Abstract
Background
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by autoanti-bodies specific for the 180-kd BP antigen-2 (BP180) (also termed “type XVII collagen”) protein. The BP180 enzyme-linked immunosorbent assay (ELISA) is specific for the immunodominant NC16A domain of the protein. However, we and others have observed patients whose reactivity to BP180 is exclusive of the NC16A domain (referred to henceforth as non-NC16A BP).
Objective
We sought to determine the incidence of non-NC16A BP and identify regions of reactivity within the BP180 protein.
Methods
Sera from 51 patients who met the clinical and histologic criteria for BP were screened for NC16A reactivity by ELISA. Sera that were negative by ELISA were screened for IgG reactivity to an epidermal extract, recombinant BP180 protein, and subregions of BP180, by immunoblot. Demographic and clinical data were also collected on all patients.
Results
Four sera (7.8%) were negative using the BP180 ELISA but positive for IgG reactivity to the extracellular domain of BP180. Further mapping identified 4 regions outside of NC16A recognized by these sera: amino acid (AA) 1280 to 1315, AA 1080 to 1107, AA 1331 to 1404, and AA 1365 to 1413. One of these sera also had IgE specific for NC16A. One patient had an atypical presentation with lesions limited to the lower aspect of the legs and scarring of the nail beds.
Limitations
The small total number of patients with non-NC16A BP limits the identification of demographic or clinical correlates.
Conclusion
It is significant that 7.8% of sera from patients with new BP react to regions of BP180 exclusively outside of NC16A and, thus, would not be identified using the currently available BP180 ELISA.
Keywords: autoantibody, blister, bullous pemphigoid, enzyme-linked immunosorbent assay, immunobullous
Bullous pemphigoid (BP) is the most common autoimmune blistering disease and is characterized by autoantibodies directed against the hemidesmosomal proteins, 230-kd BP antigen-1 (BP230) and 180-kd BP antigen-2 (BP180) (also termed “type XVII collagen”).1,2 Experimentally, it is well established that BP180-specific autoantibodies play a primary role in the pathogenesis of BP, as adoptive transfer of these antibodies reproduces many of the features typical of BP.3-5 Moreover, disease activity directly correlates with circulating levels of BP180-specific autoantibodies.6-9 The primary pathogenic significance of the BP230-specific autoantibodies remains unclear.10,11
BP180 is a type II trans-membrane protein composed of a globular cytoplasmic domain, a single transmembrane domain, and a long extracellular region that is composed of 15 interrupted collagenous domains.12 Approximately 90% of BP sera target the extracellular noncollagenous 16A region (NC16A) of the protein.1,13-15 The pathologic importance of the NC16A region was demonstrated in BP180-humanized mice where absorption of NC16A-specific antibodies from BP sera, before subcutaneous injection, resulted in a significant reduction in blistering.4
A diagnosis of BP is based on the presence of serum autoantibodies that have been historically evaluated using indirect immunofluorescence (IF) on intact or salt-split skin and, more recently, by enzyme-linked immunosorbent assay (ELISA). The currently available ELISA for detection of BP180-specific IgG-class autoantibodies targets the NC16A region of the protein with a sensitivity of 84% and a specificity of 99%.12 The clinical usefulness of this ELISA is well established14,16,17; however, we have noted a subset of patients who meet the clinical and histologic criteria for BP18 but do not recognize the NC16A region targeted in this assay. One such patient was previously described in our epitope mapping study.19 In studies examining the epitope spreading phenomenon in patients with BP, Di Zenzo et al20 also identified 3 patients with autoantibodies that reacted exclusively with non-NC16A sites. The purpose of this study was to determine the incidence of non-NC16A BP at an early stage of the disease, to identify regions of reactivity within the BP180 protein, and to ascertain if these patients with non-NC16A BP had any clinical features that differed from patients in whom the immunodominant NC16A region is targeted.
METHODS
Patients
Patients with BP (N = 54) were enrolled in this study at the University of Iowa Hospitals and Clinics between January 1, 2007, and May 1, 2011, after giving written informed consent. This study was approved by the Institutional Review Board at the University of Iowa and the Veterans Affairs Medical Center in Iowa City, IA, and was performed in adherence to the Declaration of Helsinki Guidelines. BP was confirmed by clinical (cutaneous blistering, erythematous/urticarial plaques), histologic (subepidermal blistering on skin biopsy specimen), and IF (linear IgG and/or C3 at the basement membrane zone [BMZ]) criteria18 using standard procedures. Data regarding age, sex, ethnic background, medications, and other medical history were also acquired.
