66Zn and 68Zn. Internal standards 45Sc and 89Y were used to correct for sample loss and matrix effects.
Preparation of yeasts for ICP-MS
H. capsulatum strains were cultured to log phase in HMM and washed twice with phosphate buffered saline (PBS). Yeasts (1 × 108) were then cultured in zinc deficient medium (HMM, which contains 3 μM Zn, and 2 μM TPEN) for twenty hours and intracellular metal content analyzed by ICP-MS. Yeasts (1 × 108) were washed three times with PBS and subsequently cultured in zinc replete medium HMM for two additional hours and intracellular metal content analyzed by ICP-MS.
Total metal analysis
H. capsulatum was analyzed for total metal concentration by ICP-MS. All samples were digested using TraceMetalTM Grade Nitric Acid (Fisher Scientific Pittsburg, PA, USA) and Hydrogen Peroxide, 30% (Fisher Scientific Pittsburg, PA, USA) in borosilicate digestion vials in a dry bath (Southwest Science, Hamilton, NJ). Doubly deionized water18 MΩ generated from a Milli-Q system (Bedford, MA, USA) was utilized for sample dilution.
Silencing of Zrt2 by RNA interference (RNAi)
The H. capsulatum Zrt2-silenced strains were constructed using a hairpin-loop silencing telomeric plasmid (pCR186) in a G217B-derived strain (UC7) and are described in Table 1. A 400 bp fragment of the Zrt2 coding region was amplified by PCR and cloned into the pCR186 construct in the forward and reverse directions using the respective primers: sense primer Zrt2F5′ with an Asc1 site: TGGCGCGCCTTCGGTTCAGGTGTCATTGTG; and the antisense primer Zrt2R3′ with an XhoI site: TCTCCGAGAAGACGGGAGTGGTTGTTGG; and with the sense primer Zrt2F5′ with an SpeI site: TACTAGTTTCGGTTCAGGTGTCATTGTG; and the antisense primer Zrt2R3′ with an AgeI site: TACCGGTAAGACGGGAGTGGTTGTTGG to give pCR186 Zrt2 (supplemental data). Pme1 digested pCR186 Zrt2 was transformed into UC7 and spread onto HMM plates for uracil selection to generate UC7 Zrt2 silenced.
Mouse infection
Male C57BL/6 mice (5–6 weeks old purchased from Charles River, Frederick Maryland) were housed and maintained in micro-isolator cages in a barrier facility, under pathogen-free conditions, and supplied with sterilized food and water. All animal procedures were approved by the Institutional Animal Care and Use Committee. Mice were infected intranasally with a lethal dose (2 × 107) or a sublethal dose (2 × 106) of mid-exponential phase H. capsulatum yeast cells resuspended in 20 μl PBS. Mice were infected with either G217B, RNAi-vector control, HcZrt2-RNAi#1 or HcZrt2-RNAi#2 strains. For the survival study, mice were weighed daily until 30 days post infection or until they succumbed to infection. To determine fungal burden, lungs and spleens were homogenized in PBS using a gentleMACs Dissociator. Ten-fold serial dilutions of the homogenate were used for recovery of colonies HMM plates with gentamicin. Colonies were counted after 7 days of growth in 37°C.
Statistical analysis
Significance was calculated using two-way analysis of variance or one-way analysis of variance with Bonferroni's multiple comparison or Dunnett's post hoc test for comparison of groups greater than two. All bars show mean ± SEM or mouse survival; log rank (Mantel Cox) test was performed on survival curves.
