Abstract
Previous genome-wide association studies (GWAS), conducted by our group and others, have identified loci that harbor risk variants for neurodegenerative diseases, including Alzheimer's disease (AD). Human disease variants are enriched for polymorphisms that affect gene expression, including some that are known to associate with expression changes in the brain. Postulating that many variants confer risk to neurodegenerative disease via transcriptional regulatory mechanisms, we have analyzed gene expression levels in the brain tissue of subjects with AD and related diseases. Herein, we describe our collective datasets comprised of GWAS data from 2,099 subjects; microarray gene expression data from 773 brain samples, 186 of which also have RNAseq; and an independent cohort of 556 brain samples with RNAseq. We expect that these datasets, which are available to all qualified researchers, will enable investigators to explore and identify transcriptional mechanisms contributing to neurodegenerative diseases.
Subject terms: Neurodegeneration, Genetics of the nervous system, Genome-wide association studies, RNA sequencing
Background & Summary
In the past decade GWAS identified risk loci for human diseases, including AD1–7 and other neurodegenerative diseases8,9. Despite this progress, a comprehensive understanding of the molecular mechanisms underlying these complex conditions remains elusive. This is partly due to the inability of the disease GWAS approach to identify the actual disease gene and the functional disease risk variants. We10 and others11,12 utilized combined gene expression GWAS (eGWAS) and disease GWAS to identify loci which harbor regulatory variants that confer disease risk and to nominate the actual disease genes at these loci. The underlying premise of these studies is that genetic variants that modulate expression levels of genes, which encode critical members of disease molecular pathways, will also influence disease risk13. If this is correct, then there should be significant overlap between disease GWAS and eGWAS variants, especially if assessed in the disease-relevant tissue. Indeed, in an eGWAS of brain tissue from subjects with AD and non-AD, comprised largely of other neurodegenerative diagnoses, we identified significant enrichment for disease GWAS variants for AD and other diseases10. We14–18 and others8,19–22 determined that many of the risk variants for AD and other neurodegenerative diseases influence brain levels of genes that are nearby in the genome. These studies implicate the genes that are likely to be involved in disease pathways, nominate regulatory variants as the functional disease risk factors and provide testable hypotheses for their downstream effects.
Most large-scale gene expression studies in human brains published to date10,19,20,23 utilize microarray-based gene or exon arrays. Despite the versatility, cost-effectiveness and large-scale utility, this approach has limitations, including restricted dynamic range, lack of probes for all known gene isoforms and confinement of assays to known transcripts. RNA sequencing (RNAseq) provides an attractive alternative that can surpass these limitations and provide much more in-depth information about the human transcriptome in a high-throughput manner24. To expand our prior work on the human transcriptome based on microarray approaches and to evaluate gene/exon/isoform levels in a comparative fashion between AD and other neurodegenerative diseases, we have generated RNAseq data on brain samples from both a subset of the subjects that underwent microarray transcriptome studies18 and also an independent cohort. These datasets will be of utility in performing expression quantitative trait loci (eQTL), expression profiling and network analyses to facilitate interpretation of genetic associations and further understanding of disease-mediated changes in transcriptional regulation.
The present report is a description of the large-scale human genetic, and both microarray- and RNAseq-based transcriptome datasets we generated. The datasets described in this report have been made available to the research community through the Accelerating Medicines Partnership in Alzheimer’s Disease (AMP-AD) Knowledge Portal (Data Citation 1). The portal is hosted in the Synapse software platform25 from Sage Bionetworks as part of a series of datasets developed in support of the AMP-AD Target Identification and Preclinical Validation Project. The AMP-AD consortium includes six academic teams that will be generating genomic data from human brain or blood samples collected from more than 10 cohorts. Datasets are hosted in a common environment with standardized meta-data and annotations to facilitate cross-cohort query, access, and analysis. Each dataset provides a unique perspective on AD; therefore, datasets differ in types, generation protocols, and underlying patient characteristics. Together, this collection represents to date the most comprehensive collection of human genomic data in the field and, as such, it will be invaluable to a broad set of researchers.
The datasets described herein include the following: (1) late-onset AD GWAS1 (Mayo LOAD GWAS) on 2,099 subjects (Data Citation 2); (2) Mayo eGWAS10 on 773 samples from the cerebellum (CER) and temporal cortex (TCX) brain regions from a subset of Mayo LOAD GWAS participants (Data Citations 3,4); (3) Mayo Pilot RNAseq18 generated on a subset of 186 TCX samples from the Mayo eGWAS (Data Citation 5); (4) Mayo RNAseq on an independent cohort of 556 TCX26 (Data Citation 6) and CER (Data Citation 7) samples from subjects with AD, progressive supranuclear palsy (PSP), pathologic aging and elderly controls without neurodegenerative diseases. This report provides a comprehensive understanding of these cohorts, a detailed description of subjects, samples, data generation, and quality control (QC) as well as instructions to access these rich datasets by the scientific community.
Methods
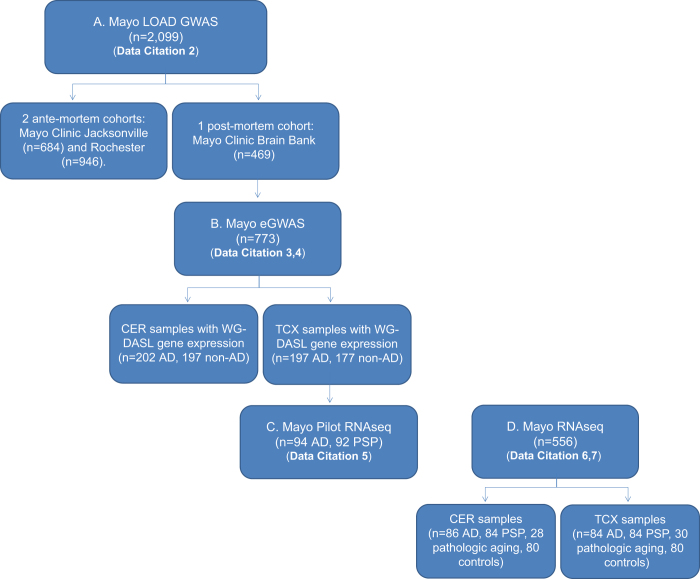
The repository of human whole genome genotype and transcriptome data described herein (Table 1,Fig. 1) consist of the following resources some of which have previously been published: Previously published datasets include whole genome genotype data from the Mayo LOAD GWAS1 (Data Citation 2) and microarray-based whole transcriptome data from the Mayo eGWAS10 (Data Citations 3,4). Next-generation RNA-sequencing (RNAseq) data from a subset of the patients from the Mayo Clinic eGWAS, referred to as the ‘Mayo Pilot RNAseq’ (Data Citation 5), was published in part18. A non-overlapping cohort with RNAseq-based transcriptome data named ‘Mayo RNAseq’ (Data Citations 6,7) has also been published in part26. For a comprehensive description of the overall repository, the data from the published studies are also described herein, albeit in an abbreviated fashion. These four study cohorts will be referred to by their names as mentioned above, preceded by letters A-D (Table 1) henceforth.
