Abstract
Purpose
Adiposity has been linked with increased breast cancer risk and mortality. It is established that etiologic associations for adiposity vary by tumor subtype, but the influence of adiposity on subtype-specific survival is unknown.
Methods
Study participants were 1,109 invasive breast cancer participants in the population-based Carolina Breast Cancer Study, diagnosed between 1993 and 2001, and with tissue blocks available for immunohistochemical subtyping. General and central adiposities were assessed by body mass index (BMI) and waist-to-hip ratio (WHR), respectively, based on in-person measurements after diagnosis. Vital status as of 2011 was determined using the National Death Index (median follow-up = 13.5 years). Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95 % confidence intervals (CIs) for breast cancer (BC)-specific and all-cause mortalities.
Results
Among all patients, high WHR (≥0.84), but not BMI, was associated with all-cause mortality (adjusted HR 1.50, 95 % CI 1.11–2.05, <0.77 as reference). No significant association between adiposity and BC-specific mortality was detected, although there was a suggestion of increased mortality risk among high-BMI (≥30 kg/m2) patients with basal-like tumors (adjusted HR 2.44, 95 % CI 0.97–6.12, <25 kg/m2 as reference). Quantitative differences in all-cause mortality were observed by subtype, with BMI associated with basal-like mortality and WHR associated with luminal mortality. The associations were attenuated by tumor characteristics.
Conclusions
Our study confirms the association of adiposity and unfavorable overall survival in breast cancer patients and suggests that this association may vary by intrinsic subtype and adiposity measure.
Keywords: Obesity, Adiposity, Body mass index, Waist hip ratio, Breast cancer subtype, Mortality, Survival
Background
The association between adiposity and breast cancer survival has been well studied. The most recently published meta-analysis shows that in comparison with lean patients, obese patients had 41 and 35 % higher risk of all-cause deaths and breast cancer (BC)-specific deaths, respectively [1]. Many mechanisms have been proposed, such as adverse disease features, deleterious hormonal and metabolic influences, chronic inflammation, undertreatment with chemotherapy, and comorbidities [2–4]. Considering the obesity epidemic in the USA [5, 6], obesity may become a critical facet of cancer management, and thus it is important to understand how obesity affects survival following breast cancer diagnosis.
Despite wide acceptance of an association between adiposity and breast cancer prognosis, inconsistent results have been observed across epidemiologic studies and population subgroups [1]. For example, in a study of 4,538 breast cancer patients aged 35–64 years, obesity (BMI ≥ 30 kg/m2) was associated with both breast cancer-specific mortality and all-cause mortality among White women, but not African-American women [7]. Another study among premenopausal women observed a negative association between obesity and breast cancer-specific mortality [8]. Given that adiposity, race, and age seem to be more strongly associated with etiology of some subtypes, such as basal-like [9–12], we hypothesized that the prognostic association of adiposity may also vary by breast cancer subtype. Thus, subtype heterogeneity, if unaccounted for, could lead to inconsistency across studies.
In recent years, it has been observed that receptor-defined subtypes are heterogeneous [13, 14]. While ER-positive tumors are predominantly of luminal subtype, strata defined by ER-negative status include a mix of tumors including HER2-positive, basal-like, and triple-negative tumors that are unclassifiable [14, 15]. Few studies have examined adiposity-associated survival after stratifying on intrinsic subtype. Among studies that have evaluated adiposity and survival by subtype, most have used only BMI to quantify obesity. Data on central adiposity (such as WHR) are less commonly available [16]. Studies conflict on whether BMI or WHR may be more strongly linked with breast cancer subtypes [17, 18].
Using data from the Carolina Breast Cancer Study (CBCS), a large population-based case–control study, we assessed the impact of BMI and WHR on all-cause and BC-specific mortality. These associations were evaluated among breast cancers as a whole and in strata defined by specific breast cancer subtypes (basal-like and luminal).
Methods
Study population
The CBCS is a population-based case–control study, the details of which have been described previously [17, 19]. Briefly, a total of 1,808 patients aged 20–74 years diagnosed with primary invasive breast cancer during 1993–1996 (Phase I) and 1996–2001 (Phase II) were identified using rapid case ascertainment from NC Central Cancer Registry, with African-American and young cases (aged 20–49 years) oversampled using randomized recruitment [19, 20]. Participants were interviewed in person within 1 year of diagnosis by trained nurses who collected anthropometric measurements and questionnaire responses. Clinicopathological data, including ER, PR, and HER2 status, were abstracted from clinical records and pathological reports. The study procedures for recruitment and enrollment into the CBCS were approved by the Institutional Review Board of the University of North Carolina. All study participants gave written informed consent.
