Abstract
Background
Pregnancy during lactation is common in Egypt and is often unplanned. Overlap between pregnancy and lactation could be associated with an increased risk for the pregnant mother, her fetus as well as her nursing child.
Aim of the study
The current study aims to compare the maternal and perinatal outcome of pregnancies occurred during lactation with those occurred after weaning in women with substandard nutrition.
Materials and methods
A prospective-cohort study was carried out in six Maternal and Child Health Centers in Assiut-Egypt. Estimated sample size was 540 women divided equally into two groups; the first included women who got pregnant during breastfeeding (PDBF), while the second included women who got pregnant after weaning (PAW). Tools were consisted of structured interview questionnaire including personal history, obstetrical data, breastfeeding, family planning histories and dietary intake during pregnancy. Pregnant women had been followed up to delivery to assess different maternal and fetal outcomes.
Results
Miscarriage rate was not statistically significant between both groups (2.2% in PDBF and 0.4% in PAW, p = 0.284). Women in PDBF group had higher prevalence of maternal anemia (54.1% versus 30.7%), intrauterine growth restriction (16.7% versus 4.8%), cesarean delivery (43.7% versus 31.5%), prolonged labor (13.3% versus 11.1%) and low birth weight infants (15.7% versus 8.8%) compared to women in PAW group.
Conclusion
Pregnancy during breastfeeding is associated with an increase in the overall complications of pregnancy as compared to PAW. Although it does not increase the miscarriage rate, it increases the prevalence of maternal anemia, delayed fetal growth, prolonged labor, cesarean section delivery and the prevalence of low birth weight infants.
Keywords: Breastfeeding, LAM, maternal anemia, miscarriage, nutrition during pregnancy, obstetrics, perinatal outcome, pregnancy-lactation overlap
Introduction
There is a great potential for using lactation as a method of contraception in Egypt and other developing countries. Lactational amenorrhea method (LAM) has been integrated in the family planning program of many developing countries including Egypt. However, LAM is limited to six months because infant-feeding guidelines recommend that supplementation with other food should begin six months after delivery (Khella et al., 2004; Shaaban and Glasier, 2008).
Lactation generally extends beyond the period of LAM, thus its prerequisites and consequently its efficacy expires unexpectedly. Reluctance of the use of a long-term method of contraception during lactation may result in unplanned pregnancy and sometimes unwanted childbirth (Fathalla et al., 2003). A previous study in the same setting showed that one in four women conceived while breastfeeding, and nearly one in three of these pregnancies were unplanned. Breastfeeding for longer than 6 months post partum should initiate other long-acting methods of contraception to avoid unplanned pregnancy (Shaaban and Glasier, 2008, Tilley et al., 2009).
Birth spacing is mandatory to make pregnancy safer and therefore the WHO endorses a minimum birth-to-pregnancy interval of two years to reduce the incidence of maternal and fetal risks in each pregnancy (Tilley et al., 2009). A short interpregnancy interval of less than 24 months increases the risk of adverse prenatal outcomes such as miscarriage, preterm birth, low birth weight, intrauterine growth restriction and fetal death. Moreover, women with shorter inter-pregnancy intervals have a higher risk of maternal mortality, hypertensive disorders during pregnancy, peripartal bleeding and maternal anemia (Smith et al., 2003).
Maternal nutritional status is considered an important risk factor for the progression of labor and birth weight. Poor birth outcomes, such as experiencing a prolonged labor or being small for gestational age represent a risk factor for maternal as well as infant morbidity and mortality. Pregnancy that occurs during lactation represents a particularly large nutritional burden, since both lactation and pregnancy are very demanding nutritional conditions. When these two physiological changes occur simultaneously, the risk of depletion of basic nutrient stores in the mother and consequently affection of the course of pregnancy and delivery might increase, particularly among women with substandard food intake (Pareja, 2007; Sengul et al., 2013).
Women in our community are having a high total fertility rate and almost inadequate dietary intake. Subsequently they need an appropriate interval between breastfeeding and the next pregnancy. Therefore we believe that it is of great significance to understand the effects of pregnancy-lactation overlap on maternal and fetal outcomes. According to our hypothesis communities with low dietary intake may show different maternal and pregnancy adverse outcome than previously demonstrated in developed communities with good and balanced dietary intake (Sengul et al., 2013). The current study aims to compare the pregnancy outcome in women who conceived during breastfeeding with those who conceived after weaning in a population with substandard nutrition.