Table 1. Meta-data for each of the four studies.
| Study Name | Brief Description | Study Cohort/Sample type | N | Cohort Characteristics | Datatype | Platform | Reference |
|---|---|---|---|---|---|---|---|
| A. Mayo LOAD GWAS (Data Citation 2) | LOAD Case control GWAS. Uses samples from 3 cohorts: Total 2,099 subjects (Post-QC). This data is used to identify loci associated with LOAD risk. | Mayo Clinic Jacksonville (JS)/Antemortem | N=353 cases, 331 controls | Clinical: AD Cases and Controls, collected at Mayo Clinic Jacksonville. Age at first diagnosis of AD or age at study entry: 60–80. | LOAD GWAS Genotypes, demographics | Illumina Hap 300 | Carrasquillo et al.1, Nature Genetics |
| Mayo Clinic Rochester (RS)/Antemortem | N=245 cases, 701 controls | Clinical: AD Cases and Controls, collected at Mayo Clinic Rochester. Age at first diagnosis of AD or age at study entry: 60–80. | |||||
| Mayo Clinic Brain Bank (AUT)/ Postmortem | N=246 cases, 223 controls | Post-mortem: AD Cases (Braak ≥4.0) and Other Pathologies (Braak ≤2.5). Age at death: 60–80. | |||||
| B. Mayo eGWAS (Data Citations 3,4) | WG-DASL gene expression measures for a subset of Mayo Brain Bank subjects that were included in the Mayo LOAD GWAS: RNA was isolated from two brain regions: TCX and CER. This data is utilized to identify loci associated with brain gene expression in subjects with AD, subjects with Other brain pathologies that do not meet criteria for AD (Non-AD), and the combined cohort. | Mayo Brain Bank/Temporal Cortex | N=202 AD, 197 Non-AD controls | Post-mortem: AD Cases (Braak ≥4.0) and Other Pathologies (Braak ≤2.5). Age at death: 60–80. | Gene expression phenotypes, eGWAS results, covariates | Illumina WG-DASL | Zou et al.10, PLoS Genetics |
| Mayo Brain Bank/Cerebellum | N=197 AD, 177 Non-AD controls | ||||||
| C. Mayo Pilot RNAseq (Data Citation 5) | RNAseq gene expression measures for a subset of Mayo Brain Bank subjects that were included in the Mayo LOAD GWAS: RNA was isolated from TCX. This data is utilized to identify loci associated with brain gene expression in subjects with AD and subjects with PSP. | Mayo Brain Bank/Temporal Cortex | N=94 AD, 92 PSP | Post-mortem: AD Cases (Braak ≥4.0) and pathologic diagnosis of PSP (Braak≤2.5). Age at death: 60–80. | Gene expression phenotypes, covariates | IlluminaHiSeq2000, 50 bp, paired end RNAseq | Allen et al.18, Neurology:Genetics |
| D. Mayo RNAseq (Data Citations 6,7) | RNAseq gene expression measures for subjects from the Mayo Brain Bank non-overlapping with the Mayo LOAD GWAS, and also from Banner Sun Health Institute. RNA was isolated from two brain regions: TCX and CER. This data is utilized to compare brain gene expression between different pairwise diagnostic groups. | Mayo Brain Bank and Banner Sun Health/Temporal Cortex | N=84 AD, 84 PSP, 30 pathologic aging, 80 controls | Post-mortem: AD Cases (Braak ≥4.0), pathologic diagnoses of PSP (Braak≤3), pathologic aging (Braak≤3) and elderly control brains (Braak≤3) without neurodegenerative diagnoses. Age at death≤60. | Gene expression phenotypes, covariates | IlluminaHiSeq2000, 101 bp, paired end RNAseq | NA |
| Mayo Brain Bank and Banner Sun Health/Cerebellum | N=86 AD, 84 PSP, 28 pathologic aging, 80 controls |
Figure 1. Overview of the relationship of the four genomic datasets herein described.
Study Populations
All of this work was approved by the Mayo Clinic Institutional Review Board. All human subjects or their next of kin provided informed consent. The characteristics of the four study populations are as follows:
Mayo LOAD GWAS
The characteristics of the cohort for this study (Data Citation 2) were previously described in detail1. Briefly, this is a LOAD case versus control study composed in total of 2,099 subjects sourced from three different series, namely: Mayo Clinic Jacksonville, Mayo Clinic Rochester and Mayo Clinic Brain Bank series. These series are respectively termed as JS, RS and AUT in the GWAS publication1 (Table 1). Subjects in the Mayo Clinic Jacksonville and Mayo Clinic Rochester series were diagnosed clinically. These series consisted of 353 LOAD cases versus 331 controls; and 245 LOAD cases versus 701 controls. The Mayo Clinic Brain Bank series is a post-mortem cohort that consists of 246 LOAD cases versus 223 controls. All subjects were North American Caucasians. All clinical LOAD subjects were diagnosed as probable or possible AD, according to NINCDS-ADRDA criteria27. All clinical controls had a clinical dementia rating score of 0. LOAD subjects in the Mayo Clinic Brain Bank series met neuropathologic criteria for definite AD and had a Braak score of ≥4.0 (ref. 28), while controls did not meet neuropathologic criteria for AD, and each had Braak score of ≤2.5, which is an intermediary level of neurofibrillary tangle pathology between Braak score of 2 and 3; but most controls had neuropathologies unrelated to AD, including vascular dementia, frontotemporal dementia, dementia with Lewy bodies, multi-system atrophy, amyotrophic lateral sclerosis, and progressive supranuclear palsy. Ages, APOE ε4 genotype and sex distribution for the Mayo LOAD GWAS cohort are shown in Table 2. This study only included subjects with ages between 60 and 80 years, based on the assumption that much of the genetic risk for LOAD will be concentrated in this age group, especially given the age-dependent effects of the strongest AD risk variant apolipoprotein E ε4 (APOE4)28. Age for the clinically diagnosed LOAD cases is defined as age at first diagnosis of AD, since age at onset is not always available. Age at entry into the study is used for the clinically diagnosed controls. Age at death is utilized for the cases and controls in the postmortem Mayo Clinic Brain Bank series, given that for this cohort, age at clinical diagnosis/ evaluation is not always available. Illumina Hap300 microarray genotypes from the subjects in these three case-control series were utilized to conduct a GWAS of LOAD risk1.
Table 2. Demographics for the cohorts included in each of the four studies.