Breast cancer subtype classification
The details of breast cancer subtyping have been published previously [15, 17]. Briefly, clinical records were abstracted for ER, PR, and HER2 status, and when unavailable, whole, formalin-fixed paraffin-embedded tumor tissues were sectioned and stained for a panel of immunohistochemical (IHC) markers in the IHC Core Laboratory at University of North Carolina. All tumors were stained for HER1 and CK 5/6+ at UNC. The following markers were used to determine breast cancer intrinsic subtypes: luminal A (ER+ and/or PR+, HER−), luminal B (ER+ and/or PR+, HER 2+), basal-like (ER−, PR−, HER2−, HER1+and/or CK 5/6+), HER2-enriched (ER−, PR−, HER2+), and unclassified (negative for all five markers). We combined luminal A and luminal B as luminal tumors due to the small number of luminal B tumors (n = 111), and more importantly, recent revisions to the IHC definition of luminal B [21, 22]. Luminal A and B tumors cannot be reliably distinguished without additional markers (such as Ki-67) or RNA expression data [23]. In the CBCS, the demographic and tumor characteristics in patients with luminal A and luminal B tumors were comparable except luminal B tumors more likely to be lymph node positive (p = 0.01). Furthermore, recent findings based on surveillance epidemiology and end result data suggest that segregating luminal A and B cancers may not be informative, specifically because two etiologic subtypes are most consistent with current national incidence and mortality patterns [24].
Exposure and outcome assessment
Waist circumference, hip circumference, height, and body weight were measured by trained nurses at the time of interview. The average time between interview and diagnosis was 145 days, without significant difference by adiposity levels (BMI, p = 0.72; WHR, p = 0.95). Tertiles of the WHR distribution in CBCS controls (with cutoff points of 0.77 and 0.83) were used in WHR categorization [17]. BMI was computed by dividing the weight in kilograms by the square of the height in meters. The World Health Organization (WHO) definition was used to classify patients as underweight (BMI < 18.5 kg/m2), normal (BMI 18.5–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥ 30 kg/m2). To explore the potentially heterogeneous influence of extreme adiposity status suggested by previous studies [25], we performed two sensitivity analyses: (1) excluding underweight patients (n = 23, 2 %) from the analysis and (2) further categorizing obesity into obesity I (BMI 30–34.9 kg/m2) and obesity II (BMI ≥ 35 kg/m2).
Linkage with the National Death Index provided vital status, dates of deaths, and cause of death on the CBCS cases through 31 December 2011. International Classification of Diseases (ICD) breast cancer codes 174.9 (ICD-9) or C50.9 (ICD-10) were used to identify deaths due to breast cancer on the death certificate. Besides BC-specific mortality, all-cause mortality was assessed to facilitate cross-study comparisons and to support indirect inference regarding the effect on non-cancer deaths.
Statistical analysis
Our analysis included 1,109 patients, after excluding nine cases with race other than White or African-American, 659 cases without subtype information, and 31 cases with missing data on anthropometric measures. Demographic, tumor characteristics, and survival of the excluded cases were compared with those of the included cases; no significant differences were detected, except that the excluded cases were more likely to have negative lymph node status, tumor size ≤2 cm, and stage I. The demographic, lifestyle, clinical, and other characteristics of the study population were evaluated by BMI and WHR using Chi-square test or Student’s t test (Supplementary Table 1, 2). The assessment and definition of these variables have been described previously [17]. Patients living as of 31 December 2011 were censored, and those who died of causes other than breast cancer were censored for breast cancer-specific analysis. Kaplan–Meier survival curves and log-rank tests were used to compare overall and breast cancer-specific survival by BMI and WHR.