ELISA for BP180 IgG and BP230 IgG, collagen VII IgG, NC16A IgE, and total IgE
In all cases, antibody levels were evaluated in patient serum by ELISA. IgG specific for BP180 and BP230 was evaluated using commercial assays (MBL International, Nagoya, Japan). To rule out epidermolysis bullosa (EBA), autoantibodies against full-length collagen VII and the first noncollagenous (NC1) domain of collagen VII were evaluated.21 The NC16A IgE ELISA was performed as described.22 Total IgE levels were quantitated using electrochemiluminescence performed by the pathology laboratory services at the University of Iowa.
Indirect IF
Circulating autoantibodies specific for proteins of the BMZ were detected by indirect IF using 1 mol/L sodium chloride-split skin as a substrate.23 Sera were diluted 1:10 and were used as a primary antibody, and antihuman IgG-fluorescein isothiocyanate (fitc) was used as a secondary antibody. The localization of antibody to the roof or base of the split skin was detected with epifluorescent microscopy.
BP180 constructs and expression of recombinant proteins
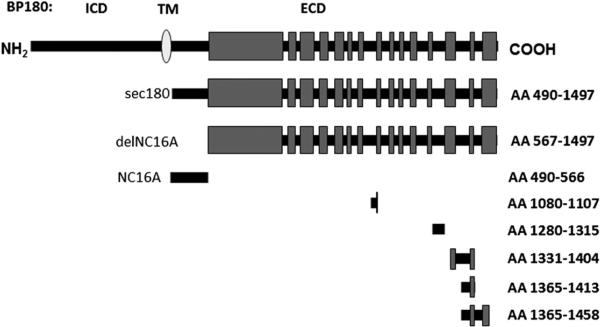
Recombinant proteins comprising the entire ecto-domain of BP180 (sec180) or the BP180 ectodomain with the NC16A region deleted (delNC16A) were generated and expressed as described.19,24 The relative positions of the BP180 recombinant and fusion proteins are indicated in Fig 1 and are referred to by the first and last amino acid (AA) of the selected protein: AA 490 to 1497 (sec180), AA 490 to 566 (NC16A), and outside the NC16A domain, AA 567 to 1497 (delNC16A), AA 1080 to 1107, AA 1280 to 1315, AA 1331 to 1404, AA 1365 to 1413, and AA 1365 to 1458.
Fig 1.
The 180-kd bullous pemphigoid (BP) antigen-2 (BP180) recombinant proteins. Schematic representation of BP180, type II transmembrane (TM) protein containing globular intracellular domain (ICD) and extracellular domain (ECD) with 15 interrupted collagenous regions. Noncollagenous 16A (NC16A) region contains immunodominant autoantibody epitope in BP. Recombinant fusion proteins are indicated by amino acid (AA) residues of protein. AA 490 to 1497 (sec180) represents entire ECD of protein and AA 567 to 1497 (delNC16A) represents ECD of BP180 with NC16A region deleted.
For the epitope mapping studies, glutathione S-transferase (GST) fusion proteins containing segments known to harbor immune reactive sites within the BP180 extracellular domain were used. The complimentary DNA cloning, expression, and purification of AA 490 to 566 (NC16A), AA 1365 to 1413, and AA 1365 to 1458 fusion proteins were described previously.15,19 The constructs of AA 1080 to 1107 and AA 1331 to 1404 were made using previously described methods.15,19,25 The corresponding GST fusion proteins and GST alone were expressed in Escherichia coli, strain DH5a (Invitrogen, Grand Island, NY) or strain Rosetta 2 (Merck KGaA, Darmstadt, Germany) and purified from bacterial lysates using glutathione-agarose affinity chromatography.15 Epitope mapping studies indicate this series of proteins will detect more than 90% of antibody reactivity with the extracellular domain.3,5,20 The intracellular domain of BP180 was not examined because no relationship between disease activity and reactivity to intracellular epitopes has been demonstrated.4
Immunoblot analysis
The autoantibody mapping studies were carried out using recombinant proteins or human epidermal extracts,26 a standard immunoblotting protocol.19 Sera of patients or control subjects were preadsorbed with a bacterial cell lysate containing recombinant GST to eliminate reactivity with this moiety of the fusion proteins. Reactivity was detected using anti-human IgG- horseradish peroxidase and enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). Densitometry was performed using analysis software (Image J, National Institutes of Health, Bethesda, MD).