|
A. Mayo LOAD GWAS (Data Citation 2) |
B. Mayo eGWAS (Data Citations 3,4) |
C. Mayo Pilot RNAseq (Data Citation 5) |
||||||
|---|---|---|---|---|---|---|---|---|
|
TCX |
CER |
TCX |
||||||
| Variables | AD (n=844) | CON (1,255) | AD (n=202) | NON-AD (n=197) | AD (n=197) | NON-AD (n=177) | AD (n=94) | PSP (N=92) |
| Mean Age±s.d. (Range) | 74.0±4.8 (60–80) | 73.2±4.4 (60–80) | 73.6±5.5 (60–80) | 71.6±5.6 (60–80) | 73.6±5.6 (60–80) | 71.7±5.5 (60–80) | 74.1±5.7 (60–80) | 71.9±5.4 (60–80) |
| APOE4 positive/negative/null (%APOE4 positive) | 549/277/18 (65%) | 344/889/22 (27%) | 123/79/0 (61%) | 49/146/2 (25%) | 126/71/0 (64%) | 45/130/2 (25%) | 58/36/0 (62%) | 20/72/0 (22%) |
| Female (%) | 482 (57%) | 641 (51%) | 108 (53%) | 78 (40%) | 101 (51%) | 63 (36%) | 41 (44%) | 37 (40%) |
| Mean RIN±s.d. (Range) | NA | NA | 6.3±0.9 (5–9) | 6.9±1.0 (5–9.3) | 7.2±1.0 (5–9.4) | 7.2±1.0 (5–9) | 7.0±0.7 (6.2–9) | 7.0±0.9 (5.7–9.3) |
|
D. Mayo RNAseq (Data Citations 6,7) |
||||||||
|---|---|---|---|---|---|---|---|---|
|
TCX |
CER |
|||||||
| Variables | AD (n=84) | PSP (n=84) | Path Aging (n=30) | Control (n=80) | AD (n=86) | PSP (n=84) | Path Aging (n=28) | Control (n=80) |
| Mean Age±s.d. (Range) | 82.4±7.7 (60–90) | 74.0±6.5 (61–89) | 85.2±4.3 (76–90) | 82.6±8.8 (53–90) | 82.5±7.7 (60–90) | 74.0±6.5 (61–89) | 84.7±4.3 (76–90) | 82.5±8.3 (58–90) |
| APOE4 positive/negative (%APOE4 positive) | 43/41 (51%) | 13/71 (15%) | 10/20 (33%) | 10/70 (13%) | 43/43 (50%) | 13/71 (15%) | 9/19 (32%) | 11/69 (14%) |
| Female (%) | 48 (57%) | 33 (39%) | 17 (57%) | 39 (49%) | 49 (57%) | 33 (39%) | 16 (57%) | 39 (49%) |
| Mean RIN±s.d. (Range) | 8.6±0.5 (7.7–10.0) | 8.5±0.5 (7.8–10.0) | 7.4±1.0 (5.3–8.9) | 7.6±1.0 (5.3–9.7) | 8.3±0.8 (5.7–10.0) | 8.4±0.9 (5.5–10.0) | 7.5±1.0 (5.7–9.0) | 7.6±1.0 (5.5–9.7) |
Mayo eGWAS
This cohort was previously described in detail10. All subjects in the Mayo eGWAS (Data Citations 3,4) are a subset of the Mayo Clinic Brain Bank series from the Mayo LOAD GWAS (Data Citation 2) (Fig. 1). The Mayo eGWAS is a whole transcriptome expression study in which brain samples from two different regions were analyzed, namely cerebellum (CER), which is relatively spared in AD, and temporal cortex (TCX), which is typically one of the first regions to be affected with AD neuropathology29. Transcriptome measurements were obtained from TCX of 202 AD subjects and from CER of 197 AD (Table 1). This study also included subjects without AD neuropathology, which are referred to as non-AD, given that many of these subjects had other neuropathologies. There were 197 non-AD subjects with TCX transcriptome measurements with the following neuropathologic diagnoses: progressive supranuclear palsy (PSP, n=107); Lewy body disease (LBD, n=25); corticobasal degeneration (CBD, n=22); frontotemporal lobar degeneration (FTLD, n=16); multiple system atrophy (MSA, n=11), vascular dementia (VaD, n=6); other (n=10). There were 177 non-AD subjects with CER transcriptome measurements that had the following neuropathologies: PSP (n=98); LBD (n=23); CBD (n=22); FTLD (n=15); MSA (n=7); VaD (n=4); other (n=8). Eighty-five percent of the subjects in the TCX cohort overlapped with those in the CER cohort. Demographics for the Mayo eGWAS subjects and samples, including RNA quality as assessed by RNA Integrity Numbers (RIN) are shown in Table 2.
Mayo Pilot RNAseq
All subjects in the Mayo Pilot RNAseq study (Data Citation 5) are a subset of the Mayo eGWAS (Data Citations 3,4), and are therefore also participants of the Mayo Clinic Brain Bank series that was included in the Mayo LOAD GWAS (Data Citation 2) (Fig. 1). The diagnostic categories in the Mayo Pilot RNAseq consist of 94 subjects with AD neuropathology and 92 PSP subjects, previously described18,26. PSP is a primary tauopathy characterized neuropathologically by neurofibrillary tangles (NFT) and tau-positive glial lesions29,30; and often presents clinically as a parkinsonian disorder. All PSP subjects were diagnosed neuropathologically by a single neuropathologist (DWD). For this study, only TCX samples were assessed (Table 2).
Mayo RNAseq
The subjects from this cohort are non-overlapping with the cohorts described above. The Mayo RNAseq cohort was utilized to generate RNAseq-based whole transcriptome data from 278 TCX26 (Data Citation 6) and 278 CER (Data Citation 7) samples. Two hundred thirty-eight subjects had both CER and TCX RNAseq and the rest had either CER or TCX RNAseq measurements based on tissue availability. CER samples were from the following diagnostic categories: 86 AD, 84 PSP, 28 pathologic aging and 80 controls without neurodegenerative diagnoses. TCX samples had the following diagnostic groups: 84 AD, 84 PSP, 30 pathologic aging and 80 controls. Control subjects each had Braak28 NFT stage of 3.0 or less, CERAD31 neuritic and cortical plaque densities of 0 (none) or 1 (sparse) and lacked any of the following pathologic diagnoses: AD, Parkinson’s disease (PD), DLB, VaD, PSP, motor neuron disease (MND), CBD, Pick’s disease (PiD), Huntington’s disease (HD), FTLD, hippocampal sclerosis (HipScl) or dementia lacking distinctive histology (DLDH). Subjects with pathologic aging also lacked the above diagnoses and had Braak NFT stage of 3.0 or less, but had CERAD neuritic and cortical plaque densities of 2 or more. None of the pathologic aging subjects had a clinical diagnosis of dementia or mild cognitive impairment. Given the presence of amyloid plaques, but not tangles and the absence of dementia, pathologic aging is considered to be either a prodrome of AD or a condition, in which there is resistance to the development of NFT and/or dementia32.
Within the Mayo RNAseq cohort (Data Citations 6,7), all AD and PSP subjects were from the Mayo Clinic Brain Bank, and all pathologic aging subjects were obtained from the Banner Sun Health Institute. Thirty-four control CER and 31 control TCX samples were from the Mayo Clinic Brain Bank, and the remaining control tissue was from the Banner Sun Health Institute. All subjects were North American Caucasians. All but control subjects, had ages at death ≥60, and a more relaxed lower age cutoff of ≥50 was applied for normal controls to achieve sample sizes similar to that of AD and PSP subjects. No upper age limit was imposed on this cohort, however when subjects had ages at death of ≥90, their ages were recorded as ‘90_or_above’ and shown as ‘90’ in Table 2 to protect patient confidentiality.
Table 2 details the demographic characteristics of the Mayo RNAseq cohort (Data Citations 6,7). PSP subjects tended to be younger than the other diagnostic groups. As expected, there was a greater frequency of APOE4 positive subjects in the AD group, followed by pathologic aging, then PSP and control subjects. AD and pathologic aging subjects had greater female sex frequency (57%), followed by controls (49%), then PSP subjects (39%). RIN for all samples were selected to be ≥5.0. Pathologic aging and control samples had slightly lower RINs than AD and PSP samples, due to limitations in availability of samples in these former diagnostic categories.
Molecular Data
Sample collection and processing
For the Mayo LOAD GWAS (A) (Data Citation 2), DNA samples were collected and processed as previously described1. For the antemortem Mayo Clinic Jacksonville and Mayo Clinic Rochester series, whole blood samples were collected in 10 ml EDTA tubes followed by DNA extraction using AutoGenFlex STAR instrument (AutoGen), whereas cerebellar tissue was used for DNA extraction from the postmortem Mayo Clinic Brain Bank series using the Wizard Genomic DNA purification kit (Promega). Given limited amounts of DNA from samples in the Mayo Clinic Rochester series and Mayo Clinic Brain Bank series, whole genome amplification (WGA) was applied using the Illustra GenomiPhi V2 DNA Amplification Kit (GE Healthcare Bio-Sciences), in four 5 ml reactions that utilized 5–15 ng genomic DNA as a template. Subsequent to the pooling of these reaction products, WGA DNA was subjected to quality control (QC) using SNP genotyping as previously described.