Patients and Methods
2.1. Study type, setting and duration
The study was a prospective-cohort study carried out during a 14-months’ period from November 1st 2013 until December 31st 2014 in six Maternal and Child Health Centers (MCH) in Assiut, Egypt.
A consultant in Foetal Medicine scanned all 21 patients trans abdominally with a Voluson E8 Expert US machine. Additionally, one observer confirmed inclusion of the entire placenta.
2.2. Study population
The study population included all consecutive multiparous pregnant women presented to the recruitment MCH centers in their first trimester of pregnancy (less than 12 weeks pregnant). However, women with a history of an abortion in the last pregnancy or a history of any chronic medical diseases had been excluded from participation. Additionally, women who did not breastfeed their last child and those who were living in districts away from recruitment centers or refused to participate in the study had also been excluded.
A sample size of 540 pregnant women was obtained and divided in two groups (270 each). This sample size was estimated to evaluate two indicators for both fetal and maternal health. Miscarriage rate was chosen as an indicator of fetal wellbeing and maternal anemia as an indicator of maternal wellbeing.
In a previous study it was reported that general miscarriage rate is about 12% (Delabaere et al., 2014). Using two sided chi-square (X2) test with α of 0.05, a total sample size of at least 270 pregnant women in each group was needed in each group with 95% power to detect a 10% difference in the miscarriage rate in both groups (odds ratio of 1.89) assuming a lost to follow up rate of 10%. The same equation was used to calculate the sample size to detect the difference in maternal anemia between the two groups.
A recent study in the same community showed that the prevalence of anemia in pregnancy was about 67.0% in the third trimester of pregnancy (Elashiry et al., 2014). A sample size of at least 287 pregnant women in each group was needed in each group, with 95% power to detect a 10% difference in the prevalence of anemia during pregnancy (odds ratio of 1.65) assuming a lost to follow up rate of 10% (Epi-info TM, CDC, USA).
Intervention
Eligible participants had stratified into two groups depending upon if they got pregnant during breastfeeding (PDBF) or after weaning (PAW).
Recruitment included to all consecutive eligible participants until the required sample size had been fulfilled. The study tool was a structured interview questionnaire introduced by trained nurses at the time of admission to the study. The questions included questions related to personal (included detailed contact details), obstetric, breast-feeding histories and the family planning history.
Daily dietary intakes were assessed to the first consecutive 100 women in each study group using a 24-hour dietary recall questionnaire. The questionnaires were done through one-to one interview by a trained nurse regarding the types and quantities of food products consumed in the last 24 hours prior to the interview. Obtained data were linked with specific food composition tables to obtain an estimate of daily intake of different essential nutrients. Nutrients estimation included also total caloric intake calculated from the percentage of carbohydrate and fat dietary intake. The amount of proteins, calcium and iron was also estimated. The results were evaluated in comparison with the dietary reference intakes tables of the institute of medicine (Institute of Medicine, 2001). Daily nutrient intakes were calculated in grams of food multiplied by the amount of each nutrient in the food and the frequency of consumption.
The study protocol had been approved from the Assiut Medical School Ethical Review Board and the Ministry of Health approval of collecting data from their affiliated centers was also obtained. The non-interventional nature of the study and respect of patients’ confidentiality were clear to the patient and their written consent to participate had been obtained.
Follow-up
Women in both groups were followed up during their routine antenatal visits to the centers until delivery to know if there were any complication occurred during the current pregnancy (miscarriage, vaginal bleeding during pregnancy, placental abruption (partially or completely), intrauterine growth restriction (IUGR), elevation of blood sugar, elevation of blood pressure, eclampsia and anemia. Placental abruption was defined as vaginal bleeding due to placental separation before fetal birth. IUGR was defined as fetal birth weight below the 10th percentile, adjusted for gestational age (Resnik, 2002). Elevation of blood sugar (gestational diabetes) was defined if fasting blood glucose ≥ 7 mmol/l by the 75 gm oral glucose tolerance test (NICE guidelines, 2008) and elevation of blood pressure (gestational hypertension) was defined if blood pressure ≥ 140/90 mmHg (ACOG, 2013).
