Abstract
Older individuals have a reduced capacity to induce muscle hypertrophy with resistance exercise (RE), which may contribute to the age-induced loss of muscle mass and function, sarcopenia. We tested the novel hypothesis that dysregulation of microRNAs (miRNAs) may contribute to reduced muscle plasticity with aging. Skeletal muscle expression profiling of protein-coding genes and miRNA was performed in younger (YNG) and older (OLD) men after an acute bout of RE. 21 miRNAs were altered by RE in YNG men, while no RE-induced changes in miRNA expression were observed in OLD men. This striking absence in miRNA regulation in OLD men was associated with blunted transcription of mRNAs, with only 42 genes altered in OLD men vs. 175 in YNG men following RE, demonstrating a reduced adaptability of aging muscle to exercise. Integrated bioinformatics analysis identified miR-126 as an important regulator of the transcriptional response to exercise and reduced lean mass in OLD men. Manipulation of miR-126 levels in myocytes, in vitro, revealed its direct effects on the expression of regulators of skeletal muscle growth and activation of insulin growth factor 1 (IGF-1) signaling. This work identifies a mechanistic role of miRNA in the adaptation of muscle to anabolic stimulation and reveals a significant impairment in exercise-induced miRNA/mRNA regulation with aging.—Rivas, D. A., Lessard, S. J., Rice, N. P., Lustgarten, M. S., So, K., Goodyear, L. J., Parnell, L. D., Fielding, R. A. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling.
Keywords: noncoding RNA, genomics, humans, resistance exercise
Aging is accompanied by a progression of skeletal muscle atrophy and reduced muscle function, termed sarcopenia (1). Sarcopenia is a predictor of physical disability, leading to loss of independence, reduced quality of life, and increased mortality (2). With an estimated prevalence of 20% in persons ≥70 yr, the total annual cost of sarcopenia to the U.S. health system is reported to be ∼$18.4 billion (3). In addition, sarcopenia is associated with the onset of metabolic diseases, such as anabolic/insulin resistance, type 2 diabetes, dyslipidemia, and obesity (4, 5). Given that the world population is aging at an unprecedented rate (6), determining the molecular mechanisms that contribute to sarcopenia is of great importance and represents a first step for the development of therapeutic interventions to treat sarcopenia and its comorbidities.
To optimize its function, skeletal muscle exhibits exceptional plasticity and possesses the fundamental capacity to adapt its metabolic and contractile properties in response to various external stimuli (e.g., external loading, nutrient availability, and humoral factors; ref. 7). The adaptability of skeletal muscle, along with its relatively large mass and high metabolic rate, makes this tissue an important contributor to whole body health and mobility. In this regard, physical exercise is one of the most effective therapies for the maintenance of healthy muscle mass and function. However, we and others recently have reported a diminished adaptability of aging skeletal muscle to exercise and other anabolic stimuli (8, 9). Age-related impairments to skeletal muscle plasticity in response to exercise, or “anabolic resistance,” may play a significant role in the onset of sarcopenia and the chronic physical and metabolic complications that accompany this condition. However, the specific molecular and cellular mechanisms that underlie the diminished capacity for aging skeletal muscle to respond to anabolic stimulation have not been fully characterized.
Recent work has revealed a novel role for small noncoding RNAs, called microRNAs (miRNAs), in the regulation of muscle phenotype via alterations in gene expression (10). miRNAs also have been identified as potential regulators of exercise-induced adaptations in skeletal muscle (11–14). However, it is unknown whether miRNAs contribute to age-related changes in muscle anabolism and sarcopenia. Thus, the objective of this study was to determine the association between muscle-specific miRNA expression and the response of aged human skeletal muscle to anabolic stimulation. This was accomplished by conducting a comprehensive expression profile of protein-coding genes and skeletal muscle-specific miRNAs in younger (YNG) and older (OLD) men to establish the response to exercise, a well-characterized anabolic stimulus.
We identified several miRNAs that were associated with a transcriptional response to exercise in YNG subjects but that exhibited a blunted exercise response in OLD individuals. Notably, miR-126 and its mRNA targets were dysregulated in OLD men following exercise, and further in vitro analysis revealed a role for miR-126 as a regulator of growth and development pathways [Akt/forkhead box protein O1 (Foxo1) and myogenic differentiation 1 (MyoD)/myogenic factor 5 (Myf5)] and insulin growth factor-1 (IGF-1) signaling. Our results demonstrate that impaired regulation of miRNA in older individuals may contribute to resistance to anabolic stimulus and loss of muscle mass in this population. Furthermore, we identify specific miRNAs and their downstream mRNA targets that have the potential to be therapeutically targeted for the prevention and treatment of sarcopenia.
MATERIALS AND METHODS
Human study design
This study included a subset of 8 healthy YNG (22±1 yr) and OLD (74±2 yr) men enrolled in an acute exercise study, as described previously (8). Briefly, the subjects underwent initial evaluation of muscle strength [1 repetition maximum (RM)] to establish the prescribed exercise intensity for the acute intervention. The 1 RM testing was performed a minimum of 1 wk before the exercise. Subjects were admitted 24 h before the exercise bout; after an overnight fast, a single baseline (BL) percutaneous needle biopsy of the left vastus lateralis muscle was performed. The acute resistance exercise (RE) protocol was implemented the following morning. Each subject performed 3 sets of bilateral knee extension exercise (10 repetitions at 80% of 1 RM) and 3 sets of bilateral leg press exercise (10 repetitions at 80% of 1 RM). Muscle biopsies were obtained 6 h following RE from the right leg. All biopsies were stored in RNAlater (Ambion, Life Technologies, Grand Island, NY, USA) for subsequent analysis.
Body composition
Total body mass and composition were measured at screening using dual-energy X-ray absorptiometry (DXA) as described previously (8).
Transcriptome analysis of human skeletal muscle at BL and after exercise
Total RNA isolation
RNA was analyzed by Qiagen Service Core for Genomics and Gene Expression (Qiagen, Valencia, CA, USA). Total RNA (including miRNA) was isolated using miRNEasy Mini Kit (74104; Qiagen). RNA quality was determined using the Agilent Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA) with RNA 6000 Nano Kits (5067-1511; Agilent Technologies). Total RNA yield, 260/280, and 260/230 ratios were measured using a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific, Inc., Waltham, MA, USA).
Global gene array analysis
Illumina
Biotin-aRNA was generated using the Epicenter TargetAmp-Nano Labeling Kit for Illumina Expression BeadChip (TAN07908; Illumina, San Diego, CA, USA) from an input of 300 ng of high-quality RNA. Illumina bead arrays were assessed using HumanHT-12 v4 Expression BeadChip Kits (BD-103–0204). Raw intensity values were acquired using the HiScan microarray scanner (Illumina) and imported to GenomeStudio using the Gene Expression Module (Illumina). Protein-coding mRNA genes were defined as differentially expressed when >±2-fold compared with control (OLD vs. YNG BL, YNG BL vs. YNG 6 h, OLD BL vs. OLD 6 h) and a value of P < 0.05.
Validation of microarray results with quantitative PCR (qPCR)
Four genes that were the most highly altered after exercise in either age were determined using a custom PrimePCR Assay Plate (Bio-Rad, Hercules, CA, USA), which included primer assays for the human genes TNFRSF12A, FOS, PRKAG2, and PPARGC1A, with GAPDH used for normalization. RNA (100 ng) was reverse transcribed with iScript cDNA Synthesis Kit for reverse-transcription qPCR (RT-qPCR; Bio-Rad). Reactions were run using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad).
Analysis of miRNAs expressed in human skeletal muscle
An RT reaction using 250 ng of total RNA was done using the miScript II RT kit (218161; Qiagen). cDNA samples were assayed using a custom miRNA PCR array from Qiagen. The array contained 60 assays for essential miRNAs that have been observed previously to function in the regulation of skeletal muscle: hsa-miR-1, hsa-miR-10b, hsa-miR-16, hsa-miR-22, hsa-miR-23a, hsa-miR-23b, hsa-miR-24, hsa-miR-25, hsa-miR-26a, hsa-miR-26b, hsa-miR-27a, hsa-miR-27b, hsa-miR-29a, hsa-miR-29c, hsa-miR-30a, hsa-miR-30d, hsa-miR-30e*, hsa-miR-95, hsa-miR-98, hsa-miR-101, hsa-miR-103, hsa-miR-107, hsa-miR-124, hsa-miR-125a-5p, hsa-miR-125b, hsa-miR-128a, hsa-miR-126, hsa-miR-130a, hsa-miR-133a, hsa-miR-133b, hsa-miR-140-3p, hsa-miR-143, hsa-miR-181a, hsa-miR-181b, hsa-miR-191, hsa-miR-193a-3p, hsa-miR-195, hsa-miR-199a-3p, hsa-miR-206, hsa-miR-208b, hsa-miR-223, hsa-miR-296-5p, hsa-miR-324-3p, hsa-miR-330-3p, hsa-miR-335, hsa-miR-378, hsa-miR-423-3p, hsa-miR-423-5p, hsa-miR-451, hsa-miR-486-5p, hsa-miR-491-5p, hsa-miR-499-3p, hsa-miR-499-5p, hsa-miR-532-5p, hsa-miR-1268, hsa-let-7a, hsa-let-7b, hsa-let-7c, hsa-let-7f, hsa-let-7g, and 2 assays for normalization (SNORD68 and SNORD96A). PCR arrays were run using miScript SYBR Green PCR Kit (218073;Qiagen). Real-time PCR was performed using an ABI 7900 SDS Real Time Instrument (Life Technologies). Fold regulation and P values were calculated using the ΔΔ cycle threshold (ΔΔCT) method by the miScript miRNA PCR Array Data Analysis tool (http://www.sabiosciences.com/mirnaArrayDataAnalysis.php).