RNA extraction methods for the Mayo eGWAS10 (B) (Data Citations 3,4) and Mayo Pilot RNAseq18 (C) (Data Citation 5) were previously described. Total RNA was extracted from frozen brain samples using the Ambion RNAqueous kit (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. Brain samples for the Mayo RNAseq (D) (Data Citations 6,7) study underwent RNA extractions via the Trizol/chloroform/ethanol method, followed by DNase and Cleanup of RNA using Qiagen RNeasy Mini Kit and Qiagen RNase -Free DNase Set. The quantity and quality of all RNA samples were determined by the Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano Chip (Agilent Technologies, Santa Clara, CA). Samples had to have an RNA Integrity Number (RIN) ≥5.0 for inclusion in either study (Table 2).
Data generation
The genotype data for the Mayo LOAD GWAS (A) (Data Citation 2) was generated using HumanHap300-Duo Genotyping BeadChips1, which were processed with an Illumina BeadLab station at the Mayo Clinic Genotyping Shared Resource (currently Mayo Clinic Medical Genome Facility=MGF, Rochester, Minnesota) according to the manufacturer’s protocols. Two samples were genotyped per chip for 318,237 SNPs across the genome. Genotype calls were made using the auto-calling algorithm in Illumina’s BeadStudio 2.0 software.
For the Mayo eGWAS study (B) (Data Citations 3,4), transcript levels were measured using the Whole Genome DASL assay (Illumina, San Diego, CA) as previously described10. Probe annotations were done based on NCBI RefSeq, Build 36.2. The RNA samples were randomized across the chips and plates using a stratified approach to ensure balance with respect to diagnosis, age, gender, RIN and APOE genotype. Raw probe mRNA expression data were exported from GenomeStudio software (Illumina Inc.) and preprocessed for background correction, variance stabilizing transformation, quantile normalization and probe filtering using the lumi package of BioConductor33.
Samples for both Mayo Pilot RNAseq (C) (Data Citation 5) and Mayo RNAseq (D) (Data Citations 6,7) studies were randomized prior to transfer to the Mayo Clinic MGF Gene Expression Core for library preparation and then the Sequencing Core for RNA sequencing. Mayo Pilot RNAseq (C) (Data Citation 5) AD and PSP samples were randomized across flowcells, taking into account age at death, sex and RIN. These samples underwent library preparation and sequencing at different times and therefore should be considered as separate datasets. Likewise, Mayo RNAseq (D) of TCX26 and CER samples (Data Citations 6,7, respectively) underwent RNAseq at different times. These samples were randomized across flowcells, taking into account age at death, sex, RIN, Braak stage and diagnosis. The TruSeq RNA Sample Prep Kit (Illumina, San Diego, CA) was used for library preparation from all samples. The library concentration and size distribution was determined on an Agilent Bioanalyzer DNA 1000 chip. All samples were run in triplicates using barcoding (3 samples per flowcell lane). For Mayo Pilot RNAseq (C) (Data Citation 5) samples, 50 base-pair, paired-end sequencing was done, whereas Mayo RNAseq (D) (Data Citations 6,7) samples underwent 101 bp, paired-end sequencing.
Data Processing
Mayo LOAD GWAS (A) (Data Citation 2) genotypes from Illumina BeadStudio 2.0 software were utilized to generate lgen, map and fam files that were imported into PLINK34 and converted to binary ped (.bed) and map (.bim) files, which are deposited together with PLINK format fam and covariate files (DOI and descriptions for each these files are provided in Table 3 (available online only)).
Table 3. Description of data files deposited in the AMP-AD Knowledge Portal.
| Study Name | doi | Data Type | File Name | doi | File Type | File Column Headers Definitions | Reference |
|---|---|---|---|---|---|---|---|
| The Genotype and Covariate data from the Mayo LOAD GWAS study (A) and the eSNP results under the Mayo eGWAS study (B) were previously made available through the NIAGADS respository: https://www.niagads.org/datasets/ng00043 and https://www.niagads.org/datasets/ng00025 respectively. | |||||||
| A. Mayo LOAD GWAS (Data Citation 2) | 10.7303/syn2910256 | Genotype and Covariate | MayoLOADGWAS_SNPGenotypes_covariates.csv | 10.7303/syn3205821.6 | Covariate information for subjects included in Mayo LOAD GWAS, comma delimited text file | FID=Family ID; IID=Individual ID; RSGWAS=‘1’ indicates if sample is part of Mayo Clinic Rochester Antemortem Cohort; AUTGWAS=‘1’ indicates if sample is part of Mayo Clinic Brain Bank Postmortem Cohort; AgeOver60=number of years beyond the age of 60 for age at death (Mayo Clinic Brain Bank Postmortem Cohort) or age at diagnosis (Mayo Clinic Rochester and Jacksonville Antemortem Cohorts); Sex=1 if Male; APOE4_Dose (+/−)=‘1’ indicates carriers of at least one copy of the APOEε4 allele; APOE4_dosage (0,1,2)= number of APOEε4 alleles ; APOE_Genotype=APOE genotype calls. For all columns ‘−9’ indicates missing data. | Carrasquillo et al., 1, Nature Genetics |
| MayoLOADGWAS_SNPGenotypes.bed | 10.7303/syn3205812.4 | LOAD GWAS binary ped file (PLINK format), genotype information | Binary File, No Column Headers. | ||||
| MayoLOADGWAS_SNPGenotypes.bim | 10.7303/syn3205814.4 | LOAD GWAS binary map file (PLINK format), variant information | Binary File, No Column Headers. | ||||
| MayoLOADGWAS_SNPGenotypes.fam | 10.7303/syn3205816.4 | LOAD GWAS fam file (PLINK format), pedigree and phenotype information | Six columns: Column 1=Family ID; Column 2= Individual ID; Column 3=Paternal ID; Column 4=Maternal ID; Column 5=Sex (1=male; 2=female); Column 6=Phenotype (1=control; 2= AD case) | ||||
| B. Mayo eGWAS (Data Citation 3,Data Citation 4) | 10.7303/syn3157225 | Array Expression and Covariate (Data Citation 3) | MayoEGWAS_arrayExpression_CBE_covariates.csv | 10.7303/syn3256502.1 | Covariate information for subjects with cerebellum expression measures, comma delimited text file. | Dxn=Diagnosis (0=Non-AD, 1=AD); Sex=1 if female and 0 if male; Age=Age at Death; E4dose=Number of APOEε4 alleles; plate1-plate4 (cerebellum) or plate 2-plate 5 (temporal cortex)=PCR plate - technical covariate; RIN=RNA intergrity number - a numerical assessment of the integrity of RNA; RINsqAdj=(RIN-RINmean)^2 - a statistical adjustment of the RIN. | Zou et al., 10, PLoS Genetics |
| MayoEGWAS_arrayExpression_TCX_covariates.csv | 10.7303/syn3617056.1 | Covariate information for subjects with temporal cortex expression measures, comma delimited text file. | |||||
| MayoEGWAS_arrayExpression_CBE.csv | 10.7303/syn3256501.1 | Gene expression phenotypes from cerebellum tissue samples, comma delimited text file. | FID=Family ID; IID=Individual ID; ILMN_1762337 to ILMN_2137536=WG-DASL Illumina Probe ID. | ||||
| MayoEGWAS_arrayExpression_TCX.csv | 10.7303/syn3617054.1 | Gene expression phenotypes from temporal cortex tissue samples, comma delimited text file. | |||||