Eclampsia was diagnosed if the patient develops fits on top of preeclampsia (ACOG, 2013). Anemia as defined by the World Health Organization as hemoglobin levels of ≤ 11 g/dl (UNICEF/UNU/WHO, 2001).
Moreover, participants were encouraged to deliver in our facility (instead of regular delivery at the MCH Centers) by giving her follow-up card to have her delivery for free in our service. Delivery data, duration of labor, any intrapartum or postpartum complications, neonatal birth weight and referral to pediatric care unit were also reported.
Postpartum Hemorrhage (PPH) was defined as a blood loss of 500 ml or more within 24 hours after birth (ACOG Practice Bulletin, 2006). Cases with prolonged labor were identified by using partograms if the action line exceeded two hours after correction of uterine inertia by AROM and/or oxytocin. Every effort had been exhausted to complete the follow-up of our participants including phone calls and home visits to complete the required data.
Study Outcomes
The primary outcome of the study was the difference in miscarriage rate as an indicator of fetal wellbeing and prevalence of maternal anemia as an indicator of maternal wellbeing in both groups.
The secondary outcomes were the difference in the rate of other maternal complications during pregnancy as bleeding, elevation of blood sugar, elevation of blood pressure, placental separation and eclampsia between both groups. Also, difference in timing, mode of delivery and occurrence of complications during delivery were secondary maternal outcomes. Fetal condition at birth, birth weight and need to referral to pediatric care unit were also considered as secondary fetal outcomes.
Statistical analysis
The collected data was coded, tabulated and analyzed using the statistical package for social science programs (SPSS) Chicago, IL, USA, version 18. Continuous data were expressed as frequency, percentage, means and standard deviation. Discrete data were expressed as frequency and percentage. Comparison between groups was done using Student’s T-test. Level of significance “P” value was evaluated, where P value < 0.05 was considered statistically significant.
Results
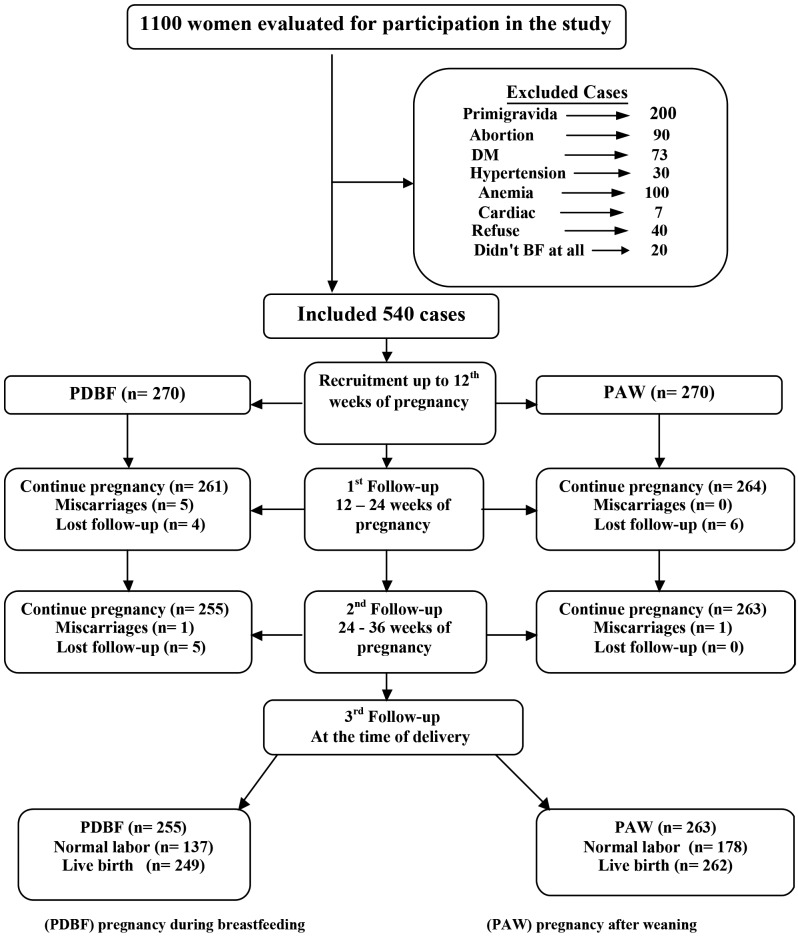
One thousand and one hundred women were approached to participate in this study. Five hundred sixty women were excluded due to presence of different exclusion criteria or a refusal to participate in the study as shown in Figure 1. The remaining 540 subjects were stratified into two groups; PDBF group and PAW group. Nine patients in PDBF group and six patients in the PAW group were lost for follow up.
