Abstract
This single center study assessed the efficacy and outcomes of radioembolization (RE) for unresectable, chemorefractory colorectal cancer liver metastases in 53 heavily pre-treated patients. Median overall survival (OS) was 12.7 months and median liver progression-free survival 4.7 months. Patients with CEA levels ≥90ng/mL at the time of RE and with lymphovascular invasion on pathology of the primary had decreased OS.
Background/Introduction
This study assessed the efficacy and factors affecting outcomes of radioembolization (RE) using yttrium-90 (90Y) resin microspheres in patients with unresectable and chemorefractory colorectal cancer liver metastases (CLM).
Patients and Methods
Following an IRB waiver of approval, review of a HIPAA-registered, prospectively created and maintained database was performed. Patient demographics, disease characteristics, RE treatment parameters and additional treatments were evaluated for significance in predicting overall survival (OS) and liver progression-free survival (LPFS). Complications were evaluated according to the NCI Common Terminology Criteria for adverse events.
Results
From September 2009 to September 2013, 53 patients underwent RE at a median 35 months after CLM diagnosis. Median OS was 12.7 months. Multivariate analysis demonstrated that CEA levels at RE ≥90 ng/mL (p=0.004) and microscopic lymphovascular invasion of the primary (p=0.002) were independent predictors of decreased OS. Median LPFS was 4.7 months. At 4-8 and 12-16 weeks after RE, the majority of patients (80% and 61% respectively) according to RECIST had stable disease; additional evaluation by PERCIST led to reclassification in 77% of these cases (response or progression). No deaths were noted within the first 30 days. Within the first 90 post-RE days, four patients (8%) developed liver failure and five patients (9%) died, all with evidence of disease progression.
Conclusion
RE in the salvage setting was well-tolerated, permitting the administration of additional therapies and leading to a median OS of 12.7 months. Evaluation by PERCIST was more likely than RECIST to document response or progression when compared to baseline pre-RE assessment.
Keywords: Radioembolization, Yttrium-90, Hepatic Malignancy, Colorectal Cancer Liver Metastases, Arterially directed therapies, Selective Internal Radiation Therapy, SIRT
Introduction
Colorectal cancer (CRC) is the most common gastrointestinal malignancy and the second most common cause of cancer-related death.1 The liver is the most common site of metastatic spread, with a significant percentage of CRC patients developing liver metastases (CLM) during the course of their disease.2, 3 Although hepatic resection is considered the gold-standard treatment of CLM, only up to 20% of patients are surgical candidates due to the extent of hepatic disease, the presence of extrahepatic metastases or comorbid conditions.3-5 For the majority of patients not candidates for resection or for patients developing recurrent disease after resection, limited therapeutic options are available for hepatic disease control and survival prolongation. These options include liver-directed therapies such as tumor ablation, hepatic artery infusion pump (HAIP) chemotherapy, irinotecan eluting beads/chemoembolization and radioembolization with yttrium-90 (90Y).6-9
90Y radioembolization (RE) is an intra-arterial brachytherapy. The treatment consists of the injection of 90Y bound to non-biodegradable resin10-19 or glass9, 20-23 microspheres in the local or regional hepatic arterial system. 90Y is a beta-emitting isotope which decays to stable zirconium-90 with a half-life of 64.2 hours. This intra-arterial treatment delivers tumoricidal radiation doses selectively in the arterial capillary bed of the target tumors while relatively sparing the normal liver parenchyma, contributing to a good safety profile.11, 24 90Y bound to resin microspheres is approved by the Food and Drug Administration (FDA) for the treatment of CLM.11
The combination of RE with systemic chemotherapy16, 25 and/or HAIP therapy26 is associated with improved tumor response rates and increased progression-free and overall survival in selected patients with liver only or liver dominant colorectal cancer metastatic disease. However, patients often present for RE at the salvage stage with progression of disease after several prior treatments, including hepatic resection, ablation and chemotherapy with cytotoxic and biologic agents. Several studies have demonstrated an acceptable safety profile of RE when used as a salvage treatment,13, 15-19 despite the concern that these heavily pre-treated patients could be at increased risk of developing treatment-related complications such as RE - induced liver disease (REILD).27
The purpose of this study is to determine the clinical and oncologic outcomes of RE with 90Y resin microspheres (SIR-Spheres, Sirtex Medical, Sydney, Australia) for the management of unresectable and chemorefractory CLM and to identify factors that influence these oncologic outcomes in a heavily pretreated population.
Patients and methods
Following IRB waiver of approval, a HIPAA-registered retrospective review was performed to investigate the oncologic outcomes of patients treated with RE for CLM not responding to systemic and/or HAIP chemotherapy. Patient demographics (age, gender), disease characteristics (extent of tumor burden, tumor distribution, presence of extrahepatic disease at the time of RE, disease intervals), prior treatments (liver surgery, HAIP chemotherapy, systemic chemotherapy), procedural details (full dose delivery before stasis) and known prognostic factors such as primary lymphovascular invasion, tumor histology, k-ras tumor status, timing of hepatic metastases -synchronous vs. metachronous- and baseline CEA values before RE were recorded.
Inclusion and exclusion criteria
Inclusion criteria were: (a) age ≥ 18 years; (b) Eastern Cooperative Oncology Group (ECOG) performance status 0-2; (c) histologically confirmed primary adenocarcinoma of the colon or rectum; (d) CLM considered unresectable or not treatable using ablative methods; (e) adequate blood cell counts (WBC > 1.5 × 109/L, platelets > 100 × 109/L), (f) adequate renal function (creatinine < 1.5 mg/dL) and (g) bilirubin ≤ 1.5 mg/dL.
Exclusion criteria were: (a) prior hepatic radiotherapy; (b) evidence of severe cirrhosis; (c) severe portal hypertension as determined by clinical, radiologic or laboratory assessments; (d) evidence of either uncorrectable flow to the gastrointestinal tract and/or e) > 20% shunting to the lungs, as determined with a technetium-99m labeled macroaggregated albumin (99mTc-MAA) hepatic arterial perfusion scintigram. The number of extrahepatic disease sites per se was not considered an absolute exclusion criterion. All patients at the time of RE had liver-dominant disease and were considered candidates for RE even in the face of oligometastatic (up to 5 sites) extrahepatic disease, that was generally stable or controlled by chemotherapy.
