Abstract
PURPOSE
Current treatments for metastatic RCC (mRCC) do not extend survival beyond a few months. Sorafenib (SF) is a targeted drug approved for mRCC, but it has modest efficacy. Hymecromone is a nontoxic dietary supplement with some antitumor activity at high doses (450 – 3000 mg/day). HC inhibits hyaluronic acid (HA) synthesis. HA promotes tumor growth and metastasis. We recently showed that HA-receptors CD44 and RHAMM are potential predictors of mRCC. We examined the anti-tumor properties of HC, SF, and their combination in RCC models.
METHODS
Using proliferation, clonogenic and apoptosis assays, effects of HC (0–32 μg/ml), SF (0–3.2 μg/ml) and HC+SF were examined in RCC cells (Caki–1, 786–O, ACHN, A498) and endothelial cells (HMVEC–L, HUVEC). Boyden chamber was used for motility and invasion assays. Apoptosis indicators, HA receptors, EGFR and c-Met were evaluated by immunoblotting. Efficacy of HC, SF and HC+SF was evaluated in the SF-resistant Caki–1 xenograft model.
RESULTS
HC+SF synergistically inhibited proliferation (>95%), motility/invasion (65%) and capillary formation (76%) in RCC and/or endothelial cells, and induced apoptosis by 8-fold (P<0.001). HC+SF inhibited HA synthesis and HA addition reversed the cytotoxicity of HC+SF. HC+SF up-regulated pro-apoptotic indicators and downregulated Mcl-1, CD44, RHAMM, phospho-EGFR and phospho-cMet levels. In all assays, HC and SF alone were ineffective. Oral administration of HC (50–200mg/kg) plus SF (30mg/kg) eradicated Caki–1 tumor growth without toxicity. HC and SF alone were ineffective.
CONCLUSION
This is the first study that demonstrates combination of SF with HC a non–toxic dietary supplement is highly effective in controlling RCC.
Keywords: Hymecromone, renal cell carcinoma, Sorafenib, Tumor growth, Invasion
INTRODUCTION
In 2012, 64,770 new cases and 13,570 deaths are estimated for kidney cancer in the United States. At initial diagnosis, about 1/4th of patients with renal cell carcinoma (RCC) have metastatic or advanced disease and ≥ 1/3rd of patients develop metastasis (1). Despite surgery and targeted therapies, including tyrosine kinase inhibitors, cytokines and proteosome inhibitors, the median survival of patients with metastatic RCC (mRCC) is ≤ 2 years (2,3). Sorafenib (Nexavar®; Bayor Corp) is an orally bioavailable, angiogenesis inhibitor which is FDA-approved for the treatment of mRCC. However it has modest efficacy; SF improves survival by 12–18% and causes disease stabilization for ≥ 8 weeks (4,5).
Hymecromone is a dietary supplement sold in Europe and Asia under several brand names, including Heparvit, Adesin C and Cantabiline for improving liver health. HC is a coumerin derivative, 7-hydroxy-4methyl coumerin or 4-methylumbelliferone (6,7). HC or 4-methylumbelliferone is usually used as a fluorogenic detector in enzyme assays and such applications account for the majority of publications in PubMed on HC. However, HC is consumed at a dose of 1500 – 2200 mg/day as a choleretic. In a double blinded placebo control trial, HC at 1200 mg/day dose effectively blocked choleretic spasms (8). HC has no known toxicity and lacks the anti-coagulant activity of Coumadin, as well as the anti-sperminogenic and anti-aromatase activities of Coumerin (9–11).
The known biological activity of HC is inhibition of hyaluronic acid (HA) synthesis (12–14). HC competitively inhibits the synthesis of UDP-glucuronic acid, which is one of the two building blocks of HA. HA is a glycosaminoglycan, which promotes tumor growth and metastasis. It regulates cell proliferation, adhesion, motility and invasion by binding to cellular HA receptors, CD44 and RHAMM (15,16). We recently showed that CD44 and RHAMM mRNA levels in RCC tissues are independent predictors of metastasis (17). Further, in our study on prostate cancer cells HC (i.e., 4-methylumbelliferone) inhibited HA synthesis and downregulated both CD44 and RHAMM expression (IC50 80-μg/ml). At 450 mg/kg/day dose, HC inhibited the growth of a prostate cancer xenograft by ~ 70% (9). HC has also shown antitumor activity in a few other models at doses (i.e., 1000 – 3000 mg/kg/day) close to the maximal tolerated dose of HC in mice, i.e., 2300 – 7200 mg/kg (NIOSH registry #: GN7000000; 12,13,18,19). Except for one study, in which HC (1000-mg/kg/day) showed modest efficacy in combination with Gemcitabine against a pancreatic cell line, HC has not been combined with any agent for the treatment of any disease (20).