Figure 1.
— Flowchart of the study participants
The mean age of study participants was 28.82 ± 6.18 in the PDBF group and 30.52 ± 5.20 in the PAW group (p = 0.001) (Table I).
Table I.
— Demographic characteristics and relevant clinical data of the study participants.
| PDBF (n = 270) | PAW (n = 270) | P-Value | |||
| No. | % | No. | % | ||
| Age (Mean ± SD) | 28.82 ± 6.18 | 30.52 ± 5.20 | 0.001a | ||
| Parity (Mean ± SD) | 2.39 ± 1.54 | 2.64 ± 1.18 | 0.036a | ||
| Level of education | 0.649 | ||||
| Illiterate or Read & write | 93 | 34.4 | 88 | 32.6 | |
| Educated | 177 | 65.6 | 182 | 67.4 | |
| Occupation | 0.643 | ||||
| Working | 87 | 32.2 | 82 | 30.4 | |
| Not working | 183 | 67.8 | 188 | 69.6 | |
| History of pregnancy during breastfeeding | 39 | 14.4 | 44 | 16.3 | 0.551 |
PDBF: Pregnancy during breastfeeding.
PAW: Pregnancy after weaning.
(a) Statistically significant difference.
The percentage of women who had had any complication in the current pregnancy was higher in the PDBF group (70.0%) compared with in PAW group (48.9%). The difference had reached a statistically significant value (p = 0.001). The miscarriage percentage was higher in the PDBF group (2.2%) compared to (0.4%) in the PAW group; however, the difference was not statistically significant (p = 0.284). Anemia had the highest percentage in both PDBF and PAW groups (54.1% and 30.7%, respectively), being significantly higher in PDBF group (p = 0.005). IUGR was higher in the PDBF group (16.7%) compared with (4.8%) in the PAW group. However, elevation of blood pressure was higher in the PAW group (15.6%) compared with (12.2%) in the PDBF group with statistical significant difference (P = 0.003) (Table II).
Table II.
— The outcome of the current pregnancy in the two study groups.
| PDBF (n = 270) | PAW (n = 270) | P-Value | |||
| No. | % | No. | % | ||
| Lost follow up | 9 | 3.33 | 6 | 2.22 | 0.09 |
| Complications during current pregnancyb | 189 | 70.0 | 132 | 48.9 | 0.001a |
| Miscarriage | 6 | 2.2 | 1 | 0.4 | 0.284 |
| Vaginal bleeding during pregnancy | 17 | 6.3 | 5 | 1.9 | 0.069 |
| Placental separation | 18 | 6.7 | 5 | 1.9 | 0.050a |
| Intrauterine growth restriction | 45 | 16.7 | 13 | 4.8 | 0.001a |
| Elevation of blood sugar | 16 | 5.9 | 15 | 5.6 | 0.387 |
| Elevation of blood pressure | 33 | 12.2 | 42 | 15.6 | 0.003a |
| Eclampsia | 17 | 6.3 | 10 | 3.7 | 0.652 |
| Maternal anemia | 146 | 54.1 | 83 | 30.7 | 0.005a |
PDBF: Pregnancy during breastfeeding.
PAW: Pregnancy after weaning.
(a) Statistically significant difference.
(b) Sum more than 100% as there was more than one complication.
Table III demonstrates that the percentage of women who had CS was higher in the PDBF group (43.7%) compared with PAW group (31.5%) (p = 0.001). Complications during vaginal delivery were significantly higher in the PDBF group. The mean birth weight of the study participants was (2844.8 ± 445.4) in the PDBF group and (2897.0 ± 414.1) in the PAW group (P = 0.171), the percentage of low birth weight was significantly higher in the PDBF group (14.4%) compared with (8.5%) in the PAW group (P = 0.017).
Table III.