Pathway analysis of human data
Lists of mRNAs and miRNAs that were differentially expressed before and after exercise in each age group (with P<0.05) were uploaded into the Ingenuity Pathway Analysis (IPA) 9.0 tool (Ingenuity Systems, Redwood, CA, USA; http://www.ingenuity.com).
Functional analysis of mRNA
To identify biological functions that were significant in skeletal muscle after exercise, the differentially expressed protein-coding genes that showed a ±2-fold change (P<0.05) when comparing each group after RE vs. BL were analyzed with IPA. Right-tailed Fisher's exact test was used to calculate a significant P value for each functional category as referenced in Ingenuity Knowledgebase. To examine associations that were considered to occur specifically in skeletal muscle, we narrowed the Ingenuity Knowledgebase database to interactions reported to occur in skeletal muscle.
mRNA/miRNA target filter analysis of pathways regulated by exercise
We used the Ingenuity miRNA Target Filter to analyze all possible miRNA/target-gene interactions from the miRNA PCR array and gene array data sets after exercise in YNG men. The data of differentially expressed mRNAs and miRNAs were integrated using the expression pairing function to obtain miRNA-mRNA relationships with expression changes in opposite directions. The miRNA-mRNA interacting networks were further explored for relevance to biological pathways related to muscle development and growth that were significantly enriched in protein-coding genes targeted by 21 miRNAs changed (P<0.10) after exercise. Both experimentally validated interactions and predictions characterized as “high” confidence were considered based on TargetScan and Ingenuity Knowledgebase. Data from OLD men were excluded from this analysis because of no observed changes in miRNA expression after exercise.
miRScore correlations
As the consequences of directionality of miRNA expression remain unknown, we calculated miRNA scores by the median absolute fold change of all expressed miRNA for each subject, as described previously (15). Associations between variables of interest were performed using Pearson's correlation, and normal distribution of the data was determined using the D'Agostino-Pearson normality test with GraphPad Prism 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Statistical significance was accepted at P < 0.05.
Clustering of relevant miRNAs and establishing functional pathways associated with skeletal muscle adaptation after exercise
To reduce the dimensionality of the data and to control type 1 error, we used principal component analysis (PCA) on miRNAs differentially expressed (P<0.10) after exercise (YNG/OLD, BL vs. 6 h). miRNAs containing PCA-derived factors were obtained using ΔCT values from random sets of up to 12 miRNAs. To determine the association between lower leg lean mass (LLgLM) with the PCA-derived factors, we used age-adjusted backward-elimination linear regression. Clustered miRNAs were then uploaded to DNA Intelligent Analysis (DIANA)-miRPath 2.0 [Alexander Fleming Biological Sciences Research Center (BSRC), Athens, Greece; http://diana.cslab.ece.ntua.gr], and relevant Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) pathways were identified using predicted targets from microT-CDS 5.0 and experimentally verified targets from TarBase 6.0 (Alexander Fleming BSRC).
Cell culture studies
Stock C2C12 (mouse) myoblasts (American Type Culture Collection, Manassas, VA, USA) were maintained at 37°C (95% O2-5% CO2) in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) culture medium, 1% penicillin–streptomycin (Sigma-Aldrich), and 5.5 mM glucose. For experimental procedures, the myoblasts and myotubes were seeded in 6-well plates. Differentiation was induced by switching to medium containing 2% horse serum (Sigma-Aldrich) when the myoblasts were ∼80% confluent. Myotube experiments were started after 7 d, by which time all of the myoblasts had fused to form myotubes.
In vitro transfections
All transfections were performed at minimum in triplicate. Cells were transfected for 24 h, using either mirVana (Life Technologies) mmu-miR-126-3p inhibitor (4464084; assay ID MH12841) or mmu-miR-126-3p mimetic (4464066; assay ID MC12841) with equal volumes of RNAiMax Lipofectamine (Life Technologies) for a final concentration of 15 nM. Transfections were performed on cells that were either 80% confluent (myoblasts) or differentiated for 7 d (myotubes).
Protein and total RNA extractions
After 24 h of transfection, the myoblasts were collected in lysis buffer as described previously (8). A separate set of myoblasts was collected and stored in RNAlater for future analysis, and total RNA was then extracted with the miRVana miRNA Isolation Kit (Life Technologies).
IGF-1 experiments
After 24 h of transfection, the myotubes were serum starved for an additional 4 h, and then medium with or without IGF-1 (long-R3-IGF-1; Sigma) was added for 30 min to stimulate IGF-1 signaling. After 30 min, the cells were collected in lysis buffer (see above).
Western blot analysis
The phosphorylation and total protein expression of insulin receptor substrate 1 (IRS1), Akt, mTOR, ribosomal s6 kinase 1 (S6K1), and ribosomal protein S6 (rpS6) were determined with SDS-PAGE and Western blot as described previously (8). The immunoreactive proteins were detected with Supersignal Chemiluminescent Substrate (ThermoFisher), and intensities were quantified by densitometry (Chemidoc XRS+ System; Bio-Rad).
qPCR
All qPCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad) using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) unless otherwise stated.
mRNA
RNA (25 ng/μl) was reverse-transcribed with the iScript cDNA Synthesis Kit for RT-qPCR (Bio-Rad). The relative levels of mRNA were determined for Abce1 (7657518a1), Atp2a1 (19548097a1), B2m (31981890a1) Ccl2 (6755430a), Fbxo32 (13385848a1), Gapdh (6679937a1), Irs1 (29825829a1), Tnnc1 (6678369a1), Tnni2 (118130404c1), Vcam1 (31981430a1), and Vegfa (6678563a1) at 1 μM final concentration using primer sequences validated by PrimerBank (16).
miRNA-specific stem-loop RT-qPCR
miR-126-3p was transcribed into cDNA at 25 ng/μl via miRNA-specific RT reaction using a stem-loop RT primer (CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCATTAT) at 100 nM final concentration and iScript Select cDNA Synthesis Kit (Bio-Rad). Quantification was performed by qPCR RT primer and forward primer (ACACTCCAGCTGGGTCGTACCGTGAGTAAT) at 1 μM final concentration. miRNA results were normalized to the U6 reference gene.
Small RNA housekeeping gene
The small nuclear RNA (snRNA) U6 was transcribed into cDNA at 25 ng/μl via miRNA-specific RT reaction using a TaqMan microRNA assay (4427975; assay ID 001973; Life Technologies) and iScript Select cDNA Synthesis Kit (Bio-Rad). snRNA quantification was performed by qPCR using the aforementioned TaqMan kit and iTaq Universal Probe Supermix (Bio-Rad).
PrimePCR assay plate
Genes associated with myogenesis and myopathy were measured using a custom PrimePCR Assay Plate (Bio-Rad), which included primer assays for 80 mouse genes (see Supplemental Table S2). Assay plates were used for pooled samples of RNA from the negative control group and the anti-miR-126 group. RNA samples were pooled based on previous results showing that gene expression from RNA pools are similar to averages of individuals that comprise the pool (17, 18).
Statistical analysis
All values are expressed as means ± se. Significance was determined by either unpaired Student's t tests or 1-way ANOVA followed by the Student-Newman-Keuls method. Significance was set at P < 0.05.
RESULTS
Loss of skeletal muscle plasticity to RE with aging
The adaptive response to exercise in muscle is reflected in gene expression changes after acute exercise (19); however, the response of an acute bout of contraction-mediated gene expression on changes across the entire transcriptome has not been well-established in YNG vs. OLD individuals. We first determined the nature of the differential transcriptional response 6 h after an acute bout of high-intensity RE between age groups. At 6 h after exercise, a total of 175 protein-coding genes were altered by RE in YNG men compared with only 42 in OLD men, including 36 genes shared by both groups (Fig. 1 and Supplemental Table S1). Our results demonstrate the transcriptional response to exercise is impaired in older individuals. Eleven notable genes exhibiting the largest magnitude of change after exercise in each age group are presented in Table 1. These include XIRP1, TNFRSF12A, RRAD, MYC, and EGR1, which showed a greater increase in expression in YNG men and are key regulators of myogenesis, fatigue resistance, muscle development, regeneration, and adaptation (20–24). In contrast, PRKAG2 and PPARGC1A, components of energy-sensing pathways that regulate mitochondrial biogenesis and lipid metabolism (25, 26), were increased to a greater extent by RE in OLD men (Table 1). At BL, only 14 genes were differentially expressed in OLD compared with YNG men, and these did not highlight any particular pathway or biological function (Supplemental Table S1). Exercise-induced changes in expression of a subset of 4 genes (TNFRSF12A, FOS, PPARGC1A, and PRKAG2) that were differentially expressed in YNG and OLD men at 6 h after exercise were validated by quantitative PCR and mirrored the gene array results.
Figure 1.