Pre-procedural work-up/Angiographic mapping
All patients were evaluated at a clinic visit within 30 days before RE. Past medical history was recorded, physical examination was performed and relevant baseline laboratory values were measured. Pre-procedural baseline imaging with liver triphasic CT (in all cases) and with PET/CT (48/53 patients) was available within 30 days from RE for accurate re-staging of disease extent and for calculation of liver and CLM volumes. All patients underwent angiographic mapping before RE. During arteriography, the hepatic arterial anatomy and the tumor vascular supply were assessed. Extrahepatic vessels with hepatofugal flow within 3 cm from desired point of 90Y administration were prophylactically coil-embolized to prevent inadvertent delivery of 90Y in extrahepatic sites. Once the desired location(s) of RE administration(s) was determined, a total of 4-5 mCi of 99mTc-MAA were injected intra-arterially and subsequent liver/lung planar γ-scintigraphy was performed to detect extrahepatic activity (e.g. extrahepatic shunting to the gastrointestinal tract) and to estimate the percentage of shunting to the lungs (mean: 6%, range: 2-14.9%).
Treatment
At a median of 14 days after mapping angiography (mean: 16.1 days; range: 6-43 days), patients underwent RE with SIR-Spheres (Sirtex Medical, Sydney, Australia). The total activity of 90Y resin microsphere (in GBq) for each patient was calculated using the body surface area (BSA) method with the following formula: Activity = (BSA - 0.2) + Tumor Involvement / (Tumor Volume + Normal Liver Volume). Four patients required a 20% reduction to the calculated dose because of shunting to the lungs between 10-15%. Adjustments were not made based on prior treatment history.
CLM confined to one lobe was treated within one session. Two patients with initial unilobar disease received an additional session of RE in the contralateral lobe after 160 and 311 days respectively to treat new CLM. Sixteen/34 patients with bilobar disease distribution and extensive tumor burden received two consecutive lobar treatments, with a mean 41 days between the two sessions (range: 34-57 days) completing bilobar, total liver treatment. Eleven/34 patients with bilobar disease were treated within one session, as the limited extent of disease permitted sublobar microsphere infusions. Seven/34 patients with bilobar disease, initially scheduled for two treatment sessions, received only one lobar infusion of RE, due to either progression of disease in the treated lobe after the first treatment (n=4), extremely diseased hepatic arterial branches (n=2) or limited disease in the contralateral lobe that was successfully controlled with systemic chemotherapy alone (n=1).
Endpoints
Study endpoints included: radiographic and laboratory response, liver progression-free survival (LPFS) and patient overall survival (OS) after initial RE. Patient demographics, disease characteristics, prior treatments, procedural details and additional therapies after RE were evaluated in relation to OS and LPFS.
Documentation of tumor response/progression and definitions
Assessment of radiologic response within the treated hepatic volume was performed with contrast-enhanced CT and FDG-PET/CT at 4-8 and 12-16 weeks post-RE and compared to the pre-RE scans. Subsequent follow-up imaging was performed every 2-4 months. Radiologic response in the liver was evaluated based on changes in tumor size (1 to 5 target lesions selected per patient) using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0.28
Response was also assessed by changes in metabolic activity using PET Response Criteria in Solid Tumors (PERCIST).29-31 Taking PERCIST as a basis, a 30% decrease from baseline indicated response to treatment. Both RECIST and PERCIST were used for documentation of response and progression any time during follow-up. Changes in CEA levels were also monitored at these time points.32
Overall survival (OS) was defined as the time period from initial RE to patient death or last follow-up. Liver progression-free survival (LPFS) was defined as the time from initial RE to documented liver progression using RECIST and/or PERCIST.
Toxicity
Acute toxicity (≤ 30 days of treatment) and late toxicity (31-90 days) were evaluated for all patients based on the National Cancer Institute's Terminology Criteria for adverse events v3.0. All patients were followed-up at 2, 4-8 and 12-16 weeks after RE with clinic visits and blood work to evaluate for possible radiation-induced hepatotoxicity or other toxic effects.
Statistical analysis
The median follow-up period was calculated using the median OS of patients alive at last follow-up. OS and LPFS were estimated by the Kaplan-Meier method. Each variable was evaluated as a potential prognostic factor of OS and LPFS using the log-rank test or Cox regression analysis (for continuous variables). For OS, significant variables in univariate analysis (p≤0.05) were used as candidate variables in a multivariate Cox model. Forward model selection was performed using the Wald test as the criterion. Data analysis was performed using software (SPSS version 22, Chicago, IL).
Results
Fifty-three patients (median age 54 years, range 24-86 years) received a total of 71 RE sessions during the study period. Patient demographics are depicted in Table 1. Forty (76%) patients had synchronous liver metastases. Extrahepatic disease was documented in 41 (77%) patients at the time of RE. The median time from diagnosis of CLM to RE was 35.1 months (mean: 40.9, range: 5.9-161.7 months). All patients were heavily pretreated and all progressed after receiving systemic chemotherapy (28% of patients receiving three or more chemotherapy regimens). Twenty-nine (55%) patients had prior HAIP chemotherapy. Twenty-six (49%) patients had prior liver surgery. Thirty-four (64%) patients received additional therapy after RE: 32 (60%) received systemic chemotherapy, 12 (23%) HAIP chemotherapy and 5 (9%) thermal ablation.
Table 1.
Patient demographics and disease characteristics.