| 10.7303/syn3157249 | eSNP Results (Data Citation 4) | MayoEGWAS_analysis_eQTL_CBE_results_AD.gz | 10.7303/syn3207163.3 | Brain expression GWAS (eGWAS) results obtained using Hap300 genotypes in the cerebellar samples of the AD subjects, text file with 444,372 rows. | CHR=Chromosome; SNP=SNP rs number; BP=Physical position (base-pair) according to NCBI Ref Seq, Build 36.2; A1=Tested Allele; TEST=The ‘additive’ SNP genotype test; NMISS=Number of non-missing individuals included in analysis; BETA=Regression coefficient. Based on the SNP minor allele using an additive model; STAT=Coefficient t-statistic; P=Asymptotic p-value for t-statistic (uncorrected); PROBE=WG-DASL Illumina Probe ID; txStart=Starting base pair for the RefSeq gene the brain level of which is tested for associations. (Based on Build 36.2); txEnd=Ending base pair for the RefSeq gene the brain level of which is tested for associations. (Based on Build 36.2); SYMBOL=Gene symbol for the RefSeq gene tested in the eGWAS; hasSNP=If the probe sequence harbors ≥1 SNPs, then this is shown as ‘TRUE’ under the ‘SNP-In-Probe’ column, and ‘FALSE’, otherwise. | ||
| MayoEGWAS_analysis_eQTL_CBE_results_nonAD.gz | 10.7303/syn3207167.3 | Brain expression GWAS (eGWAS) results obtained using Hap300 genotypes in the cerebellar samples of the non-AD subjects, text file with 443,171 rows | |||||
| MayoEGWAS_analysis_eQTL_CBE_results.gz | 10.7303/syn3207165.3 | Brain expression GWAS (eGWAS) results obtained using Hap300 genotypes in the cerebellar samples of the combined AD and non-AD subjects, text file with 443,784 rows | |||||
| MayoEGWAS_analysis_eQTL_TCX_results_AD.gz | 10.7303/syn3207169.3 | Brain expression GWAS (eGWAS) results obtained using Hap300 genotypes in the temporal cortex samples of the AD subjects, text file with 450,813 rows. | |||||
| MayoEGWAS_analysis_eQTL_TCX_results_nonAD.gz | 10.7303/syn3207175.3 | Brain expression GWAS (eGWAS) results obtained using Hap300 genotypes in the temporal cortex samples of the non-AD subjects, text file with 440,065 rows. | |||||
| MayoEGWAS_analysis_eQTL_TCX_results.gz | 10.7303/syn3207173.3 | Brain expression GWAS (eGWAS) results obtained using Hap300 genotypes in the temporal cortex samples of the combined AD and non-AD subjects, text file with 445,356 rows. | |||||
| MayoEGWAS_analysis_eQTL_CBE_imputedResults_AD.gz | 10.7303/syn3207177.3 | Brain expression GWAS (eGWAS) results obtained using HapMap2 imputed genotypes in the cerebellar samples of the AD subjects, text file with 4,427,924 rows. | CHR=Chromosome; SNP=SNP rs number; BP=Physical position (base-pair) according to NCBI Ref Seq, Build 36.2; A1=Tested Allele; TEST=The ‘additive’ SNP genotype test; NMISS=Number of non-missing individuals included in analysis; BETA=Regression coefficient. Based on the SNP minor allele using an additive model; STAT=Coefficient t-statistic; P=Asymptotic p-value for t-statistic (uncorrected); PROBE=WG-DASL Illumina Probe ID; txStart=Starting base pair for the RefSeq gene the brain level of which is tested for associations. (Based on Build 36.2); txEnd=Ending base pair for the RefSeq gene the brain level of which is tested for associations. (Based on Build 36.2); SYMBOL=Gene symbol for the RefSeq gene tested in the eGWAS; hasSNP=If the probe sequence harbors ≥1 SNPs, then this is shown as ‘TRUE’ under the ‘SNP-In-Probe’ column, and ‘FALSE’, otherwise. We also calculated for each probe within each analytic group, percent detection rate above background. Probes that are detected in >12.5%, >25%, >50% and >75% of the subjects in each analytic group are annotated by four separate columns labeled respectively and indicated with ‘TRUE’ and ‘FALSE’ statetments. | ||||
| MayoEGWAS_analysis_eQTL_CBE_imputedResults_nonAD.gz | 10.7303/syn3207181.3 | Brain expression GWAS (eGWAS) results obtained using HapMap2 imputed genotypes in the cerebellar samples of the non-AD subjects, text file with 4,419,055 rows | |||||
| MayoEGWAS_analysis_eQTL_CBE_imputedResults.gz | 10.7303/syn3207179.3 | Brain expression GWAS (eGWAS) results obtained using HapMap2 imputed genotypes in the cerebellar samples of the combined AD and non-AD subjects, text file with 4,483,512 rows | |||||
| MayoEGWAS_analysis_eQTL_TCX_imputedResults_AD.gz | 10.7303/syn3207183.3 | Brain expression GWAS (eGWAS) results obtained using HapMap2 imputed genotypes in the temporal cortex samples of the AD subjects, text file with 4,426,363 rows. | |||||
| MayoEGWAS_analysis_eQTL_TCX_imputedResults_nonAD.gz | 10.7303/syn3207187.3 | Brain expression GWAS (eGWAS) results obtained using HapMap2 imputed genotypes in the temporal cortex samples of the non-AD subjects, text file with 4,425,955 rows. | |||||
| MayoEGWAS_analysis_eQTL_TCX_imputedResults.gz | 10.7303/syn3207185.3 | Brain expression GWAS (eGWAS) results obtained using HapMap2 imputed genotypes in the temporal cortex samples of the combined AD and non-AD subjects, text file with 4,484,344 rows. | |||||
| C. Mayo Pilot RNAseq (Data Citation 5) | 10.7303/syn3157268 | RNA-seq Alignment, Expression and Covariate | MayoPilotRNAseq Alzheimers Disease RNAseq BAMs | 10.7303/syn5580964 | Folder of BAM alignment files, 1 file per sample. Sample ID (IID) for each subject is provided in the first 12 digits of each file name. | NA | Allen et al., 18, Neurology:Genetics |
| MayoPilotRNAseq Progressive Supranuclear Palsy RNAseq BAMs | 10.7303/syn5584594 | Folder of BAM alignment files, 1 file per sample. Sample ID (IID) for each subject is provided in the first 12 digits of each file name. | |||||
| MayoPilotRNAseq_RNAseq_TCX_AD_covariates.csv | 10.7303/syn3607480.2 | Covariate information for AD subjects with temporal cortex expression measures, comma delimited text file. | IlluminaSampleID=Individual ID (matches corresponding IID in Mayo LOAD GWAS files); Sex=M if Male and F if Female; Age at death; RIN=RNA intergrity number - a numerical assessment of the integrity of RNA; RINsqAdj=(RIN-RINmean)^2 - a statistical adjustment of the RIN; Library Batch=RNAseq library preparation batch; FCC1MR9ACXX - FCD1LUUACXX (AD) or FCD1GH3ACXX -FCC1CDJACXX (PSP)=‘1’ indicates sample was on sequencing flowcell - technical covariate; Flowcell=sequencing flowcell name. | ||||
| MayoPilotRNAseq_RNAseq_TCX_PSP_covariates.csv | 10.7303/syn3607506.1 | Covariate information for PSP subjects with temporal cortex expression measures, comma delimited text file. | |||||
| MayoPilotRNAseq_RNAseq_TCX_AD_geneCounts.tsv | 10.7303/syn3607482.2 | Gene expression phenotypes from AD temporal cortex tissue samples, space delimited text file with 64,254 rows. | Column 1=Ensemble Gene IDs; 1823480105_B - 1796012340_B=Illumina Sample ID's (matches corresponding IID in Mayo LOAD GWAS files). | ||||
| MayoPilotRNAseq_RNAseq_TCX_AD_geneCounts_normalized.tsv | 10.7303/syn3607497.1 | Normalized gene expression phenotypes from AD temporal cortex tissue samples, tab delimited text file with 64,254 rows | |||||