Since SF is an anti-angiogenic drug used for the treatment of metastatic RCC, CD44 and RHAMM are independent predictors of RCC metastasis and HC downregulates CD44 and RHAMM expression and has antitumor activity, we hypothesized that at minimum HC and SF combination should have an additive effect on inhibiting RCC and EC growth in vitro and in xenografts. Therefore, we evaluated the effect of SF and HC combination on RCC and endothelial cell functions in vitro and in RCC xenograft.
MATERIALS AND METHODS
Cell lines
Human RCC cell lines Caki-1, ACHN, 786-O, A498, murine RCC cell line RENCA, normal human kidney epithelial cells (HK2) and primary human lung fibroblast (HLF) were purchased from ATCC. Primary Human Microvessel Endothelial Cells –Lung (HMVEC-L) and Human Umbilical Vein Endothelial Cells (HUVEC) were purchased from Clontech. RCC cells were cultured in RPMI+10% fetal bovine serum (FBS) + gentamicin. Endothelial cells (ECs) and HLF were cultured in EGM-V and DMEM+10% FBS+gentamicin media, respectively.
Reagents
SF and HC were purchased from LC Laboratories and Sigma-Aldrich, respectively. All other reagents, antibodies and kits were purchased as described before (9,21).
Cell proliferation, colony assay, apoptosis and HA level measurement
RCC cells (12,500) were plated in growth medium and exposed to SF (0–3.2 μg/ml), HC (0–32 μg/ml), HC+SF and/or HA (50-μg/ml) for 72-h; cells were then counted. Apoptosis was measured using the Cell Death ELISA kit (Roche Diagnostics). For colony assay, 786-O cells (0.25x103) were plated in 6-well plates and exposed to HC, SF or HC+SF. Colonies were stained and counted after 7-days. For HA level measurement, cells (1x105) were exposed to SF (0, 3.2-μg/ml), HC (0, 16-μg/ml) or SF+HC for 24-h. HA levels in media were measured by the HA test (22).
Co-culture
In a 2D-chamber assay with 3-μm insert, HLF, HUVEC or HLF+HUVEC cells were plated in the bottom inserts. Following 24-h incubation, 786-O cells were plated in top inserts. The cultures were exposed on HC, SF or HC+SF and the cytotoxicity was determined by the MTT assay 48-h later.
Motility and invasion
Motility and Matrigel™ invasion assays were carried out as described previously, using the MTT assay (9,21), except that HC (16-μg/ml), SF (3.2-μg/ml) or HC+SF were added in both chambers of the Transwell. Motility and invasion were evaluated after 18-h and 48-h, respectively. For co-cultures, 786-O or HMVEC-L cells were plated in the top insert and the bottom well contained confluent cultures of HLF and HUVEC or 786-O cells, respectively. Percent invasion or motility was calculated as [O.D. of the cells on the bottom of the filter ÷ O.D. (top chamber + bottom filter)] x100.
Capillary formation assay
HUVEC cells were plated on Matrigel™ coated wells in EGM-V. The cells were exposed to SF (1.6-μg/ml), HC (16-μg/ml) or HC+SF. Following 12-h incubation, capillary formation was photographed and capillaries were counted under 100X magnification.
Immunoblot analysis
786-O and HUVEC cells treated with SF (0–3.2-μg/ml), HC (0–32-μg/ml) or HC+SF for 48h. Cell lysates (~20,000 cell equivalent) were analyzed by immunoblotting using specific antibodies (9); β-actin was used as a loading control.
Tumor xenografts
Caki-1 cells (2x106) was mixed 1:1 with Matrigel™ and implanted subcutaneously on the dorsal flank of 5–6 week old athymic mice. Mice (10/group) were orally gavaged daily with SF (30-mg/kg), HC (200 mg/kg) or SF (30mg/kg) plus HC (at 50,100 or 200-mg/kg) starting on day 6. At day 37 all mice in vehicle, SF, HC groups and 50% from the HC+SF group (with HC at 100- or 200-mg/kg) were euthanized. For the remaining 50% of mice in the HC+SF groups, treatment was stopped on day 37 and the mice were observed until day 52. Tumor volume was measured twice weekly, as described before (9).
Statistical analysis
Differences between control and treatment groups for in vitro and xenograft studies were determined by Tukey’s multiple comparison test.