| Age (y), median (range) | 54 (24-86) |
| Sex, M:F (n) | 30:23 |
| Stage at primary diagnosis of CRC (I/ II/III/IV; n) | 2/3/8/40 |
| Synchronous Liver Disease, n (%) | 40 (75.5) |
| Pathologic evidence of vascular invasion from primary tumor (yes/no/unknown; n) | 35/22/6 |
| K-ras mutation, n (%) | 16 (30.2) |
| Surgical resection of primary disease, n (%) | 47 (88.7) |
| CEA levels at time of diagnosis of liver metastases (median, SD) | 119.9, 2218.2 |
| CEA levels before RE (median, SD; ng/mL) | 90.5, 1181.9 |
| Time from diagnosis of CRC to diagnosis of CLM (median, mean, range; months) | 0, 7, 0-64.2 |
| Time from diagnosis of CLM to RE (median, mean, range; months) | 35.1, 40.9, 5.9-161.7 |
| Prior liver surgery, n (%) | 26 (49.1) |
| Prior HAIP chemotherapy, n (%) | 29 (54.7) |
| Prior systemic chemotherapy (≥3 lines vs. <3) | 15/38 |
| Prior bevacizumab, n (%) | 41 (77.4) |
| Extent of hepatic replacement by tumor at time of RE (< 25% vs. ≥25%) | 45/8 |
| Presence of extrahepatic disease at time of RE, n (%) | 41 (77.4) |
| Number of sites with extrahepatic disease per patient at time of RE (median, range) | 1 (0-5) |
| - Lungs, n (%) | 32 (60.4) |
| - Nodes (thoracic/abdominal), n (%) | 26 (49.1) |
| - Bone, n (%) | 6 (11.3) |
| - Peritoneum, n (%) | 3 (5.7) |
| - Adrenal Gland, n (%) | 3 (5,7) |
| ECOG Performance Status at time of RE, n (Grade 0, 1, 2) | 39/13/1 |
| Distribution of hepatic disease at time of RE (unilobar/unilobar in patients with one surgical hepatic lobe/bilobar) | 12/7/34 |
| Full 90Y dose delivered before stasis, n (% of sessions) | 48 (67.6) |
| No. of RE sessions per patient (one vs. two) | 35/18 |
| Post-RE HAIP therapy, n (%) | 12 (22.6) |
| Post-RE systemic chemotherapy (%) | 32 (60.4) |
| Post-RE ablation, n (%) | 5 (9.4) |
Tumor response
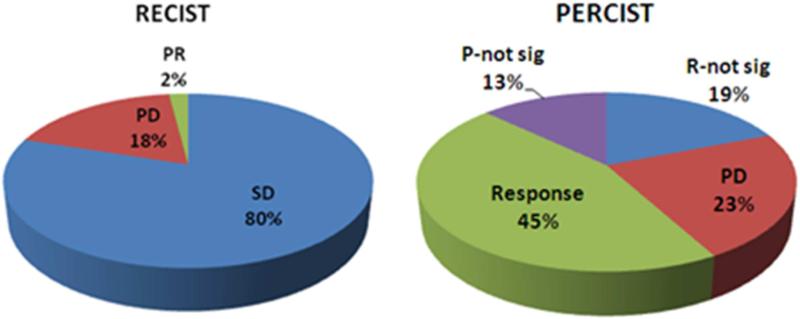
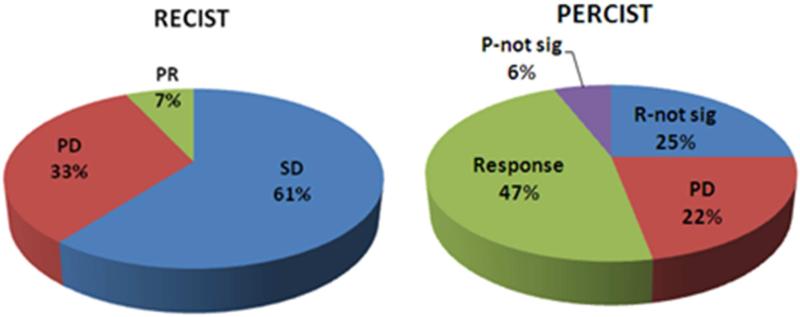
Patient response to treatment was evaluated by assessing target lesions with RECIST and PERCIST at the 4-8 and 12-16 week time points after RE. Ten patients died before repeat imaging (five before 12 weeks and five between 12 and 16 weeks). Response data at 4-8 and 12-16 weeks is summarized in Figure 1.
Figure 1.
Tumor Response at (A) 4-8 weeks (B) and 12-16 weeks post-RE. Abbreviations: PR: partial response, SD: stable disease, PD: progressive disease, R: response, P: progression, not-sig: not significant.
Using RECIST, stable disease (SD) was observed in the majority of patients at both time points (80% and 61% respectively). Additional evaluation however of these patients by PERCIST led to disease reclassification in 77% (at 4-8 weeks) and 76% (at 12-16 weeks) of cases (Figure 2). These patients were found to have either response to treatment (46% and 47%) or disease progression (31% and 29%) at 4-8 and 12-16 weeks respectively, when assessed with PERCIST.
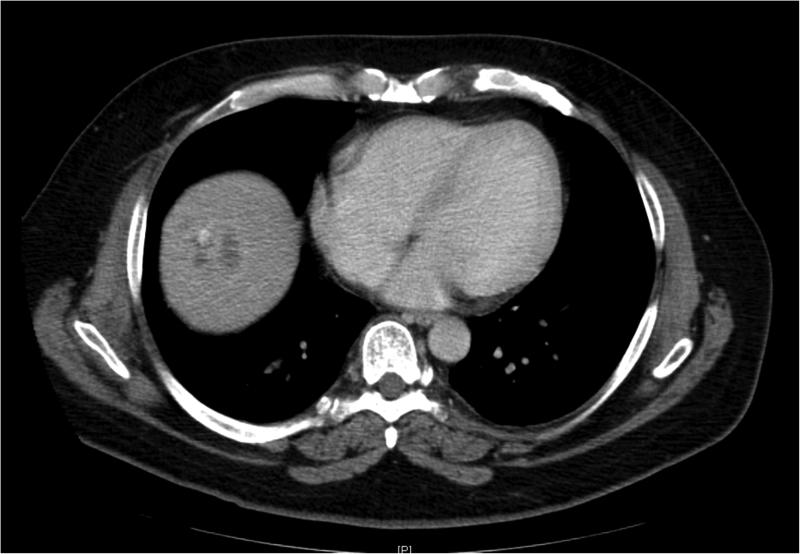
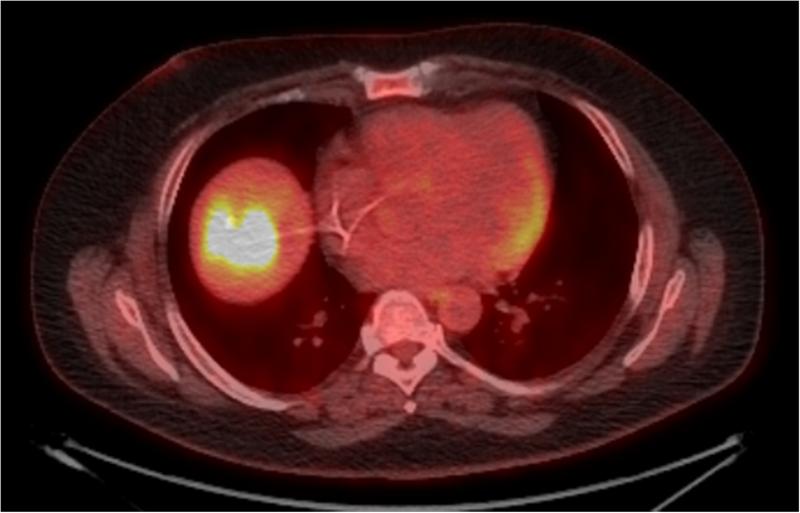
Figure 2.