| MayoPilotRNAseq_RNAseq_TCX_AD_transcriptCounts.tsv | 10.7303/syn3607485.2 | Transcript expression phenotypes from AD temporal cortex tissue samples, space delimited text file with 208,245 rows. | Column 1=Ensemble Transcript IDs; 1823480105_B - 1796012340_B=Illumina Sample ID's (matches corresponding IID in Mayo LOAD GWAS files). | ||||
| MayoPilotRNAseq_RNAseq_TCX_AD_transcriptCounts_normalized.tsv | 10.7303/syn3607502.1 | Normalized transcript expression phenotypes from AD temporal cortex tissue samples, tab delimited text file with 208,245 rows. | |||||
| MayoPilotRNAseq_RNAseq_TCX_PSP_geneCounts.tsv | 10.7303/syn3607487.2 | Gene expression phenotypes from PSP temporal cortex tissue samples, space delimited text file with 64,254 rows | Column 1=Ensemble Gene IDs; 1811024586_A - 1811024534_A=Illumina Sample ID's (matches corresponding IID in Mayo LOAD GWAS files). | ||||
| MayoPilotRNAseq_RNAseq_TCX_PSP_geneCounts_normalized.tsv | 10.7303/syn3607513.1 | Normalized gene expression phenotypes from PSP temporal cortex tissue samples, tab delimited text file with 64,254 rows | |||||
| MayoPilotRNAseq_RNAseq_TCX_PSP_transcriptCounts.tsv | 10.7303/syn3607489.2 | Transcript expression phenotypes from PSP temporal cortex tissue samples, space delimited text file with 208,245 rows. | Column 1=Ensemble Transcript IDs; 1811024586_A - 1811024534_A=Illumina Sample ID's (matches corresponding IID in Mayo LOAD GWAS files). | ||||
| MayoPilotRNAseq_RNAseq_TCX_PSP_transcriptCounts_normalized.tsv | 10.7303/syn3607519.1 | Normalized transcript expression phenotypes from PSP temporal cortex tissue samples, tab delimited text file with 208,245 rows. | |||||
| D. Mayo RNAseq (Data Citation 6,Data Citation 7) | 10.7303/syn3163039 | TCX RNA-seq Alignment, Expression and Covariate (Data Citation 6) | MayoRNAseq Temporal Cortex BAMs | 10.7303/syn4894912 | Folder of BAM alignment files, 1 file per sample. Sample ID (IID) for each subject is provided in the first 6 to 8 digits of each file name. | NA | NA |
| MayoRNAseq_RNAseq_TCX_covariates.csv | 10.7303/syn3817650.5 | Covariate information for subjects with temporal cortex expression measures, comma delimited text file. | Sample ID=3 to 5 digit unique sample identifier followed by _TCX; Source=Pathology lab that provided the tissue; Tissue=Brain region sampled; RIN=RNA intergrity number - a numerical assessment of the integrity of RNA; Diagnosis=Pathological diagnosis at time of death (AD=Alzheimer's Disease; PSP=Progressive Supranuclear Palsy; Pathologic Aging; Control); Sex=M if Male, F if Female; AgeAtDeath; Flowcell=sequencing flowcell. | ||||
| MayoRNAseq_RNAseq_TCX_geneCounts.tsv | 10.7303/syn4650257.8 | Gene expression phenotypes, temporal cortex tissue samples, space delimited text file with 64,254 rows | Column 1=Ensemble Gene IDs; 11344_TCX to 1047_TCX etc.=Sample ID's (matches corresponding SampleID in Covariate file). | ||||
| MayoRNAseq_RNAseq_TCX_geneCounts_normalized.tsv | 10.7303/syn4650265.4 | Normalized gene expression phenotypes, temporal cortex tissue samples, tab delimited text file with 64,254 rows | |||||
| MayoRNAseq_RNAseq_TCX_transcriptCounts.tsv | 10.7303/syn5600752.2 | Transcript expression phenotypes, temporal cortex tissue samples, space delimited text file with 208,245 rows | Column 1=Ensemble Transcript IDs; 11344_TCX to 1047_TCX etc.=Sample ID's (matches corresponding SampleID in Covariate file). | ||||
| MayoRNAseq_RNAseq_TCX_transcriptCounts_normalized.tsv | 10.7303/syn5600755.2 | Normalized Transcript expression phenotypes, temporal cortex tissue samples, tab delimited text file with 208,245 rows | |||||
| 10.7303/syn5049298 | CER RNA-seq Alignment, Expression and Covariate (Data Citation 7) | MayoRNAseq Cerebellum BAMs | 10.7303/syn5049322 | Folder of BAM alignment files, 1 file per sample. Sample ID for each subject is provided in the first 6 to 8 digits of each file name. | NA | ||
| MayoRNAseq_RNAseq_CBE_covariates.csv | 10.7303/syn5223705.2 | Covariate information for subjects with cerebellum expression measures, comma delimited text file. | Sample ID=3 to 5 digit unique sample identifier followed by _CER; Source=Pathology lab that provided the tissue; Tissue=Brain region sampled; RIN=RNA intergrity number - a numerical assessment of the integrity of RNA; Diagnosis=Pathological diagnosis at time of death (AD=Alzheimer's Disease; PSP=Progressive Supranuclear Palsy; Pathologic Aging; Control); Sex=M if Male, F if Female; AgeAtDeath; ApoE=APOE genotype; Flowcell=sequencing flowcell. | ||||
| MayoRNAseq_RNAseq_CBE_geneCounts.tsv | 10.7303/syn5201012.4 | Gene expression phenotypes, cerebellum tissue samples, tab delimited text file with 64,254 rows | Column 1=Ensemble Gene IDs; 1000_CER to 991_CER= Sample ID's (matches corresponding SampleID in Covariate file). | ||||
| MayoRNAseq_RNAseq_CBE_geneCounts_normalized.tsv | 10.7303/syn5201007.4 | Normalized gene expression phenotypes, cerebellum tissue samples, tab delimited text file with 64,254 rows | |||||
| MayoRNAseq_RNAseq_CBE_transcriptCounts.tsv | 10.7303/syn5600773.2 | Transcript expression phenotypes, cerebellum tissue samples, space delimited text file with 208,245 rows | Column 1=Ensemble Transcript IDs; 1000_CER to 991_CER=Sample ID's (matches corresponding SampleID in Covariate file). | ||||
| MayoRNAseq_RNAseq_CBE_transcriptCounts_normalized.tsv | 10.7303/syn5600772.2 | Normalized Transcript expression phenotypes, cerebellum tissue samples, tab delimited text file with 208,245 rows |
The Mayo eGWAS WG-DASL microarray expression dataset from TCX and CER (B) includes covariates and probe expression levels (Data Citation 3), which are preprocessed as published10 and described above. The Mayo eGWAS ‘eSNP Results’ (Data Citation 4) are the eQTL results from the test of association between the Mayo LOAD GWAS (Data Citation 2) genotypes and the WG-DASL gene expression measures analyzed by multivariable linear regression using an additive model in PLINK34, as published previously10 (DOI and descriptions for each these files are provided in Table 3 (available online only)). These analysis used preprocessed probe transcript levels as traits, SNP minor allele dosage as the independent variable, and adjusted for the following covariates: APOE ε4 dosage (0, 1, 2), age at death, sex, PCR plate, RIN and adjusted RIN squared (RIN-RINmean)2. Analyses were limited to SNP-probe pairs that were in-cis, defined as +/−100 kb of the targeted gene according to NCBI Build 36. The ADs and non–ADs were analyzed both separately and jointly. The joint analyses included diagnosis as an additional covariate (AD=1, non–AD=0). Results of analyses for both the genotyped SNPs as well as genotypes imputed to HapMap2 reference are provided. HapMap2 imputations were done as described10. The eGWAS results were previously made available through the NIAGADS repository (https://www.niagads.org/datasets/ng00025).