Cox regression analysis was used to estimate the hazard ratio (HR) and 95 % confidence interval (CI) for overall death and BC-specific death, with lowest adiposity as the reference category. In multivariable analyses, three models were assessed: model 1 adjusted for study design factors (age, race, and study phase), selected socioeconomic factors (education and income), and lifestyle factors (smoking, alcohol intake, physical activity, and parity); model 2 additionally adjusted for WHR (in model of BMI) or BMI (in model of WHR); model 3 adjusted for tumor stage, tumor size, lymph node status, histological type, and the variables in model 1. Model 1 was considered as the primary model, with the potential confounders selected based on prior knowledge and DAGs. Neither tumor characteristics nor BMI/WHR was considered as confounders in the adiposity-prognosis association, because they are not risk factors for adiposity and consequently do not meet the definition of confounders. The objective of model 2 and 3 is to understand the dependence of general and central adiposity and to explore the additional prognostic value of adiposity, respectively. Treatment data were not collected in Phases I and II of the CBCS and therefore not included in the models.
Stratified analyses were performed to evaluate effect modification by intrinsic subtype. Only basal-like and luminal strata are presented because unclassified tumors are of biologically uncertain subtype and because too few patients (n = 73) were HER2-enriched for stable estimation. The difference in HRs by race and menopausal status within luminal and basal-like tumors was also assessed in sensitivity analyses. In the analysis by menopausal status, perimenopausal women (defined as women aged 41–53 years with hormone replacement therapy, or unilateral oophorectomy, or hysterectomy) were excluded to avoid misclassification (n = 95). In addition, because studies have suggested that factors predicting survival in early years after diagnosis may differ from those in later years (e.g., with tumor biological and pathologic characteristics dominant in early years and lifestyle dominant in later years [26]), analyses were conducted conditioned on follow-up length: data were truncated at 5 years to evaluate 5-year mortality, and then survival was assessed conditional upon surviving the first 5 years.
The proportional hazards assumption in each Cox model was assessed using log–log plots of survival and time-dependent cross-product terms of the survival time (years) and the variables of interest, and showed no violation of the assumptions. All statistical tests were two-sided with α = 0.05, all analyses were performed using SAS version 9.2 (SAS Institute), and all figures were generated using R 3.0.0.
Results
Patient and tumor characteristics
Among 1,086 breast cancer patients in this study, the average age at diagnosis was 51 years (SD 12 years, range 23–74 years). Approximately, half of patients were African-American (45 %) and premenopausal (42 %). The mean BMI for the entire study population was 28.5 kg/m2 (SD 6.9 kg/m2, range 14.3–57.9 kg/m2), with 37 % (n = 410) considered obese. The mean WHR was 0.82 (SD 0.08, range 0.60–1.34), with 40 % (n = 445) having WHR ≥0.84. Consistent with previous reports [27], BMI and WHR showed relatively low correlation (Pearson’s r = 0.40, p < 0.01) and low agreement for adiposity classification (kappa coefficient = 0.32, p < 0.01). Fifteen percentage of patient with BMI <25 kg/m2 had WHR ≥0.84; conversely, 12 % of patient with WHR <0.77 had BMI ≥30 kg/m2. Patients with higher BMI or WHR tended to be older, African-American, alcohol abstainers, lower socioeconomic status (SES) (measured by education and family income), OC users, and have more births. In addition, the high-WHR group (≥0.84) had a higher proportion of current smokers (Supplementary Table 1, 2).
Luminal tumors comprised the majority of breast cancers (n = 714, 64 %), followed in prevalence by basal-like tumors (n = 197, 18 %), unclassified (n = 126, 11 %), and HER2-enriched tumors (n = 72, 6 %). Consistent with the previous CBCS data [17], women with higher adiposity level (both BMI and WHR) were more likely to have basal-like tumors, but less likely to have luminal tumors. Compared with BMI, WHR was more strongly related to tumor characteristics, with high-WHR group (≥0.84) having higher prevalence of large (>5 cm, p < 0.01) and high-stage (stage III and IV, p = 0.01) tumors (Supplementary Table 1, 2).