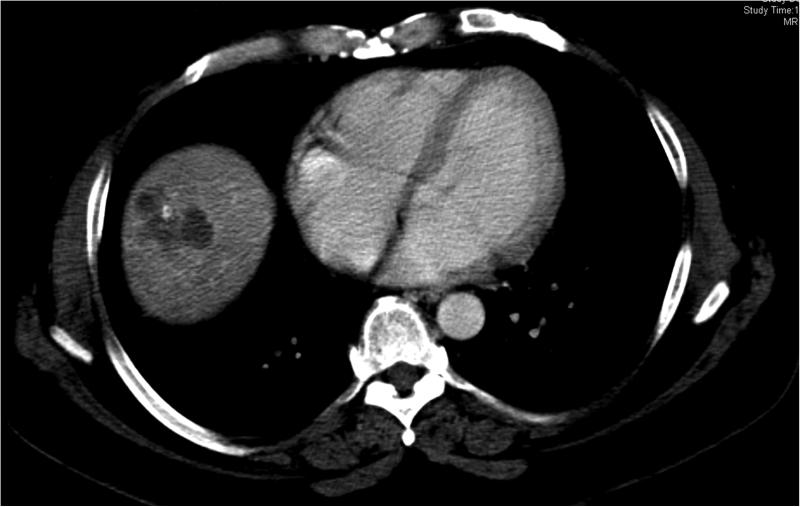
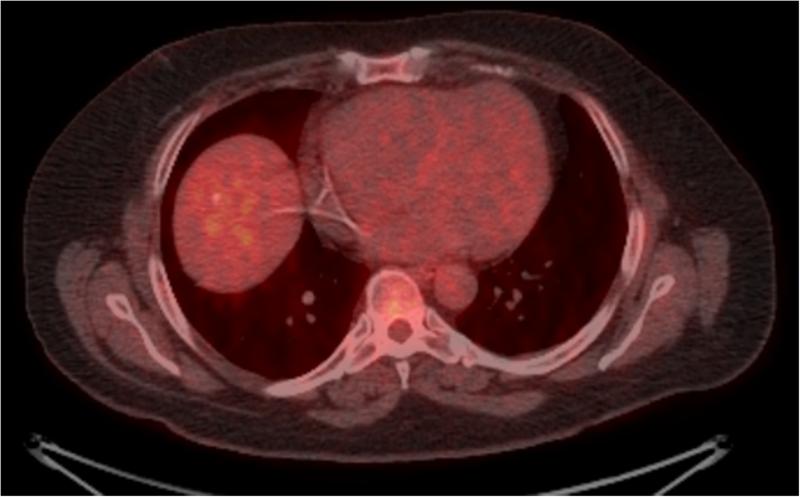
45 year old patient treated with RE for CLM in the hepatic dome. Images (A) and (B) are preprocedural contrast enhanced CT and PET/CT images at the level of the target lesions. Images (C) and (D) were obtained 7 weeks post-RE. Evaluation by RECIST showed stable disease (from 4.5 cm to 4.3 cm), however evaluation based on changes in metabolic activity was characteristic of response to treatment (SUVmax change: from 13.7 to 3.3).
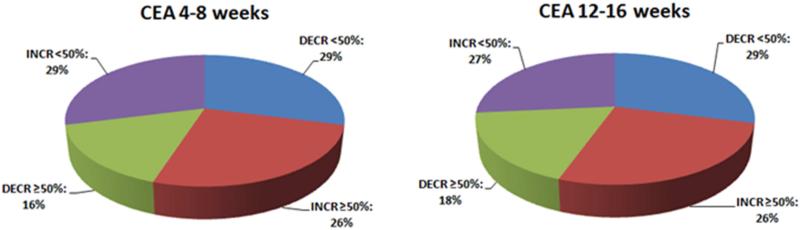
Changes in CEA levels, as compared to baseline levels before RE, were also monitored. These are summarized in Figure 3.
Figure 3.
Changes in CEA levels, compared to baseline levels before RE.
Overall survival
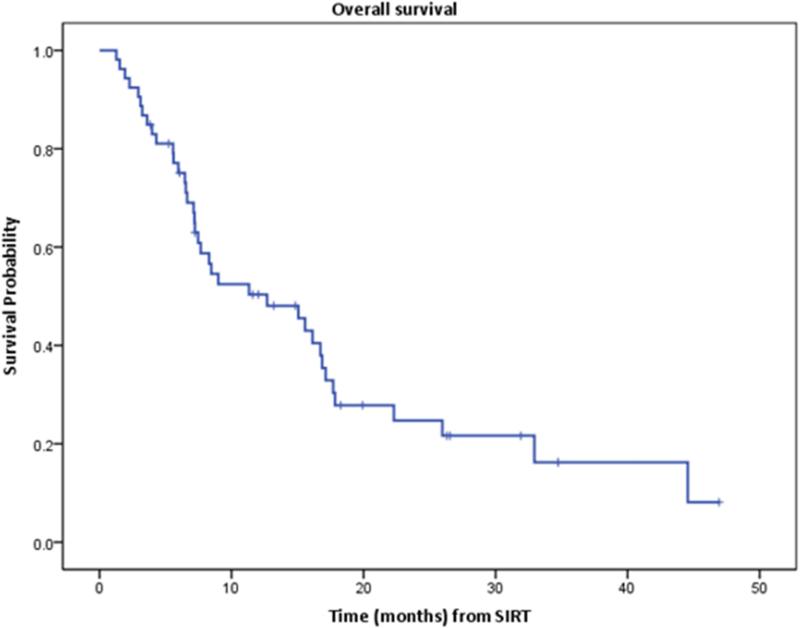
Within the median follow-up period of 15 months after RE, median OS was 12.7 months (95% CI: 5.2-20.2 months). One and 2 year survival was 50% (95% CI: 35%-63%) and 25% (95% CI: 13%-39%) respectively (Figure 4).