RESULTS
HC+SF combination inhibits RCC and EC proliferation
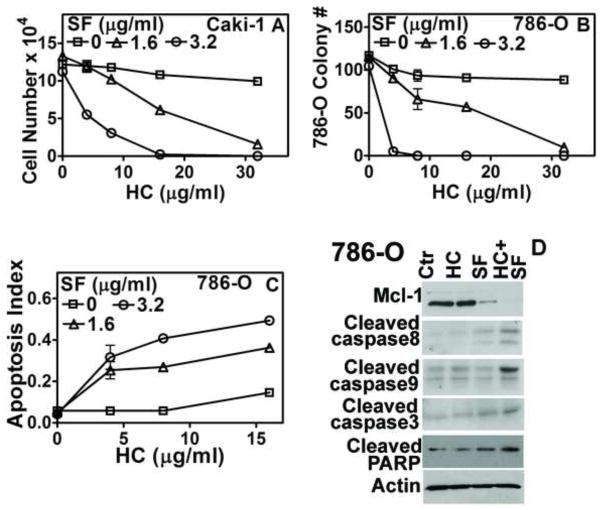
In a cell proliferation assay, the HC+SF combination inhibited Caki-1 cell growth in a dose-dependent manner, but HC and SF, individually, were ineffective (Fig 1A; P<0.001). HC (16-μg/ml) and SF (3.2-μg/ml) combination inhibited RCC cell (Caki-1, 786-O, ACHN, A498, RENCA) and EC (HMVEC-L, HUVEC) growth by ≥ 90% (Table 1). Combination indices for RCC cells and ECs were < 0.005, indicating very strong synergy between HC and SF for growth inhibition (Table 1). Quiescent normal cells (HK2, HLF) were resistant to HC+SF (Table 1). The combination also inhibited clonogenic survival of 786-O cells by 100% (P<0.001; Fig 1B). The growth inhibition by HC+SF was due to apoptosis induction. The combination (HC: 16-μg/ml + SF: 3.2-μg/ml) caused ~ 7-fold induction of apoptosis in 786-O cells (Fig 1 C). Further, HC+SF increased the levels of apoptosis indicators- activated caspase-3, -8, -9 and cleaved PARP- but downregulated anti-apoptotic protein Mcl-1 (Fig 1D). HC (16-μg/ml) and SF (3.2-μg/ml) combination also induced apoptosis in Caki-1 cells by 3.4-fold (apoptosis indices: Ctr: 0.055±0.002; SF:0.053±0.01; HC:0.059±0.001; HC+SF:0.189±0.02).
Figure 1. Effect of HC, SF and HC+SF on RCC cells.
A: Caki-1 cell viability was determined after 72-h exposure to HC, SF or HC+SF at indicated concentrations. Live cells were counted; B: Clonogenic survival of 786-O cells was determined after 7-days. C: Apoptosis was measured following the exposure of 786-O cells to HC, SF or HC+SF for 72 h. Y-axis: O.D. 405 nm. Data for A–C: Mean±sd. D: Immunoblotting of 786-O cells for apoptosis indicators following 48-h exposure to HC (16-μg/ml), SF (3.2-μg/ml) and HC+SF.
Table 1. Analysis of cytotoxic effects of HC+SF combination.
RCC, EC, and normal cells were exposed to HC, SF or HC+SF for 72 h and the cells were counted. Inhibition of cell proliferation, as shown here is for HC (16-μg/ml) + SF (3.2-μg/ml) combination. Calcusyn program was used to evaluate drug synergy for the HC+SF combination. Combination index Combination is a measure of synergy. Combination indices <0.01 reflect very strong synergy (Calcusyn manual).