The Mayo Pilot RNAseq18 (Data Citation 5), Mayo RNAseq TCX26 and CER data (Data Citations 6,7, respectively) were processed using the same analytic pipeline. Read alignments were done using the SNAPR software35, an RNA sequence aligner based on SNAP, using GRCh38 reference and Ensembl v77 gene models. Outputs include per-sample gene and transcript counts, which are merged into a single file per data type (gene or transcript) that contains data for all samples across all genes/transcripts (DOI and descriptions for each these files are provided in Table 3 (available online only)). Alignment with SNAPR starts with the creation of hash indices built from both a reference genome GRCh38 and transcriptome GRCh38.77. SNAPR filters fastq reads by Phred score (>80% of the read must have a Phred score >= 20) and simultaneously aligns each read (or read pair) to both the genome and transcriptome. The best alignment is written to a sorted BAM file with read counts simultaneously tabulated and written for each sample. Read counts are given by gene ID and transcript ID (two separate files). We have previously tested the read counts generated by SNAPR to the read counts generated by HT-Seq and found them to be very comparable.
Post-processing was also performed using the same pipeline for these three RNAseq datasets as follows: The individual read count files produced by SNAPR are merged into a single file using two scripts: merge_count_files.R and a dataset-specific read-count merge script. These scripts generate the corresponding _counts.txt.gz files. The merged count files are normalized with the normalize_readcounts.R script, which uses the edgeR implementation of the trimmed mean of M-values (TMM) normalization method to calculate counts per million (CPM). These normalized counts are saved for both gene and transcript levels (DOI and descriptions for each these files are provided in Table 3 (available online only)).
Code Availability
The R script called merge_count_files.R36 was used to merge the RNAseq read count files produced by SNAPR into a single file, and can be found at https://github.com/CoryFunk/AMP-AD-scripts/blob/master/combine_count_files.pl. Also, the R script used to normalize the merged RNAseq read counts, called normalize_readcounts.R36, can be found at https://github.com/CoryFunk/AMP-AD-scripts/blob/master/tmm_normalization.R.
Data Records
Data available for studies A-D (, , , , , ; Table 3 (available online only)) consists of a set of files that contain genomic, genetic or covariate data for a defined set of samples; analytic results are also provided when available. Data files can be found in the Sage Bionetworks AMP-AD Knowledge Portal (Data Citation 1) in study specific folders (and subfolders). Users can identify and search for data files and data descriptions using the unique Synapse ID and corresponding DOI provided in Table 3 (available online only). Each sample within a study has a unique sample ID, this sample ID is consistent across all files within the study, and files in other studies where applicable. The relationship between studies and sample overlaps is illustrated in Fig. 1. The samples in study C (Data Citation 5) are a subset of the samples in study B (Data Citation 3) which are likewise a subset of the samples in study A (Data Citation 2); the samples in study D (Data Citations 6,7) are independent of those in studies A-C. The Usage Notes section describes the data accession conditions, and the steps for requesting access.
Technical Validation
Data QC
Mayo LOAD GWAS (A) (Data Citation 2) QC methods were previously published1. Briefly, using PLINK34, subjects with genotyping call rates of <90%, duplicate genotyping and/or sex-mismatches between recorded and deduced sex were eliminated from the dataset. All SNPs with genotyping call rates <90%, minor allele frequencies <0.01, and/or Hardy-Weinberg p values <0.001 were also eliminated. Prior to QC, 318,237 SNPs were genotyped in 2,465 subjects. The available data includes the 313,504 SNP genotypes from 2,099 subjects that passed these QC parameters.
The Mayo eGWAS10 (B) (Data Citations 3,4) data was generated as follows: We annotated probes for presence of genetic variants by comparing their positions according to NCBI RefSeq, Build 36.3 to those of all variants within dbSNP131 and identified the list of probes that have ≥1 variants within their sequence. We depict this information in the files for the Mayo eGWAS, ‘eSNP Results’ (Data Citation 4) (Table 3 (available online only)), by including ‘SNP-In-Probe’ column, which has ‘TRUE’ if the probe sequence harbors ≥1 SNP, and ‘FALSE’, otherwise. We also calculated for each probe within each analytic group, percent detection rate above background. Probes that are detected in >12.5%, >25%, >50% and >75% of the subjects in each analytic group are annotated by four separate columns within the ‘eSNP Results’ (Data Citation 4) from the eGWAS that included HapMap2 imputed genotypes, described below. The purpose of these annotation columns is to enable others the flexibility to impose cutoffs based on presence/absence of variants within probe sequence and/or probe detection rates while providing the full dataset for completeness. The Mayo eGWAS (Data Citation 3,Data Citation 4) also included replicate samples as described for QC and to estimate intraclass coefficients (ICC), which is the between-subject variance, as a percentage of the total variance in probe expression10. There were 4 AD and 4 non-AD temporal cortex samples that were measured in 5 replicates; and 10 AD and 5 non-AD cerebellar sample replicates across five plates. Universal human RNA (UHR) samples were also run on each PCR plate as part of QC. The expression phenotypes include results from only one of the replicate subjects selected randomly and exclude UHR results. It should be noted that 3 AD and 9 non-AD subjects for TCX, and 4 AD subjects for CER, do not have associated GWAS genotypes as they did not pass≥1 GWAS QC parameter described above.
For the Mayo Pilot RNAseq18 (C) (Data Citation 5) data principal components analysis (PCA) identified 2 outliers in the AD and 4 in the PSP cohort. The covariates for these subjects were set to missing (=NA) in the respective covariate files (DOI and descriptions for these files are provide in Table 3 (available online only)). Hence, although 96 AD and 96 PSP subjects underwent sequencing in the Mayo Pilot RNAseq study, 94 AD and 92 PSP subjects were retained for analyses. It should be noted that of these subjects, 1 AD and 7 PSP subjects lack GWAS data due to either having genotype counts <90% or failing sex checks. PCA identified no outliers in the Mayo RNAseq (D) of TCX26 samples (Data Citation 6) but 2 such subjects in the CER analyses (Data Citation 7). The covariate data in the relevant CER files for these two subjects were set to missing. We likewise assessed the RNASeq data for sex discrepancies based on Y chromosome gene expression and documented sex and identified 2 subjects with mis-matched sex for both TCX and CER, plus a third subject in the CER cohort. These were also set to missing in the covariate files. At the time of this publication, the Mayo RNAseq subjects did not have GWAS genotypes deposited on Synapse.
Usage Notes
The data described herein is available for use by the research community and has been deposited in the AMP-AD Knowledge Portal (). Table 3 (available online only) provides a detailed description of the files deposited for the four studies, their specific Synapse identifiers (IDs), DOIs, the types of files and definitions of the column headers. These files (, , , , , ), and their assigned DOIs will be maintained in perpetuity in the AMP-AD Knowledge Portal (). Access to all of these files is enabled through the Sage Bionetworks, Synapse repository; and a subset of the files for the Mayo LOAD GWAS () and the Mayo eGWAS (, ) are also available via NIAGADS (www.niagads.org).
The AMP-AD Knowledge Portal hosts data derived from multiple cohorts that were generated as part of or used in support of the AMP-AD Target Identification and Preclinical Validation project (). The portal uses the Synapse software platform25 for backend support, providing users with both web-based and programmatic access to data files. All data files in the portal are annotated using a standard vocabulary to enable users to search for relevant content across the AMP-AD datasets using programmatic queries. Data is stored in a cloud based manner hosted by Amazon web services (AWS), which enables user to execute cloud-based compute. Detailed descriptions including data processing, QC metrics, and assay and cohort specific variables are provided for each file as applicable.