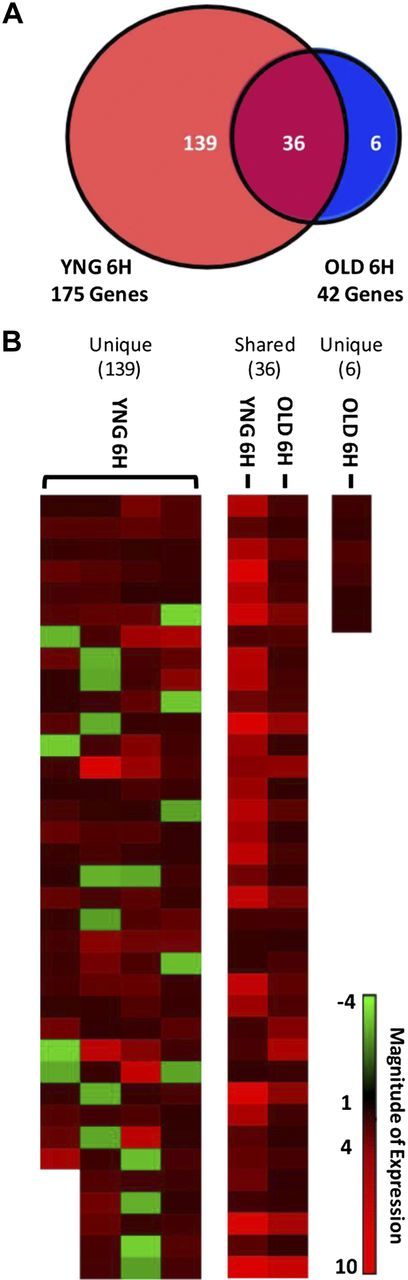
Gene expression profile of YNG and OLD men. Muscle biopsies were taken from the vastus lateralis of YNG and OLD men after an overnight fast at rest (BL) and 6 h after a single-bout of high-intensity RE (n=8/age group). A) Venn diagram represents overlapping genes changed ≥±2-fold and P < 0.05 at 6H in YNG and OLD men. B) Heatmap represents the magnitude of gene expression changed ≥±2-fold and P < 0.05 6 h after exercise unique to YNG men, shared between age groups, and unique to OLD men.
Table 1.
Top regulated genes after exercise
| YNG |
OLD |
|||||
|---|---|---|---|---|---|---|
| Top upregulated |
Top downregulated |
Top upregulated |
Top downregulated | |||
| Gene | FC | Gene | FC | Gene | FC | |
| XIRP1 | 16.2 | RHOBTB1 | −3.1 | XIRP1 | 6.8 | — |
| TNFRSF12A | 15.1 | DDIT4 | −3.0 | PRKAG2 | 5.2 | — |
| ATF3 | 14.2 | PLEKHF1 | −2.9 | TNFRSF12A | 4.9 | — |
| FOS | 10.0 | SBK1 | −2.8 | EGR1 | 3.9 | — |
| RRAD | 9.4 | TLE1 | −2.6 | RRAD | 3.8 | — |
| CDKN1A | 7.0 | PFKFB3 | −2.6 | PPARGC1A | 3.6 | — |
| KBTBD5 | 6.5 | IL17D | −2.4 | CDKN1A | 3.5 | — |
| MYC | 6.4 | PITX2 | −2.4 | KBTBD5 | 3.4 | — |
| IFRD1 | 6.2 | DDIT4L | −2.3 | MYC | 3.0 | — |
| HBEGF | 6.1 | IRS1 | −2.2 | ASB5 | 2.9 | — |
| PDK4 | 6.0 | FBXO32 | −2.2 | GADD45A | 2.8 | — |
Muscle biopsies were taken from the vastus lateralis of YNG and OLD men after an overnight fast, at rest (BL), and at 6 h after a single bout of high-intensity RE. Top up- and down-regulated genes at 6 h (vs. BL) expressed ≥±2-fold are presented for YNG and OLD men; n = 8/age group. FC, fold change.
Molecular pathways associated with acute exercise in YNG and OLD men
To determine the disparities in mRNA transcriptional networks and functional pathways in skeletal muscle of YNG and OLD men after RE, we performed a comparative analysis using IPA. Exercise affected several common functional pathways in both age groups (Table 2). However, there was a substantial reduction in number of genes changed by exercise in critical functional pathways in OLD compared with YNG men (Table 2). Although there were 36 shared differentially expressed mRNAs, no exercise-regulated canonical pathways were shared between OLD and YNG men. For example, IGF-1 and p38 MAPK pathways, regulators of muscle growth and development (27, 28), were found to be modulated by exercise in YNG but not in OLD men. In contrast, an inflammatory response was established in OLD men, as observed by the modulation of the IL17 and PXR/RXR pathways that control these events (29, 30). Therefore, our data suggest that impaired stimulation of pathways that regulate growth and development, combined with an increased inflammatory response, may contribute to the impaired anabolic response to exercise in OLD individuals.
Table 2.
Comparison analysis of gene expression changes
| YNG |
OLD |
||||
|---|---|---|---|---|---|
| IPA pathway | P | Genes (n) | IPA pathway | P | Genes (n) |
| Molecular and cellular functions | |||||
| Cellular growth and proliferation | 2.67E-17–1.63E-05 | 115 | Cellular development | 3.30E-12–1.56E-03 | 29 |
| Cell death and survival | 3.68E-16–1.63E-05 | 115 | Cellular growth and proliferation | 3.30E-12–1.46E-03 | 29 |
| Cellular development | 1.12E-13–1.63E-05 | 113 | Cell cycle | 9.73E-12–1.61E-03 | 24 |
| Cellular movement | 2.56E-13–1.58E-05 | 76 | Cell death and survival | 3.41E-08–1.38E-03 | 31 |
| Cell cycle | 9.77E-12–1.05E-05 | 56 | Lipid metabolism | 2.25E-07–1.60E-03 | 18 |
| Physiological system development and function | |||||
| Tumor morphology | 1.12E-13–1.22E-05 | 43 | Connective tissue development and function | 3.30E-12–1.55E-03 | 20 |
| Connective tissue development and function | 1.92E-12–1.15E-05 | 71 | Cardiovascular system development and function | 9.08E-08–1.56E-03 | 20 |
| Organismal survival | 7.16E-12–1.85E-07 | 83 | Organismal development | 9.08E-08–1.36E-03 | 25 |
| Tissue morphology | 1.97E-11–1.58E-05 | 74 | Tissue development | 1.35E-07–1.36E-03 | 23 |
| Cardiovascular system development and function | 2.19E-11–1.63E-05 | 65 | Tumor morphology | 2.38E-07–1.01E-03 | 17 |
| Top canonical pathways | |||||
| Hepatic fibrosis/hepatic stellate cell activation | 5.85E-06 | 11/155 (0.071) | Glucocorticoid receptor signaling | 8.74E-04 | 9/299 (0.03) |
| Role of tissue factor in cancer | 7.55E-06 | 10/130 (0.077) | PXR/RXR activation | 1.03E-03 | 3/92 (0.033) |
| Ilk signaling | 1.52E-05 | 13/205 (0.063) | IL-17 signaling | 1.07E-03 | 3/75 (0.04) |
| P38 MAPK signaling | 7.76E-05 | 8/120 (0.067) | Xenobiotic metabolism signaling | 1.24E-03 | 6/304 (0.02) |
| Igf-1 signaling | 1.68E-04 | 8/107 (0.075) | Acute myeloid leukemia signaling | 1.3E-03 | 3/84 (0.036) |
Comparison analysis of functional and canonical pathways with IPA reveals the disparities of transcriptional regulation between YNG and OLD men at 6 h after an acute bout of high-intensity RE. Italics denote relevant pathways.
Skeletal muscle miRNA expression profile after acute RE
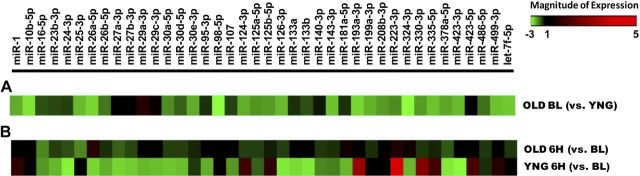
Early changes to skeletal muscle gene expression in response to exercise are important for the adaptations of muscle and mediate the hypertrophic effects of RE (31). However, miRNAs recently have emerged as potentially important regulators of exercise adaptations in skeletal muscle (32–34). To determine whether altered miRNA expression contributes to the altered response to exercise between ages, we measured 60 miRNAs that were highly expressed in skeletal muscle and have prominent roles in regulating muscle growth and metabolism, as well as miRNAs with less-established roles (11–15, 32–39). At baseline, all 60 miRNAs were expressed in OLD men, and the expression of 14 of these was significantly lower compared with YNG men, with a further 9 miRNAs tending (P≤0.10) to be diminished vs. YNG men (Fig. 2 and Table 3). Exercise did not alter the expression of any miRNA in OLD men. In contrast, 17 miRNAs were significantly altered by RE in YNG men, with an additional 4 miRNAs tending (P≤0.10) to change (Fig. 2 and Table 3). The striking absence of exercise-induced miRNA regulation in OLD men mirrored the blunted transcription of mRNA observed after exercise compared with YNG men, and suggests that impaired miRNA regulation may be a novel contributor to blunted transcriptional and adaptive responses of OLD individuals in response to exercise.
Figure 2.
Diminished skeletal muscle miRNA expression in OLD men. Heatmaps represent changes of skeletal muscle miRNA expression at rest (BL) in OLD men (A) and 6 h after exercise in YNG and OLD men (B); n = 8/age group.