Results
Identification of HcZrt2 in H. capsulatum
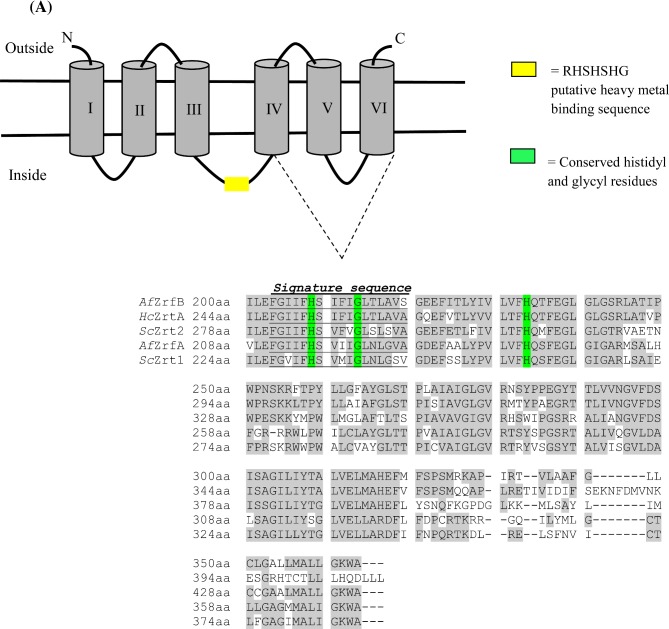
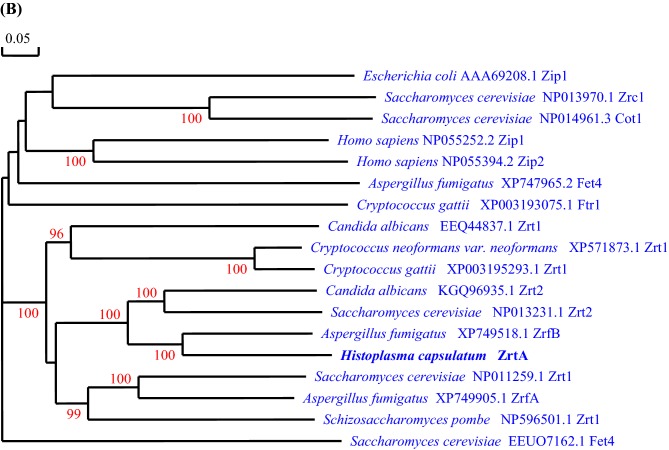
To find potential zinc transporters in H. capsulatum we queried the genome of G217B, for orthologs of S. cerevisiae Zrt1 and Zrt2 using an online genome database http://histo.ucsf.edu/. From the database we identified a putative H. capsulatum zinc transporter gene (HISTO_FX.Contig167.Fgenesh_histo.44.final_new), and it is referred to as HcZrt2. We conducted bioinformatics analyses to identify characteristics of ZIP family transporters.15 Topology analysis revealed that there are 6 predicted transmembrane regions and highly conserved amino acid regions occur in transmembrane regions IV–VII as seen in the multiple alignment (Fig. 1). The fungal zinc transporters contain a signature sequence that is a unique identifier for some ZIP family proteins in transmembrane region IV.15 Between the transmembrane regions III and IV is a putative heavy metal binding sequence, HXHXH, with histidines present in the hydrophilic region of the protein also found in A. thaliana Irt, and S. cerevisiae Zrt1 and Zrt2. We compared HcZrt2 with zinc regulated transporter sequences from fungal, bacterial and mammalian species, fungal iron transporters and zinc exporters, S. cerevisiae Zrc1 and Cot1 using phylogenetic analysis. HcZrt2 shares 34.77% and 37.66% amino acid sequence identity with S. cerevisiae Zrt1 and Zrt2, respectively. We observed that HcZrt2 is most similar to A. fumigatus ZrfB in the phylogenetic tree with 57.68% identity and is 32% identical to ZrfA (Fig. 1).
Figure 1.
A) Topology model of H. capsulatum Zrt2 using HMMTOP http://www.enzim.hu/hmmtop/ (Prediction of transmembrane helices and topology of proteins) Version 2. Multiple alignment of well conserved regions, transmembrane regions (shaded in gray) of the S. cerevisiae (Sc), A. fumigatus (Af), and H. capsulatum (Hc) zinc regulated transporters of the ZIP family using DNAman. Area highlighted in yellow indicate the putative metal ion binding sequence motif found in some ZIP family proteins. Conserved histidyl and glycyl residues are highlighted in green. B) Phylogenetic analysis demonstrating the relationship of HcZrt2 to other ZIP family proteins. (HcZrt2, H. capsulatum predicted zinc transporter 1 & 2- HistoBase microarray spot unique IDs- G217Borfs1.15b8, G217Borfs1.5d12, G217Borfs35o1, respectively). This Figure is reproduced in color in the online version of Medical Mycology.
Figure 1.
– Continued.