RESULTS
Identification of patients with non-NC16A BP
In all, 54 patients who met the clinical and diagnostic criteria outlined for BP18 were identified; of these 54, 7 patients did not react with BP180 using the commercially available ELISA (MBL International). Sera from 2 of these patients labeled the roof of salt-split skin using IF, but their sera did not react with sec180 (the recombinant extracellular domain of BP180), nor did either produce a band of appropriate size for either BP180 or BP230 by immunoblotting with an epidermal lysate. One additional patient had no detectable circulating auto-antibodies to intact or salt-split skin and did not react with an epidermal lysate on immunoblot, and thus could not be further characterized. The remaining 4 patients showed immunoreactivity with sec180 and delNC16A by immunoblotting. Thus, of the 51 patients who met immunologic criteria for a diagnosis of BP, 4 (7.8%) appeared to react with BP180 epitopes exclusive of the NC16A domain. None of these 4 patients acquired NC16A reactivity during the course of their therapy, with follow-up ranging from 13 to 23 months (average 18 months). None of the 4 patients had detectable antibodies specific for full-length collagen VII or the first noncollagenous (NC1) domain of collagen VII.
Demographics and clinical characteristics
The demographics of the patients with non-NC16A BP are shown in Table I. The average age of these 4 patients with non-NC16A BP, 67 years, was slightly younger than the mean in our BP database (N = 54, 76 years). Two of the patients were male and 2 were female. One patient was of Latino descent, and the other 3 were Caucasian with no further ethnic group identified. Other autoimmune diseases were common in this cohort, including 1 each of Graves disease, rheumatoid arthritis, and psoriasis. Patient 316 reported that the blistering reaction began after receiving a contrast agent, to which he had previously had an allergic reaction; however, this sequence of events could not be confirmed. Finally, the onset of blistering in patient 345 correlated with initiation of acitretin for psoriasis, and the referring physician suggested that this condition might have been contributory. Acitretin was stopped but the blistering continued, and he was referred to the University of Iowa at that time.
Table I.
Clinical characterization of patients with non-NC16A bullous pemphigoid
| Patient | Age at onset, y | Sex | Other medical conditions | Disease extent before therapy | Pathology and immunopathology | Therapy and response |
|---|---|---|---|---|---|---|
| 233 | 77 | F | Graves disease, DM2 | Multiple vesicles and small, tense bullae scattered over trunk and bilateral upper and lower extremities | Subepidermal blister with eosinophils DIF—linear IgG and C3 IIF—positive | Prednisone and azathioprine initially; 5 mg of prednisone and 25 ng of azathioprine with good control at 20 mo duration |
| 294 | 61 | F | RA, CHF, lower aspect of leg stasis ulcers | Tense bullae confined to lower aspect of legs and feet; onycholysis of all 10 toenails was also present | Subepidermal blister with eosinophils DIF—linear IgG and C3 IIF—positive | Prednisone and minocycline initially; minocycline 200 mg/d and clobetasol at 22 mo duration |
| 316 | 60 | M | DM2, iodine contrast allergy, aspirin allergy | Urticarial rash that transitioned to bullous eruptions on flanks, legs, face, and tongue days after receiving unknown noniodinated contrast agent | Subepidermal blister with eosinophils DIF—linear IgG and C3 IIF—negative | Prednisone, doxycycline, and azathioprine initially; prednisone 2.5 mg/d and azathioprine 50 mg/d at 17 mo duration |
| 345 | 76 | M | Psoriasis, hypertension, numerous NMSCs | Tense bullae of groin, lower aspect of abdomen, and inner thigh that began 3 mo after starting acitretin | Subepidermal blister with eosinophils DIF—linear IgG and C3 IIF—positive | Discontinued acitretin; minocycline 200 mg/d and topical steroids with good control at 13 mo duration |
CHF, Congestive heart failure; DIF, direct immunofluorescence; DM2, diabetes mellitus type 2; F, female; IIF, indirect immunofluorescence; M, male; NMSC, nonmelanoma skin cancer; RA, rheumatoid arthritis.
The clinical characteristics of these 4 patients are shown in Table I. Three of the patients had clinical findings that were consistent with typical BP with classic urticarial plaques and tense bullae. Histologically, biopsy specimens from all 4 patients revealed subepidermal blistering with eosinophilic infiltrate, and direct IF revealed linear deposition of IgG and C3 at the BMZ as is generally observed with BP. Indirect IF onsalt-split skin was negativefor patient 316.