Table 3.
miRNA expression profile of human subjects
| miRNA | BL |
Postexercise (6 h) |
||||
|---|---|---|---|---|---|---|
| OLD vs. YNG |
OLD vs. BL |
YNG vs. BL |
||||
| FC | P | FC | P | FC | P | |
| miR-1 | 0.58 ↓ | 0.022 | 1.04 | ND | 1.72 | ND |
| miR-10b-5pa | 0.45 ↓ | 0.042 | 1.04 | ND | 1.26 | ND |
| miR-16-5p | 0.81 | ND | 0.66 | ND | 0.61 ↓ | 0.086 |
| miR-23b-3p | 0.82 | ND | 0.83 | ND | 0.54 ↓ | 0.004 |
| miR-24-3p | 0.84 | ND | 0.79 | ND | 0.38 ↓ | 0.002 |
| miR-25-3p | 0.56 ↓ | 0.084 | 0.65 | ND | 1.14 | ND |
| miR-26a-5pa | 0.47 ↓ | 0.071 | 1.62 | ND | 0.45 ↓ | 0.021 |
| miR-26b-5pa | 0.71 | ND | 0.88 | ND | 0.55 ↓ | 0.007 |
| miR-27a-3p | 1.06 | ND | 0.75 | ND | 0.45 ↓ | 0.001 |
| miR-27b-3p | 1.15 | ND | 0.81 | ND | 0.48 ↓ | 0.023 |
| miR-29a-3p | 1.58 | 0.118 | 1.06 | ND | 0.53 ↓ | 0.062 |
| miR-29c-3p | 1.33 | ND | 1.18 | ND | 0.52 ↓ | 0.020 |
| miR-30a-5pa | 0.66 ↓ | 0.090 | 0.83 | ND | 0.50 ↓ | <0.001 |
| miR-30d-5p | 0.77 | ND | 0.83 | ND | 0.51 ↓ | 0.002 |
| miR-30e-3p | 0.58 ↓ | 0.020 | 0.89 | ND | 0.86 | ND |
| miR-95-3p | 0.87 | ND | 0.96 | ND | 0.56 ↓ | 0.042 |
| miR-98-5p | 0.36 ↓ | 0.067 | 1.11 | ND | 1.10 | ND |
| miR-107a | 0.84 | ND | 1.09 | ND | 0.62 ↓ | 0.065 |
| miR-124-3p | 0.58 ↓ | 0.007 | 0.84 | ND | 1.92 | ND |
| miR-125a-5pa | 0.65 ↓ | 0.038 | 0.83 | ND | 0.76 | ND |
| miR-125b-5pa | 0.63 ↓ | 0.043 | 0.92 | ND | 1.74 | ND |
| miR-126-3pa | 0.56 ↓ | 0.056 | 0.84 | ND | 0.39 ↓ | 0.005 |
| miR-133a | 0.88 | ND | 1.08 | ND | 0.44 ↓ | 0.042 |
| miR-133b | 0.80 | ND | 1.07 | ND | 0.39 ↓ | 0.027 |
| miR-140-3p | 0.94 | ND | 0.86 | ND | 0.65 ↓ | 0.016 |
| miR-143-3pa | 0.53 ↓ | 0.049 | 0.88 | ND | 0.68 | 0.111 |
| miR-181a-5p | 0.87 | ND | 1.35 | ND | 0.53 ↓ | 0.011 |
| miR-193a-3pa | 0.48 ↓ | <0.001 | 1.07 | ND | 3.10 | ND |
| miR-199a-3p | 0.57 ↓ | 0.025 | 0.88 | ND | 1.27 | ND |
| miR-208b-3p | 0.62 ↓ | 0.077 | 1.06 | ND | 1.10 | ND |
| miR-223-3p | 0.66 ↓ | 0.066 | 1.52 | ND | 5.42 | 0.137 |
| miR-324-3p | 0.36 ↓ | 0.004 | 1.39 | ND | 0.53 ↓ | 0.079 |
| miR-330-3p | 0.63 ↓ | 0.015 | 0.86 | ND | 2.81 | 0.191 |
| miR-335-5p | 0.54 ↓ | 0.058 | 0.89 | ND | 1.96 | ND |
| miR-378a-5p | 0.61 ↓ | 0.029 | 0.78 | ND | 0.40 ↓ | 0.002 |
| miR-423-3p | 0.55 | ND | 1.14 | ND | 0.33 | ND |
| miR-423-5p | 1.04 | ND | 1.25 ↑ | 0.053 | 2.05 ↑ | 0.023 |
| miR-486-5p | 0.74 ↓ | 0.037 | 0.8512 | ND | 0.87 | ND |
| miR-499-3p | 0.54 ↓ | 0.006 | 0.982 | ND | 1.61 | ND |
| let-7f-5p | 0.51 ↓ | 0.058 | 0.8526 | ND | 1.19 | ND |
Muscle biopsies were taken from the vastus lateralis of YNG men at rest after an overnight fast and at 6 h after a single bout of high-intensity RE. miRNA expression at BL in OLD men and at 6h after RE in OLD and YNG men. FC, fold change; ↑, upregulated; ↓, downregulated; ND, no difference.
Clustered miRNA in miR factor analysis; n = 8/age group.
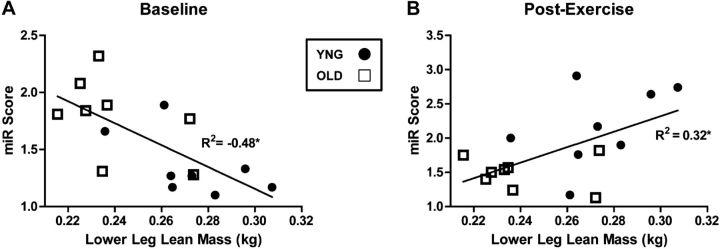
Direct relationship between miR score and lean mass
The combined median fold change of all expressed miRNAs from baseline vs. exercise was used to calculate a miRNA (miR) score for each subject, as described previously (15). We observed a significant negative association (P=0.003, R2=−0.48) between miR score and LLgLM in the YNG and OLD subjects combined (Fig. 3A). The miR score determined after exercise was positively associated (P=0.023, R2=0.31) with LLgLM (Fig. 3B). These results demonstrate that a diminished capacity for the transcription of miRNA at rest and in response to exercise is associated with decreased skeletal muscle mass.
Figure 3.
miR score at BL and postexercise are associated with LLgLM. Correlations of miR score and LLgLM at BL (A) and at 6 h postexercise in YNG and OLD men (B); n = 8/age group. Open squares, OLD; solid circles, YNG. Significant differences between groups. *P < 0.05 for OLD vs. YNG.
mRNA/miRNA interactions after exercise
To identify the contribution of miRNA regulation after exercise to differential gene expression in YNG men, the transcripts (21 miRNA and 201 mRNA) that were altered in response to exercise were filtered using IPA miRNA target filter to predict pathways regulated by these direct binding events. We found that 17 of the 21 miRNAs that were altered with exercise in YNG men had mRNA target information and were predicted to have a direct influence on 63% (102 of 164) of the protein-coding genes that we determined as significantly changed with exercise (Table 4). Pathway analysis indicated that our identified miRNA/mRNA interactions were predicted to regulate cellular and multiple molecular functions including cell growth and proliferation and cell development (Table 4). Notably, one of the top regulated canonical pathways and upstream regulators included IGF-1, which was similarly observed in the comparative analysis of the gene expression in YNG men (Table 2), a critical regulator of muscle growth and metabolism.
Table 4.
miRNA/mRNA interaction analysis
| IPA pathway | P | Genes (n) |
|---|---|---|
| Molecular and cellular functions | ||
| Cellular growth and proliferation | 1.09E-14–8.24E-04 | 69 |
| Cellular development | 4.07E-13–8.18E-04 | 68 |
| Cell Death and Survival | 3.56E-12–8.40E-04 | 65 |
| Cellular movement | 1.89E-09–8.04E-04 | 44 |
| Cell cycle | 6.52E-09–8.47E-04 | 29 |
| Physiological system development and function | ||
| Organismal survival | 2.88E-09–4.93E-04 | 47 |
| Organismal development | 3.02E-09–7.64E-04 | 52 |
| Hematological system development and function | 8.16E-09–7.77E-04 | 38 |
| Tumor morphology | 2.40E-08–7.64E-04 | 25 |
| Connective tissue development and function | 2.44E-08–8.47E-04 | 37 |
| Top canonical pathways | ||
| ILK signaling | 2.13E-05 | 8/205 (0.039) |
| IGF-1 signaling | 3.56E-04 | 5/107 (0.047) |
| Role of tissue factor in cancer | 6.53E-04 | 5/130 (0.038) |
| NRF2-mediated oxidative stress response | 8.44E-04 | 6/195 (0.031) |
| Role of JAK2 in hormone-like cytokine signaling | 1.16E-03 | 3/37 (0.081) |
| Top upstream regulators | ||
| TNF | 4.60E-22 | |
| IL1B | 1.57E-18 | |
| β-Estradiol | 7.62E-17 | |
| IGF1 | 4.02E-16 | |
| F2 | 4.72E-16 | |
Functional analysis of miRNA-mRNA data interactions using IPA with the miRNA target filter. Italics denote relevant pathways.
Identification of miRNA associated with LLgLM and age
PCA using 21 myo-miRs that were differentially expressed ±1.5-fold (P<0.10) at BL between age groups, derived 5 miRNA containing factors (data not shown). Use of backward elimination regression modeling on these 5 miRNA containing factors established that 70% of the total variance in LLgLM was significantly accounted for by age and miR factor 1. Of this variance, age accounted for 46%, and miR factor 1 (eigenvalue of 8.0) accounted for 24%. These included 10 miRs with component loading values of ≥0.4 with miR factor 1 (Table 5). These 10 significantly clustered miRs were then uploaded into DIANA-miRPath 2.0, and relevant KEGG pathways were identified using predicted targets. We observed 26 essential pathways potentially related to skeletal muscle that contained ≥8 of the clustered miRs (Table 6). Similar to the mRNA/miRNA interaction analysis, we observed that these clustered miRs had a predicted role in the regulating pathways associated with growth and metabolism, such as PI3K-Akt, mTOR, TGFβ, and VEGF, demonstrating the potential for miRNAs to act as critical regulators of skeletal muscle adaptability.