Figure 4.
Kaplan-Meier curve of overall survival. Median survival was 12.7 months.
In univariate analysis (Table 2), increased time from diagnosis of CRC to detection of CLM (p=0.024), increased age (>60 years; p=0.034), post-RE HAIP chemotherapy (p=0.04) and post-RE ablation (p=0.045) were associated with increased OS. Extent of liver replacement by tumor >25% (median survival: 5.6 months vs. 15.6 months, p=0.009), evidence of vascular invasion on pathology of primary disease (median survival: 7.1 vs. 16.9 months, p=0.002), baseline CEA levels before RE ≥ 90 ng/mL (median survival: 7.2 vs. 16.9 months, p=0.012), synchronous liver metastases (median survival: 8.3 vs. 17.8 months, p=0.036), three or more sites of extrahepatic disease (median survival: 5.6 vs. 15.6 months, p=0.028) and bilobar tumor disease (vs. unilobar) for patients without prior hepatectomy (median survival: 7.2 vs. 26 months, p=0.007) were associated with decreased overall survival.
Table 2.
Univariate analysis for overall survival and LPFS (log-rank test, Cox regression for continuous variables). CRC: colorectal cancer, CLM: colorectal liver metastases, EhD: extrahepatic disease, RE: radioembolization, HAIP: hepatic arterial infusion pump.
| Variable | OS p value | LPFS p value | |
|---|---|---|---|
| Patient-related variables | Gender | 0.407 | 0.005 |
| Age (continuous*) | 0.140 | 0.057 | |
| Age ≥ 60 vs. <60 | 0.034 | 0.026 | |
| Time variables | Time from diagnosis of CRC to diagnosis of CLM (continuous*) | 0.024 | 0.123 |
| Time from diagnosis of CLM to RE (continuous*) | 0.189 | 0.099 | |
| Primary disease-related variables | Primary tumor resection | 0.842 | 0.982 |
| Node status of the primary | 0.185 | 0.818 | |
| Vascular invasion on pathology of primary | 0.002 | 0.367 | |
| Primary tumor histology | 0.130 | 0.833 | |
| K-ras mutation | 0.536 | 0.053 | |
| Liver metastases at diagnosis of primary (synchronous vs. metachronous) | 0.036 | 0.434 | |
| Disease-related variables at time of RE | Extent of liver replacement by tumor (≥25% vs. <25%) | 0.009 | 0.062 |
| EhD | 0.430 | 0.498 | |
| Number of EhD sites (≥3 vs <3) | 0.028 | 0.935 | |
| CEA levels at time of RE (≥90 vs. <90 ng/mL) | 0.012 | 0.577 | |
| Tumor distribution | |||
| - Bilobar vs. unilobar disease in patients with two lobes | 0.007 | 0.113 | |
| - Unilobar disease in patients with 1 surgical lobe (due to prior hepatectomy) vs. patients with unilobar disease (with two lobes) | 0.631 | 0.678 | |
| Prior to RE treatment variables | Prior HAIP therapy | 0.859 | 0.801 |
| Prior systemic (≥3 lines vs. <3) | 0.251 | 0.151 | |
| Prior bevacizumab | 0.865 | 0.240 | |
| Prior liver surgery | 0.667 | 0.714 | |
| RE-related variables | Full dose delivery | 0.998 | 0.084 |
| No. of RE sessions (two vs. one) | 0.178 | 0.956 | |
| Post-RE additional treatment variables | Post-RE HAIP therapy | 0.042 | 0.099 |
| Post-RE chemotherapy | 0.352 | 0.061 | |
| Post RE ablation | 0.020 | 0.446 | |
| Progression after RE, as documented by: | RECIST at 4-8 weeks | 0.076 | N/A |
| RECIST at 12-16 weeks | 0.703 | N/A | |
| PERCIST at 4-8 weeks | 0.348 | N/A | |
| PERCIST at 12-16 weeks | 0.359 | N/A | |
| CEA increase >50% from baseline at 4-8 weeks | 0.080 | 0.069 | |
| CEA increase >50% from baseline at 12-16 weeks | 0.045 | 0.009 | |
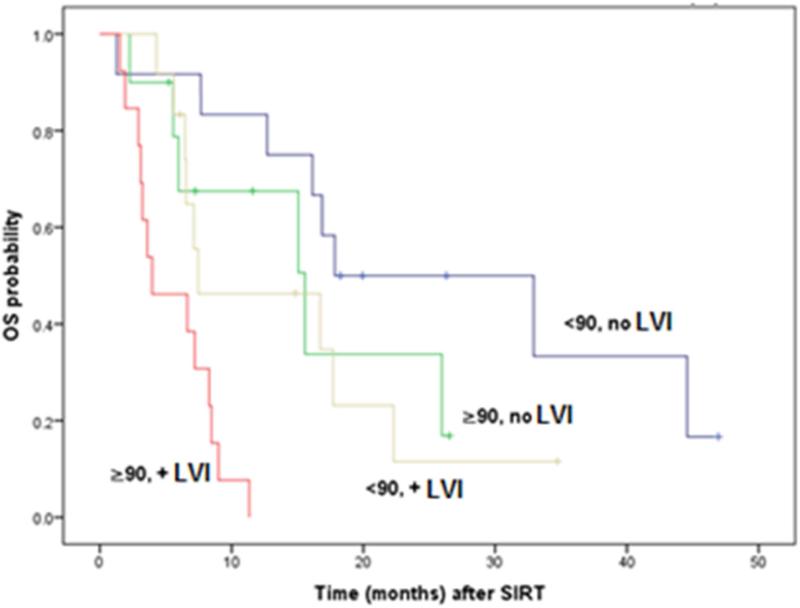
In multivariate analysis, only CEA levels above 90 ng/mL (HR: 2.52, p=0.004) and vascular invasion on pathology of primary disease (HR: 2.87, p=0.002) were confirmed as independent predictors of decreased overall survival (Table 3). Using these two independent predictors Kaplan-Meier curves were plotted for each subgroup (Figure 5).
Table 3.