Zinc deficiency induces HcZrt2 gene expression
To determine whether HcZrt2 functions similarly to other characterized zinc regulated transporters of the ZIP family we determined whether HcZrt2 is transcriptionally regulated by zinc availability. We analyzed gene expression by quantitative RT-PCR from yeast cells grown in zinc deficient media. We found that in the presence of TPEN, HcZrt2 gene expression is upregulated while the addition of zinc to medium containing TPEN blunts expression of this transporter (Fig. 2A). TPEN is an effective zinc chelator but also binds iron and copper. The addition of iron or copper TPEN-containing medium decreases HcZrt2 expression but does not return expression to baseline (Fig. 2A). Expression of HcZrt2 is not induced by the addition of zinc or iron alone (Supplementary Fig. S1). To investigate whether iron deficiency induces expression of HcZrt2, we analyzed gene expression after exposing yeast cells to an iron chelator, deferoxamine. Restricting iron with deferoxamine did not upregulate HcZrt2 (Fig. 2B). These results demonstrate that HcZrt2 is transcriptionally regulated by zinc deficiency and partially regulated by iron and copper.
Figure 2.
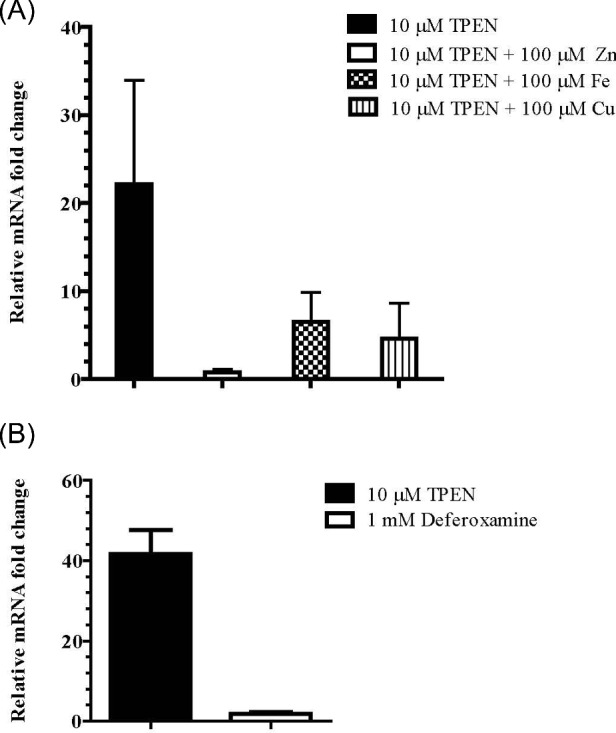
Quantitative real time RT-PCR of HcZrt2 mRNA expression. Yeasts were cultured in A) HMM + 10 μM TPEN alone, with respective metals added or B) HMM + 1 mM of Deferoxamine for 2 hours at 37°C. Transcript levels were normalized to Ct values obtained from control samples (HMM only) and GAPDH gene. Data are shown as the mean with SEM. **P ≤ .01 and ***P ≤ .001. Statistical analyses performed using two-way ANOVA.