Table 5.
Clustered miRNAs by PCA
| miRNA | Component loading | Eigenvalue | Cumulative variance |
|---|---|---|---|
| MIR factor 1 | 8.0 | 0.67 | |
| miR-126 | 0.94 | ||
| miR-30a | 0.93 | ||
| miR-10b | 0.90 | ||
| miR-143 | 0.87 | ||
| miR-125a-5p | 0.84 | ||
| miR-26b | 0.82 | ||
| miR-107 | 0.81 | ||
| miR-26a | 0.80 | ||
| miR-125b | 0.78 | ||
| miR-193a-3p | 0.75 |
PCA clustering of 23 miRNAs that were different at 6 h after exercise in YNG men revealed a miR factor containing 10 miRNAs that were significantly associated with lean mass when accounting for age.
Table 6.
KEGG pathways associated with miR factor 1 clustered with PCA
| KEGG pathway | P | Genes (n) | miRNAs (n) |
|---|---|---|---|
| Ubiquitin-mediated proteolysis | 1.96E-18 | 55 | 9 |
| Regulation of actin cytoskeleton | 2.33E-12 | 70 | 10 |
| Focal adhesion | 3.15E-12 | 66 | 10 |
| MAPK signaling pathway | 3.12E-11 | 81 | 10 |
| Wnt signaling pathway | 9.10E-11 | 55 | 9 |
| PI3K-Akt signaling pathway | 1.94E-10 | 97 | 10 |
| mRNA surveillance pathway | 1.33E-09 | 34 | 9 |
| Transcriptional misregulation in cancer | 5.28E-09 | 61 | 10 |
| TGF-β signaling pathway | 9.40E-09 | 30 | 8 |
| mTOR signaling pathway | 2.61E-07 | 24 | 10 |
| ErbB signaling pathway | 1.07E-06 | 32 | 10 |
| Insulin signaling pathway | 1.24E-06 | 42 | 10 |
| Type II diabetes mellitus | 3.57E-05 | 17 | 8 |
| VEGF signaling pathway | 0.000382 | 21 | 9 |
| p53 signaling pathway | 0.000559 | 22 | 10 |
| Basal transcription factors | 0.000731 | 15 | 9 |
| Adipocytokine signaling pathway | 0.005166 | 20 | 8 |
| Cell cycle | 0.005487 | 35 | 10 |
| Phosphatidylinositol signaling system | 0.008304 | 26 | 9 |
| HIF-1 signaling pathway | 0.024575 | 29 | 9 |
| Chemokine signaling pathway | 0.045806 | 44 | 10 |
Identification of relevant KEGG pathways regulated by 10 miRNAs clustered into miR factor 1 as determined with PCA and identified with DIANA-miRPath 2.0 using experimentally verified targets from TarBase 6.0. Italics denote relevant pathways.
miR-126 expression and skeletal muscle growth pathways
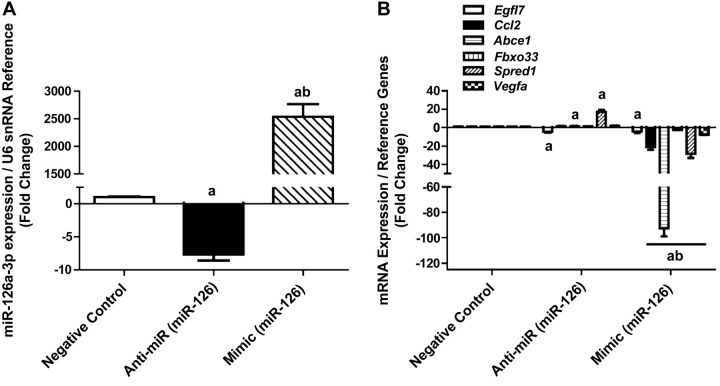
PCA revealed a possible role for the growth suppressor miR-126 in exercised-induced changes to gene expression in YNG men. In addition, we observed a significant 2-fold decrease of miR-126 after exercise (Table 3) likely leading to observed changes in 7 predicted mRNA targets (Supplemental Table S1). We therefore hypothesized that miR-126 may be a critical regulator of muscle growth and IGF-1 signaling and tested this hypothesis by manipulating miR-126 expression in myocytes. Transfection of miR-126 inhibitor and mimic in myocytes for 24 h resulted in a 7-fold decrease of miR-126 expression with inhibition and a 2500-fold increase of miR-126 in mimic-transfected myocytes (Fig. 4A). Inhibition of miR-126 increased the expression of its predicted mRNA targets Abce1, Ccl2, Fbxo33, and Vegfa, while Irs1 and Spred1 were unchanged (Fig. 4B). In contrast, overexpression of miR-126 diminished the expression of Abce1, Ccl2, Fbxo33, Irs1, Spred1, and Vegfa (Fig. 4B). These results indicate that manipulating expression of miR-126 in skeletal muscle in vitro affected known targets that were also changed in YNG after exercise.
Figure 4.
Manipulation of miR-126 expression in skeletal muscle cells. C2C12 myocytes were transfected with a negative control (CON), a miR-126 antisense oligonucleotide (anti-miR), or a miR-126 mimic (mimic). A) miRNA expression levels of miR-126 normalized to U6 snRNA after transfection. B) mRNA expression of select miR-126 predicted gene targets after transfection. aP < 0.05 vs. CON, bP < 0.05 vs. anti-miR.
When determining the effect of miR-126 inhibition on the expression of 80 genes that are known modulators of myogenesis and myopathy, we observed that 21 of these genes were differentially expressed ±2-fold compared with negative control-transfected cells (Fig. 5A and Supplemental Table S2). Nine of these differentially expressed gene targets were validated in negative control, inhibitor, and mimic-transfected myocytes with qPCR. miR-126 inhibition increased 2- to 6-fold the expression of Atp2a1 and Bag3 and decreased Fbxo32, Ikba, Myf6, and Tnnc1 2- to 6-fold (Fig. 5B). miR-126 overexpression increased expression of Atp2a1, Neb, Trim63, Tnnc1, and Fbxo32 3- to 56-fold and decreased expression of Myf6 5-fold (Fig. 5B). These data provide evidence that manipulation of miR-126 levels in skeletal muscle cells imparts downstream effects on genes involved in skeletal muscle development.
Figure 5.
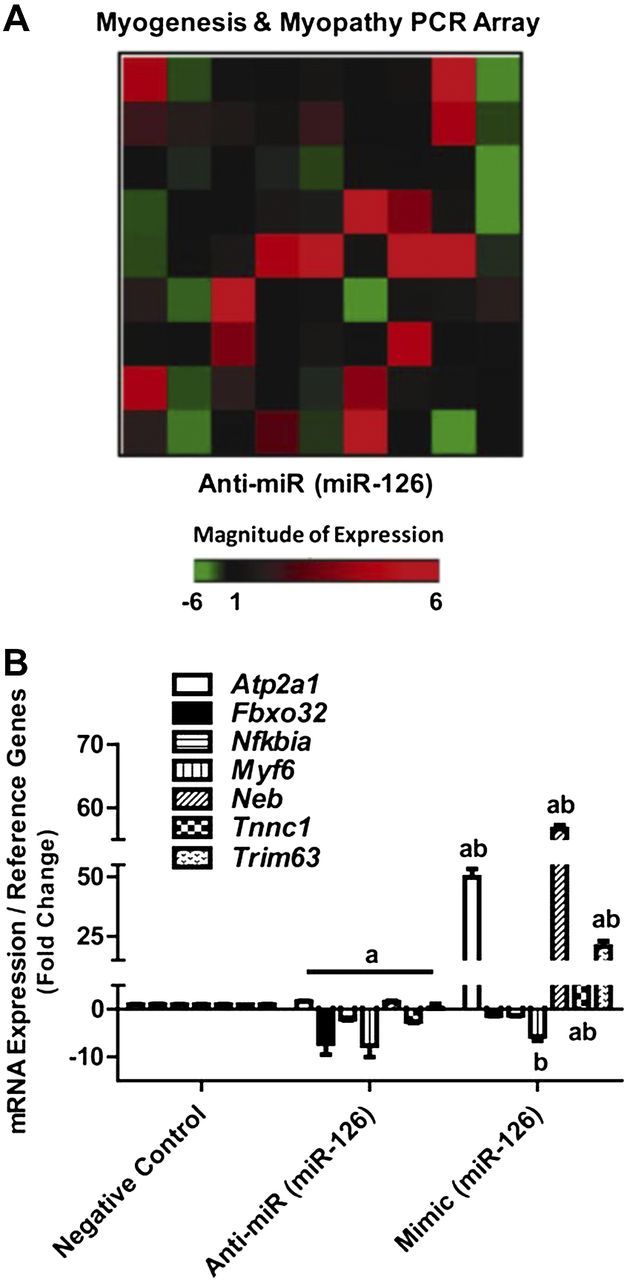
mRNA expression of myogenic genes in skeletal muscle cells. C2C12 myocytes were transfected with a negative control (CON), a miR-126 antisense oligonucleotide (anti-miR), or a miR-126 mimic (mimic). A) Fold changes of 80 genes associated with myogenesis or myopathy were measured with PCR array in CON and anti-miR pooled samples. B) Selected myogenic genes were validated after inhibition (anti-miR) and overexpression (mimic) of miR-126 in skeletal muscle cells. aP < 0.05 vs. CON, bP < 0.05 vs. anti-miR; n = 3/group.