Multivariate analysis of OS with significant disease-related variables on univariate analysis.
| Variablea | P value | HR | 95% CI |
|---|---|---|---|
| CEA levels before RE (≥90 vs. <90) | 0.004 | 3.0 | 1.4-6.2 |
| Lymphovascular invasion on pathology of primary | 0.002 | 3.3 | 1.6-6.9 |
Variables entered: (1) Lymphovascular invasion of primary, (2) CEA levels ≥90 ng/mL, (3) Synchronous disease and (4) >25% liver replacement by tumor at time of RE. Forward model selection was performed using the Wald test as the criterion. The final model included the first two variables, confirming these as independent predictors of OS.
Note: The variable time from diagnosis of CRC to CLM was considered similar to the variable indicating presence or not of synchronous disease and thus was not entered in the multivariate model despite displaying statistical significance in univariate analysis. The variable bilobar disease vs. unilobar disease in patients with two hepatic lobes was not entered in the multivariate model in order to not exclude the seven patients with unilobar disease within one surgical lobe from analysis. The variable ≥3 vs. <3 sites of extrahepatic disease was not entered since the presence of extrahepatic disease was not statistically significant in univariate analysis.
Figure 5.
Overall survival (OS) curves stratified for CEA levels at time of RE (< or ≥ 90 ng/mL) and presence of microscopic lymphovascular invasion (VI) on pathology of the primary.
Liver Progression-Free Survival
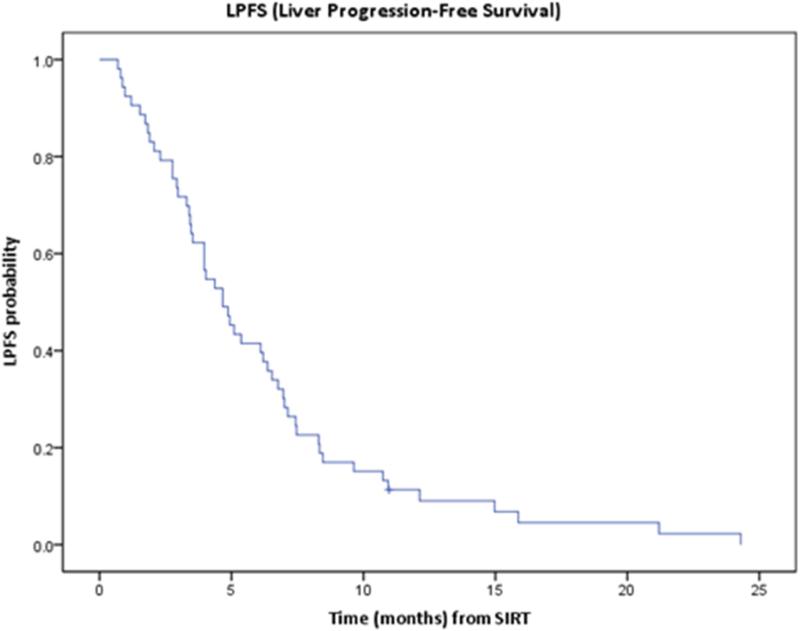
Median LPFS was 4.7 months (95% CI: 3.5-5.8). One year LPFS was 11% (95% CI: 5%-21%) (Figure 6).
Figure 6.
Kaplan-Meier curve of liver progression free survival (LPFS).
In univariate analysis (Table 2), female patients (p=0.005) and patients above 60 years (p=0.026) were associated with increased LPFS. K-ras mutation and extent of liver replacement by tumor > 25% were associated with shorter LPFS, although these were not statistically significant by a short margin (p=0.053 and 0.062 respectively). Multivariate analysis of LPFS did not lead to clinically relevant results.
Complications/Toxicities
Complications occurring up to 16 weeks after RE were recorded. Table 4 lists all recorded clinical and biochemical toxicities. The majority of patients (60%) experienced self-limited fatigue lasting up to 14 days.
Table 4.
Complications
| Grade 1-2: | ||
|---|---|---|
| Complication | No of patients | Percentage |
| Fatigue/Weakness | 32 | 60% |
| Abdominal distention/Abdominal Pain | 15 | 28% |
| Shortness of breath | 9 | 17% |
| Fever | 9 | 17% |
| Change in appetite/Anorexia/ Weight changes | 8 | 15% |
| Hyperbilirubinemia | 8 | 15% |
| Nausea | 6 | 11% |
| Vomiting | 3 | 6% |
| Constipation/Diarrhea | 12 | 23% |
| Grade 3-4 Toxicities: | |||
|---|---|---|---|
| Event that required hospitalization | Number of patients | Days post RE (range) | Comments |
| Grade 3 Hyperbilirubinemia | 2 (4%) | 20-21 | |
| Grade 4 Hyperbilirubinemia | 5 (9%) | 14-76 | All 5 patients had POD and 4/5 evidence of liver failure |
| Grade 3 Epigastric Pain | 5 (9%) | 7-36 | |
| Grade 3 Shortness of Breath | 2 (4%) | 20-30 | Attributed to POD |
| Grade 4 Shortness of Breath | 1 (2%) | 0 | Immediately post-procedure |
Twenty-nine patients (55%) retained their pre-procedural ECOG performance status when evaluated at one and three months after RE. Eight patients (15%) had a transient decline in status at one month by one grade that resolved at 3 months after RE. Nine patients (17%) had a sustained decline by one grade (two with evidence of disease progression within the first three months). Five patients (9%) died before 12 weeks and two patients (4%) had a 2 grade decline, all with evidence of disease progression.
Significant hyperbilirubinemia (range: 2.1-14.4 mg/dL; median: 7.1 mg/dL) was reported in 7 patients (Grade 3: n=2; Grade 4: n=5) after a median of 29 days after RE (range: 14-76 days), all with imaging displaying disease progression in the liver. One patient that developed grade 4 hyperbilirubinemia received additional radiofrequency ablation 28 days after RE that resulted in this complication; the hyperbilirubinemia was considered to represent liver failure as a result of RE and thermal ablation in the background of a heavily pretreated hepatic parenchyma in a patient that had received prior hepatic resections, lengthy systemic chemotherapy and HAIP chemotherapy. This patient died 2.3 months post-RE.
Five patients experienced grade 3 abdominal pain. In one case presenting one week after RE, immediate post-procedural Bremmstrahlung imaging showed duodenal uptake, however evaluation with endoscopy did not show gastrointestinal ulceration. This patient was placed on proton pump inhibitors and sucralfate, leading within 3 weeks to significant clinical improvement not requiring further therapy.