— The outcome of labor and neonatal outcomes in the two study groups.
| Items | PDBF (n = 255) | PAW (n = 263) | P-Value | ||
| No. | % | No. | % | ||
| Time of delivery | 0.496 | ||||
| Full term delivery (37-40 weeks) | 185 | 68.5 | 179 | 66.3 | |
| Preterm labor (< 37 weeks) | 42 | 15.6 | 53 | 19.6 | |
| Post date (> 40 weeks) | 28 | 10.4 | 31 | 11.5 | |
| Mode of delivery | 0.001a | ||||
| Vaginal | 137 | 50.7 | 178 | 65.9 | |
| CS | 118 | 43.7 | 85 | 31.5 | |
| Complications of delivery | |||||
| Ante-partum hemorrhage | 11 | 4.1 | 4 | 1.5 | 0.017a |
| Post-partum hemorrhage | 14 | 5.2 | 8 | 3.0 | 0.048a |
| Prolonged labor | 36 | 13.3 | 30 | 11.1 | 0.042a |
| Live birth | 249 | 97.7 | 262 | 99.6 | 0.118 |
| Birth weight | |||||
| Normal ≥ 2500 gm | 210 | 84.3 | 239 | 91.2 | 0.017a |
| < 2500 gm | 39 | 15.7 | 23 | 8.8 | |
| (Mean ± SD) | 2844.8 ± 445.4 | 2897.0 ± 414.1 | 0.171 | ||
| Referred to PCU | 117 | 43.3 | 106 | 39.3 | 0.137 |
PDBF: Pregnancy during breastfeeding.
PAW: Pregnancy after weaning.
PCU: Pediatric.
(a) Statistically significant difference.
Table IV shows an analysis of the 24-hours dietary recall in both groups. The mean total energy intake is significantly higher than the reference value in both groups (p = 0.001). Protein dietary contribution was within the recommendations while the carbohydrate contribution was significantly higher. Moreover, fat contribution was significantly lower (p = 0.001 for each) than the international standards No significant difference between both groups in total caloric intake (p = 0.077), proteins (p = 0.063), carbohydrates (p = 0.582), or fat (p = 0.390).
Table IV.
— Analysis of mean nutrients intake consumed by pregnant women in comparison with the reference values.
| Items | Reference valueb | PDBF group | PAW group | Intergroup P-Value | ||
| Mean ± SD | P-value | Mean ± SD | P-value | |||
| Calories (Kcl) | 2000 | 2731.6 ± 637.2 | 0.001a | 2609.1 ± 566.8 | 0.001a | 0.077 |
| Carbohydrate (%) | 50 | 53.8 ± 9.9 | 0.001a | 54.4 ± 8.1 | 0.001a | 0.582 |
| Fat (%) | 35 | 31.1 ± 9.0 | 0.001a | 30.3 ± 7.2 | 0.001a | 0.582 |
| Protein (grams) | 71 | 74.6 ± 15.4 | 0.142 | 68.2 ± 13.7 | 0.208 | 0.053 |
| Calcium (mg) | 1000 | 909.2 ± 407.5 | 0.007a | 939.9 ± 799.9 | 0.356 | 0.673 |
| Iron (mg) | 27 | 25.9 ± 12.2 | 0.312 | 24.6 ± 11.9 | 0.013a | 0.308 |
PDBF: Pregnancy during breastfeeding.
PAW: Pregnancy after weaning.
(a) Statistically significant difference.
(b) Reference values according to the standards of institute of medicine, 2001.
As regard calcium, the intake was far below the recommended values of 1000 mg/day for pregnant women in both groups, but there was significant lower calcium intake in PDBF group in comparison to the control (p = 0.007). The mean iron intake for both groups was also lower than the reference value, but significant difference was found in PAW group (p = 0.013).
Discussion
Pregnancy during lactation is a prevailing event in Egypt as women overrely on lactation in birth spacing without adequate education and information about requirements of LAM, which usually leads to unplanned pregnancy (Shaaban and Glasier, 2008). The current study investigated the effect of pregnancy-lactation overlap on the maternal and neonatal outcomes of the current pregnancy. The study demonstrated that PDBF was not associated with a significant increase of miscarriage rate, but increases the risk of maternal anemia, delayed fetal growth, prolonged labor, low birth weight and finally associated with higher incidence of having a CS.