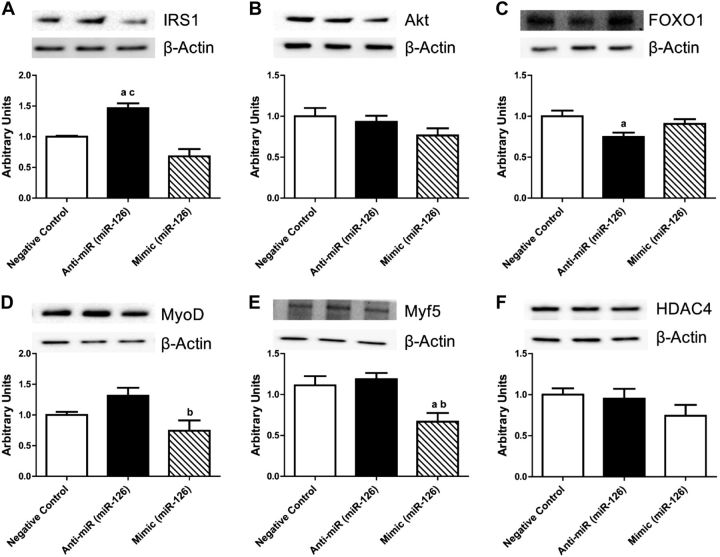
At the translational level, we observed that miR-126 had a significant role in regulating proteins involved in skeletal muscle growth. Inhibition of miR-126 increased the expression of IRS1 50 and 70% compared with control and overexpression, respectively (Fig. 6A), while expression of Akt was unchanged (Fig. 6B), and Foxo1 was decreased 25% compared with control (Fig. 6C). There were no significant changes in protein expression of IRS1, Akt, and FOXO1 with overexpression. The protein expression of key myogenic regulators was decreased, MyoD 60% with overexpression compared with inhibition and Myf5 50% compared with control and miR-126 inhibition. No changes in histone deacetylase 4 (HDAC4) expression were noted (Fig. 6D).
Figure 6.
Protein expression of myogenic regulators in skeletal muscle cells after miR-126 inhibition and overexpression. Total protein content of IRS1 (A), protein kinase B (Akt; B), FOXO1 (C), MyoD (D), Myf5 (E), and HDAC4 (F) in negative control (CON), miR-126 inhibition (anti-miR), and miR-126 overexpression (mimic). Relative protein levels were quantified using Western blot analysis. aP < 0.05 vs. CON, bP < 0.05 vs. anti-miR, cP < 0.05 vs. mimic; n = 3/group.
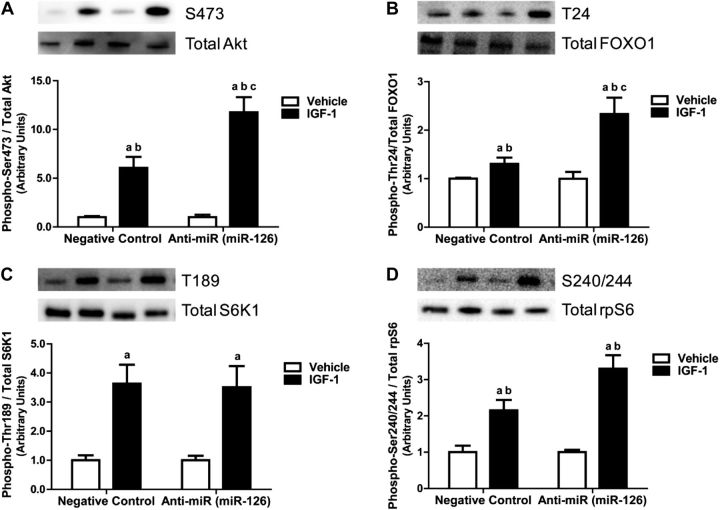
To characterize the effect of miR-126 on anabolic signaling, we transfected C2C12 myotubes with either negative control or miR-126 inhibitor and treated with IGF-1 for 30 min to replicate in vitro the exercise condition of our human study. IGF-1 stimulation significantly increased Akt activation 5-fold in negative control-transfected myotubes compared with cells treated with vehicle. In addition, Akt activation was significantly increased 15-fold in miR-126 inhibitor-transfected cells compared with vehicle and control (Fig. 7A). Phosphorylation of Foxo1, an Akt target and myogenic regulator, was increased 20% on IGF-1 stimulation in negative control and was significantly increased 120% in inhibitor-transfected myotubes compared with vehicle and control (Fig. 7B). IGF-1 treatment similarly increased S6K1 phosphorylation in negative control and with miR-126 inhibition compared with vehicle. The downstream target of S6K1, phosphorylation of rpS6, was significantly increased 2-fold with IGF-1 stimulation in negative control and 3-fold with inhibition compared with vehicle and control (Fig. 7C, D). Therefore, we demonstrate a role for miR-126 in the regulation of growth signaling in response to anabolic stimulation by IGF-1.
Figure 7.
IGF-1 signaling increased with miR-126 inhibition in skeletal muscle cells. Phosphorylation and total protein content of protein kinase B (Akt; A), FOXO1 (B), S6K1 (C), and ribosomal protein S6 (D) after vehicle and IGF-1 treatment for 30 min in negative control (CON) and miR-126 inhibited (anti-miR) skeletal muscle cells. Fold change of relative protein levels were quantified using Western blot analysis. aP < 0.05 vs. CON, bP < 0.05 vs. anti-miR, cP < 0.05 vs. mimic; n = 3/group.
DISCUSSION
This study provides compelling mechanistic and translational evidence that miRNAs expressed in skeletal muscle have a key role in muscle plasticity following anabolic stimulation. We show in healthy OLD males, compared with healthy YNG males, a significant blunting of transcriptional regulation after an acute bout of high-intensity RE. We further elucidate that the decline of transcriptional changes following exercise in OLD individuals may result from deregulation of miRNAs expressed in skeletal muscle, which, in the context of aging, are critical posttranscriptional regulators of metabolism, growth, and whole-body health. Notably, we identified the growth suppressor miR-126 as dysregulated with age with a novel role in the molecular control of exercise-induced adaptation of skeletal muscle. Lastly, using gain- and loss-of-function experiments, we demonstrate that miR-126 functionally regulates skeletal muscle cells by modulating signal transduction in response to IGF-1.
RE is a powerful anabolic stimulus that can modify the expression of critical regulatory genes associated with skeletal muscle growth and function (40–42), but this response may be impaired in aged individuals (40, 43). In line with this assertion, by performing a comprehensive exercise-induced gene expression profiling of our subjects, we observed significant decreases in both the number and magnitude of genes changed by exercise in OLD individuals, demonstrating a reduction in adaptability of aging skeletal muscle to exercise. In addition, the limited transcriptional response that was observed in OLD subjects was associated with an up-regulation of inflammatory pathways, in contrast to the up-regulation of growth pathways observed in YNG subjects. Increases in inflammatory markers are associated with frailty and loss of function in OLD adults (44), a relationship that is consistent with evidence from master's athletes who display lower levels of inflammation than their sedentary counterparts (45). Thus, our observation of a progression toward transcription of inflammatory to the exclusion of growth pathways in the skeletal muscle of the elderly may be a contributing factor leading to sarcopenia.
With the goal of developing an improved understanding about the molecular events that influence the dissimilar transcriptional changes observed in older adults after exercise, we then investigated muscle-specific changes in miRNAs. miRNAs expressed in skeletal muscle are implicated in the regulation of metabolism (36, 37), insulin secretion, muscle disorders (35, 46), and adaptation of skeletal muscle (11–14). Recent work also has demonstrated a deregulation of miRNA expression with human aging (38, 47), coronary artery disease (48), metabolic disease (38, 49), muscle disorders (15), and in elderly individuals after anabolic stimulation (50, 51). Furthermore, a scan of genome-wide association studies for single-nucleotide polymorphisms (SNPs) associating with disease-relevant phenotypes showed that thousands of SNPs could influence mRNA-miRNA interactions with disease consequences, indicating that the influence of miRNAs indeed could be widespread (52). In the current study, we measured the expression of 60 specific miRNAs, which are highly linked to the regulation of skeletal muscle development, following a single bout of high-intensity RE. We demonstrated a significant attenuation of exercise-induced changes to miRNA expression in OLD compared with YNG men. This striking absence of exercise-induced miRNA regulation with age mirrored the blunted transcription of mRNA observed after exercise. Therefore, our results suggest the blunted transcriptional response to exercise in aging muscle may be, at least in part, a consequence of deregulated miRNA expression. Indeed, miRNA-mRNA interactions indicated that exercise-induced changes to miRNA were predicted to influence >63% of the protein-coding genes changed with exercise and these were significantly grouped with functional and canonical pathways associated with growth and development in the YNG subjects. Together, these data provide strong evidence for deregulated miRNA as a key factor, in concomitance with signaling events, for the loss of skeletal muscle plasticity with age. Notably, SNPs linked to disease risk and affecting mRNA-miRNA interactions (52) and the recent finding that physical activity is a factor in all-cause mortality in the National Health and Nutrition Examination Survey cohort (53) combined with our data have mechanistic implications beyond sarcopenia and metabolic disorders linked to muscle function.