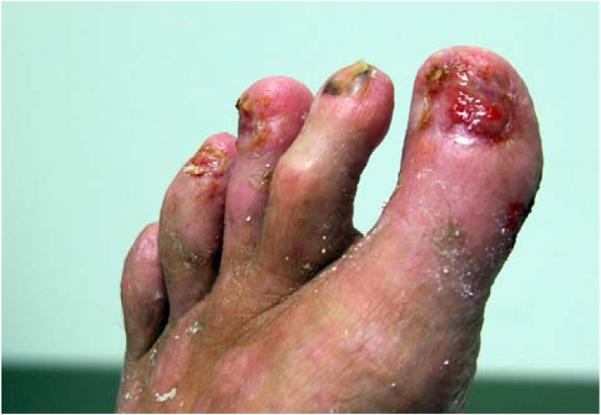
Patients 233, 316, and 345 exhibited a generalized distribution of lesions whereas patient 294 had a somewhat atypical appearance with lesions predominantly on the lower aspect of the legs. In addition, patient 294 shed her toenails during flares of disease activity, which left an eroded nail bed (Fig 2). This shedding did not resemble Beau lines but involved blistering of the nail bed with acute loss of the plate. After therapy, the nails regrew in 9 of 10 toenails, although some were slightly dystrophic.
Fig 2.
Toenail shedding in patient 294. Shedding of nail plate was seen after periungual blistering during flares of disease activity. After several episodes, permanent loss of plate occurred in 1 nail and dystrophy was seen in several others.
Total IgE was evaluated in 3 of our test patients before the initiation of their treatment course. Of these, only 1 had elevated total IgE at 1874 IU.
Mapping of immunoreactive regions in these patients
Serum reactivity to epidermal lysates and recombinant forms of BP180 was evaluated using immunoblot analysis as shown in Table II. Sera from all 4 patients recognized a band corresponding with 180 kd in epidermal lysate. In addition, these sera showed specific reactivity to the entire recombinant extracellular domain of BP180 (sec180) and the recombinant extracellular domain of BP180 from which the NC16A domain was deleted (delNC16A). These results confirmed the diagnosis of BP and indicated that all 4 sera had reactivity outside of NC16A. To further characterize the pattern of auto-antibody reactivity, we generated a series of bacterial fusion proteins spanning the major antigenic sites within the BP180 molecule (Fig 1). These fragments are indicated by AA residue and are clustered largely in the C-terminus of the molecule reported to contain additional antigenic sites within BP180. After preadsorbtion of sera with lysates of bacteria expressing recombinant GST, reactivity to GST was not detectable in any of the test sera (Table II). For comparison, sera from 2 female patients and 1 male patient with BP known to have high reactivity to NC16A by ELISA and sera from 3 age- and sex-matched control subjects were also included in analysis with identical results. None of the control sera reacted to any of the BP180 proteins. As expected, the positive control sera reacted strongly with a 180-kd band in the epidermal lysate, the secreted portion of BP180 (sec180); weakly with delNC16A; and very strongly with the NC16A fusion protein. None of the positive control sera reacted to any of the other regions of the BP180 molecule examined.
Table II.
Immunoblot epitope mapping of patients who are negative for 180-kd bullous pemphigoid antigen-2 IgG by enzyme-linked immunosorbent assay
| IgE ELISA* |
IgG immunoblot analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Total, IU | NC16A (index value) |
Epidermal lysate |
490-1479 (sec180) |
567-1497 (delNC16A) |
-GST | 490-566 (NC16A) | 1080-1107 | 1280-1315 | 1331-1404 | 1365-1413 | 1365-1458 |
| 233 | na | 8 | + + | ++ | ++ | − nd |
+ 2764† |
− nd |
+ 3478 |
+ 4046 |
++ 7605 |
− nd |
| 294 | 6 | 14 | + | + | + | − nd |
− nd |
− nd |
++ 6468 |
− nd |
− nd |
− nd |
| 316 | 1874 | 0 | + | + | + | − nd |
− nd |
++ 6806 |
− nd |
++ 6655 |
− nd |
− nd |
| 345 | 89 | 125 | + | + | + | − nd |
− nd |
− nd |
++ 5616 |
+ 2466 |
+ 1995 |
− nd |
| NHS‡ N = 3 |
20 ± 6 | 9 ± 5 | − | − | − | − nd |
− nd |
− nd |
− nd |
− nd |
− nd |
− nd |
| BP§ N = 3 |
2289 ± 987 | 79 ± 21 | ++ | +++ | + | − nd |
+++ 12,531 |
− nd |
− nd |
− nd |
− nd |
− nd |
BP, Bullous pemphigoid; ELISA, enzyme-linked immunosorbent assay; GST, glutathione-S-transferase; na, not assayed; NHS, normal human serum; nd, not detectable.
Serum samples were assayed in duplicate by ELISA.
Densitometric analysis of immunoblots was performed where indicated with NIH ImageJ software. The arbitrary densitometric value of the GST control protein was subtracted from the arbitrary densitometric value obtained with each of the test proteins on the same membrane. The resultant value is indicated and scored as − (no net reactivity), + (0-5000), ++ (5001-10,000), +++ (10,001-15,000).