Associations between adiposity and mortality
The median follow-up time was 13.5 years, ranging from 0.2 to 18.7 years. By the end of follow-up, there were 435 deaths, and 62 % of deaths were due to breast cancer (n = 268). Among breast cancer deaths, 155 (58 %) occurred within 5 years of diagnosis, and 76 (28 %) occurred between 5 and 10 years. Kaplan–Meier analysis indicated that patients with higher BMI or WHR had poorer overall survival (p value for log-rank test < 0.01, Fig. 1). The survival difference by BMI group became insignificant and smaller after adjusting for age, race, study phase, SES factors, and lifestyle factors (BMI ≥ 30 kg/m2 vs. <25 kg/m2, adjusted HR 1.19, 95 % CI 0.91–1.55, Table 1). Compared with BMI, WHR showed a stronger association with all-cause mortality, which was independent of potential confounders (WHR ≥0.84 vs. <0.77, adjusted HR 1.50, 95 % CI 1.11–2.05). To evaluate the effect dependency of general and central adiposities, WHR and BMI were mutually adjusted. Only minor reductions in HRs were observed (Table 1). The prognostic association of both general and central adiposity was attenuated after further adjustment for tumor characteristics (BMI: adjusted HR 1.11, 95 % CI 0.84–1.45; WHR: adjusted HR 1.25, 95 % CI 0.91–1.72). Associations of obesity with BC-specific mortality were weak and insignificant in multivariate analyses (Table 2). In sensitivity analysis, the main results did not change significantly when excluding underweight patients; however, patients with extreme obesity (BMI ≥ 35 kg/m2) had the worst survival. The adjusted HRs were 1.14 (95 % CI 0.88–1.48, adjusted for age, race, study phase, SES factors, and lifestyle factors) for overweight, 1.04 (95 % CI 0.78–1.39) for obesity I (BMI 30–34.9 kg/m2), and 1.30 (95 % CI 0.95–1.79) for obesity II (BMI ≥ 35 kg/m2) (Fig. 2).
Fig. 1.

Overall survival by BMI and WHR, overall, among luminal tumors, and among basal-like tumors
Table 1.
HRs for overall mortality associated with BMI and WHR, in the CBCS Phases I and II
| Variable | Deaths/N | Model 1 HR (95 % CI) |
Model 2 HR (95 % CI) |
Model 3 HR (95 % CI) |
|---|---|---|---|---|
| BMI | ||||
| All BC patients | ||||
| <25 kg/m2 | 129/395 | 1 | 1 | 1 |
| 25 to <30 kg/m2 | 125/308 | 1.28 (0.98, 1.68) | 1.17 (0.89, 1.55) | 1.14 (0.86, 1.50) |
| ≥30 kg/m2 | 184/411 | 1.19 (0.91, 1.55) | 1.07 (0.81, 1.41) | 1.11 (0.84, 1.45) |
| Basal-like | ||||
| <25 kg/m2 | 16/53 | 1 | 1 | 1 |
| 25 to <30 kg/m2 | 27/62 | 1.90 (0.93, 3.85) | 2.04 (0.95, 4.37) | 1.65 (0.79, 3.45) |
| ≥30 kg/m2 | 41/83 | 2.25 (1.14, 4.46) | 2.73 (1.28, 5.80) | 2.04 (1.01, 4.13) |
| Luminal | ||||
| <25 kg/m2 | 84/268 | 1 | 1 | 1 |
| 25 to <30 kg/m2 | 80/198 | 1.33 (0.95, 1.85) | 1.14 (0.80, 1.62) | 1.24 (0.88, 1.74) |
| ≥30 kg/m2 | 111/251 | 1.12 (0.80, 1.57) | 0.91 (0.63, 1.31) | 1.01 (0.71, 1.44) |
| WHR | ||||
| All BC patients | ||||
| <0.77 | 79/284 | 1 | 1 | 1 |
| 0.77 to <0.84 | 142/389 | 1.25 (0.92, 1.68) | 1.24 (0.91, 1.68) | 1.08 (0.79, 1.47) |
| ≥0.84 | 221/451 | 1.50 (1.11, 2.05) | 1.44 (1.04, 1.99) | 1.25 (0.91, 1.72) |
| Basal-like | ||||
| <0.77 | 13/36 | 1 | 1 | 1 |
| 0.77 to <0.84 | 37/81 | 1.43 (0.69, 2.93) | 0.95 (0.44, 2.05) | 1.26 (0.61, 2.62) |
| ≥0.84 | 34/83 | 0.88 (0.40, 1.95) | 0.52 (0.22, 1.23) | 0.87 (0.39, 1.93) |
| Luminal | ||||
| <0.77 | 53/201 | 1 | 1 | 1 |
| 0.77 to <0.84 | 77/240 | 1.09 (0.74, 1.60) | 1.10 (0.75, 1.63) | 0.89 (0.60, 1.33) |
| ≥0.84 | 148/282 | 1.75 (1,20, 2.56) | 1.79 (1.20, 2.68) | 1.33 (0.89, 1.97) |
Model 1 was adjusted for age, race, study phase, income, education, physical activity, alcohol intake, smoking, and parity; model 2 was additionally adjusted for WHR (in model of BMI) or BMI (in model of WHR); model 3 was adjusted for tumor stage, tumor size, lymph node status, histological-type, and variables in model 1
Table 2.