An integrated bioinformatics analysis identified miR-126 as a potentially important regulator of the blunted transcriptional response to exercise and reduced lean mass in OLD individuals. miR-126 is a highly conserved miRNA initially described as endothelial specific that since has been shown to be expressed in other tissues, including skeletal muscle and hepatocytes (39). Located at intron 5 of the EGFL7 gene, miR-126 is an important regulator of growth and angiogenesis in highly vascularized tissues, such as lung, heart, brain, and skeletal muscle (54–57). Interestingly, after exercise in YNG men, we observed that miR-126 was decreased by 60%, leading to a 2-fold increase in the expression of 7 of its predicted mRNA targets. The magnitude of these changes is in line with the fact that interactions between miRNA and mRNA are subtle, and most targets are expected to be downregulated by ∼50% (58). These data provide evidence for directionality of miR-126 in skeletal muscle, making it a major target of interest in our analysis. miR-126 levels were manipulated in C2C12 myoblasts to determine whether we could directly regulate the expression of specific mRNA targets in skeletal muscle. These results to some extent mirrored the changes to the expression of miR-126 predicted mRNA targets that were altered with exercise in YNG subjects. To further illustrate the effects of manipulating miR-126 levels on muscle related pathways, genes associated with muscle development were screened, and we report significant changes in the expression of 21 genes with miR-126 inhibition, compared with control, thus identifying potential downstream effects of miR-126 in muscle.
Inhibiting miR-126 levels in proliferating myoblasts led to significantly increased IRS1 and decreased FOXO1 protein expression, 2 important regulators of muscle growth. Overexpression of miR-126 decreased protein levels of 2 markers of myogenic determination, MyoD and Myf5 (59–61). These results show that manipulating miR-126 had differential consequences on posttranscriptional regulation of important regulators of skeletal muscle growth. To better imitate our current human experiments in vitro, we next determined the response of mature myotubes to IGF-1 stimulation, a powerful anabolic factor, after miR-126 inhibition. We show that the inhibition of miR-126 increased phosphorylation of Akt, FOXO1 and rpS6 to a larger extent than control. This is in agreement with previous studies where manipulating miR-126 levels in other tissue types showed a significant change in growth signaling (39, 62), demonstrating miR-126 as a potential intermediate of skeletal muscle adaptation and anabolic response to growth stimulation.
In this study, we were able to convincingly demonstrate a directional association between changes in miRNA and changes in mRNA expression after exercise in YNG men, an effect that is lost in OLD men. Although the current study includes a small sample size, we believe that this overlap of data from both protein-coding gene expression and muscle-specific miRNA expression in the two age groups provides strong evidence for the effects of miRNA on skeletal muscle transcriptional adaptations to exercise. Furthermore, confirming the mechanistic consequences of miRNA expression by manipulating their levels in skeletal muscle cells in vitro, we believe, shows a convincing directional effect. Our data indicate a role for inadequate IGF-1 activation as a mediator of impaired transcriptional response to OLD individuals to exercise. However, the role of IGF-1 in exercise-mediated muscle hypertrophy is contentious; as it has been reported in previous studies there is no beneficial effect to muscle hypertrophy when using GH/IGF-1 treatment alone or synergistically with exercise (63). The timing of the biopsies is an important consideration possibly explaining some of the observed differences in gene expression because it appears at least there is no intrinsic difference in aged or young muscle progenitor cells (64). However, changes in the response of protein synthesis and gene expression 6H after exercise have been reported (65).
In summary, this work identifies a potential mechanistic role of miRNA in the adaptation of skeletal muscle to anabolic stimulation and reveals a significant impairment in exercise-induced miRNA/mRNA regulation with aging. Hence, dysregulation of miRNA expression could be involved centrally in pathogenic mechanisms associated with sarcopenia. Although RE is one of the most potent clinical modalities to induce hypertrophy thereby reducing losses of skeletal muscle mass and function associated with aging, our data provide translational evidence that introducing an additional stimulus, such as nutrients, that can either directly modulate specific miRNAs or influence the expression of multiple miRNAs in skeletal muscle may have greater beneficial effects than exercise alone. Interestingly, current investigations on miRNA-based gene therapies, in different phases of clinical trial, have shown potential for the treatment of multiple diseases (66–68). Studies characterizing the roles of miRNAs in response to such treatments are lacking for skeletal muscle growth, but as our study suggests are a logical next step in developing improved clinical treatments for sarcopenia.
Supplementary Material
Acknowledgments
This material is based on the work supported by the U.S. Department of Agriculture (USDA), under agreement 58-1950-0014.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The study was also supported by the Boston Claude D. Pepper Center Older American Independence Centers (OAIC; 1P30AG031679). D.A.R. is supported by a Research Career Development Core Fellowship from the Boston Claude D. Pepper Center OAIC. S.J.L. is supported by the William Randolph Hearst Fellowship in Clinical and Translational Research.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- BL
- baseline
- CT
- cycle threshold
- DIANA
- DNA Intelligent Analysis
- DMEM
- Dulbecco's modified Eagle's medium
- Foxo1
- forkhead box protein O1
- HDAC4
- histone deacetylase 4
- IPA
- Ingenuity Pathway Analysis
- IRS1
- insulin receptor substrate 1
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- LLgLM
- lower leg lean mass
- miR
- microRNA
- miRNA
- microRNA
- Myf5
- myogenic factor 5
- MyoD
- myogenic differentiation 1
- OLD
- older
- PCA
- principal component analysis
- qPCR
- quantitative PCR
- RE
- resistance exercise
- RM
- repetition maximum
- rpS6
- ribosomal protein S6
- RT-qPCR
- reverse-transcription quantitative PCR
- S6K1
- s6 kinase 1
- SNP
- single-nucleotide polymorphism
- snRNA
- small nuclear RNA
- YNG
- younger
REFERENCES
- 1. Fielding R. A., Vellas B., Evans W. J., Bhasin S., Morley J. E., Newman A. B., Abellan van Kan G., Andrieu S., Bauer J., Breuille D., Cederholm T., Chandler J., De Meynard C., Donini L., Harris T., Kannt A., Keime Guibert F., Onder G., Papanicolaou D., Rolland Y., Rooks D., Sieber C., Souhami E., Verlaan S., Zamboni M. (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 12, 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hwee D. T., Baehr L. M., Philp A., Baar K., Bodine S. C. (2014) Maintenance of muscle mass and load-induced growth in muscle RING finger 1 null mice with age. Aging Cell 13, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssen I., Shepard D. S., Katzmarzyk P. T., Roubenoff R. (2004) The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 52, 80–85 [DOI] [PubMed] [Google Scholar]
- 4. Moon S. S. (2014) Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr. J. 61, 61–70 [DOI] [PubMed] [Google Scholar]
- 5. Akasaki Y., Ouchi N., Izumiya Y., Bernardo B. L., Lebrasseur N. K., Walsh K. (2013) Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism. Aging Cell 13, 80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen K., Doblhammer G., Rau R., Vaupel J. W. (2009) Ageing populations: the challenges ahead. Lancet 374, 1196–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Egan B., Zierath J. R. (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17, 162–184 [DOI] [PubMed] [Google Scholar]
- 8. Rivas D. A., Morris E. P., Haran P. H., Pasha E. P., Morais Mda S., Dolnikowski G. G., Phillips E. M., Fielding R. A. (2012) Increased ceramide content and NFkappaB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J. Appl. Physiol. 113, 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durham W. J., Casperson S. L., Dillon E. L., Keske M. A., Paddon-Jones D., Sanford A. P., Hickner R. C., Grady J. J., Sheffield-Moore M. (2010) Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 24, 4117–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ameres S. L., Zamore P. D. (2013) Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell. Biol. 14, 475–488 [DOI] [PubMed] [Google Scholar]
- 11. Timmons J. A. (2011) Modulation of microRNAs during exercise and disease in human skeletal muscle. Exerc. Sport Sci. Rev. 39, 218; author reply 219 [DOI] [PubMed] [Google Scholar]
- 12. Nielsen S., Scheele C., Yfanti C., Akerstrom T., Nielsen A. R., Pedersen B. K., Laye M. J. (2010) Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J. Physiol. 588, 4029–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCarthy J. J., Esser K. A. (2007) MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J. Appl. Physiol. 102, 306–313 [DOI] [PubMed] [Google Scholar]
- 14. Safdar A., Abadi A., Akhtar M., Hettinga B. P., Tarnopolsky M. A. (2009) miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS One 4, e5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greco S., Perfetti A., Fasanaro P., Cardani R., Capogrossi M. C., Meola G., Martelli F. (2012) Deregulated microRNAs in myotonic dystrophy type 2. PLoS One 7, e39732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spandidos A., Wang X., Wang H., Seed B. (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kendziorski C., Irizarry R. A., Chen K. S., Haag J. D., Gould M. N. (2005) On the utility of pooling biological samples in microarray experiments. Proc. Natl. Acad. Sci. U. S. A. 102, 4252–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaillou T., Lee J. D., England J. H., Esser K. A., McCarthy J. J. (2013) Time course of gene expression during mouse skeletal muscle hypertrophy. J. Appl. Physiol. (1985) 115, 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fluck M. (2006) Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J. Exp. Biol. 209, 2239–2248 [DOI] [PubMed] [Google Scholar]