The socio-demographic characteristics of the two study groups; PDBF group and PAW were a little different. Women who got pregnant after weaning are significantly older than those who got pregnant during breastfeeding. Women in PAW group had higher parity, so they were more concerned about the use of contraceptive methods. This may not be the case in PDBF group who was usually of younger age and parity with probably not yet completed their family life. Our findings, contradicted studies in our setting, which compared contraceptive use among women with unplanned pregnancies less than 2 years after delivery, were comparing between PDBF group and PAW group and reported that there was no significant difference between the ages of the two groups (Tilley et al., 2009). The present results disagreed with the study done in Turkey to assess the outcomes of the pregnancies of lactating women who found no significant difference between the mean ages in the two study groups (Sengul et al., 2013). The difference may be secondary to the traditional difference between the two communities.
Concerning with the history of previous miscarriages, the present study revealed higher percentage of women with history of one or more miscarriage in PDBF group (22.2%) compared with PAW group (14.4%). Simply this may be secondary of having younger women in PDBF group and of less experience of self-care during pregnancy. The above results agreed with Sengul et al. who found significant increase in abortion rate in PDBF group compared with PAW group (0.79 ± 0.86 and 0.05 ± 0.21) respectively (p = 0.01) (Sengul et al., 2013)
The present work revealed that 45.2% of women in PDBF group had resumed their menses before the current pregnancy. These results are consistent with the study in the same setting by Shaaban and Glassier, which demonstrated the menstrual cycle resumed in about 84.9% in this group of women, that got pregnant during breastfeeding (Shaaban and Glasier, 2008).
Maternal substandard nutrition affects woman’s chances of having good health during pregnancy and may affect the health of their infants. Our study population had higher caloric carbohydrate and fat intake, but lower calcium and iron intake as compared to the ideal international values. This unbalanced dietary intake is particularly detrimental while a woman is pregnant and during a child’s first two years of life. During this period, nutritional imbalance may pose a significant threath to mothers and to children’s survival, growth and development, which in turn negatively affects children’s learning ability, and to work and grow as adults (UNICEF, 2009).
The present work disclosed a statistically significant higher incidence of having more overall pregnancy complications in women who got pregnant during breastfeeding compared with those got pregnant after weaning (70.0% and 48.9%, respectively). The above results were contradicting the results of two previous studies (Madarshahian and Hassanabadi, 2012; Sengul et al., 2013). They found no statistical significant difference in the problems during pregnancy between the lactation and pregnancy overlap group and the non-overlap group in their population. The difference between our study and the above two studies could be basically the difference on the basal nutritional point from where women in the different study started their pregnancies. The results of the present study showed that the majority of women included in the present study had substandard nutritional care. This is an important point in the rationally of this study. As based upon the fact that, women in our community either in PDBF or PAW group are of unbalanced dietary intake, we can hypothesis that extra burden of pregnancy-lactation overlap can add to the bad nutritional status of women and possibly responsible upon hazards effect on the current pregnancy.
Miscarriage rate (about 2.0%) in this cohort was lower than reported in previous studies probably because of our inclusion criteria that recruit participants at any time during first trimester, some early miscarriages had been lost before recruitment in the study. Miscarriage rate was not significantly different between both groups. These results agreed with the results of two previous studies of Madarshahian and Hassanabadi (2012) and Ishii (2009). On the other hand Albadran described a significantly higher frequency of miscarriage among those who got pregnant after weaning (10.35%) compared to patients who breastfed during pregnancy (5.12%) (Albadran, 2013). Moreover, unplanned pregnancy is expected to occur more commonly in PDBF group leading to seeking induced miscarriage. This type of illegal miscarriage may be hidden among women leading to underestimation of the effect of PDBF on the miscarriage rate.
Moreover, more than half of PDBF group (54.1%) was suffering from anemia, a factor that is probably contributed. This finding agreed with Dairo and Lawoyin (2004) who found an association between interpregnancy intervals shorter than 24 months and an increased risk of anemia during pregnancy. Short intervals could indirectly increase the risk of adverse neonatal/infant outcomes through changes in breastfeeding patterns or the composition and/or quantity of breast milk secondary to breastfeedingpregnancy overlap.