HRs for BC-specific mortality associated with BMI and WHR, in the CBCS Phases I and II
| Variable | Deaths/N | Model 1 HR (95 % CI) |
Model 2 HR (95 % CI) |
Model 3 HR (95 % CI) |
|---|---|---|---|---|
| BMI | ||||
| All BC patients | ||||
| <25 kg/m2 | 84/395 | 1 | 1 | 1 |
| 25 to <30 kg/m2 | 79/308 | 1.27 (0.91, 1.78) | 1.21 (0.86, 1.71) | 1.07 (0.76, 1.52) |
| ≥30 kg/m2 | 106/411 | 1.06 (0.75, 1.48) | 1.01 (0.71, 1.44) | 0.97 (0.68, 1.37) |
| Basal-like | ||||
| <25 kg/m2 | 10/53 | 1 | 1 | 1 |
| 25 to <30 kg/m2 | 20/62 | 2.03 (0.85, 4.84) | 2.18 (0.85, 5.54) | 1.40 (0.56, 3.48) |
| ≥30 kg/m2 | 30/83 | 2.21 (0.94, 5.21) | 2.44 (0.97, 6.12) | 1.67 (0.69, 4.05) |
| Luminal | ||||
| <25 kg/m2 | 53/268 | 1 | 1 | 1 |
| 25 to <30 kg/m2 | 42/198 | 1.16 (0.74, 1.81) | 1.07 (0.68, 1.70) | 1.04 (0.66, 1.64) |
| ≥30 kg/m2 | 55/251 | 0.95 (0.60, 1.49) | 0.86 (0.53, 1.39) | 0.84 (0.52, 1.36) |
| WHR | ||||
| All BC patients | ||||
| <0.77 | 61/284 | 1 | 1 | 1 |
| 0.77 to < 0.84 | 94/389 | 1.09 (0.77, 1.54) | 1.08 (0.76, 1.55) | 0.95 (0.66, 1.36) |
| ≥0.84 | 118/451 | 1.14 (0.79, 1.65) | 1.12 (0.76, 1.64) | 0.91 (0.62, 1.34) |
| Basal-like | ||||
| <0.77 | 8/36 | 1 | 1 | 1 |
| 0.77 to <0.84 | 29/81 | 1.49 (0.64, 3.46) | 1.05 (0.43, 2.56) | 1.35 (0.58, 3.15) |
| ≥0.84 | 24/83 | 0.98 (0.38, 2.49) | 0.64 (0.24, 1.74) | 0.94 (0.37, 2.41) |
| Luminal | ||||
| <0.77 | 41/201 | 1 | 1 | 1 |
| 0.77 to <0.84 | 44/240 | 0.92 (0.57, 1.47) | 0.94 (0.58, 1.53) | 0.75 (0.46, 1.22) |
| ≥0.84 | 67/282 | 1.15 (0.71, 1.85) | 1.21 (0.73, 2.08) | 0.82 (0.50, 1.36) |
Model 1 was adjusted for age, race, study phase, income, education, physical activity, alcohol intake, smoking, and parity; model 2 was additionally adjusted for WHR (in model of BMI) or BMI (in model of WHR); model 3 was additionally adjusted for tumor stage, tumor size, lymph node status, histological-type, and variables in model 1
Fig. 2.