- 20. Feng H. Z., Wang Q., Reiter R. S., Lin J. L., Lin J. J., Jin J. P. (2013) Localization and function of Xinalpha in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. 304, C1002–C1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhatnagar S., Kumar A. (2012) The TWEAK-Fn14 system: breaking the silence of cytokine-induced skeletal muscle wasting. Curr. Mol. Med. 12, 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moyers J. S., Bilan P. J., Zhu J., Kahn C. R. (1997) Rad and Rad-related GTPases interact with calmodulin and calmodulin-dependent protein kinase II. J. Biol. Chem. 272, 11832–11839 [DOI] [PubMed] [Google Scholar]
- 23. Wright W. E., Sassoon D. A., Lin V. K. (1989) Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 56, 607–617 [DOI] [PubMed] [Google Scholar]
- 24. Pardo P. S., Mohamed J. S., Lopez M. A., Boriek A. M. (2011) Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J. Biol. Chem. 286, 2559–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narkar V. A., Downes M., Yu R. T., Embler E., Wang Y. X., Banayo E., Mihaylova M. M., Nelson M. C., Zou Y., Juguilon H., Kang H., Shaw R. J., Evans R. M. (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134, 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jager S., Handschin C., St-Pierre J., Spiegelman B. M. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 104, 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrini S., Laviola L., Carreira M. C., Cignarelli A., Natalicchio A., Giorgino F. (2010) The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J. Endocrinol. 205, 201–210 [DOI] [PubMed] [Google Scholar]
- 28. Keren A., Tamir Y., Bengal E. (2006) The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 252, 224–230 [DOI] [PubMed] [Google Scholar]
- 29. Kliewer S. A., Goodwin B., Willson T. M. (2002) The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr. Rev. 23, 687–702 [DOI] [PubMed] [Google Scholar]
- 30. Miossec P., Korn T., Kuchroo V. K. (2009) Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361, 888–898 [DOI] [PubMed] [Google Scholar]
- 31. Hawley J. A., Lessard S. J. (2008) Exercise training-induced improvements in insulin action. Acta Physiol. (Oxf.) 192, 127–135 [DOI] [PubMed] [Google Scholar]
- 32. Hulsmans M., Holvoet P. (2013) MicroRNAs as early biomarkers in obesity and related metabolic and cardiovascular diseases. Curr. Pharm. Des. 19, 5704–5717 [DOI] [PubMed] [Google Scholar]
- 33. Bates D. J., Liang R., Li N., Wang E. (2009) The impact of noncoding RNA on the biochemical and molecular mechanisms of aging. Biochim. Biophys. Acta 1790, 970–979 [DOI] [PubMed] [Google Scholar]
- 34. Davidsen P. K., Gallagher I. J., Hartman J. W., Tarnopolsky M. A., Dela F., Helge J. W., Timmons J. A., Phillips S. M. (2011) High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J. Appl. Physiol. 110, 309–317 [DOI] [PubMed] [Google Scholar]
- 35. Wang X. H. (2013) MicroRNA in myogenesis and muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16, 258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boehm M., Slack F. J. (2006) MicroRNA control of lifespan and metabolism. Cell Cycle 5, 837–840 [DOI] [PubMed] [Google Scholar]
- 37. Moore K. J. (2013) microRNAs: small regulators with a big impact on lipid metabolism. J. Lipid Res. 54, 1159–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olivieri F., Rippo M. R., Procopio A. D., Fazioli F. (2013) Circulating inflamma-miRs in aging and age-related diseases. Front. Genet. 4, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ryu H. S., Park S. Y., Ma D., Zhang J., Lee W. (2011) The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS One 6, e17343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dennis R. A., Przybyla B., Gurley C., Kortebein P. M., Simpson P., Sullivan D. H., Peterson C. A. (2008) Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol. Genomics 32, 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raue U., Slivka D., Jemiolo B., Hollon C., Trappe S. (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J. Appl. Physiol. 101, 53–59 [DOI] [PubMed] [Google Scholar]
- 42. Jozsi A. C., Dupont-Versteegden E. E., Taylor-Jones J. M., Evans W. J., Trappe T. A., Campbell W. W., Peterson C. A. (2000) Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech. Ageing Dev. 120, 45–56 [DOI] [PubMed] [Google Scholar]
- 43. Raue U., Trappe T. A., Estrem S. T., Qian H. R., Helvering L. M., Smith R. C., Trappe S. (2012) Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J. Appl. Physiol. 112, 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schaap L. A., Pluijm S. M., Deeg D. J., Harris T. B., Kritchevsky S. B., Newman A. B., Colbert L. H., Pahor M., Rubin S. M., Tylavsky F. A., Visser M. (2009) Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mikkelsen U. R., Couppe C., Karlsen A., Grosset J. F., Schjerling P., Mackey A. L., Klausen H. H., Magnusson S. P., Kjaer M. (2013) Life-long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech. Ageing Dev. 134, 531–540 [DOI] [PubMed] [Google Scholar]
- 46. Wei R., Yang J., Liu G. Q., Gao M. J., Hou W. F., Zhang L., Gao H. W., Liu Y., Chen G. A., Hong T. P. (2013) Dynamic expression of microRNAs during the differentiation of human embryonic stem cells into insulin-producing cells. Gene 518, 246–255 [DOI] [PubMed] [Google Scholar]
- 47. Serna E., Gambini J., Borras C., Abdelaziz K. M., Belenguer A., Sanchis P., Avellana J. A., Rodriguez-Manas L., Vina J. (2012) Centenarians, but not octogenarians, up-regulate the expression of microRNAs. Sci. Rep. 2, 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fichtlscherer S., De Rosa S., Fox H., Schwietz T., Fischer A., Liebetrau C., Weber M., Hamm C. W., Roxe T., Muller-Ardogan M., Bonauer A., Zeiher A. M., Dimmeler S. (2010) Circulating microRNAs in patients with coronary artery disease. Circ. Res. 107, 677–684 [DOI] [PubMed] [Google Scholar]
- 49. Zampetaki A., Kiechl S., Drozdov I., Willeit P., Mayr U., Prokopi M., Mayr A., Weger S., Oberhollenzer F., Bonora E., Shah A., Willeit J., Mayr M. (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 107, 810–817 [DOI] [PubMed] [Google Scholar]
- 50. Drummond M. J., McCarthy J. J., Fry C. S., Esser K. A., Rasmussen B. B. (2008) Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am. J. Physiol. Endocrinol. Metab. 295, E1333–E1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Drummond M. J., McCarthy J. J., Sinha M., Spratt H. M., Volpi E., Esser K. A., Rasmussen B. B. (2011) Aging and microRNA expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol. Genomics 43, 595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richardson K., Lai C. Q., Parnell L. D., Lee Y. C., Ordovas J. M. (2011) A genome-wide survey for SNPs altering microRNA seed sites identifies functional candidates in GWAS. BMC Genomics 12, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel C. J., Rehkopf D. H., Leppert J. T., Bortz W. M., Cullen M. R., Chertow G. M., Ioannidis J. P. (2013) Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the United States National Health and Nutrition Examination Survey. Int. J. Epidemiol. 42, 1795-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. (2002) Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 [DOI] [PubMed] [Google Scholar]
- 55. Wienholds E., Kloosterman W. P., Miska E., Alvarez-Saavedra E., Berezikov E., de Bruijn E., Horvitz H. R., Kauppinen S., Plasterk R. H. (2005) MicroRNA expression in zebrafish embryonic development. Science 309, 310–311 [DOI] [PubMed] [Google Scholar]
- 56. Cho I. S., Kim J., Seo H. Y., Lim do H., Hong J. S., Park Y. H., Park D. C., Hong K. C., Whang K. Y., Lee Y. S. (2010) Cloning and characterization of microRNAs from porcine skeletal muscle and adipose tissue. Mol. Biol. Rep. 37, 3567–3574 [DOI] [PubMed] [Google Scholar]
- 57. Muroya S., Taniguchi M., Shibata M., Oe M., Ojima K., Nakajima I., Chikuni K. (2013) Profiling of differentially expressed microRNA and the bioinformatic target gene analyses in bovine fast- and slow-type muscles by massively parallel sequencing. J. Anim. Sci. 91, 90–103 [DOI] [PubMed] [Google Scholar]
- 58. Bartel D. P. (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Long Y. C., Cheng Z., Copps K. D., White M. F. (2011) Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol. Cell. Biol. 31, 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kamei Y., Miura S., Suzuki M., Kai Y., Mizukami J., Taniguchi T., Mochida K., Hata T., Matsuda J., Aburatani H., Nishino I., Ezaki O. (2004) Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 279, 41114–41123 [DOI] [PubMed] [Google Scholar]
- 61. Kitamura T., Kitamura Y. I., Funahashi Y., Shawber C. J., Castrillon D. H., Kollipara R., DePinho R. A., Kitajewski J., Accili D. (2007) A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J. Clin. Invest. 117, 2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang J., Du Y. Y., Lin Y. F., Chen Y. T., Yang L., Wang H. J., Ma D. (2008) The cell growth suppressor, mir-126, targets IRS-1. Biochem. Biophys. Res. Commun. 377, 136–140 [DOI] [PubMed] [Google Scholar]
- 63. Lange K. H., Andersen J. L., Beyer N., Isaksson F., Larsson B., Rasmussen M. H., Juul A., Bulow J., Kjaer M. (2002) GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J. Clin. Endocrinol. Metab. 87, 513–523 [DOI] [PubMed] [Google Scholar]
- 64. Alsharidah M., Lazarus N. R., George T. E., Agley C. C., Velloso C. P., Harridge S. D. (2013) Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell 12, 333–344 [DOI] [PubMed] [Google Scholar]
- 65. Zambon A. C., McDearmon E. L., Salomonis N., Vranizan K. M., Johansen K. L., Adey D., Takahashi J. S., Schambelan M., Conklin B. R. (2003) Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 4, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nana-Sinkam S. P., Croce C. M. (2013) Clinical applications for microRNAs in cancer. Clin. Pharmacol. Ther. 93, 98–104 [DOI] [PubMed] [Google Scholar]
- 67. Janssen H. L., Reesink H. W., Lawitz E. J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A. J., Patick A. K., Chen A., Zhou Y., Persson R., King B. D., Kauppinen S., Levin A. A., Hodges M. R. (2013) Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368, 1685–1694 [DOI] [PubMed] [Google Scholar]
- 68. Ling H., Fabbri M., Calin G. A. (2013) MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 12, 847–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.