Thirty-day mortality and incidence of post-procedural radiation pneumonitis were both zero. There was no incidence of liver failure in the absence of disease progression in the liver.
Discussion
The safety and efficacy of RE with 90Y resin microspheres in the salvage setting has been evaluated by several studies: RE for chemorefractory CLM appears to extend overall survival, as compared to best supportive care17, 33 and to extend liver progression-free survival, as compared to patients receiving systemic chemotherapy alone.16, 25 The concomitant use of RE and systemic chemotherapy (either fluorouracil16 or irinotecan34) appears to be safe and well-tolerated for the treatment of chemorefractory disease. Specifically, a small randomized controlled trial of 90Y with systemic fluorouracil versus protracted fluorouracil alone demonstrated a significant prolongation of progression-free survival in the combination arm in patients that had already failed previous chemotherapy regimens containing fluorouracil.16 This was attributed to the chemosensitization effect of RE as has been observed in other studies.12, 25, 34
Recent evidence also suggests that RE has an acceptable safety profile and does not prohibit subsequent treatment in heavily pre-treated patients with CLM progressing after multiple therapies including, surgery, ablation, HAIP chemotherapy and systemic chemotherapy. A recent phase I trial indicated that there is no need for 90Y dose reduction even in heavily pretreated patients with CLM.18 Similarly, the patient population in the present study was subjected to several treatments prior to RE and had a relatively long history of CLM (median time from CLM diagnosis to RE: 35 months). All patients received prior systemic chemotherapy, with 28% progressing after 3 or more different regimens of chemotherapy. After RE, 60% of patients received additional chemotherapy at some point due to disease progression. As a result, all patients included in the present study had at least some degree of chemotherapy induced steatosis35 and were at a relatively increased risk for the development of REILD.27
Disease progression within a post-hepatectomy liver remnant was addressed with treatment of the entire liver volume in a single session. For patients with bilobar disease, sequential lobar treatment or sublobar infusions were preferred in an effort to reduce treatment-related complications. Twenty-nine (55%) patients received HAIP chemotherapy prior to RE and twelve of these patients resumed HAIP chemotherapy after RE to address subsequent hepatic disease progression. In fact, patients in our study that received HAIP chemotherapy after RE had improved OS (p=0.042). Thus, similarly to what it was seen in earlier small series, RE did not prohibit re-initiation of systemic chemotherapy or HAIP chemotherapy in the post-procedural setting.26 Ablation for residual CLM after RE was associated with increased OS. This correlation is potentially confounding because all five patients that underwent subsequent ablation had limited replacement of liver volume by tumor (<25%) and received lobar infusions of 90Y resin microspheres in an effort to downsize CLM within acceptable criteria for ablation. This approach may be preferred for selected patients with limited disease that can be ablated with adequate margins, even in the salvage setting.36, 37 Downsizing to resection or ablation has been previously reported in 10-20% of patients managed with RE in earlier stages of the disease.12, 38 The current data suggests that a similar rate can be achieved in the salvage setting in selected patients with limited disease.
In our study, median OS after RE was 12.7 months, similar to median OS rates reported in other studies that evaluated the treatment of chemorefractory CLM with RE in the salvage setting (12-12.6 months).15, 17, 19, 34 We consider this to represent an encouraging finding when taking into consideration the characteristics and the prognosis of this specific patient population. Median patient survival since initial diagnosis of CRC and CLM was 48.2 (95% CI: 35.3-61.1) and 47.1 (95% CI: 39.5-54.7) months respectively. A number of factors demonstrated to negatively impact patient survival in univariate analysis (i.e.: extent of liver replacement by tumor >25%, evidence of vascular invasion on pathology of primary disease, CEA levels ≥ 90 ng/mL before RE, synchronous liver metastases, three or more sites of extrahepatic disease and bilobar tumor disease). The small number of patients and events (deaths) impacted the multivariate analysis. We plan to update our results in a larger cohort with longer follow-up to assess further the impact of these factors on patient survival.
Lymphovascular invasion on pathology of the primary tumor – a well recognized risk factor carrying poor prognosis and indicating aggressive tumor biology39–, and a CEA level ≥ 90 ng/mL were independent predictors of short survival in multivariate analysis.
Evaluation of response to catheter-directed liver therapies, including RE, with the use of imaging is extremely challenging.40 In our study, evaluation of response and liver progression was performed using RECIST and PERCIST. This approach was preferred to address the inherent weaknesses of RECIST, where response/progression is solely assessed with changes of the maximum diameter of target lesions without taking into account other factors that may indicate tumor response such as degree of enhancement or metabolic activity of the target tumor.29, 41 In particular after RE, treatment-related changes such as edema and tumor necrosis may actually make lesions appear larger than before treatment, without indicating progression, similarly to what has been seen after other arterially directed therapies.42, 43 Additionally, response assessment in relatively hypovascular tumors such as CLM is even more challenging because modified RECIST cannot be directly applied as done for hepatocellular carcinoma or other hypervascular tumors.41 For these lesions, metabolic imaging with PET/CT appears to provide an earlier maker of response.29, 44, 45
The results of our study show that evaluation of response using RECIST did not show in the majority of cases differentiation from pre-RE assessment. Our experience supports that changes in SUV activity appear to be an earlier marker of response to RE, especially when assessing response on PET/CT scans performed 12-16 weeks after the procedure when post treatment changes have subsided. This finding suggests that the appropriate post-procedural follow-up protocol of these patients should include routine metabolic/functional imaging, to assist subsequent clinical decisions.
Limitations of our study include the relatively small patient population, the retrospective nature of the work and the fact that all procedures were performed at a single center. The lack of randomization to no therapy and the additional treatment with several agents and approaches after RE, including HAIP chemotherapy and ablation, makes assessment of the value of RE difficult to establish. However it is highly unlikely, if not impossible, that such a trial will ever be available in the salvage setting. The lack of pathologic examinations of CLM before and after RE is another limitation since there is no information on marker status that may contribute significantly to differences in observed outcomes.
Conclusion
Based on our findings, RE for CLM was well-tolerated in the salvage setting. In the majority of cases, patient general condition did not prevent the administration of additional systemic or locoregional therapies. Oncologic outcomes and in particular patient survival (median: 12.7 months) were encouraging in this cohort. Decreased OS was observed with lymphovascular invasion on pathology of the primary tumor and with CEA levels ≥ 90 ng/mL before RE. Evaluation by PERCIST was more likely to show differentiation from baseline pre-RE assessment, in comparison to evaluation by RECIST.