| Cell line | Inhibition | Combination Index |
|---|---|---|
| 786-O | 92.1% | 0.001 |
| Caki-1 | 93.2% | 0.004 |
| RENCA | 95% | 0.001 |
| ACHN | 90.5% | 0.002 |
| A498 | 94.1% | 0.001 |
| HMVEC-L | 89.5% | 0.001 |
| HUVEC | 92.3% | 0.001 |
| HK2 | 11% | 1.0 |
| HLF | 7% | 1.0 |
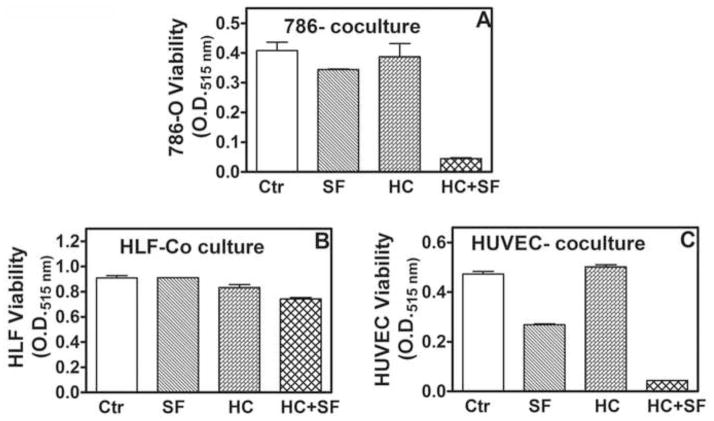
In tumor tissues, tumor-associated stroma can influence the response of tumor cells to drugs. Further, SF is a known inhibitor of angiogenesis, however, in tumors RCC cells may modulate the response of ECs to SF. Therefore, we examined the efficacy of SF, HC and their combination in co-cultures of 786-O cells, HLF and HUVEC. HC+SF inhibited 786-O viability > 90% in co-cultures (Fig 2A; Supplement). HLF were resistant to growth inhibition by HC+SF even in co-culture (Fig 2B; Supplement). While SF alone inhibited HUVEC viability by 43%, HC+SF inhibited the viability by 93% (Fig 2B; Supplement).
Figure 2. Effect of HC+SF combination on RCC, fibroblast and EC viability in co-cultures.
Co-cultures of 786-O, HLF and HUVEC were exposed to HC, SF or HC+SF. Viability of each cell type was measured by MTT assay at 48-h. A: 786-O; B: HLF; C: HUVEC.
HC+SF combination inhibits motility and invasion
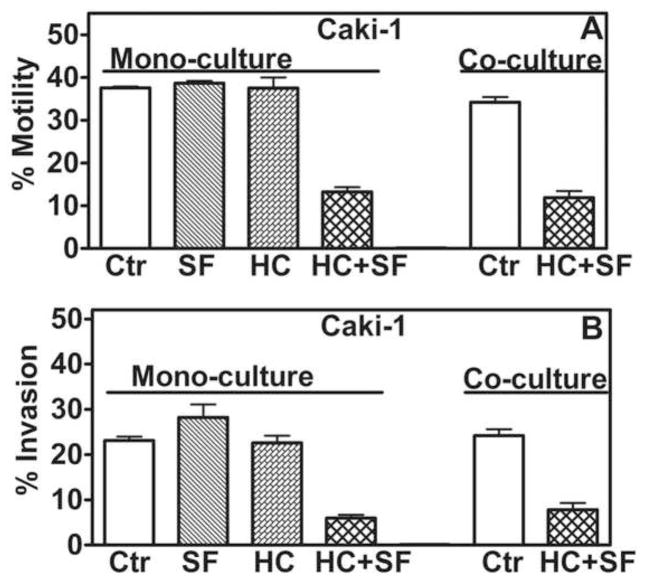
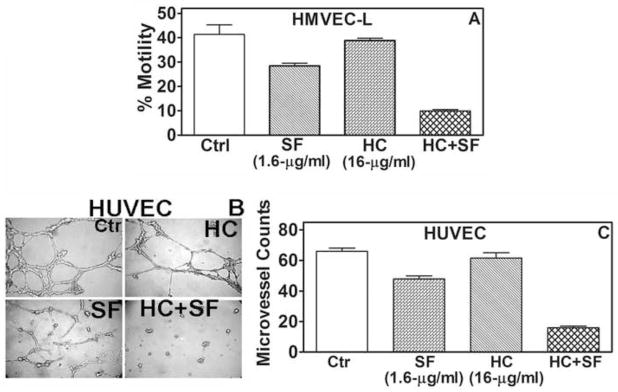
The HC+SF combination, but not HC and SF individually, inhibited chemotactic motility and invasive activity of Caki-1 cells (≥ 65% inhibition; Fig 3A, B). In HMVEC-L, HC+SF inhibited chemotactic motility in both mono- and co-cultures by ~70% (P<0.001; Fig 4A); similar results were obtained for HUVEC (data not shown). SF alone (1.6-μg/ml) inhibited capillary formation in HUVEC by 28%, but HC+SF caused 76% inhibition (P<0.001; Fig 4B, C).
Figure 3. Effect of HC+SF on motility and invasion of Caki-1 cells.
A and B: Motility and invasion of Caki-1 cells was measured in mono- or in co-cultures with HLF+ HUVEC in modified Boyden chambers and calculated as describe in “Materials and Methods”. Data: Mean±sd.
Figure 4. Effect of HC+SF on EC functions.