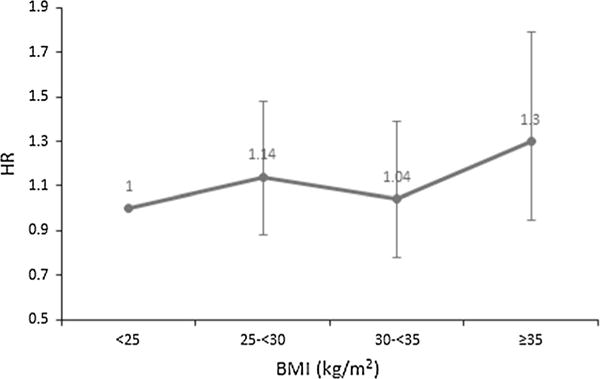
Adjusted HR estimates of all-cause mortality by categories of BMI
In subtype-stratified analyses, BMI and WHR demonstrated different prognostic association by subtype (p value of the interaction term in model 1 for all-cause mortality was 0.24 and 0.04 for BMI and WHR, respectively). As shown in Fig. 1 and Table 1, among patients with basal-like tumors, there was a significant difference in overall survival by BMI. This difference remained after further adjustment for WHR (Table 1, BMI ≥ 30 kg/m2 vs. <25 kg/m2, adjusted HR 2.73, 95 % CI 1.28–5.80) or tumor characteristics (adjusted HR 2.04, 95 % CI 1.01–4.13). In contrast, WHR had a stronger influence on all-cause mortality in patients with luminal tumors (WHR ≥ 0.84 vs. < 0.77, adjusted HR 1.75, 95 % CI 1.20–2.56). Although this association cannot be explained by BMI (adjusted HR 1.79, 95 % CI 1.20–2.68), it was not independent of tumor characteristics (adjusted HR 1.33, 95 % CI 0.89–1.97). For breast cancer-specific mortality, a strong but insignificant association with BMI was observed among patients with basal-like tumors (Table 2, BMI ≥ 30 kg/m2 vs. <25 kg/m2, adjusted HR 2.21, 95 % CI 0.94–5.21), which was partially explained by tumor characteristics (adjusted HR 1.67, 95 % CI 0.69–4.05). The analysis of obesity I and obesity II by subtype did not generate reliable estimates due to small sample size.
We further evaluated subtype-specific HRs according to follow-up period, menopausal status, and race. The influence of obesity on all-cause mortality appeared slightly stronger among patients who survived at least 5 years after diagnosis, particularly among 5-year survivors with basal-like tumors (≤5 years: HR 1.44, 95 % CI 0.72–2.87; >5 years: HR 3.15, 95 % CI 1.13–8.79; BMI ≥ 30 kg/m2 vs. BMI < 25 kg/m2, adjusted for age, race, and study phase). No differences in these associations by follow-up length were observed in BC-specific mortality. The association estimates by menopausal status or race were imprecise due to small sample size, but no substantial or significant effect modification was suggested.
Discussion
Our study was in agreement with previous reports of an association between BMI or WHR and all-cause mortality among breast cancer cases overall [1, 28, 29]. The association with all-cause deaths was independent of age, race, lifestyle, and SES factors, and was attenuated, but not fully explained by tumor characteristics. The influence on all-cause mortality was suggested to vary by intrinsic subtype and adiposity measure. While BMI predicted mortality in patients with basal-like tumors, WHR predicted mortality in patients with luminal tumors.
A few previous studies have assessed the relationship between adiposity and breast cancer mortality by intrinsic or molecular subtype [30–36], and these have suggested a heterogeneous effect of obesity. Five studies examined BMI in triple-negative breast cancer (TNBC) [31–35]. One of these studies, among premenopausal women, reported increased breast cancer-specific mortality associated with BMI (obese vs. normal BMI: HR 1.4, 95 % CI 1.0–2.1) among TNBC, but not among luminal tumors [32]. In another study among predominantly African-American TNBC cases (n = 183), and including both pre- and postmenopausal women, BMI was suggested to be associated overall survival (HR 1.36, 95 % CI 0.77–2.42), but not relapse-free survival (HR 1.01, 95 % CI 0.67–1.52) [31]. The other three studies, with a majority of White women (both pre- and postmenopausal), did not detect any association between BMI and breast cancer prognosis among TNBC [34, 35, 37]. Although the sparse data and differences in population characteristics and covariates limit direct comparison across these studies, our results seem consistent with the findings of previous studies with high proportions of young or African-American patients.