HcZrt2 functionally replaces S. cerevisiae's zinc regulated transporters
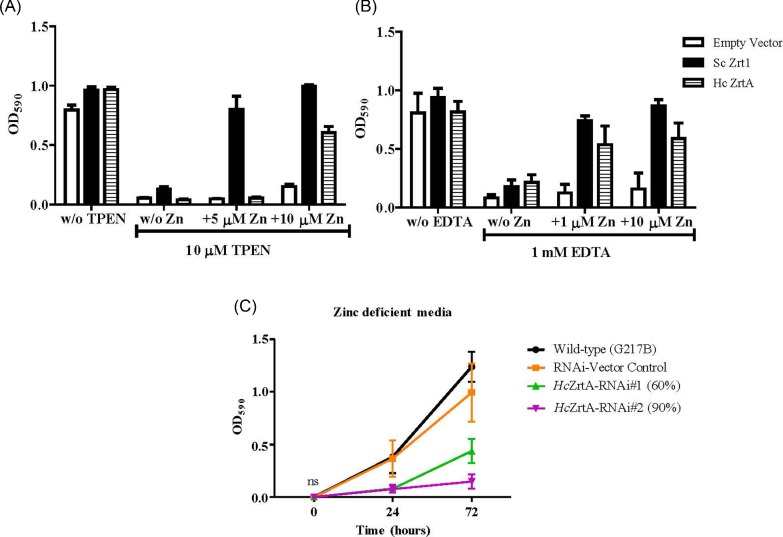
We next asked whether HcZrt2 can rescue growth of S. cerevisiae strain, ZHY3, which lacks plasma membrane zinc regulated transporters, Zrt1 and Zrt2. We separately cloned HcZrt2 and ScZrt1 (positive control) under control of the endogenous S. cerevisiae Zrt1 regulatory sequence in a low copy number vector to assess growth of the strains in zinc deficient medium. Zinc deficient media was created using either 10 μM TPEN (cell permeable) or 1 mM EDTA (non-cell permeable) alone. Transfection of the HcZrt2 gene restored growth of ZHY3 similar to that of ScZrt1 in zinc deficient media (Fig. 3). In the presence of TPEN, HcZrt2 required at least 10 μM Zn in order to detect growth of the HcZrt2 (Fig. 3A). However, with EDTA added to the media, 1 μM Zn restored growth of HcZrt2 and ScZrt1 comparably (Fig. 3B).
Figure 3.
Analysis of HcZrt2 for growth in zinc limiting conditions. Complementation analysis of growth in zinc deficient media by the HcZrt2 gene in S. cerevisiae ZHY3 strain. Transformants with the HcZrt2 and ScZrt1 genes under the control of the S. cerevisiae Zrt1 promoter were grown in medium with either A) 10 μM TPEN or B) 1 mM EDTA. Optical density readings were recorded after 24 hours of growth at 37°C. Growth compared between media without zinc or zinc added in the presence of TPEN or EDTA. Data shown as the mean with SEM ***P ≤ .001. Statistical analyses performed using two-way ANOVA and Bonferroni post hoc test. Empty Vector compared to ScZrt1 and HcZrt2 in 4 independent experiments for each. C) Optical density readings of H. capsulatum yeasts (Wild-type, silenced vector control and RNAi-HcZrt2) grown in Histoplasma Macrophage Media only or treated with 2 μM TPEN (zinc deficient media). Statistical analyses performed using two-way ANOVA and Bonferroni post tests. Silenced strains compared to Empty Vector controls in four independent experiments. This Figure is reproduced in color in the online version of Medical Mycology
H. capsulatum requires HcZrt2 for growth and zinc accumulation
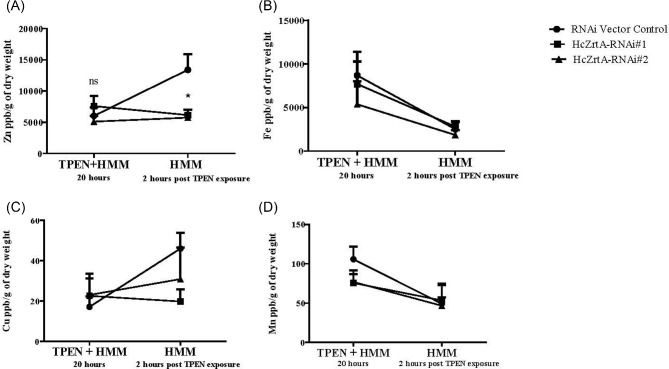
To investigate the role of HcZrt2 for survival in zinc deficient media, we used RNA interference to reduce the expression of HcZrt2. We designed a stable hairpin RNAi plasmid and confirmed the degree of silencing of two individual RNAi strains (HcZrt2-RNAi#1- 60% and HcZrt2-RNAi#2- 90%) through quantitative real time RT-PCR (Supplementary Fig. S2). Growth of RNAi strains was not significantly different from control strains in HMM medium (Supplementary Fig. S3). Wild-type (G217B), RNAi-vector control, HcZrt2-RNAi#1 and HcZrt2-RNAi#2 strains were cultured in HMM or HMM + 2 μM TPEN for 72 hours at 37°C. The decreased expression of HcZrt2 was associated with impaired growth in media containing 2 μM TPEN compared to wild-type and RNAi-vector control strains (Fig. 3C). HcZrt2-RNAi#2 failed to increase in optical density readings whereas HcZrt2-RNAi#1 demonstrated a slight increase at 72 hours (Fig. 3C). The higher degree of gene silencing correlated with a reduced ability to grow in zinc deficient media. To determine whether HcZrt2 is involved in zinc accumulation, we used inductively coupled plasma mass spectrometry (ICP-MS) to measure total intracellular zinc in HcZrt2-RNAi strains at zinc deprived and zinc replete time points. We analyzed total Zn and also Fe, Cu, and Mn in yeasts after 20 hours of zinc deprivation and in yeasts that were cultured for an additional 2 hours in zinc replete medium (Fig. 4). Zinc accumulation in HcZrt2-RNAi#1 was reduced by 55%, while the HcZrt2-RNAi#2 was reduced by 60% compared to wild-type vector control after two hours in zinc replete medium (Fig. 4A). The accumulation of iron, copper, and manganese did not exhibit a difference between HcZrt2 silenced strains and the wild-type vector control (Fig. 4B–D).