A: HMVEC-L motility was determined after 18-h exposure to HC (16-μg/ml) plus SF (3.2-μg/ml). B and C: Effect of HC (16-μg/ml), SF (1.6-μg/ml) and HC+SF on capillary formation was examined as described in “Materials and Methods”. B: Pictures were taken at 12-h. C: microvessels were counted; data: Mean±sd.
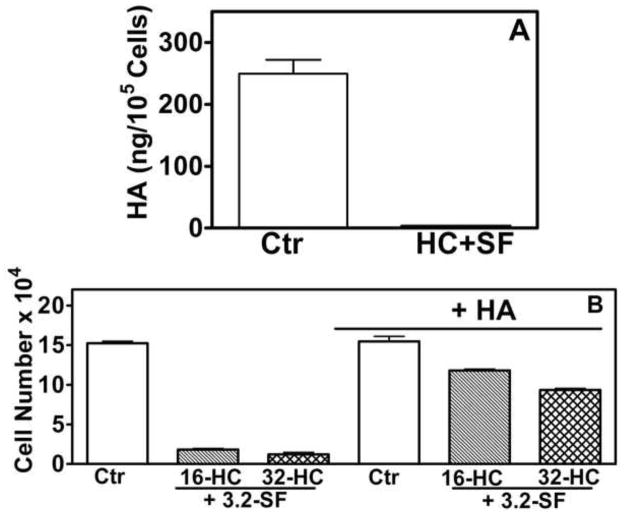
HC+SF mediate cytotoxic effects by inhibiting HA synthesis
HC is known inhibitor of HA synthesis (11,12); IC50 of HC to block HA synthesis is 80-μg/ml (9). As expected, HC at 16 or 32-μg/ml concentrations did not inhibit HA synthesis in RCC cells (data not shown). However, HC (16-μg/ml) and SF (3.2-μg/ml) combination inhibited HA synthesis by 100% (Fig 5A; Supplement). Further, in the presence of HA, cytotoxic effects of HC+SF on 786-O cells were reversed; 20–30% versus 90% cytotoxicity by HC+SF in the presence or absence of HA, respectively (Fig 5B; Supplement).
Figure 5. Effect of HA on the cytotoxicty of HC+SF.
A: HA levels were measured in Caki-1 cells exposed to HC (16-μg/ml) + SF (3.2-μg/ml) for 24-h by the HA-test. B: Effect of HC (16-μg/ml) + SF (3.2-μg/ml) on the growth of 786-O cells in the presence or absence of HA (50-μg/ml) was tested after 72-h exposure. Data: Mean±sd.
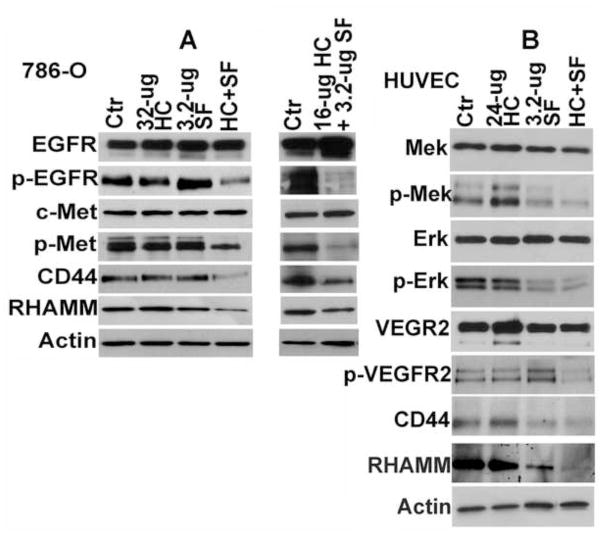
We have shown that inhibition of HA synthesis downregulates the expression of HA receptors - CD44 and RHAMM (9). In tumor cells, binding of HA to HA receptors induces a signaling complex between HA receptors, EGF-receptor and hepatocyte growth factor receptor, c-Met; which in turn, activates EGF-receptor and c-Met signaling (15,16, 23,24). Consistent with the inhibition of HA synthesis, HC+SF downregulated CD44, RHAMM, p-EGFR and p-Met levels by > 3-fold in 786-O cells (Fig 6A). In HUVEC, HC+SF downregulated CD44 and RHAMM along with p-Mek, p-Erk and p-VEGFR levels (Fig 6B). HC or SF alone did not significantly affect the levels of respective signaling molecules in 786-O cells or HUVEC (Fig 6A, B). Similar results were obtained in HMVEC-L (data not shown).
Figure 6. Immunoblot analysis of 786-O and HUVEC cells.