From 2 female and 1 male patients, age-matched ±5 y.
BP sera from 3 individual patients (2 female and 1 male) known to have IgG reactivity to NC16A.
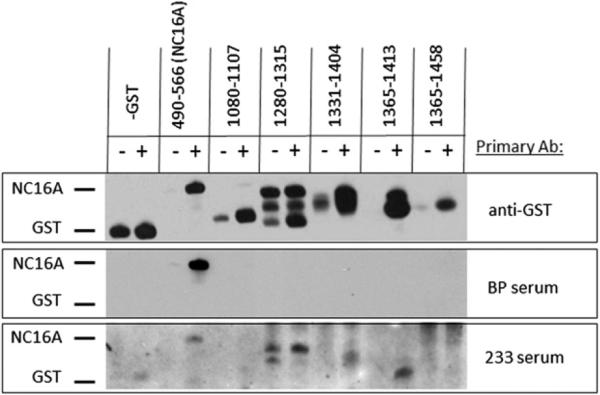
Interestingly, 1 serum (patient 233) that was repeatedly negative in the BP180 ELISA showed weak reactivity with denatured NC16A by immunoblotting (Fig 3 and Table II). However, this serum had much stronger reactivity to 3 regions of BP180 outside of NC16A: AA 1280 to 1315, AA 1331 to 1404, and AA 1365 to 1413, as determined by densitometric analysis. Serum from patient 294, with the unusual nail shedding, showed strong reactivity to only 1 of the fusion proteins examined representing AA 1280 to 1315. Because of the unusual appearance of the nails, and our previous report of a patient who showed intermolecular epitope spreading from BP180 (collagen XVII) to collagen VII,27 this sample was also tested for reactivity with collagen VII by ELISA and was negative. Serum obtained from patient 316 had strong reactivity to 2 of the fusion proteins tested: AA 1080 to 1107 and AA 1331 to 1404. Serum from patient 345 was repeatedly negative for IgG reactivity to NC16A by both ELISA and immunoblot but did have IgE reactivity to NC16A (125 U/mL, normal <17 U/mL). Although we have studied the role of IgE-class autoantibodies in BP for the past 10 years, this is the first patient we have identified with IgE against NC16A without corresponding IgG. In addition, the patient's total IgE level was within the normal range. Serum from this patient also recognized other regions of BP180, including strong reactivity AA 1280 to 1315 and, more weakly, AA 1331 to 1404 and AA 1365 to 1413. In sum, all but 1 of the patients (patient 294) had serum reactivity to more than 1 of the fusion proteins tested, and none of the sera examined bound the distal AA 1365 to 1458 protein.
Fig 3.
IgG reactivity of serum from patient 233 with antigenic regions of 180-kd bullous pemphigoid (BP) antigen-2 (BP180). Sera were screened for IgG reactivity to either affinity-purified glutathione-S-transferase (GST) fusion proteins spanning BP180 molecule or GST control protein. Bacterial protein production was induced (1) using isopropylb -D-1 thiogalactopyranoside (IPTG) (250 μmol/L) or no IPTG was added (−). Proteins were electrophoresed and transferred to nitrocellulose membrane and identical membranes were probed with the following primary antibodies (Ab): anti-GST antibodies (top), BP serum known to have high reactivity to NC16A by enzyme-linked immunosorbent assay (ELISA) (middle), or serum from patient 233 with BP negative by ELISA (bottom). Appropriate locations for GST and NC16A are indicated based on molecular weight markers.
Therapeutic regimen and clinical response
Three of the 4 patients started prednisone at 0.5 mg/kg/d, with their dose tapered as tolerated. Patients 233 and 316 had azathioprine (75 mg) added to their regimens initially to try to minimize the prednisone dose because of pre-existing insulin-dependent diabetes. The fourth patient was treated with minocycline and topical clobetasol. A summary of their clinical status as defined by the recently reported consensus definitions is found in Table I.28
DISCUSSION
IgG reactivity to the immunodominant NC16A region of BP180 is associated with disease severity in BP.6,7,20,29 It is also well known that, in addition to NC16A, the majority of BP sera recognize other regions of the BP180 protein.25,30,31 It has been proposed that reactivity to these other non-NC16A epitopes is a secondary event resulting from epitope spreading, a process in which inflammation triggered by autoimmunity damages the target tissue, which induces antibodies on the same or different antigens.25,32 By definition, the initial reactivity with the NC16A region of BP180 would accompany, or even precede, reactivity to other sites in the molecule or other antigens, such as BP230.25 Our study indicates that, at the time of diagnosis, there is a cohort of BP with autoantibody reactivity exclusively outside of NC16A, indicating that antibodies to these regions can also be pathogenic.