Figure 4.
Total concentration of Zn, Fe, Cu, and Mn by ICP-MS in vector control and HcZrt2-RNAi silenced strains after culture in zinc deficient medium (2 μM TPEN) and compared to total concentrations in zinc replete medium. Zinc content represented as parts per billion (ppb) indicating ng of metal per gram and calculated based on mass of yeasts in sample. Statistical analyses performed using two-way ANOVA and Bonferroni post test. P < .05.
Growth of HcZrt2 mutants in vivo and in vitro
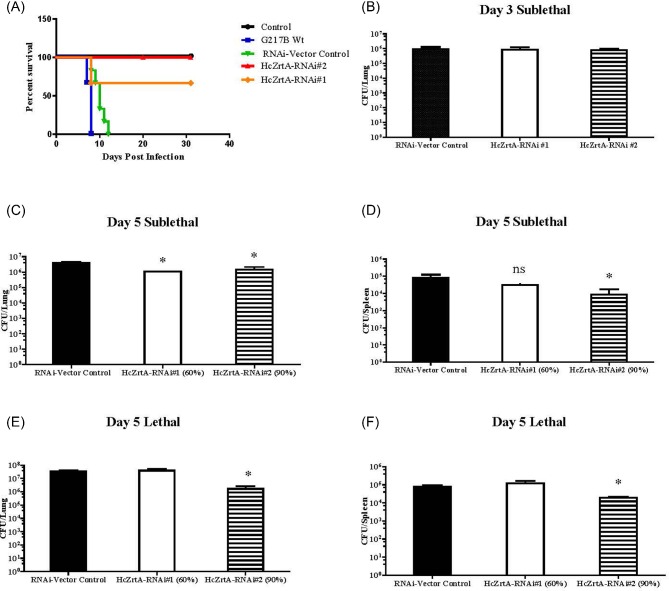
To ascertain whether HcZrt2 was essential for virulence in vivo, mice were intranasally infected with a lethal inoculum of HcZrt2-RNAi and wild-type RNAi-vector control yeasts. Mice infected with the RNAi-vector control strain succumbed to infection and died by day 10 (Fig. 5A). Sixty-seven percent (8 out of 12) of the mice infected with HcZrt2-RNAi#1 and all mice infected with HcZrt2-RNAi#2 survived to day 30 and regained weight. (Fig. 5A and Supplementary Fig. S4).
Figure 5.