786-O (A) and HUVEC (B) cells were exposed to HC, SF or HC+SF for 48-h and the cell lysates were analyzed by immunoblot analysis. Actin: loading control.
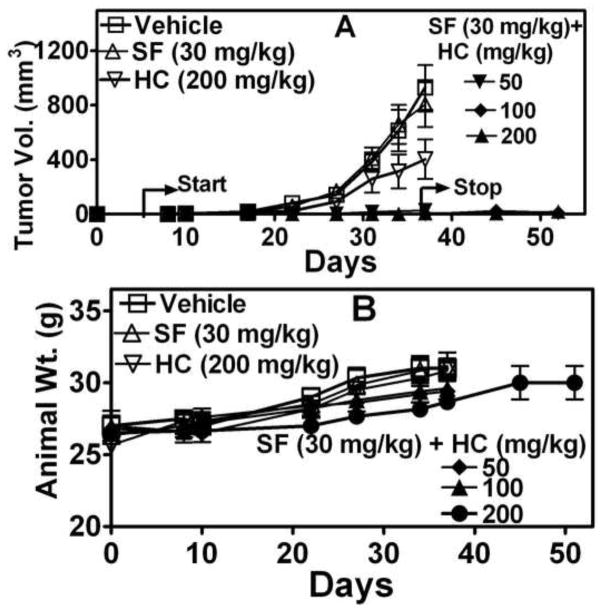
HC+SF combination inhibits Caki-1 tumor growth
Caki-1 tumors are resistant to SF treatment at 60-mg/kg, which is close to the maximum tolerated dose for SF (25). As shown in Fig 7A, Caki-1 tumor growth was not inhibited by SF alone. Inhibition of tumor growth by HC alone was not statistically significant (P<0.05). However, in all three HC+SF combination groups, tumor growth was completely inhibited. Further, even after treatment was stopped on day 37, only two animals in HC+SF-treated groups formed palpable tumors. In none of the treatment groups animals lost weight (Fig 7B). No toxicity was observed upon histological examination of liver, lung and lung and analysis of serum chemistry (Table 2; Supplement). Since no tumors were generated in the HC+SF combination group, no tissues were available to determine whether the combination induced the same growth inhibitory signaling (i.e., apoptosis, downregulation of HA receptors, etc) in vivo.
Figure 7. Examination of the antitumor activity of HC, SF and HC+SF in Caki-1 xenograft.
Caki-1 xenograft was generated and treated as described in Materials and Methods. A: Tumor volume in various treatment groups. B: Animal weight.
Table 2. Analysis of serum chemistry.
Serum specimens from animals in various treatment groups (as described in Figure 6) were analyzed for various serum markers of toxicity. ND: not determined due to low sample volume.
| Serum Marker | Ctr | SF | HC | SF+HC | Normal Range |
|---|---|---|---|---|---|
| Glucose | 189±49 | 157±26 | 207±47 | 167±15 | 90 – 193 mg/dL |
| Lipidemia | 0 | 0 | 0 | 0 | 0 |
| BUN | 14±17 | 18±2.3 | 16±1.5 | 21.7±2 | 18–29 mg/dL |
| Creatinine | 0.2±0 | 0.13±0.06 | 0.2±0 | 0.2±0 | 0.1–0.4 mg/dL |
| Calcium | 10.5±0.4 | 10.2±1.0 | 10.4±0.06 | 10.4±0.25 | 8.7–10.7 mg/dL |
| Protein | 5.2±0.1 | 6.9±1.1 | 5.2±0.2 | 5.7±0.7 | 4.6–6.9 mg/dL |
| Albumin | 2.7±0.2 | ND | 2.7±0.06 | 3.2±0.7 | 2.5–4.8 mg/dL |
| AST | 160±20.1 | ND | 94.7±16 | 167±15 | 54–298 U/I |
| ALT | 44.7±9.0 | 38.3±7.6 | 36.7±1.2 | 59±25 | 29–191 U/L |
| Clot time | 2.33 ±0.1 | ND | ND | 2.25±0.09 | ~2.3 min/0.3 cc |
DISCUSSION
SF is currently used as a second-line treatment for mRCC and has modest efficacy (2,3). Our study is the first to show that combination of SF with a dietary supplement HC has potent antitumor and anti-angiogenic efficacy in pre-clinical models of RCC. An important aspect of our study is the demonstration of synergy between HC and SF, at the concentrations at which both agents individually are ineffective in inhibiting RCC and EC functions. Another noteworthy aspect of the study is that while SF is an anti-angiogenic agent, when combined with HC, the combination becomes a potent antitumor agent with cytotoxic and anti-invasive activities against RCC cells. At the daily dose of SF (i.e., 400 mg b.i.d) steady state plasma levels of SF are between 2.9- and 5.4-μg/ml (25,26). Therefore, the concentrations at which SF synergizes with HC are easily plasma achievable. Similarly, the concentrations at which HC synergizes with SF are 3–5-fold less than the concentrations at which HC alone has biological effects in vitro (9,27).