The results of present study revealed significant higher occurrence of hypertensive disorders with pregnancy in PAW group as compared with PDBF group (p = 0.003) this result disagreed with Madarshahian and Hassanabadi, who found that there was no relation between overlap and non overlap groups with hypertension, this finding may be explained by low dietary intake of calcium in our community (Madarshahian and Hassanabadi, 2012). However, agreed with Murphy et al. who found that calcium intakes in Kenya and Egypt were, well, below recommended amounts in his study, which estimated mineral intakes of toddlers; in Egypt, Kenya, and Mexico (Murphy et al., 1992).
Our women in PDBF suffered from more delayed fetal growth, (p = 0.001) as compared with women in the PAW group. This result is very similar to the results reported by Merchant et al. (1990). They suggested that the overlap could produce suboptimal outcome for both pregnancy and subsequent lactation, such as IUGR of the fetus. Moreover, this study agreed with Van Eijsden et al. (2008) who suggested that folate depletion contributes to the risk of IUGR, which is associated with short interpregnancy intervals. However, these results disagreed with Sengul et al. (2013) who reported that there was no difference in IUGR between the two groups. The basic nutritional status of women in our culture is much different from others.
According to the findings of the current study, it can be observed that women who got pregnant during breastfeeding had higher incidence of cesarean delivery (43.7%) as compared with women who got pregnant after weaning (31.5%) (p = 0.001). This may be explained by a shorter inter-pregnancy interval in women who got pregnant during breastfeeding with less time for adequate healing of a CS scar.
The present study revealed that there was increase incidence of having more prolonged labor in women who got pregnant during breastfeeding as compared with those who got pregnant after weaning. These results agreed with a previous study showing that PDBF mothers have a higher incidence of prolonged labor (p = 0.042) compared to PAW mothers (Marquis et al., 2002). On the other hand, this result disagreed with Pareja (2007) who found that there no differences were found in the proportion of mothers who breastfed during late pregnancy between the cases of prolonged active Phase of labor and their respective controls.
With regard to neonatal outcome, the present work showed a higher incidence of low birth weight in women who got pregnant during breastfeeding. This result confirms the data of with Sengul et al. (2013) reporting a significant decrease in birth weight observed in women who became pregnant during lactation. The lower birth weight of infants in the overlap group may be caused by maternal anemia and a lack of maternal milk consumption during pregnancy. However this result disagrees with Madarshahian and Hassanabadi (2012) and Albadran (2013) that showed no difference in neonatal birth weight between PDBF and PAW groups.
Although the presence of some confounding factors that should be taken into consideration when examining the above results, the study is alarming against short inter-pregnancy interval and pregnancy and lactation overlap in communities with substandard nutrition. The inter-pregnancy interval should be well spaced to ensure healthier mothers and children in society. Women should be better educated about contraceptive methods and they should not accept lactation only as a contraceptive method in order to have more planned pregnancies. Nutrition consultation should be offered to all women before and during pregnancy and during lactation.
In conclusion, pregnancy during breastfeeding is associated with an increase in the overall complications of pregnancy as compared to PAW. Although it does not increase the miscarriage rate, it increases the prevalence of maternal anemia, delayed fetal growth, prolonged labor, cesarean delivery and low birth weight infants. More studies are needed to evaluate the effect of pregnancylactation overlap in each outcome independently.