Access for the data described herein is controlled in a manner set forth by the institutional review board (IRB) at the Mayo Clinic. All data use terms include: (1) maintenance of data in a secure and confidential manner, (2) respect for the privacy of study participants, (3) citation of the data contributors in any publications resulting from data use, and (4) informing data contributors of resultant publications. Specific data use terms are provided for each dataset (, , , ) under the header ‘Terms of use’; users must register for a Synapse account and provide electronic agreement to these terms prior to accessing the study files. Access to the Mayo LOAD GWAS data (A) () requires a data use certificate (doi:10.7303/syn2954402.2). User approvals are managed by the Synapse Access and Compliance Team (ACT).
Data on the AMP-AD Knowledge Portal are annotated with a common dictionary of terms (doi:10.7303/syn5478487.2) to enable querying of the data using the Synapse analytical clients (R client: syn1834618, python client: syn1768504, command line client: syn2375225). Fields, their allowable values specific to the datasets described herein and the dictionary of annotations are shown in Table 3 (available online only). These annotations can be used to identify files of interest within the available datasets and to filter on any of the fields using the allowable values from the dictionary (an example is shown here: doi:10.7303/syn5585666.1).
Additional Information
How to cite this article: Allen, M et al. Human whole genome genotype and transcriptome data for Alzheimer's and other neurodegenerative diseases. Sci. Data 3:160089 doi: 10.1038/sdata.2016.89 (2016).
Supplementary Material
Acknowledgments
We thank the patients and their families for the sample and tissue donations. Without their generosity, this research would not be possible. The Mayo Clinic Alzheimer's Disease Genetic Studies were led by Dr Nilüfer Ertekin-Taner and Dr Steven G. Younkin, Mayo Clinic, Jacksonville, FL using samples from the Mayo Clinic Study of Aging, the Mayo Clinic Alzheimer's Disease Research Center, and the Mayo Clinic Brain Bank. Data collection was supported through funding by NIA grants P50 AG016574, R01 AG032990, U01 AG046139, R01 AG018023, U01 AG006576, U01 AG006786, R01 AG025711, R01 AG017216, R01 AG003949, NINDS grant R01 NS080820, the GHR foundation, CurePSP Foundation, and support from Mayo Foundation. Samples collected through the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. We thank Mrs. Kelly Viola for her assistance with revisions of this manuscript.
Footnotes
Below are the disclosures for R.C.P.: Pfizer, Inc., and Janssen Alzheimer Immunotherapy: Chair, Data Monitoring Committee. Hoffman-La Roche, Inc.: Consultant. Merck, Inc.: Consultant. Genentech, Inc.: Consultant. Biogen, Inc.: Consultant. Eli Lilly & Co.: Consultant. N.R.G.-R. has multicenter treatment study grants from Lilly and TauRx and consulted for Cytox. N.E.-T. has consulted for Cytox. The remaining authors declare no competing financial interests.
Data Citations
- 2016. Synapse. http://dx.doi.org/10.7303/syn2580853
- Carrasquillo M. M. 2016. Synapse. http://dx.doi.org/10.7303/syn2910256
- Zou F. 2016. Synapse. http://dx.doi.org/10.7303/syn3157225
- Zou F. 2016. Synapse. http://dx.doi.org/10.7303/syn3157249
- Allen M. 2016. Synapse. http://dx.doi.org/10.7303/syn3157268
- Allen M. 2016. Synapse. http://dx.doi.org/10.7303/syn3163039
- Allen M. 2016. Synapse. http://dx.doi.org/10.7303/syn5049298
References
- Carrasquillo M. M. et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer's disease. Nat Genet 41, 192–198 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nature genetics 41, 1088–1093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. C. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature genetics 41, 1094–1099 (2009). [DOI] [PubMed] [Google Scholar]
- Seshadri S. et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. Jama 303, 1832–1840 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj A. C. et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature genetics 43, 436–441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth P. et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nature genetics 43, 429–435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature genetics 45, 1452–1458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger G. U. et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature genetics 43, 699–705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J. et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nature genetics 41, 1308–1312 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou F. et al. Brain expression genome-wide association study (eGWAS) identifies human disease-associated variants. PLoS Genet 8, e1002707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon A. L. et al. A genome-wide association study of global gene expression. Nature genetics 39, 1202–1207 (2007). [DOI] [PubMed] [Google Scholar]
- Emilsson V. et al. Genetics of gene expression and its effect on disease. Nature 452, 423–428 (2008). [DOI] [PubMed] [Google Scholar]
- Saykin A. J. et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans. Alzheimers Dement 11, 792–814 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou F. et al. Gene expression levels as endophenotypes in genome-wide association studies of Alzheimer disease. Neurology 74, 480–486 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. et al. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology 79, 221–228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. et al. Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: an association study with mechanistic implications. Mol Neurodegener 7, 13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. et al. Association of MAPT haplotypes with Alzheimer's disease risk and MAPT brain gene expression levels. Alzheimers Res Ther 6, 39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M et al. Late-onset Alzheimer disease risk variants mark brain regulatory loci. Neurology: Genetics 1, e15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. J. et al. A survey of genetic human cortical gene expression. Nature genetics 39, 1494–1499 (2007). [DOI] [PubMed] [Google Scholar]
- Webster J. A. et al. Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet 84, 445–458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J. et al. Increased expression of BIN1 mediates Alzheimer genetic risk by modulating tau pathology. Mol Psychiatry 18, 1225–1234 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati L. N. et al. Genetic association of CR1 with Alzheimer's disease: a tentative disease mechanism. Neurobiol Aging 33, 2949 e5–2949 e12 (2012). [DOI] [PubMed] [Google Scholar]
- Ramasamy A. et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci 17, 1418–1428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S. B. et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 464, 773–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry J. M. et al. Developing predictive molecular maps of human disease through community-based modeling. Nature genetics 44, 127–130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. et al. Gene expression, methylation and neuropathology correlations at progressive supranuclear palsy risk loci. Acta Neuropathol 132, 197–211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944 (1984). [DOI] [PubMed] [Google Scholar]
- Farrer LA et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama 278, 1349–1356 (1997). [PubMed] [Google Scholar]
- Braak H. & Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 82, 239–259 (1991). [DOI] [PubMed] [Google Scholar]
- Hauw J. J. et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44, 2015–2019 (1994). [DOI] [PubMed] [Google Scholar]
- Mirra S. S. et al. Interlaboratory comparison of neuropathology assessments in Alzheimer's disease: a study of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD). J Neuropathol Exp Neurol 53, 303–315 (1994). [DOI] [PubMed] [Google Scholar]
- Wang J., Dickson D. W., Trojanowski J. Q. & Lee V. M. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol 158, 328–337 (1999). [DOI] [PubMed] [Google Scholar]
- Du P., Kibbe W. A. & Lin S. M. lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 (2008). [DOI] [PubMed] [Google Scholar]
- Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magis A. T., Funk C. C. & Price N. D. SNAPR: A Bioinformatics Pipeline for Efficient and Accurate RNA-Seq Alignment and Analysis. IEEE Life Sciences Letters 1, 22–25 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C. AMP-AD-scripts: AMP-AD Fl-Mayo-ISB. in Zenodo https://dx.doi.org/10.5281/zenodo.56828 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2016. Synapse. http://dx.doi.org/10.7303/syn2580853
- Carrasquillo M. M. 2016. Synapse. http://dx.doi.org/10.7303/syn2910256
- Zou F. 2016. Synapse. http://dx.doi.org/10.7303/syn3157225
- Zou F. 2016. Synapse. http://dx.doi.org/10.7303/syn3157249
- Allen M. 2016. Synapse. http://dx.doi.org/10.7303/syn3157268
- Allen M. 2016. Synapse. http://dx.doi.org/10.7303/syn3163039
- Allen M. 2016. Synapse. http://dx.doi.org/10.7303/syn5049298