In our study and in previous literature, the effect of BMI on breast cancer-specific mortality has often been weaker than that for all-cause mortality [1], with several null findings [35, 38, 39]. These results indicate that non-cancer causes of death contribute to the less favorable outcomes noted for obese patients. Adiposity is a compound exposure [40], and likely the obesity-associated factors may both increase risk of specific subtypes and affect long-term survival. When we stratified by intrinsic subtype, an influence of BMI on breast cancer-specific mortality among patients with basal-like tumors was suggested, but was not statistically significant. Another study among premenopausal breast cancer patients also reported higher breast cancer-specific mortality among obese triple-negative patients [32]. These data highlight the important role of adiposity in the disease history of basal-like tumors, indicating that the tumor-promoting effect of adiposity may extend from etiology through the clinical expression period, ultimately affecting disease prognosis. This hypothesis is supported by a recent study where breast tumors of extremely obese (BMI ≥ 35 kg/m2) women demonstrated a similar gene expression pattern of basal-like tumors (high expression of proliferation genes, but low expression of ESR1) [41]. This work provides novel insights into molecular pathways by which obesity affects prognosis, while more studies are needed to elucidate the subtype-specific mechanisms.
BMI and WHR are the most commonly used anthropometric measures of general obesity and central obesity, respectively. There is an increasing body of evidence that different adipose tissue depots (e.g., visceral and subcutaneous adipose tissues) differ in both cellular composition and physiology, resulting in distinct roles in disease development and progression [42, 43]. Generally, visceral/abdominal adipose is considered more metabolically active and plays more important roles in pathological processes [42, 44–46]. In the CBCS population, patients with basal-like tumors had higher WHR than patients with luminal tumors [17, 47], but it was BMI that predicted all-cause mortality. Both BMI and WHR are indirect measures of obesity. WHR is determined by both visceral and subcutaneous adiposities; therefore, its effect is actually a mixed effect of the two types of adiposity. To better disentangle the different effect of adiposity types on mortality, future studies with finer measures describing adipose distribution or body shape are needed.
Our study should be interpreted in light of some limitations. First, despite the fact that the CBCS oversampled young and African-American patients and therefore had larger proportions of basal-like tumors, stratified analyses by subtype suffered from small sample size and imprecise estimates. Moreover, the curvilinear association between BMI and all-cause mortality has been well recognized and debated intensively [25, 48]. Our analysis suggested a nonlinear relation, with the worst survival observed in extremely obese women, but our sample size was insufficient to fully explore this association or differences by intrinsic subtype. Second, obesity status in our study was assessed shortly after diagnosis (<1 year). Anthropometry is likely to change following diagnosis and treatment and may vary by subtype. However, based on an analysis of 12,915 breast cancer patients from four prospective cohorts, the mean weight change was 1.6 kg during a follow-up averaging 8.1 years [49]. Thus, weight change may not cause considerable misclassification of categorical adiposity status. Third, treatment data were not collected in Phases I and II of the CBCS, preventing evaluation of the effect of treatment–obesity interactions. Finally, although distinct biological features and prognosis by subtype have been established in gene expression studies, classification of luminal A and B in epidemiologic studies remains problematic. Recent data show that definition of luminal A versus B using HER2 status (as has been done previously in the CBCS study) results in misclassification of both tumor types [32]. To avoid misclassification, we combined luminal A and B in this analysis. However, if there are only two distinct epidemiologic subtypes of breast cancer as recent work suggests [24], then combining luminal A, B, and even ER−, HER2+ tumors may be justified.
In conclusion, we found a negative association between adiposity and overall survival in all and by subtypes, while some effect on breast cancer-specific survival among patients with basal-like tumors were suggested. Basal-like and luminal breast cancer patients, particularly those that have longer-term survival, may have greater risk of mortality due to comorbidities. Future research should seek to validate the prognostic differences by subtype and adiposity measures and should evaluate underlying mechanisms of adiposity-prognosis association. Better understanding of mechanisms could help determine whether weight loss after breast cancer diagnosis can improve breast cancer prognosis.
Supplementary Material
Acknowledgments
This project was supported by the National Cancer Institute (U01-ES019472), the Breast SPORE Project (P50CA058223) Career Development Award to M.A.T., and the University Cancer Research Fund from the University of North Carolina at Chapel Hill.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10552-015-0673-6) contains supplementary material, which is available to authorized users.
Conflict of interest None.