A) Kinetics of mouse survival and loss of body weight as a percentage after infection with a lethal dose (1 × 107) of Histoplasma yeasts. Six mice per group in two independent experiments. Total n = 12. Statistical analyses were performed using the log-rank test. B) Fungal burden analysis of mice infected with vector control strains and HcZrt2-RNAi strains at day 3. Limit of detection 5 × 102 (lung) 2 × 102 (spleen). Data represented as mean with SEM. Statistical analyses performed using one-way ANOVA and Dunnett's multiple comparison test P < .05. C–F) Fungal burden analysis of mice infected with vector control strains and HcZrt2-RNAi strains at day 5 for sublethal and lethal inocula. Limit of detection 5 × 102 (lung) 2 × 102 (spleen). Data represented as mean with SEM. Statistical analyses performed using one-way ANOVA and Dunnett's multiple comparison test. P < .05. This Figure is reproduced in color in the online version of Medical Mycology
We investigated whether the degree of silencing affected fungal burden by infecting mice with HcZrt2-RNAi#1, HcZrt2-RNAi#2 and wild-type RNAi vector control strains. In addition, we wanted to determine whether the inoculum size was a factor in the phenotype of the HcZrt2 mutant strains. Lungs and spleens were harvested at days 3 and 5 following sublethal infection and day 5 post lethal inoculum. We observed no difference in fungal burden between control and silenced strains at day 3 (Fig. 5B). In contrast, at day 5 with a sublethal dose, both HcZrt2#1 and HcZrt2#2 RNAi silenced strains demonstrated approximately a 70% and 60% decrease in the lung fungal burden, respectively, compared to the wild-type RNAi vector control strain. In the spleen, fungal burden decreased in the HcZrt2#1 and HcZrt2#2 RNAi silenced strains about 60% and 90% respectively (Fig. 5C–D). However, when a lethal inoculum was administered, only HcZrt2-RNAi#2 demonstrated a ten-fold decrease in lung and spleen burden compared to RNAi vector control and HcZrt2-RNAi#1 strains (Fig. 5E–F).
To analyze the impact of the loss of HcZrt2 on growth within macrophages we infected BMDMs with HcZrt2-RNAi and wild-type RNAi vector control strains with 0.5 yeast to 1 macrophage. The growth of the HcZrt2 silenced strains was similar to the wild-type RNAi vector control at 2, 24, and 48 hours (Fig. 6).
Figure 6.
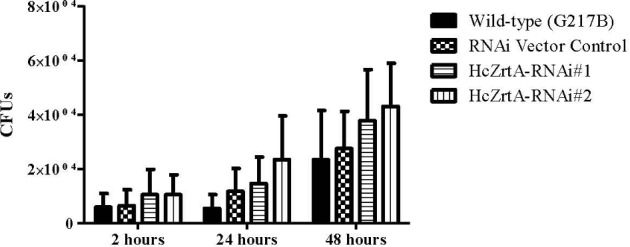
Colony-forming units of yeasts from infected bone marrow derived macrophages. Macrophages were lysed with water at 2, 24, and 48 hours and plated on HMM plates. Data represented as mean with SEM. P < .05.
Discussion
In this study we explored the mechanism utilized by H. capsulatum to adjust to a zinc limited environment. Low zinc triggers the upregulation of HcZrt2 and when this is interrupted through RNAi, zinc accumulation and in vitro growth decreases. Furthermore, the knockdown of HcZrt2 results in fungal growth retardation in murine lungs and spleens. We have also demonstrated that differences in the degree of gene suppression result in functional differences in virulence and pathogenesis.
Chelation of zinc by TPEN, DTPA (diethylene triamine pentaacetic acid), and EDTA is an effective method to observe the transcriptional response to low zinc in S. cerevisiae, C. gattii, C. albicans, and E. coli.6,7,9,17–19 In C. albicans, TPEN was shown to be the most effective inducer of Zap1 expression when compared to extracellular zinc chelators, EDTA and DTPA, because of its higher affinity for zinc and ability to scavenge intracellular and extracellular zinc.19 In addition to zinc, TPEN chelates iron, copper and manganese and this binding can impact results.18 Previously, we demonstrated that TPEN inhibits the growth of H. capsulatum and zinc can fully restore growth while iron alone can partially restore growth.20 Similarly, in TPEN supplemented media the expression of HcZrt2 can be repressed by zinc, iron, and copper. Evidence of iron regulating transcription of ZIP zinc transporters is seen in C. gattii in which the addition of iron with DTPA returned expression to baseline.17 These results suggest a role for fungal ZIP zinc transporters in iron homeostasis. Conversely, we found that the chelation of iron with deferoxamine does not induce expression of HcZrt2. This finding suggests that HcZrt2 is only induced by low ferrous iron (Fe2+) not low ferric iron (Fe3+) because these ions are chelated by TPEN and deferoxamine, respectively. One explanation for these results is that metal transporters interact with ions of the same oxidation state and this may be due to size of ions and binding properties.21 In H. capsulatum, ferric iron (Fe3+) is reduced by reductases to soluble ferrous iron (Fe2+) that is then transported across the plasma membrane.22 While our data demonstrate that HcZrt2 may be regulated by Fe2+, we did not observe a decrease in iron accumulation in HcZrt2 mutants after culture in TPEN supplemented media, indicating that it is not essential for iron acquisition.