In the current study, 4 of 51 (7.8%) of our BP serum samples recognized regions of BP180 exclusive of the immunodominant NC16A region, which is similar to our previous report (1 of 10, 10%) and that of Di Zenzo et al20 (3 of 35, 8.6%). Together, these studies identify a population, ranging from 8% to 10%, of patients with BP whose sera would yield a negative result using the current commercially available ELISA test. Thus, use of the BP180 ELISA alone for the confirmation of the diagnosis of BP will miss a significant number of patients with BP. Use of the BP230 ELISA would only have detected 1 of the 4 patients with non-NC16A BP described in this study. Recently several articles have pointed out the enhanced sensitivity of the ELISA,33 and recommendations have been made to use ELISA rather than indirect IF for confirmation of the diagnosis of BP.34 However, clinicians need to recognize that this ELISA will fail to identify patients whose autoantibodies target regions outside of NC16A or who generate autoantibodies of classes other than IgG.
We recognize that both treatment and intramolecular epitope spreading could result in loss of reactivity to the immunodominant NC16A region of BP180. However, all patients had active blistering when first examined and 3 of 4 were observed before receiving any treatment. Moreover, reactivity to the immunodominant epitope was not observed at any point during the 13 to 23 months of the study duration. This is consistent with the recent report that indicated that epitope spreading and acquisition of new epitopes is most common within the first 6 months of the disease.20
It has been proposed that the pattern of epitope recognition influences the course of disease.20 In the current study, only patient 294 had atypical disease presentation, with lesions predominantly on the lower aspect of the legs, and shedding of the toenails that was associated with flares of her disease activity. To our knowledge, there are only 4 previous reports of well-documented nail involvement in BP35-38; however, epitope mapping was not performed on sera from any of these patients. The condition of patient 294 was also curious in that her serum reactivity was restricted to only 1 region of the BP180 protein, AA 1280 to 1315, the fourth noncollagenous region of the protein. Because patients 233 and 345 also had reactivity to AA1280 to 1315 without nail involvement, it is unlikely that reactivity to this epitope led to the unusual nail changes observed in patient 294. BP180 is clearly expressed in BMZ of the nail bed, proximal nailfold, nail matrix, and the hyponychium,39 and patients with junctional epidermolysis bullosa harboring mutations in BP180 have nail loss.40 Thus, it is possible that antibody reactivity to an additional region outside of those we tested is responsible for the nail changes observed in patient 294. Study of a larger number of these rare patients will be necessary to determine if there are distinct correlations between epitope recognition and clinical features of the disease.
In BP, both clinical and experimental studies support a critical role for NC16A-reactive IgG in disease pathogenesis, whereas the development of additional sites of reactivity within BP180 and BP230 are thought to be secondary. Our study demonstrates a small percentage of patients with untreated BP whose autoantibody reactivity is restricted to sites exclusively outside the NC16A region of BP180. These studies suggest that recognition of other non-NC16A regions of BP180 is pathologically relevant for disease initiation. Examination of additional patients with non-NC16A BP will better define the clinical characteristics of this subgroup.
CAPSULE SUMMARY.
Most patients with bullous pemphigoid (BP) have autoantibodies targeting the NC16A region of 180-kd BP antigen-2; however, occasional patients are observed whose sera do not react to this region.
Herein, 7% to 10% of BP sera react to regions of 180-kd BP antigen-2 exclusively outside the NC16A domain.
This study indicates that: (1) use of the enzyme-linked immunosorbent assay for confirmation of BP, rather than indirect immunofluorescence, will not identify this patient subset; and (2) antibodies targeting areas other than NC16A can be pathogenic.
Acknowledgments
Based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development 1BX001680-01 and the National Insitutes of Health CTSA: 2 UL1 TR000442-06. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
Abbreviations used
- AA
amino acid
- BMZ
basement membrane zone
- BP
bullous pemphigoid
- BP180
180-kd bullous pemphigoid antigen-2
- BP230
230-kd bullous pemphigoid antigen-1
- delNC16A
BP180 ectodomain with NC16A region deleted
- ELISA
enzyme-linked immunosorbent assay
- GST
glutathione-S-transferase
- IF
immunofluorescence
- NC1
first noncollagenous domain of collagen VII
- NC16A
noncollagenous 16A domain
- non-NC16A BP
reactivity to BP180 exclusive of NC16A domain
- sec180
the entire BP180 ectodomain of BP180
Footnotes
Conflicts of interest: None declared.