The mechanism by which HC and SF synergistically become a potent antitumor and anti-angiogenic combination is currently under investigation. However, the combination inhibits HA synthesis and HA-mediated signaling in RCC cells, which involves downregulation of CD44, RHAMM and phosphorylation of EGF-receptor and c-Met. Since the cytotoxic effects of HC+SF on RCC cells are attenuated in the presence of HA, it indicates that inhibition of HA synthesis and subsequent HA-mediated signaling are very likely the reasons for the potent cytotoxic, and anti-invasive activity of HC+SF in RCC cells.
Three signaling pathways in ECs contribute to angiogenesis; two of these are the VEGF-R – Mek- Erk axis and c-Met signaling (28,29). Induction of EC functions through binding of angiogenic HA fragments to RHAMM is the third angiogenic pathway (30). Therefore, by inhibiting VEGF-R and c-Met activation and downregulating CD44 and RHAMM, the combination plausibly inhibits all three angiogenic pathways.
In this study, HC+SF combinations eradicated tumor growth, without toxicity. Further, the doses of HC used in the combinations are 5 to >10-fold less than the doses at which HC alone has antitumor activity (12,13,18,19). One limitation of the study is the use of the subcutaneous model. However, this model allowed us to establish the proof-of-principle regarding the efficacy of the combination at non-toxic doses in a SF-resistant model. The second limitation is that since no tumors were generated in the combination groups, tumor tissues could not be evaluated for the signaling events that were inhibited by HC+SF in vitro.
Taken together, although SF has modest efficacy as a single agent against mRCC, its combination with a non-toxic and dietary supplement, HC, has potent antitumor activity in RCC models, which are SF-resistant. Since both HC and SF are orally bioavailable, and are already in use, their combination at non-toxic doses should be useful in the treatment, and more importantly, in the prevention of mRCC in high-risk patients.
Take home message.
This is the first study that shows combination of Sorafenib with Hymecromone, a non-toxic dietary supplement is potentially an effective strategy to control and prevent RCC growth and progression.
Acknowledgments
Support: R01 CA 72821–11 (VBL), The Woman’s Cancer Association of the University of Miami (VBL–VGB).
Abbreviations used
- ECs
Endothelial cells
- HA
Hyaluronic acid
- HC
Hymecromone
- HLF
Human lung fibroblast
- HMVEC-L
Human Microvessel Endothelial Cells
- HUVEC
Human Umbilical Vein Endothelial Cells
- mRCC
metastatic renal cell carcinoma
- SF
Sorafenib
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen DY, Uzzo RG. Evaluation and management of the renal mass. Med Clin North Am. 2011;95:179–89. doi: 10.1016/j.mcna.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Hu B, Lara PN, Jr, Evans CP. Defining an individualized treatment strategy for metastatic renal cancer. Urol Clin North Am. 2012;39:233–49. doi: 10.1016/j.ucl.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Kenney PA, Wood CG. Integration of surgery and systemic therapy for renal cell carcinoma. Urol Clin North Am. 2012;39:211–31. doi: 10.1016/j.ucl.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Arranz JA, Climent MA, González-Larriba JL, León L, Maroto JP. Sorafenib in renal cell carcinoma. Crit Rev Oncol Hematol. 2011;80:314–22. doi: 10.1016/j.critrevonc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B. Sorafenib for the management of advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2011;11:825–36. doi: 10.1586/era.11.55. [DOI] [PubMed] [Google Scholar]
- 6.Keating GJ, O’Kennedy R. The chemistry and occurrence of coumarins. In: O’Kennedy R, Thornes RD, editors. Coumarins Biology, Applications and Mode of action. Wiley and Sons; Chichister: 1997. pp. 23–65. [Google Scholar]
- 7.Crooke D, Fitzpatrick B, O’Kennedy R, McCormack T. Coumarins-multifaceted molecules with many analytical and other applications. In: O’Kennedy R, Thornes RD, editors. Coumarins Biology, Applications and Mode of action. Wiley and Sons; Chichister: 1997. pp. 303–31. [Google Scholar]
- 8.Stacchino C, Spano R, Pettiti A. Spasmolytic activity of some 4-methylumbelliferone derivatives. Boll Chim Farm. 1983;122:158–60. (and references there in) [PubMed] [Google Scholar]