References
- 1. American College of Obstetricians and Gynecologists, ACOG, (Task Force on Hypertension in Pregnancy). Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Albadran MM. Effect of Breastfeeding during Pregnancy on the Occurrence of Miscarriage and Preterm Labour. Iraq J of Med Sci. 2013;11:285–289. [Google Scholar]
- 3.Dairo MD, Lawoyin TO. Socio-demo-graphic determinants of anemia in pregnancy at primary care level:A study in urban and rural Oyo State, Nigeria. Afr J Med Sci. 2004;33:213–217. [PubMed] [Google Scholar]
- 4.Delabaere A, Huchon C, Deffieux X, et al. Epidemiology of loss pregnancy. J Gynecol Obstet Biol Reprod. 2014;43:764–775. doi: 10.1016/j.jgyn.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Elashiry A, El ghazali S, Habil I, et al. Prevalence and determinants of anaemia in third trimester pregnancy in fayoum governorate-Egypt. Acta Medica Mediterranea. 2014;30:1045–1051. [Google Scholar]
- 6.Fathalla MF, Abdel-Raheem MS, Amin A, et al. The prevalence, determinants and outcome of unintended pregnancy:a hospital-based study. J Egypt Soc Obstet Gynecol. 2003;29:945–954. [Google Scholar]
- 7. Institute of Medicine (US) Panel on Micronutrients. National Academy Press:Washington DC. 2001. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. [DOI] [PubMed] [Google Scholar]
- 8.Ishii H. Does breastfeeding induce Spontaneous abortion? J Obstet Gynaecol Res. 2009;35:864–868. doi: 10.1111/j.1447-0756.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 9.Khella AK, Fahim HI, Issa AH, et al. Lactational amenorrhea as a method of family planning in Egypt. Contraception. 2004;69:317–322. doi: 10.1016/j.contraception.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Madarshahian F, Hassanabadi M. A comparative study of breastfeeding during pregnancy: impact on maternal and newborn outcomes. J Nurs Res. 2012;20:74–80. doi: 10.1097/JNR.0b013e31824777c1. [DOI] [PubMed] [Google Scholar]
- 11.Marquis GS, Penny ME, Diaz JM, et al. Postpartum Consequences of an overlap of breastfeeding and pregnancy:reduced breast milk intake and growth during early infancy. Pediatrics. 2012;109(4): doi: 10.1542/peds.109.4.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchant K, Martorell R, Haase J. Maternal and fetal responses to the stresses of lactation concurrent with pregnancy and of short recuperative intervals. Am J Clin Nutr. 1990;3:280–288. doi: 10.1093/ajcn/52.2.280. [DOI] [PubMed] [Google Scholar]
- 13.Murphy SP, Beaton GH, Calloway DH. Estimated mineral intakes of toddlers: predicted prevalence of inadequacy in village populations in Egypt, Kenya, and Mexico. Am J Clin Nutr. 1992;56(3):565–572. doi: 10.1093/ajcn/56.3.565. [DOI] [PubMed] [Google Scholar]
- 14.Walker JD. NICE clinical guideline 63: Diabetes in pregnancy. Management of diabetes and its complications from pre-conception to the postnatal period. Diabet Med. 2008;25:1025–1027. doi: 10.1111/j.1464-5491.2008.02532.x. [DOI] [PubMed] [Google Scholar]
- 15.Pareja R. The association between breastfeeding during late pregnancy and the occurrence of small for gestational age and prolonged active phase of labor among Peruvian women. Retrospective Theses and Dissertations. 2007;21(4):1–14. [Google Scholar]
- 16. American College of Obstetricians and Gynecologists, ACOG, ACOG Practice Bulletin No. 76: Postpartum Hemorrhage. Obstet Gynecol. 2006;108:1039–1048. [Google Scholar]
- 17.Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 18.Sengul O, Sivaslioglu Aa, Kokanali Mk, et al. The outcomes of the pregnancies of lactating women. Turk J Med Sci. 2013;43:251–254. [Google Scholar]
- 19.Shaaban OM, Glasier AF. Pregnancy during breastfeeding in rural Egypt. Contraception. 2008;77:350–354. doi: 10.1016/j.contraception.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death:retrospective cohort study. BMJ. 2003;327(7410):313–327. doi: 10.1136/bmj.327.7410.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilley EB, Shaaban OM, Wilson M, et al. Breastfeeding and contraception use among women with unplanned pregnancies less than 2 years after delivery. Int J Gynaecol Obstet. 2009;105:127–130. doi: 10.1016/j.ijgo.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 22. United Nations International Children's Emergency Fund, UNICEF, United Nations University, UNU, World Health Organization, WHO, World Health Organization. 2001. Iron deficiency anemia: assessment, prevention, and control. [Google Scholar]
- 23. United Nations International Children's Emergency Fund, UNICEF, UNICEF, Division of Communication. 2009. Tracking progress on child and maternal nutrition:A survival and development priority. [Google Scholar]
- 24.Van Eijsden M, Smits LJ, Van der Wal MF, et al. Association between short interpregnancy intervals and term birth weight, the role of folate depletion. Am J Clin Nutr. 2008;88:147–153. doi: 10.1093/ajcn/88.1.147. [DOI] [PubMed] [Google Scholar]