We are indebted to George Giudice, PhD, for the gift of cells expressing the sec180 protein and for critical advice regarding the epitope mapping studies.
REFERENCES
- 1.Labib RS, Anhalt GJ, Patel HP, Mutasim DF, Diaz LA. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J Immunol. 1986;136:1231–5. [PubMed] [Google Scholar]
- 2.Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–8. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, et al. Humanization of autoantigen. Nat Med. 2007;13:378–83. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 5.Fairley JA, Burnett CT, Fu C-L, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. 2007;127:2605–11. doi: 10.1038/sj.jid.5700958. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid [see comment]. Arch Dermatol. 2000;136:174–8. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koeb-nick C, Stauber A, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2-and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. 2002;119:1065–73. doi: 10.1046/j.1523-1747.2002.19529.x. [DOI] [PubMed] [Google Scholar]
- 8.Amo Y, Ohkawa T, Tatsuta M, Hamada Y, Fujimura T, Katsuoka K, et al. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoanti-bodies in patients with bullous pemphigoid. J Dermatol Sci. 2001;26:14–8. doi: 10.1016/s0923-1811(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura KC, Shimizu H. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. 2005;37:145–9. doi: 10.1016/j.jdermsci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Tsuruta D, Dainichi T, Hamada T, Furumura M, Ishii N. Demonstration of epitope spreading in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol. 2011;131:2175–7. doi: 10.1038/jid.2011.276. [DOI] [PubMed] [Google Scholar]
- 11.Hall RP, III, Murray JC, McCord MM, Rico MJ, Streilein RD. Rabbits immunized with a peptide encoded for by the 230-kD bullous pemphigoid antigen cDNA develop an enhanced inflammatory response to UVB irradiation: a potential animal model for bullous pemphigoid. J Invest Dermatol. 1993;101:9–14. doi: 10.1111/1523-1747.ep12358276. [DOI] [PubMed] [Google Scholar]
- 12.Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–50. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Diaz LA, Giudice GJ. Autoimmune response against the bullous pemphigoid 180 autoantigen. Dermatology. 1994;189(Suppl):34–7. doi: 10.1159/000246925. [DOI] [PubMed] [Google Scholar]
- 14.Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997;109:679–83. doi: 10.1111/1523-1747.ep12338088. [DOI] [PubMed] [Google Scholar]
- 15.Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997;109:573–9. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002;30:224–32. doi: 10.1016/s0923-1811(02)00109-3. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma-Oyama Y, Powell AM, Oyama N, Albert S, Bhogal BS, Black MM. Evaluation of a BP180-NC16a enzyme-linked immunosorbent assay in the initial diagnosis of bullous pemphigoid. Br J Dermatol. 2004;151:126–31. doi: 10.1111/j.1365-2133.2004.06082.x. [DOI] [PubMed] [Google Scholar]
- 18.Vaillant L, Bernard P, Joly P, Prost C, Labeille B, Bedane C, et al. Evaluation of clinical criteria for diagnosis of bullous pemphigoid: French bullous study group. Arch Dermatol. 1998;134:1075–80. doi: 10.1001/archderm.134.9.1075. [DOI] [PubMed] [Google Scholar]
- 19.Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J Invest Dermatol. 2005;125:467–72. doi: 10.1111/j.0022-202X.2005.23853.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Zenzo G, Thoma-Uszynski S, Calabresi V, Fontao L, Hofmann SC, Lacour J-P, et al. Demonstration of epitope-spreading phenomena in bullous pemphigoid: results of a prospective multicenter study. J Invest Dermatol. 2011;131:2271–80. doi: 10.1038/jid.2011.180. [DOI] [PubMed] [Google Scholar]
- 21.Chen M, Chan LS, Cai X, O'Toole EA, Sample JC, Woodley DT. Development of an ELISA for rapid detection of anti-type VII collagen autoantibodies in epidermolysis bullosa acquisita. J Invest Dermatol. 1997;108:68–72. doi: 10.1111/1523-1747.ep12285634. [DOI] [PubMed] [Google Scholar]