The restriction of zinc is a host defense mechanism to hamper the growth of many pathogens in vivo.14,23,24 One way this occurs is through the binding of zinc by calprotectin, a heterodimer with a high affinity for zinc, which is secreted by neutrophils that are stimulated by extracellular fungi such as A. fumigatus or large C. albicans hyphae.24 For phagocytosed C. albicans cells and H. capsulatum yeasts the zinc limiting environment within the macrophage's phagolysosome inhibits growth.14,25 A prior study demonstrated that granulocyte macrophage colony-stimulating factor-activated (GM-CSF) macrophages limit zinc availability to H. capsulatum yeasts by sequestering zinc from the phagolysosome into the Golgi apparatus.14 In addition, this growth inhibiting mechanism relies on an upregulation metalliothioneins 1 and 2 which bind free zinc and localize to the Golgi.14 The net effect of this sequestration was lower quantities of zinc in yeast cells recovered from activated macrophages versus resting macrophages.14
Based on those results, we hypothesized that the HcZrt2-RNAi mutants would not replicate well even in resting macrophages since the absence of this transporter would alter the ability of yeast cells to accumulate zinc. However, the silenced strains did not manifest a growth defect in resting macrophages or in the lungs of mice at day 3 of a sublethal inoculum. This finding implies that a decrement in the ability to import zinc is insufficient to render the yeast cells vulnerable to the innate antifungal activity of resting macrophages. However, elimination of the silenced strains was accelerated coincident with the onset of adaptive immunity.26 These data provide evidence that impairment of expression of a zinc transporter does not cause the yeast cells to be highly susceptible innate immune function, but rather as with wild-type isolates adaptive immunity must be engaged to limit the replicative capacity of yeast cells.
We utilized RNA interference to functionally characterize HcZrt2 due to the inefficiency of homologous recombination in H. capsulatum.27 The knockout of zinc acquisition mechanisms in A. fumigatus and C. gattii established a relationship between zinc transporters and fungal virulence.9,13 In our study RNAi allowed us to silence HcZrt2 by 60 and 90% and compare the degree of suppression on zinc accumulation and virulence. Unexpectedly, we observed a 5% difference in zinc accumulation between the HcZrt2-RNAi strains, suggesting that small changes in zinc levels in the absence of an essential zinc transporter can have a significant effect on pathogenesis and virulence. Moreover, in the HcZrt2-RNAi#1 strain we were able to demonstrate that a 60% reduction in zinc accumulation was the threshold for survival of a strain in vivo.
Functional characterization of HcZrt2 revealed a zinc homeostatic mechanism that is essential for growth in vitro and in vivo. Replacing Zrt1 and Zrt2 in S. cerevisiae with HcZrt2 demonstrated that it can function similarly to zinc plasma membrane transporters when extracellular zinc is depleted. Within H. capsulatum, HcZrt2 mutant strains do not accumulate enough zinc to support growth in zinc deficient conditions. Removing this zinc accumulating mechanism limits H. capsulatum's ability to overcome the host restriction of zinc and sustain growth during infection.
Acknowledgments
This work was supported by NIH grant AI-106269 awarded to G. S. D. and by Merit Review Award 5I01BX000335 from the United States (US) Department of Veterans Affairs Biomedical Laboratory Research and Development Program from the Office of Research and Development awarded to A. G. S.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Supplementary material
Supplementary material is available at Medical Mycology online (http://www.mmy.oxfordjournals.org/).