Clinical Practice Points.
- RE has a good safety profile when used in the salvage setting, permitting the administration of additional chemotherapy in the post-procedural setting.
- Factors related to tumor biology were found to be independent predictors of OS (CEA levels at time of RE, lymphovascular invasion on pathology of primary tumor).
- Median OS (12.7 months) and median LPFS (4.7 months) in our study is encouraging. Patients with unresectable and chemorefractory liver-dominant colorectal cancer metastases should be evaluated as potential candidates for RE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adam R, Hoti E, Folprecht G, Benson AB. Accomplishments in 2008 in the management of curable metastatic colorectal cancer. Gastrointest Cancer Res. 2009;3:S15–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna NN. Radiofrequency ablation of primary and metastatic hepatic malignancies. Clin Colorectal Cancer. 2004;4:92–100. doi: 10.3816/ccc.2004.n.012. [DOI] [PubMed] [Google Scholar]
- 3.Alberts SR, Poston GJ. Treatment advances in liver-limited metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10:258–265. doi: 10.1016/j.clcc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Annals of Oncology. 2004;15:103–106. doi: 10.1093/annonc/mdh912. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of Clinical Oncology. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. New England Journal of Medicine. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 7.Martin RC, Joshi J, Robbins K, et al. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18:192–198. doi: 10.1245/s10434-010-1288-5. [DOI] [PubMed] [Google Scholar]
- 8.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 9.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres--safety, efficacy, and survival. Radiology. 2008;247:507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 11.Campbell AM, Bailey IH, Burton MA. Analysis of the distribution of intra-arterial microspheres in human liver following hepatic yttrium-90 microsphere therapy. Phys Med Biol. 2000;45:1023–1033. doi: 10.1088/0031-9155/45/4/316. [DOI] [PubMed] [Google Scholar]
- 12.Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 13.Jakobs TF, Hoffmann RT, Dehm K, et al. Hepatic yttrium-90 radioembolization of chemotherapy-refractory colorectal cancer liver metastases. J Vasc Interv Radiol. 2008;19:1187–1195. doi: 10.1016/j.jvir.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol. 2009;19:951–959. doi: 10.1007/s00330-008-1211-7. [DOI] [PubMed] [Google Scholar]
- 15.Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–331. doi: 10.1038/sj.bjc.6605770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 17.Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23:96–105. doi: 10.1016/j.jvir.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13:27–36. doi: 10.1016/j.clcc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Cianni R, Urigo C, Notarianni E, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol. 2009;32:1179–1186. doi: 10.1007/s00270-009-9658-8. [DOI] [PubMed] [Google Scholar]
- 20.Mulcahy MF, Lewandowski RJ, Ibrahim SM, et al. Radioembolization of colorectal hepatic metastases using yttrium-90 microspheres. Cancer. 2009;115:1849–1858. doi: 10.1002/cncr.24224. [DOI] [PubMed] [Google Scholar]
- 21.Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol. 2005;16:1641–1651. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- 22.Memon K, Lewandowski RJ, Kulik L, Riaz A, Mulcahy MF, Salem R. Radioembolization for primary and metastatic liver cancer. Semin Radiat Oncol. 2011;21:294–302. doi: 10.1016/j.semradonc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging. 2014 doi: 10.1007/s00259-014-2799-2. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. doi: 10.1016/j.ijrobp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres (R) plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. Journal of Surgical Oncology. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 26.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres((R)) plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Annals of Oncology. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 27.Sangro B, Gil-Alzugaray B, Rodriguez J, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haug AR, Tiega Donfack BP, Trumm C, et al. 18F-FDG PET/CT predicts survival after radioembolization of hepatic metastases from breast cancer. J Nucl Med. 2012;53:371–377. doi: 10.2967/jnumed.111.096230. [DOI] [PubMed] [Google Scholar]
- 31.Zerizer I, Al-Nahhas A, Towey D, et al. The role of early (1)(8)F-FDG PET/CT in prediction of progression-free survival after (9)(0)Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging. 2012;39:1391–1399. doi: 10.1007/s00259-012-2149-1. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338–351. doi: 10.1081/cnv-58878. [DOI] [PubMed] [Google Scholar]
- 33.Seidensticker R, Denecke T, Kraus P, et al. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35:1066–1073. doi: 10.1007/s00270-011-0234-7. [DOI] [PubMed] [Google Scholar]
- 34.van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol. 2009;27:4089–4095. doi: 10.1200/JCO.2008.20.8116. [DOI] [PubMed] [Google Scholar]
- 35.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 36.Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. Journal of Vascular and Interventional Radiology. 2011;22:755–761. doi: 10.1016/j.jvir.2011.01.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann RT, Jakobs TF, Kubisch CH, et al. Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease-Is it feasible? Eur J Radiol. 2010;74:199–205. doi: 10.1016/j.ejrad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 40.Janne d'Othee B, Sofocleous CT, Hanna N, et al. Development of a research agenda for the management of metastatic colorectal cancer: proceedings from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2012;23:153–163. doi: 10.1016/j.jvir.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 42.Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl 1):S41–55. doi: 10.1148/rg.25si055515. [DOI] [PubMed] [Google Scholar]
- 43.Atassi B, Bangash AK, Bahram A, et al. Multimodality imaging following Y-90 radioembolization: A comprehensive review and pictorial essay. Radiographics. 2008;28:81–99. doi: 10.1148/rg.281065721. [DOI] [PubMed] [Google Scholar]
- 44.Szyszko T, Al-Nahhas A, Canelo R, et al. Assessment of response to treatment of unresectable liver tumours with 90Y microspheres: value of FDG PET versus computed tomography. Nucl Med Commun. 2007;28:15–20. doi: 10.1097/MNM.0b013e328011453b. [DOI] [PubMed] [Google Scholar]
- 45.Wong CY, Salem R, Raman S, Gates VL, Dworkin HJ. Evaluating 90Y-glass microsphere treatment response of unresectable colorectal liver metastases by [18F]FDG PET: a comparison with CT or MRI. Eur J Nucl Med Mol Imaging. 2002;29:815–820. doi: 10.1007/s00259-002-0787-4. [DOI] [PubMed] [Google Scholar]