- 22.Messingham KA, Noe MH, Chapman MA, Giudice GJ, Fairley JA. A novel ELISA reveals high frequencies of BP180-specific IgE production in bullous pemphigoid. J Immunol Methods. 2009;346:18–25. doi: 10.1016/j.jim.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gammon WR, Fine JD, Forbes M, Briggaman RA. Immunofluorescence on split skin for the detection and differentiation of basement membrane zone autoantibodies. J Am Acad Dermatol. 1992;27:79–87. doi: 10.1016/0190-9622(92)70161-8. [DOI] [PubMed] [Google Scholar]
- 24.Olague-Marchan M, Twining SS, Hacker MK, McGrath JA, Diaz LA, Giudice GJ. A disease-associated glycine substitution in BP180 (type XVII collagen) leads to a local destabilization of the major collagen triple helix. Matrix Biol. 2000;19:223–33. doi: 10.1016/s0945-053x(00)00070-6. [DOI] [PubMed] [Google Scholar]
- 25.Di Zenzo G, Grosso F, Terracina M, Mariotti F, De Pita O, Owaribe K, et al. Characterization of the anti-BP180 autoantibody reactivity profile and epitope mapping in bullous pemphigoid patients. J Invest Dermatol. 2004;122:103–10. doi: 10.1046/j.0022-202X.2003.22126.x. [DOI] [PubMed] [Google Scholar]
- 26.Noe MH, Messingham KA, Brandt DS, Andrews JI, Fairley JA. Pregnant women have increased incidence of IgE autoanti-bodies reactive with the skin and placental antigen BP180 (type XVII collagen). J Reprod Immunol. 2010;85:198–204. doi: 10.1016/j.jri.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fairley JA, Woodley DT, Chen M, Giudice GJ, Lin MS. A patient with both bullous pemphigoid and epidermolysis bullosa acquisita: an example of intermolecular epitope spreading. J Am Acad Dermatol. 2004;51:118–22. doi: 10.1016/j.jaad.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 28.Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66:479–85. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haase C, Budinger L, Borradori L, Yee C, Merk HF, Yancey K, et al. Detection of IgG autoantibodies in the sera of patients with bullous and gestational pemphigoid: ELISA studies utilizing a baculovirus-encoded form of bullous pemphigoid antigen 2. J Invest Dermatol. 1998;110:282–6. doi: 10.1038/sj.jid.5602955. [DOI] [PubMed] [Google Scholar]
- 30.Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008;128:415–26. doi: 10.1016/j.clim.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Egan CA, Reddy D, Nie Z, Taylor TB, Schmidt LA, Meyer LJ, et al. IgG anti-LABD97 antibodies in bullous pemphigoid patients’ sera react with the mid-portion of the BPAg2 ectodomain. J Invest Dermatol. 2001;116:348–50. doi: 10.1046/j.1523-1747.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 32.Chan LS, Vanderlugt CJ, Hashimoto T, Nishikawa T, Zone JJ, Black MM, et al. Epitope spreading: lessons from autoimmune skin diseases. J Invest Dermatol. 1998;110:103–9. doi: 10.1046/j.1523-1747.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 33.Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. 2011;147:286–91. doi: 10.1001/archdermatol.2011.23. [DOI] [PubMed] [Google Scholar]
- 34.Chan LS. ELISA instead of indirect IF in patients with BP: comment on “Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid.”. Arch Dermatol. 2011;147:291–2. doi: 10.1001/archdermatol.2011.24. [DOI] [PubMed] [Google Scholar]
- 35.Gualco F, Cozzani E, Parodi A. Bullous pemphigoid with nail loss. Int J Dermatol. 2005;44:967–8. doi: 10.1111/j.1365-4632.2004.02358.x. [DOI] [PubMed] [Google Scholar]
- 36.Tomita M, Tanei R, Hamada Y, Fujimura T, Katsuoka K. A case of localized pemphigoid with loss of toenails. Dermatology. 2002;204:155. doi: 10.1159/000051839. [DOI] [PubMed] [Google Scholar]
- 37.Namba Y, Koizumi H, Kumakiri M, Hashimoto T, Muramatsu T, Ohkawara A. Bullous pemphigoid with permanent loss of the nails. Acta Derm Venereol. 1999;79:480–1. doi: 10.1080/000155599750010003. [DOI] [PubMed] [Google Scholar]
- 38.Delaporte E, Piette F, Janin A, Cozzani E, Joly P, Thomine E, et al. Pemphigoid mimicking epidermolysis bullosa acquisita. Ann Dermatol Venereol. 1995;122:19–22. [PubMed] [Google Scholar]
- 39.Sinclair RD, Wojnarowska F, Leigh IM, Dawber RP. The basement membrane zone of the nail. Br J Dermatol. 1994;131:499–505. doi: 10.1111/j.1365-2133.1994.tb08550.x. [DOI] [PubMed] [Google Scholar]
- 40.Yancey KB, Hintner H. Non-herlitz junctional epidermolysis bullosa. Dermatol Clin. 2010;28:67–77. doi: 10.1016/j.det.2009.10.008. [DOI] [PubMed] [Google Scholar]