- 9.Lokeshwar VB, Lopez LE, Munoz D, Chi A, Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N, Altman N. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010;70:2613–23. doi: 10.1158/0008-5472.CAN-09-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Cho M, Karlsberg K, Zhou D, Yuan YC. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J Biol Chem. 2004;279:48071–8. doi: 10.1074/jbc.M406847200. [DOI] [PubMed] [Google Scholar]
- 11.Omarbasha B, Fair WR, Heston WD. Effect of coumarin on the normal rat prostate and on the R-3327H prostatic adenocarcinoma. Cancer Res. 1989;49:3045. [PubMed] [Google Scholar]
- 12.Yoshihara S, Kon A, Kudo D, Nakazawa H, Kakizaki I, Sasaki M, Endo M, Takagaki K. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005;579:2722–6. doi: 10.1016/j.febslet.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 13.Urakawa H, Nishida Y, Wasa J, Arai E, Zhuo L, Kimata K, Kozawa E, Futamura N, Ishiguro N. Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Br J Cancer. 2011;105:1839–49. doi: 10.1038/bjc.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kultti A, Pasonen-Seppänen S, Jauhiainen M, Rilla KJ, Kärnä R, Pyöriä E, Tammi RH, Tammi MI. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–23. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18:288–95. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM. Hyaluronan in human malignancies. Exp Cell Res. 2011;317:383–91. doi: 10.1016/j.yexcr.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Chi A, Shirodkar SP, Escudero DO, Ekwenna OO, Yates TJ, Ayyathurai R, Garcia-Roig M, Gahan JC, Manoharan M, Bird VG, Lokeshwar VB. Molecular characterization of kidney cancer: Association of Hyaluronic Acid Family With Histological Subtypes and Metastasis. Cancer. 2012;118:2394–402. doi: 10.1002/cncr.26520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya SS, Paul S, Mandal SK, Banerjee A, Boujedaini N, Khuda-Bukhsh AR. A synthetic coumarin (4-methyl-7 hydroxy coumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur J Pharmacol. 2009;614:128–36. doi: 10.1016/j.ejphar.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Twarock S, Freudenberger T, Poscher E, Dai G, Jannasch K, Dullin C, Alves F, Prenzel K, Knoefel WT, Stoecklein NH, Savani RC, Homey B, Fischer JW. Inhibition of oesophageal squamous cell carcinoma progression by in vivo targeting of hyaluronan synthesis. Mol Cancer. 2011;10:30. doi: 10.1186/1476-4598-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazawa H, Yoshihara S, Kudo D, Morohashi H, Kakizaki I, Kon A, Takagaki K, Sasaki M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother Pharmacol. 2006;57:165–70. doi: 10.1007/s00280-005-0016-5. [DOI] [PubMed] [Google Scholar]
- 21.Benitez A, Yates TJ, Lopez LE, Cerwinka WH, Bakkar A, Lokeshwar VB. Targeting hyaluronidase for cancer therapy: antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res. 2011;71:4085–95. doi: 10.1158/0008-5472.CAN-10-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lokeshwar VB, Obek C, Pham HT, Wei D, Young MJ, Duncan RC, Soloway MS, Block NL. Urinary hyaluronic acid and hyaluronidase: markers for bladder cancer detection and evaluation of grade. J Urol. 2000;163:348–56. doi: 10.1016/s0022-5347(05)68050-0. [DOI] [PubMed] [Google Scholar]
- 23.Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–41. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- 24.Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–83. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 25.Nexavar-INN-Sorafenib @ EMEA 2006
- 26.Hilger RA, Richly H, Grubert M, Kredtke S, Thyssen D, Eberhardt W, Hense J, Schuler M, Scheulen ME. Pharmacokinetics of sorafenib in patients with renal impairment undergoing hemodialysis. Int J Clin Pharmacol Ther. 2009;47:61–4. doi: 10.5414/cpp47061. [DOI] [PubMed] [Google Scholar]
- 27.Kultti A, Pasonen-Seppänen S, Jauhiainen M, Rilla KJ, Kärnä R, Pyöriä E, Tammi RH, Tammi MI. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–23. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, Law PY, Hebbel RP. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999;247:495–504. doi: 10.1006/excr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 29.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 30.Lokeshwar VB, Selzer MG. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel, and vein-derived human endothelial cells. J Biol Chem. 2000;275:27641–9. doi: 10.1074/jbc.M003084200. [DOI] [PubMed] [Google Scholar]