References
- 1.Broadley MR, White PJ, Hammond JP, et al. Zinc in plants. New Phytol. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine, Panel on Micronutrients . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. 2001. Subcommittee on Upper Reference Levels of Nutrients, Subcommittee on Interpretation and Uses of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board. doi:10.1111/j.1753-4887.1997.tb01621.x. [Google Scholar]
- 3.Fukada T, Yamasaki S, Nishida K, et al. Zinc homeostasis and signaling in health and diseases. JBIC J Biol Inorg Chem. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder H, Arnold K, Ulrich AS, et al. Interaction of Zn2+ with phospholipid membranes. Biophys Chem. 2001;90:57–74. doi: 10.1016/s0301-4622(01)00130-2. [DOI] [PubMed] [Google Scholar]
- 5.Eide D, Broderius M, Fett J, et al. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H, Eide D. The yeast ZRT1 gene encodes the zinc transporter protein of a. Proc Natl Acad Sci U S A. 1996;93:2454–2458. doi: 10.1073/pnas.93.6.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Eide D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J Biol Chem. 1996;271:23203–23210. doi: 10.1074/jbc.271.38.23203. [DOI] [PubMed] [Google Scholar]
- 8.MacDiarmid CW, Gaither LA, Eide D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000;19:2845–2855. doi: 10.1093/emboj/19.12.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Oliveira Schneider R, de Souza Süffert Fogaça N, Kmetzsch L, et al. Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS One. 2012;7:e43773. doi: 10.1371/journal.pone.0043773. doi:10.1371/journal.pone.0043773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Citiulo F, Jacobsen ID, Miramón P, et al. Candida albicans scavenges host zinc via Pra1 during endothelial invasion. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002777. doi:10.1371/journal.ppat.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MJ, Kil M, Jung JH, et al. Roles of zinc-responsive transcription factor Csr1 in filamentous growth of the pathogenic yeast Candida albicans. J Microbiol Biotechnol. 2008;18:242–247. [PubMed] [Google Scholar]
- 12.Vicentefranqueira R, MÁ Moreno, Leal F, et al. The zrfA and zrfB genes of Aspergillus fumigatus encode the zinc transporter proteins of a zinc uptake system induced in an acid, zinc-depleted environment. Eukaryot Cell. 2005;4:837–848. doi: 10.1128/EC.4.5.837-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MÁ Moreno, Ibrahim-Granet O, Vicentefranqueira R, et al. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol. 2007;64:1182–1197. doi: 10.1111/j.1365-2958.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 14.SubramanianVignesh K, LanderoFigueroa JA, Porollo A, et al. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng BH, Guerinot ML, Eide D, et al. Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. J Membr Biol. 1998;166:1–7. doi: 10.1007/s002329900442. [DOI] [PubMed] [Google Scholar]
- 16.Rose, Mark D, Wintson, Fred, Hieter P. Methods in Yeast Genetics. 1990 [Google Scholar]
- 17.Schneider RDO, Diehl C, dos Santos FM, et al. Effects of zinc transporters on Cryptococcus gattii virulence. Sci Rep. 2015;5:10104. doi: 10.1038/srep10104. doi:10.1038/srep10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigdel TK, Easton JA, Crowder MW. Transcriptional response of Escherichia coli to TPEN. J Bacteriol. 2006;188:6709–6713. doi: 10.1128/JB.00680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simm C, Luan CH, Weiss E, et al. High-throughput screen for identifying small molecules that target fungal zinc homeostasis. PLoS One. 2011;6:1–9. doi: 10.1371/journal.pone.0025136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winters MS, Chan Q, Caruso JA, et al. Metallomic analysis of macrophages infected with Histoplasma capsulatum reveals a fundamental role for zinc in host defenses. J Infect Dis. 2010;202:1136–1145. doi: 10.1086/656191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philpott CC. Iron uptake in fungi: A system for every source. Biochim Biophys Acta. 2006;1763:636–645. doi: 10.1016/j.bbamcr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Zarnowski R, Cooper KG, Brunold LS, et al. Histoplasma capsulatum secreted γ-glutamyltransferase reduces iron by generating an efficient ferric reductant. Mol Microbiol. 2009;70:352–368. doi: 10.1111/j.1365-2958.2008.06410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ammendola S, Pasquali P, Pistoia C, et al. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun. 2007;75:5867–5876. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbin BD, Seeley EH, Raab A, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz MC, Bender JA FG. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–1087. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroetz DN, Deepe GS. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine. 2012;58:112–117. doi: 10.1016/j.cyto.2011.07.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rappleye CA, Engle JT, Goldman WE. RNA interference in Histoplasma capsulatum demonstrates a role for alpha-(1,3)-glucan in virulence. Mol Microbiol. 2004;53:153–165. doi: 10.1111/j.1365-2958.2004.04131.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.