References
- 1.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–165. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 6.Baskin ML, Ard J, Franklin F, Allison DB. Prevalence of obesity in the United States. Obes Rev. 2005;6:5–7. doi: 10.1111/j.1467-789X.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29:3358–3365. doi: 10.1200/JCO.2010.34.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enger SM, Bernstein L. Exercise activity, body size and premenopausal breast cancer survival. Br J Cancer. 2004;90:2138–2141. doi: 10.1038/sj.bjc.6601820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomark Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 11.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130:587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18:123–133. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 15.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 16.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 17.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control. 2009;20:1071–1082. doi: 10.1007/s10552-009-9331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman B, Moorman PG, Millikan R, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 20.Weinberg CR, Wacholder S. The design and analysis of case–control studies with biased sampling. Biometrics. 1990;46:963–975. [PubMed] [Google Scholar]
- 21.Prat A, Cheang MC, Martin M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastien RR, Rodriguez-Lescure A, Ebbert MT, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics. 2012;5:44-8794-5-44. doi: 10.1186/1755-8794-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: Two, three, four, or more? J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 26.Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 28.George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat. 2014;146:647–655. doi: 10.1007/s10549-014-3048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borugian MJ, Sheps SB, Kim-Sing C, et al. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol. 2003;158:963–968. doi: 10.1093/aje/kwg236. [DOI] [PubMed] [Google Scholar]
- 30.Mazzarella L, Disalvatore D, Bagnardi V, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. Eur J Cancer. 2013;49:3588–3597. doi: 10.1016/j.ejca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res. 2013;184:253–259. doi: 10.1016/j.jss.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 32.Turkoz FP, Solak M, Petekkaya I, et al. The prognostic impact of obesity on molecular subtypes of breast cancer in premenopausal women. J BUON. 2013;18:335–341. [PubMed] [Google Scholar]
- 33.Ademuyiwa FO, Groman A, O’Connor T, Ambrosone C, Watroba N, Edge SB. Impact of body mass index on clinical outcomes in triple-negative breast cancer. Cancer. 2011;117:4132–4140. doi: 10.1002/cncr.26019. [DOI] [PubMed] [Google Scholar]
- 34.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Impact of body mass index on survival outcome among women with early stage triple-negative breast cancer. Clin Breast Cancer. 2012;12:364–372. doi: 10.1016/j.clbc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Sparano JA, Wang M, Zhao F, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118:5937–5946. doi: 10.1002/cncr.27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson PJ, Bell RJ, Davis SR. Obesity is associated with a poorer prognosis in women with hormone receptor positive breast cancer. Maturitas. 2014;79:279–286. doi: 10.1016/j.maturitas.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomark Prev. 2006;15:1871–1877. doi: 10.1158/1055-9965.EPI-06-0356. [DOI] [PubMed] [Google Scholar]
- 38.Kawai M, Minami Y, Nishino Y, Fukamachi K, Ohuchi N, Kakugawa Y. Body mass index and survival after breast cancer diagnosis in Japanese women. BMC Cancer. 2012;12:149-2407-12-149. doi: 10.1186/1471-2407-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95:1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernán M, Taubman S. Does obesity shorten life? the importance of well-defined interventions to answer causal questions. Int J Obes. 2008;32:S8–S14. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- 41.Kwan ML, Kroenke CH, Sweeney C, et al. Association of high obesity with PAM50 breast cancer intrinsic subtypes and gene expression. BMC Cancer. 2015;15:278-015-1263-4. doi: 10.1186/s12885-015-1263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 44.Savastano S, Di Somma C, Belfiore A, et al. Growth hormone status in morbidly obese subjects and correlation with body composition. J Endocrinol Invest. 2006;29:536–543. doi: 10.1007/BF03344144. [DOI] [PubMed] [Google Scholar]
- 45.Bruning PF, Bonfrer JM, Hart AA, et al. Body measurements, estrogen availability and the risk of human breast cancer: a case–control study. Int J Cancer. 1992;51:14–19. doi: 10.1002/ijc.2910510104. [DOI] [PubMed] [Google Scholar]
- 46.Lee SW, Jo HH, Kim MR, You YO, Kim JH. Association between metabolic syndrome and serum leptin levels in postmenopausal women. J Obstet Gynaecol. 2012;32:73–77. doi: 10.3109/01443615.2011.618893. [DOI] [PubMed] [Google Scholar]
- 47.Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993–2001. Cancer Causes Control. 2014;25:1101–1117. doi: 10.1007/s10552-014-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes V. The big fat truth. Nature. 2013;497:428–430. doi: 10.1038/497428a. [DOI] [PubMed] [Google Scholar]
- 49.Ades F, Zardavas D, Bozovic-Spasojevic I, et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


